Abstract
Hematopoietic stem cell transplantation (HSCT) is an effective treatment for patients with refractory lymphomas. Nucleoside analogues (NA) and DNA alkylating agents are efficacious in treating hematologic malignancies. To design an efficacious and more economical pre-transplant regimen for lymphoma patients, we analyzed the cytotoxicity of cladribine (Clad), gemcitabine (Gem), busulfan (Bu) and suberoylanilide hydroxamic acid (SAHA) in lymphoma cell lines. J45.01 and U937 lymphoma cell lines were exposed to drugs, alone or in combination, for 48 hrs and analyzed by the MTT and Annexin V assays, Western blotting and flow cytometry. Using the IC5–10 values of the drugs, [Clad+Gem+Bu] combination inhibited the proliferation of both cell lines to ~55%–60%. Addition of SAHA to this combination further decreased proliferation to ~30%. Exposure to [Clad+Gem+Bu+SAHA] combination activated the DNA damage response and ATM-CHK2 pathway, modified histones, decreased the mitochondrial membrane potential which caused leakage of apoptosis-inducing factors, and activated apoptosis. Pre-treatment of cells with a pan-caspase inhibitor Z-VAD-FMK blocked the phosphorylation of histone 2AX and cleavage of PARP-1 and caspases. The [Clad+Gem+Bu+SAHA] combination provides synergistic cytotoxicity in lymphoma cell lines. Our results may be used as a basis for using this combination as a pre-transplant conditioning regimen in a clinical trial for lymphoma patients undergoing hematopoietic stem cell transplantation, replacing the more expensive nucleoside analog clofarabine.
Introduction
Hematopoietic stem cell transplantation (HSCT) is a potential curative approach for most hematologic diseases including lymphoma [Salit et al., 2010; Nieto et al., 2015]. However, relapse is still a major concern. To circumvent this problem, high intensity myeloablative conditioning regimens have been used but they were associated with high treatment-related morbidity and mortality [Loberiza et al., 2005; Cornelissen et al., 2012] especially in elderly patients [de Lima et al., 2004; Sorror et al., 2007]. Another problem with HSCT involves the high cost of conditioning agents. To alleviate these problems, more efficacious and economical regimens need to be developed.
Our previous studies have confirmed the anti-tumor efficacy of dual nucleoside analogs (eg. gemcitabine, fludarabine and clofarabine) combined with busulfan (Bu) both in pre-clinical and clinical settings [Andersson et al., 2011; Valdez et al., 2012a; Valdez et al., 2012b]. However, clofarabine (Clo) widespread use is hampered by its excessive cost, especially in countries outside of the United States. In order to find an alternative, efficacious and safe, yet relatively more economical combination, we designed a study in which Clo was replaced with cladribine (Clad) and combined it with gemcitabine (Gem) and Bu.
To further improve the efficacy of this combination, vorinostat (suberanilohydroxamic acid, SAHA), a deacetylase inhibitor (DACi). DAC inhibitors act on chromatin remodeling via histone modifications. Acetylation of lysine residues in the histone tails interferes with their binding to DNA, allowing a more open, or relaxed chromatin configuration that favors gene expression including tumor suppressive genes [Mersfelder and Parthun, 2006; Rosato and Grant, 2005]. DACi can also induce terminal differentiation, apoptosis and cell cycle arrest [Marks et al., 2000; Bolden et al., 2006] SAHA was the first DACi approved by the U.S. Food and Drug Administration (FDA) for the treatment of cutaneous T cell lymphoma [Marks and Breslow, 2007].
In the present study, we report the synergistic cytotoxicity of relatively low concentrations of [Clad+Gem+Bu] combination in lymphoma cell lines. Addition of SAHA to this combination further enhanced its cytoxicity. Pre-exposure of lymphoma cells to SAHA prior to exposure to either [Clad+Gem+Bu] or [Clad+Gem+Bu+SAHA] combination was found to be more efficacious than concurrent exposures, showing the importance of sequential drug exposure.
Materials and methods
Reagents
Busulfan (Sigma-Aldrich, St. Louis, MO) was dissolved in dimethyl sulfoxide (DMSO). Clad and Gem (Sigma-Aldrich) were dissolved in phosphate-buffed saline (PBS), and SAHA (Cayman Chemical Co., Ann Arbor, MI) was dissolved in ethanol.
Cell Culture
J45.01 and U937 lymphoma cell lines were grown in RPMI 1640 medium (Mediatech, Manassas, VA) supplemented with 10% fetal bovine serum (FBS) (Atlanta Biologicals, Inc., Lawrenceville, GA) and 100 U/mL of penicillin and 100 μg/mL of streptomycin. All cells were maintained in a humidified incubator at 37°C in an atmosphere of 5% CO2 in air.
Cytotoxicity and apoptosis assay
Cells (5x105 cells/ml) were exposed to Clad, Gem, Bu and SAHA alone, or in combination, for 48 h. Cells were analyzed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay [Mosmann, 1983]. All cytotoxicity data are expressed as the average ± SD of at least three independent experiments.
