Abstract
One of the factors that can result in musculoskeletal injuries, and time off work, is exposure to repetitive motion. The goal of this study was to determine if skeletal muscle injury induced by exposure to injurious stretch-shortening cycles (iSSCs), resulted in hyperalgesia in the hind limb and changes in calcitonin-gene related peptide (CGRP) immunolabeling in the dorsal root ganglia (DRG) in young and old male rats.
Methods
Young (3 mo) and old (30 mo) male Fisher 344 × BN F1 rats were anesthetized with isoflurane and the left hind limbs were exposed to 15 sets of 10 SSCs. Control animals were exposed to a single bout of SSCs of equal intensity. Sensitivity to mechanical stimulation was assessed using von Frey filaments prior to beginning the experiment, and on days 2 and 9 following exposure to iSSCs. Rats were euthanized one, 3 or 10 days after the exposure. The ipsilateral DRG were dissected from the L4-5 region of the spine, along with the left tibialis anterior (LTA) muscle.
Results
Rats exposed to iSSCs were more sensitive to mechanical stimulation than control rats 2 days after the exposure, and showed a reduction in peak force 3 days after exposure. Changes in sensitivity to pressure were not associated with increases in CGRP labeling in the DRG at 3 days. However, 9 days after exposure to iSSCs, old rats still displayed an increased sensitivity to mechanical stimulation, and this hyperalgesia was associated with an increase in CGRP immunolabeling in the DRG. Young rats exposed to iSSC did not display a change in CGRP immunolabeling and sensitivity to mechanical stimulation returned to control levels at 10 days.
Conclusions
These findings suggest that hyperalgesia seen shortly after exposure to iSSC is not influenced by CGRP levels. However, in cases where recovery from injury may be slower, as it is in older rats, CGRP may contribute to the maintenance of hyperalgesia.
Introduction
Exposure to repetitive muscle contractions of high velocity, either in occupational settings or as a result of exercise, can result in a strain injury to the muscle. If not alleviated this injury can result in the development of chronic pain. According to the Bureau of Labor Statistics (BLS), approximately 33% of all non-fatal injuries that occur in the workplace are the result of injuries and strains to the musculoskeletal system (2014). Most of these injuries are due to overexertion and the repetitive use of the upper-limbs, or overexertion of the lower back. Although workers usually regain function following injury, time away from work is often extended in workers with musculoskeletal disorders (MSDs) because of persisting pain that may be present even after the injury has appeared to heal. This pain may be more prominent in older workers (i.e., >45 years of age), and keep them out of work for longer periods (BLS 2014).
Animal models have been developed to determine how various work-related exposure factors (i.e., velocity, duty cycle, repetitive motion) contribute to the risk of developing an MSD (Baker and others 2007; Cutlip and others 2007a; Kehl and others 2000). One of the models that has been used and characterized involves exposing the hind-limbs of rats and mice to repeated bouts of injurious eccentric contractions or stretch-shortening contractions, or more physiologically-relevant injurious stretch-shortening contractions (iSSCs; reciprocal eccentric/concentric contractions; (Baker and others 2006; Brooks and Faulkner 1990; Brooks and Faulkner 1996; Cutlip and others 2004; Cutlip and others 1997). When the repetition number is high (e.g., ≥ 70 repetitions) there is injury to the muscle that is characterized by an increase in edema, inflammation and myofiber degeneration 2-3 days after the exposure (Baker and others 2006). As the muscle heals and performance recovers, inflammation is reduced and there is an increase in central nuclei within regenerating myofibers, and the presence of satellite cells (Krajnak and others 2006). The studies describing the effects of iSSCs were performed in Sprague Dawley rats. A companion study (Rader and others 2015) analyzed the effects of iSSCs in Fisher 344 × Brown Norway F1 rats, a model commonly used to study the effects of aging, to determine if exposure also resulted in anatomical, physiological and molecular changes associated with muscle injury and dysfunction. This study found that 3 days after exposure to iSSCs, there was a reduction in force production in both young and old rats. By 10 days after the exposure, muscle forces had returned to baseline levels in young rats, but there was still a force deficit in old rats. In addition, changes in gene transcription were different between young and old rats (Rader and others 2015). These age-related changes may be due to a delayed response to healing in older animals, or they could be the result of changes due to pain or discomfort that may occur as a result of the exposure.