Cell death by apoptosis following a 48-h drug exposure was determined by flow cytometric measurements of phosphatidylserine externalization [Martin et al.,1995] with Annexin-V-FLUOS (Roche Diagnostics, Indianapolis, IN), and 7-aminoactinomycin D (BD Biosciences, San Jose, CA), using a MUSE Cell Analyzer (EMD Millipore, Billerica, MA).
Preparation of cytosolic and mitochondrial extracts
Control and drug-treated cells were collected and washed with ice-cold PBS, resuspended in buffer A (10 mM HEPES (pH 7.6), 10 mM KCl, 100 μM EDTA, 100 μM EGTA, 1 mM DTT, 500 μM phenylmethylsulfonyl fluoride (PMSF) with proteinase inhibitors) and incubated on ice for 30 min. Cell disruption was completed by passing the cells 20 times in a homogenizer (Kontes Glass Co. Vineland, NJ) on ice. Cell homogenates were centrifuged at 800 x g for 5 min at 4°C to separate nuclei from the mitochondrial and cytosolic fractions. The supernatant was further subjected to 8 min of centrifugation at 12,500 × g at 4°C to pellet mitochondria and the resulting supernatant was saved as the cytosolic fraction. The pelleted mitochondria were washed with ice-cold buffer A, and then resuspended in ice-cold lysis buffer C (10 mM HEPES (pH 7.6), 300 mM KCl, 1 mM EDTA, 0.5% Triton X-100, 5 % glycerol, 1 mM DTT with proteinase inhibitors).
Western blot analysis
Cells were incubated with drug(s) at 37°C for 48 h, centrifuged, washed with ice-cold PBS, lysed with cell lysis buffer, and protein concentrations were determined using a BCA (bicinchoninic acid) Protein Assay kit (Thermo Fisher Scientific, Rockford, IL). Western blot analysis was done by resolving proteins in SDS-PAGE gels, transferring onto nitrocellulose membranes (Bio-Rad, Hercules, CA), and probing with primary antibodies. Signals were detected using Immobilon Western Chemiluminescent HRP (horseradish peroxidase) substrate (Millipore, Bedford, MA).
Analysis of mitochondrial transmembrane potential
Changes in the mitochondrial transmembrane potential (ΔΨm) were measured using a JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbo cyanine iodide) mitochondrial membrane potential detection kit (Cayman Chemical Co., Ann Arbor, MI). Cells were exposed to 1 μM valinomycin for 2 h prior to incubation with the JC-1 reagent as a positive control. Cells were incubated with the mitochondrial membrane potential-sensitive fluorescent dye JC-1 for 20 min at 37°C, and then immediately analyzed with flow cytometry, using the 530 nm (FL-1 channel, green) and 585 nm (FL-2 channel, red) band-pass filters simultaneously. Healthy cells with functional mitochondria and high ΔΨm exhibit red fluorescence while apoptotic or dying cells with collapsed mitochondria or low ΔΨm show green fluorescence.
Statistical analyses
All experiments were conducted at least 3 times, and results are expressed as mean ± SD. P values (Student t test) less than 0.05 are considered statistically significant.
Results
Combination of Clad, Gem and Bu provides synergistic toxicity on lymphoma cell lines and SAHA further enhances this effect
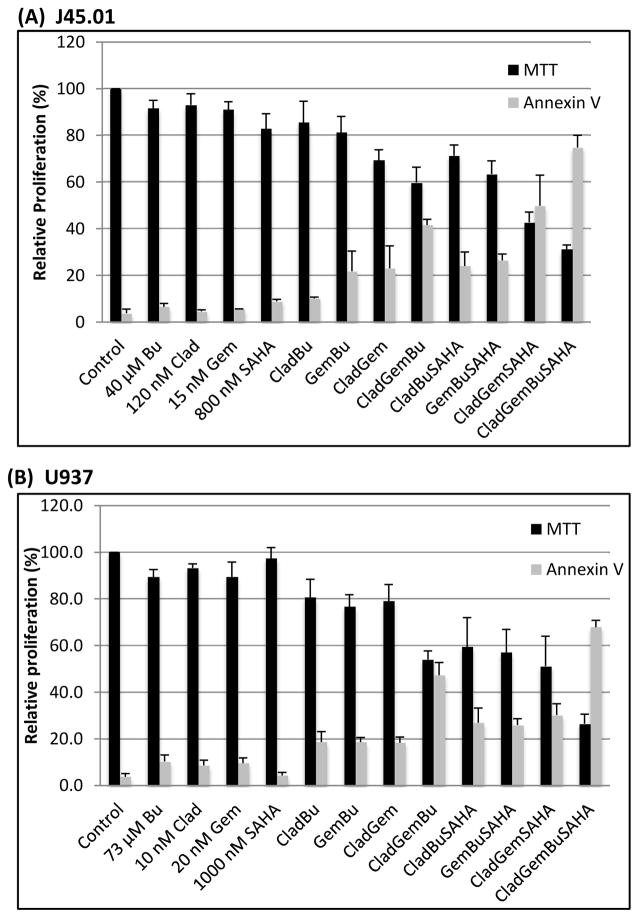
Cytotoxicity was determined with the MTT assay. Two lymphoma cell lines were exposed to single agents of Clad, Gem, Bu and SAHA or their combinations for 48 h. Exposure of J45.01 cells to 120 nM Clad, 15 nM Gem or 40 μM Bu (equivalent to their IC5–10 values showed minimal effects on cell proliferation (Figure 1A). The combination of both nucleoside analogs (NAs) with Bu resulted in ~40% inhibition of proliferation relative to control. Addition of 800 nM SAHA to the combination of [Clad+Gem+Bu] led to further inhibition of proliferation to ~70% of control, indicating strong synergistic cytotoxicity.
Figure 1.
Effects of Clad, Gem, Bu, SAHA and their combinations on cell proliferation and programmed cell death. J45.01 (A) and U937 (B) cells were exposed to drugs alone or their combinations. Results are expressed as the mean±SEM of at least 3 independent experiments. Clad: cladribine; Gem: gemcitabine; Bu: busulfan; SAHA: suberoylanilide hydroxamic acid
Similar effects were observed in another lymphoma cell line; exposure of U937 cells to 10 nM Clad, 20 nM Gem or 73 μM Bu only yielded 10% inhibition of proliferation relative to control. The combination of two NAs with Bu resulted in ~45% inhibition of proliferation. When 1000 nM SAHA was added to [Clad+Gem+Bu] combination, the inhibition increased to ~74%.
The extent of apoptosis was measured by the Annexin V assay and the results are summarized in Figure 1. Approximately 75% and 68% Ann V-positive J45.01 and U937 cells, respectively, were observed in the presence of [Clad+Gem+Bu+SAHA] combination. These results correlate with the MTT data, further suggesting that SAHA made both lymphoma cell lines more sensitive to [Clad+Gem+Bu] combination.
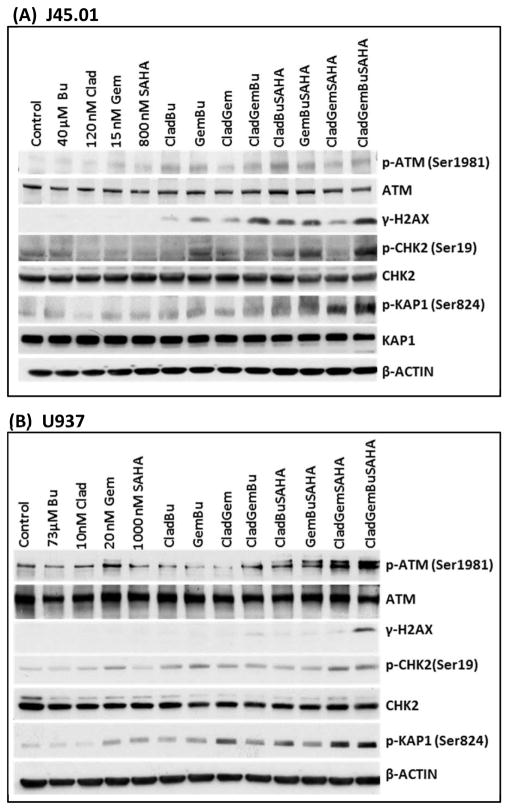
The 4-drug combination activates the DNA-damage signaling pathway
Our previous studies revealed that the combination of Gem and Bu with another nucleoside analogue clofarabine (Clo) activated the ATM pathway in J45.01 cell line [Valdez et al., 2012a]. We hypothesized that exposure of J45.01 and U937 cells to [Clad+Gem+Bu+SAHA] combination would show similar results. As shown in Figure 2, ATM phosphorylation at Ser1981 increased in cells exposed to various drug combinations, and more markedly in U937 cells exposed to [Clad+Gem+SAHA] and [Clad+Gem+Bu+SAHA]. One of the earliest substrates of the ATM kinase is the histone 2AX, which was highly phosphorylated in cells exposed to the 4-drug combination. The observed phosphorylation of CHK2 at Ser19 is another evidence for activation of the ATM pathway [Rogakou et al., 1998; Kastan and Lim, 2000]. Increased phosphorylation of KRAB-associated protein-1 (KAP-1) at Ser824 was also observed in cells exposed to [Clad+Gem+Bu+SAHA], suggesting sustained activity of ATM at an unrepaired site of DNA damage [Ziv et al., 2006]. All these results indicate drug-mediated activation of the DNA-damage response.
Figure 2.
Drug combinations activate the DNA-damage response and induce apoptosis in J45.01 (A) and U937 (B). Cells were exposed to Clad, Gem, Bu and SAHA alone, or in combination, for 48 h and changes in the level and modification of proteins involved in the ATM pathway were analyzed by Western blotting. Clad: cladribine; Gem: gemcitabine; Bu: busulfan; SAHA: suberoylanilide hydroxamic acid
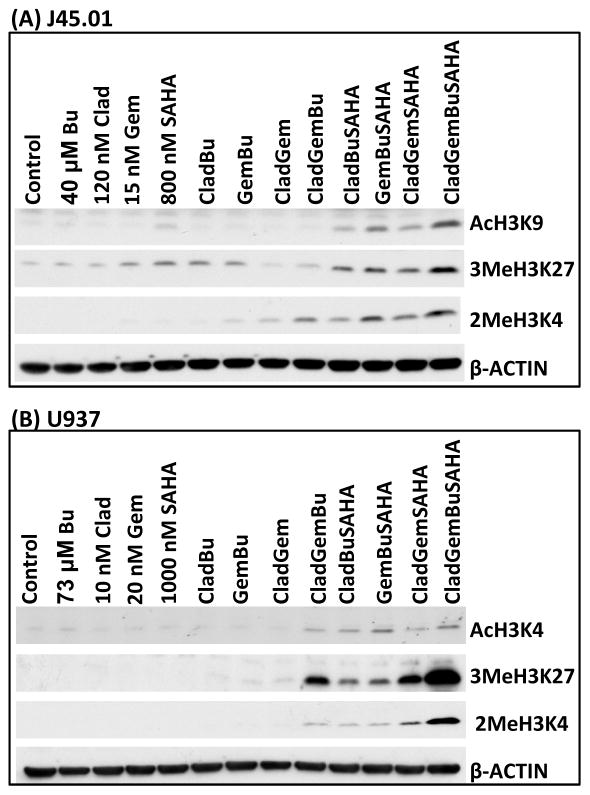
Drug combinations increase histone modifications
Histone modifications relax chromatin and render DNA more accessible to chemotherapeutic agents. To test if Clad, Gem, Bu and SAHA, or their combinations, mediate histone modifications, J45.01 and U937 cells were exposed to drugs for 48 h. As shown in Figure 3, a marked increase in the acetylation of histone 3 at Lys 9 was observed in J45.01 cells exposed to [Clad+Gem+Bu+SAHA]. Methylations of histone 3 at Lys 4 and Lys 27 dramatically increased in cells exposed to the 4-drug combination. Similar results were obtained using U937 cells, including acetylation of histone 3 at Lys 4 (Figure 3B). These results suggest drug-mediated histone modifications in lymphoma cell lines, which may result in chromatin remodeling, greater exposure of genomic DNA to alkylation, and exacerbation of DNA damage.
Figure 3.
Histone modifications in lymphoma cells. J45.01 (A) and U937 (B) cells were exposed to Clad, Gem, Bu and SAHA alone, or in combination, for 48 h and changes in levels of acetylation and methylation of histone 3 were determined by Western blotting. Clad: cladribine; Gem: gemcitabine; Bu: busulfan; SAHA: suberoylanilide hydroxamic acid
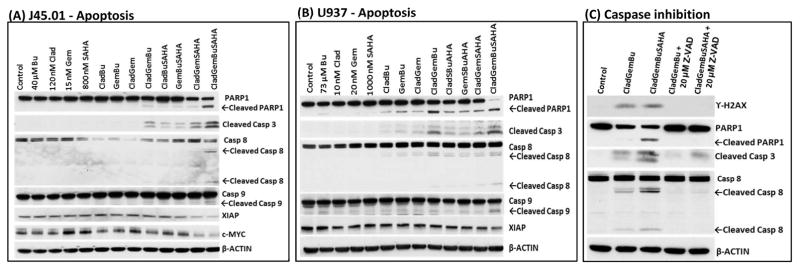
[Clad+Gem+Bu+SAHA] induces apoptosis
When cells are overwhelmed by damaged DNA, they commit to apoptosis. Since the results of Ann V assays suggest drug-mediated apoptosis (Figure 1), we sought to find additional evidence of programmed cell death. Analysis by Western blotting shows increased cleavage of poly-ADP ribose polymerase 1 (PARP1) and caspases 3, 8, and 9 (Figure 4A and 4B), suggesting activation of apoptosis in cells exposed to [Clad+Gem+Bu+SAHA]. These results correlate with decreased levels of anti-apoptosis protein XIAP and pro-survival c-MYC (Figure 4A and 4B).
Figure 4.
Induction of apoptosis in J45.01 (A) and U937 (B) cell lines. Cells were exposed to single drug, or in combination, for 48 h and analyzed by Western blotting. (C) J45.01 cells were pre-exposed to 20 μM Z-VAD-FMK for 2 h prior to exposure to the indicated drug combinations, then analyzed by Western blotting after 48 h. Clad: cladribine; Gem: gemcitabine; Bu: busulfan; SAHA: suberoylanilide hydroxamic acid; Casp: caspase
To further prove the relevance of caspases in the observed drug-mediated cell death, J45.01 cells were pre-exposed to pan caspase inhibitor Z-VAD-FMK (carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone) for 2 h prior to drug exposure. As shown in Figure 4C, Z-VAD-FMK almost completely abrogated the phosphorylation of histone 2AX, cleavage of PARP1, and cleavage of caspases 3 and 8, suggesting caspase-dependent cell death by the [Clad+Gem+Bu+SAHA] combination.
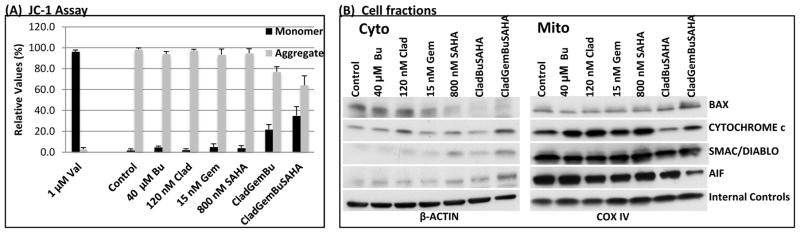
[Clad+Gem+Bu+SAHA] combination increases mitochondrial membrane permeability
Anti-cancer drugs are known to trigger cell apoptosis through intrinsic and extrinsic pathways. The intrinsic pathway may emanate from decreased mitochondrial membrane potential (ΔΨm), which results in its altered permeability [Green and Reed, 1998; Ly et al., 2003] and release of pro-apoptotic factors into the cytoplasm. Therefore, we investigated changes in the ΔΨm in lymphoma cells exposed to various drug combinations. Indeed, [Clad+Gem+Bu] decreased ΔΨm in J45.01 cells by ~23%, and [Clad+Gem+Bu+SAHA] decreased it by ~36% as shown by increased level of the monomeric form of the JC-1 detection reagent (Figure 5A). To further test possible leakage of the mitochondrial membrane, cytosolic and mitochondrial extracts were prepared and analyzed by Western blotting. BAX, which is known to translocate to the mitochondrial membrane in response to apoptotic stimuli, increased in the mitochondrial extract in cells exposed to the 4-drug combination and decreased in the cytoplasmic extract. The levels of pro-apoptotic proteins CYTOCHROME c, SMAC/DIABLO and AIF increased in the cytoplasm with a concomitant decrease in the mitochondria (Figure 5B), suggesting the release of these death factors that would in turn trigger downstream events in the apoptotic cascade.
Figure 5.
Changes in mitochondrial membrane potential in J45.01 cells (A) and analysis of fractionated cell extracts (B). Cells were exposed to Clad, Gem, Bu and SAHA alone, or in combination, for 48 h and analyzed by the JC-1 assay (A) or fractionated and analyzed by Western blotting (B). In (A) results are expressed as percentage of change of calculated ratio of red/green fluorescence relative to control and as the mean±SEM of three independent experiments. Valinomycin (Val) was used as a positive control. In (B) β-ACTIN, and COX IV were used as loading controls for the cytoplasmic (Cyto) and mitochondrial (Mito) fractions, respectively. Clad: cladribine; Gem: gemcitabine; Bu: busulfan; SAHA: suberoylanilide hydroxamic acid
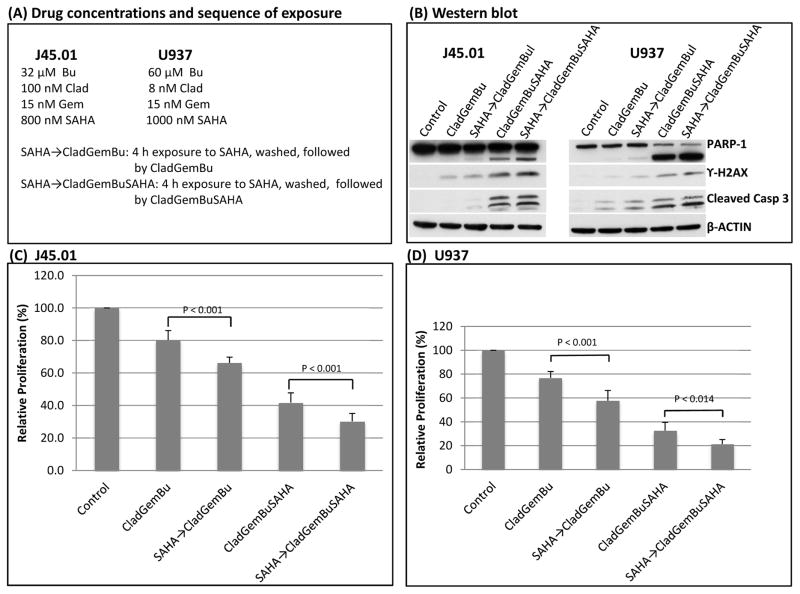
Pre-exposure to SAHA increases the cytotoxicity of [Clad+Gem+Bu] and [Clad+Gem+Bu+SAHA] combinations
To explore if pre-exposure to SAHA would further increase the cytotoxicity of the [Clad+Gem+Bu] and [Clad+Gem+Bu+SAHA] combinations, cells were pre-exposed to SAHA for 4 h, washed with growth medium, then re-suspended in fresh medium containing either [Clad+Gem+Bu] or [Clad+Gem+Bu+SAHA] (Figure 6A). Cells were then analyzed after 44 h of additional exposure for changes in the status of proteins involved in DNA-damage response and apoptosis. Pre-exposure to SAHA showed slight increase in the level of phosphorylated H2AX and cleaved PARP1 and caspase 3 (Figure 6B). More significant differences were observed when cell proliferation was analyzed. As shown in Figure 6C, concurrent exposure of J45.01 cells to [Clad+Gem+Bu] resulted in ~80% proliferation but pre-exposure to SAHA followed by the [Clad+Gem+Bu] combination significantly decreased proliferation to ~65% (P<0.001). Concurrent exposure to [Clad+Gem+Bu+SAHA] resulted in ~42% proliferation which decreased to ~30% when cells were pre-exposed to SAHA. Similar results were obtained using another lymphoma cell line U937 (Figure 6D). These results suggest the relevance of exposing the cells to an epigenetic modifier prior to, and concomitant with, nucleoside analogs and DNA alkylators.
Figure 6.
Pre-exposure of lymphoma cells to SAHA enhances toxicity of [Clad+Gem+Bu] and [Clad+Gem+Bu+SAHA] combinations. Cells were exposed to SAHA for 4 h, washed, then exposed to [Clad+Gem+Bu] or [Clad+Gem+Bu+SAHA] combination using the indicated drug concentrations (A). Changes in the level of proteins involved in DNA-damage response and apoptosis were analyzed by Western blotting (B). Cell proliferation was determined by the MTT assay (C and D). Clad: cladribine; Gem: gemcitabine; Bu: busulfan; SAHA: suberoylanilide hydroxamic acid; Casp: caspase
Discussion
The safety and efficacy of various combinations of nucleoside analogs with alkylating agents as pre-transplant conditioning regimens are well established. Busulfan, as a prototype DNA alkylator, has been used to treat hematologic neoplasm for decades. It forms DNA crosslinks, which lead to apoptosis [Andersson et al., 2008; Valdez and Andersson, 2010]. Nucleoside analogs, on the other hand, inhibit ribonucleotide reductase, deplete cellular deoxynucleotides, get incorporated into DNA strands, and inhibit DNA synthesis and repair [Parker 1991; Plunkett and Gandhi, 2001]. In the present study, we showed strong synergistic cytotoxicity of low concentrations of Clad, Gem, Bu and SAHA in lymphoma cell lines. The combination of [Clad+Gem+Bu+SAHA] resulted in the activation of the ATM pathway and DNA-damage response, histone modifications, down-regulation of anti-apoptotic proteins, and decrease in mitochondrial membrane potential which caused leakage of apoptosis-inducing factors such as CYTOCHROME c, SMAC/DIABLO and AIF.
The [Clad+Gem+Bu+SAHA] combination provoked profound DNA-damage response and triggered a series of biochemical events leading to programmed cell death. Phosphorylation of ATM, known to be mediated by DNA damage, correlated with phosphorylation of its downstream targets such as H2AX, CHK2, and KAP1. Changes in the status of these protein modifications closely parallel the observed degree of drug toxicity, with the strongest response in [Clad+Gem+Bu+SAHA] combination.
Apoptotic stimuli are known to change the conformation of BAX. This pro-apoptotic protein normally localizes to the cytoplasm or loosely bound to the mitochondrial surface in non-apoptotic cells [Wolter et al., 1997], but it is inserted in the outer mitochondrial membrane after exposure of cells to stress-inducing agents and increases the permeability of the mitochondrial membrane [Balakrishnan et al., 2013]. Indeed, exposure of lymphoma cell lines to [Clad+Gem+Bu+SAHA] increased the level of BAX in the mitochondrial extract, which was consistent with increased CYTOCHROME c, SMAC/DIABLO and AIF in the cytosol (Figure 5B). These death factors are known to activate the caspase system, ultimately resulting in chromosome condensation and DNA fragmentation, which are indicators of programmed cell death [Bratton et al., 2002].
The synergism contributed by SAHA most likely relates to its DAC inhibition activity. The increased levels of acetylation of H3K9, di-methylation of H3K4 and tri-methylation of H3K27 correlated with the toxicity of the 4-drug combination. These histone modifications are known to alter the transcription of specific genes. For example, H3K27 tri-methylation is a characteristic of polycomb group target genes and is associated with transcriptional repression [Barski et al., 2007; Ferrari et al., 2014]. Less methylation at H3K27 is often correlated with up-regulation of oncogenes and repression of tumor suppressor genes in lymphoid neoplasms [McCabe et al., 2012; Ntziachristos et al., 2014]. The observed increased in 3MeH3K27 in our study indicated that it might be one of the mechanisms that enhanced the effect of [Clad+Gem+Bu+SAHA] combinations. SAHA is also known to induce replication-mediated DNA damage and stall or slow down replication forks, which inadvertently results in recombination and genomic instability [Conti et al., 2010]. SAHA may allow nucleoside analogs and Bu to access the DNA more easily and enhance DNA damage. In fact, pre-exposure to SAHA may provide epigenetic changes that enhance the cytotoxicity of nucleoside analogs and DNA alkylators as suggested by our results in Figure 6.
In summary, our findings suggest that the cytotoxicity of [Clad+Gem+Bu+SAHA] combination in lymphoma cell lines could be attributed to chromatin relaxation mediated by Clad, Gem and SAHA, thereby increasing the susceptibility of genomic DNA to Bu alkylation. Our study provides a strong basis for considering Clad as a valid alternative to Clo and for enhancing efficacy with the addition of epigenetic modifiers in the pretransplant conditioning regimen in future clinical trials in patients with lymphoma. Moreover, [Gem+Bu+SAHA] in combination with DNA alkylating agent melphalan was found to be a safe, highly active pre-transplant conditioning regimen for refractory/relapsed lymphoma patients [Nieto et al., 2015], and inclusion of cladribine with busulfan and antithymocyte globulin as a preparative regimen for allogeneic transplantation for acute leukemia/lymphoma was well tolerated and induced early complete donor chimerism [Saito et al., 2002]. Based on these previous clinical trials, [Clad+Gem+Bu+SAHA] combination is expected to be safe for lymphoma patients undergoing HSCT. The substitution of Clo with Clad will also mean a significant reduction of cost for the overall procedure. In a more general setting, our in vitro data also suggest the possibility of using the [Clad+Gem+Bu+SAHA] combination to enhance conventional salvage regimens for patients with chemotherapy refractory lymphoma.
Highlights.
Cladribine, gemcitabine, busulfan and SAHA exert synergistic effects in lymphoma cell lines
This combination activates DNA damage response and apoptosis
Pre-exposure to SAHA improves drug efficacy.
This combination may be used as basis for clinical trial for stem cell transplantation for lymphoma patients.
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health (CCSG Core CA16672), and the Stephen L. and Lavinia Boyd Fund for Leukemia Research, and by funds donated by grateful patients.
Footnotes
Disclosure statement
The authors declare no financial conflict(s) of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson BS, de Lima M, Thall PF, et al. Once daily i.v. busulfan and fludarabine (i.v. Bu-Flu) compares favorably with i.v. busulfan and cyclophosphamide (i.v. BuCy2) as pretransplant conditioning therapy in AML/MDS. Biol Blood Marrow Transplant. 2008;14:672–684. doi: 10.1016/j.bbmt.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson BS, Valdez BC, de Lima M, et al. Clofarabine +/− fludarabine with once daily i.v. busulfan as pretransplant conditioning therapy for advanced myeloid leukemia and MDS. Biol Blood Marrow Transplant. 2011;17:893–900. doi: 10.1016/j.bbmt.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan K, Aggarwal S, Wierda W, Gandhi V. Bax and Bak are required for apogossypolone, a BH3-mimetic, induced apoptosis in chronic lymphocytic leukemia cells. Leuk Lymphoma. 2013;54:1097–1100. doi: 10.3109/10428194.2012.718344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- Barski A, Cuddapah S, Cui K, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Conti C1, Leo E, Eichler GS, et al. Inhibition of histone deacetylase in cancer cells slows down replication forks, activates dormant origins, and induces DNA damage. Cancer Res. 2010;70:4470–4480. doi: 10.1158/0008-5472.CAN-09-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratton SB, Lewis J, Butterworth M, Duckett CS, Cohen GM. XIAP inhibition of caspase-3 preserves its association with the Apaf-1 apoptosome and prevents CD95- and Bax-induced apoptosis. Cell Death Differ. 2002;9:881–892. doi: 10.1038/sj.cdd.4401069. [DOI] [PubMed] [Google Scholar]
- Cornelissen JJ, Gratwohl A, Schlenk RF, Sierra J, et al. The European LeukemiaNet AML Working Party consensus statement on allogeneic HSCT for patients with AML in remission: an integrated-risk adapted approach. Nat Rev Clin Oncol. 2012;9:579–590. doi: 10.1038/nrclinonc.2012.150. [DOI] [PubMed] [Google Scholar]
- de Lima M, Anagnostopoulos A, Munsell M, et al. Nonablative versus reduced-intensity conditioning regimens in the treatment of acute myeloid leukemia and high-risk myelodysplastic syndrome: dose is relevant for long-term disease control after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104:865–872. doi: 10.1182/blood-2003-11-3750. [DOI] [PubMed] [Google Scholar]
- Ferrari KJ, Scelfo A, Jammula S, et al. Polycomb-dependent H3K27me1 and H3K27me2 regulate active transcription and enhancer fidelity. Mol Cell. 2014;53:49–62. doi: 10.1016/j.molcel.2013.10.030. [DOI] [PubMed] [Google Scholar]
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Kastan MB, Lim DS. The many substrates and functions of ATM. Nat Rev Mol Cell Biol. 2000;1:179–186. doi: 10.1038/35043058. [DOI] [PubMed] [Google Scholar]
- Loberiza FR, Jr, Zhang MJ, Lee SJ, et al. Association of transplant center and physician factors on mortality after hematopoietic stem cell transplantation in the United States. Blood. 2005;105:2979–2987. doi: 10.1182/blood-2004-10-3863. [DOI] [PubMed] [Google Scholar]
- Ly JD, Grubb DR, Lawen A. The mitochondrial membrane potential (deltapsi(m)) in apoptosis; an update. Apoptosis. 2003;8:115–128. doi: 10.1023/a:1022945107762. [DOI] [PubMed] [Google Scholar]
- Marks PA, Breslow R. Dimethyl sulfoxide to vorinostat: development of this histone deacetylase inhibitor as an anticancer drug. Nat Biotechnol. 2007;25:84–90. doi: 10.1038/nbt1272. [DOI] [PubMed] [Google Scholar]
- Marks PA, Richon VM, Rifkind RA. Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells. J Natl Cancer Inst. 2000;92:1210–1216. doi: 10.1093/jnci/92.15.1210. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Reutelingsperger CP, McGahon AJ, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe MT, Graves AP, Ganji G, et al. Mutation of A677 in histone methyltransferase EZH2 in human B-cell lymphoma promotes hypertrimethylation of histone H3 on lysine 27 (H3K27) Proc Natl Acad Sci U S A. 2012;109:2989–2994. doi: 10.1073/pnas.1116418109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mersfelder EL, Parthun MR. The tale beyond the tail: histone core domain modifications and the regulation of chromatin structure. Nucleic Acids Res. 2006;34:2653–2662. doi: 10.1093/nar/gkl338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nieto Y, Valdez BC, Thall PF, et al. Vorinostat combined with high-dose gemcitabine, busulfan, and melphalan with autologous stem cell transplantation in patients with refractory lymphomas. Biol Blood Marrow Transplant. 2015;21:1914–1920. doi: 10.1016/j.bbmt.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntziachristos P, Tsirigos A, Welstead GG, et al. Contrasting roles of histone 3 lysine 27 demethylases in acute lymphoblastic leukaemia. Nature. 2014;514:513–517. doi: 10.1038/nature13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker WB, Shaddix SC, Chang CH, et al. Effects of 2-chloro-9-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl)adenine on K562 cellular metabolism and the inhibition of human ribonucleotide reductase and DNA polymerases by its 5′-triphosphate. Cancer Res. 1991;51:2386–2394. [PubMed] [Google Scholar]
- Plunkett W, Gandhi V. Purine and pyrimidine nucleoside analogs. Cancer Chemother Biol Response Modif. 2001;19:21–45. [PubMed] [Google Scholar]
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- Rosato RR, Grant S. Histone deacetylase inhibitors: insights into mechanisms of lethality. Expert Opin Ther Targets. 2005;9:809–824. doi: 10.1517/14728222.9.4.809. [DOI] [PubMed] [Google Scholar]
- Saito T, Kanda Y, Kami M, et al. Therapeutic potential of a reduced-intensity preparative regimen for allogeneic transplantation with cladribine, busulfan, and antithymocyte globulin against advanced/refractory acute leukemia/lymphoma. Clin Cancer Res. 2002;8:1014–1020. [PubMed] [Google Scholar]
- Salit RB, Bishop MR, Pavletic SZ. Allogeneic hematopoietic stem cell transplantation: does it have a place in treating Hodgkin lymphoma? Curr Hematol Malig Rep. 2010;5:229–238. doi: 10.1007/s11899-010-0065-7. [DOI] [PubMed] [Google Scholar]
- Sorror ML, Sandmaier BM, Storer BE, et al. Comorbidity and disease status based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoietic cell transplantation. J Clin Oncol. 2007;25:4246–4254. doi: 10.1200/JCO.2006.09.7865. [DOI] [PubMed] [Google Scholar]
- Valdez BC, Andersson BS. Interstrand crosslink inducing agents in pretransplant conditioning therapy for hematologic malignancies. Environ Mol Mutagen. 2010;51:659–668. doi: 10.1002/em.20603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez BC, Murray D, Nieto Y, et al. Synergistic cytotoxicity of the DNA alkylating agent busulfan, nucleoside analogs and suberoylanilide hydroxamic acid in lymphoma cell lines. Leuk Lymphoma. 2012a;53:973–981. doi: 10.3109/10428194.2011.634043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez BC, Nieto Y, Murray D, et al. Epigenetic modifiers enhance the synergistic cytotoxicity of combined nucleoside analog-DNA alkylating agents in lymphoma cell lines. Exp Hematol. 2012b;40:800–810. doi: 10.1016/j.exphem.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y, Bielopolski D, Galanty Y, et al. Chromatin relaxation in response to DNA double-strand breaks is modulated by a novel ATM- and KAP-1 dependent pathway. Nat Cell Biol. 2006;8:870–876. doi: 10.1038/ncb1446. [DOI] [PubMed] [Google Scholar]