To determine if age-related differences in recovery from iSSC exposure were the result of changes in responsiveness to pain, the present study used iSSCs to assess whether this exposure resulted in muscle injury and hyperalgesia in Fisher 344 × Brown Norway F1 rats. This study also determined if aging affects either the expression of, or the recovery from iSSC-induced hyperalgesia. Studies in older humans suggest that depending on the exposure, recovery of muscle function and development of pain may vary, and be resolved at a slower rate in younger humans (Lilje and others 2015; Reid and others 2015). To begin to understand the mechanisms underlying prolonged hyperalgesia, or the development of neuropathic pain, we also examined the effects of this exposure on calcitonin-gene related peptide (CGRP) labeling in the muscle and dorsal root ganglia (DRG) of these animals. Previous studies have demonstrated an increase in CGRP in the DRG of cells innervating muscles after exposure to eccentric contractions (Dessem and others 2010). There is also evidence to suggest that muscle injury and inflammation are associated with an increase in CGRP and the development of allodynia and/or hyperalgesia (Reinert and others 1998). Tracing studies performed in rats have also demonstrated that the majority of sensory neurons innervating the gastrocnemius muscle contain CGRP (Barry and others 2015). Hyperalgesia due to muscle inflammation via adjuvant injection or eccentric contractions has been associated with changes in CGRP concentrations in injured muscle and DRG (Bulling and others 2001; Dessem and others 2010). Because aged animals often show a delayed or attenuated response to muscle injury (Cutlip and others 2009; Hollander and others 2010; Rader and others 2015), there may be changes in the development of hyperalgesia in response to iSSCs, and changes in CGRP may accompany or be a marker of this hyperalgesia. Therefore, in this study, we tested the hypothesis that iSSC-induced muscle injury would result in hyperalgesia in the exposed limb, and that aging may affect the development of hyperalgesia. We also examined the relationship between hyperalgesia and CGRP-labeling in both the exposed muscle and in the DRG.
Materials and Methods
Animals
Young (n = 32; 3 months old; 309.1 ± 27.9 g) and old (n = 30; 30 months old; 587.7 ± 42.2 g) male Fischer 344 Brown Norway hybrid (F344 × BN F1) rats were obtained from the National Institutes on Aging colony. Rats were single housed in an AAALAC accredited animal facility where room temperature and humidity were held constant, with a reversed light/dark cycle (dark cycle was from 7:00 a.m. to 7:00 p.m.). Food and water was provided ad libitum. After one week of acclimatization, rats underwent exposure to an acute iSSC protocol (Baker and others 2006). All procedures were approved by the National Institute for Occupational Safety and Health (NIOSH) Animal Care and Use Committee.
Exposure
Rats were anesthetized with iso?urane gas using a small animal anesthetic system (Surgivet Anesco, Waukesha, Wisconsin). The knee was secured in ?exion (90°) with a knee holder. The left foot was secured in the load cell ?xture using a custom-built foot holder with the ankle axis (assumed to be between the medial and lateral malleoli) aligned with the axis of rotation of the load cell ?xture. Each animal was monitored during the protocol to ensure proper anesthetic depth and body temperature.
After being placed on the dynamometer, the joint position of each rat was defined by the angle between the tibia and the plantar surface of the foot. The angular position of the load cell fixture corresponded with the angular position of the ankle. A calibrated potentiometer measured the angular position of the load cell fixture in real-time during testing. Vertical forces applied to an aluminum sleeve fitted over the dorsum of the foot were translated to a load cell transducer (Sensotec, Columbus, Ohio) in the load cell ?xture. The force produced by the dorsi?exor muscles was measured at the interface of the aluminum sleeve and the dorsum of the foot. Platinum stimulating electrodes (Grass Medical Instruments, Quincy, Massachusetts) were placed subcutaneously to span the peroneal nerve. The first electrode was placed lateral to the tibial notch and the second electrode was placed 5 mm distal and 3 mm posterior to the first electrode. Activation of the electrical stimulator resulted in muscle contraction of the dorsi?exor muscle group. We optimized muscle length for the dorsiflexor muscles via multi-positional isometric contraction and the stimulator settings (i.e., frequency and voltage) were titrated to the minimum value to elicit maximal dorsiflexor isometric force (unpublished data). Muscle stimulation for all protocols was a 120-Hz square-wave pulse at 0.2-ms pulse duration and 4 V.
The iSSC exposure protocol consisted of 15 sets of 10 continuous high-velocity (i.e., 500 deg/sec) stretch-shortening contractions of the left limb (for a total of 150 SSCs). Each set was administered at 1-min intervals. This protocol previously was used to generate injury in the tibialis anterior (TA) muscle and reductions in force in young Sprague Dawley rats (Cutlip and others 2009). Dynamic performance of the dorsiflexors was assessed both before and after iSSC exposure and on day 3 and 10, prior to euthanasia. To assess dynamic performance, dorsiflexor muscles were exposed to a single SSC which maximally activates the dorsiflexor muscle for 300 ms, then the ankle was rotated from 70° to 140° at 500° per second and returned to 70° at the same velocity. (Baker and others 2007; Cutlip and others 2004). After cessation of ankle rotation, activation continued for an additional 300 ms. Following this test the rats were exposed to the iSSC protocol. Control rats underwent the dynamic performance test (i.e., a single set of SSCs), but were not exposed to iSSCs (or repetitive SSCs). After the post-exposure dynamic performance test, rats were allowed to recover, placed in their cages, and put back into the colony room. Rats were euthanized 3 or 10 days after the exposure. These time points were chosen because at 3 days after the exposure there is an intense inflammatory response, and at 10 days muscle performance and fiber morphology return to control levels in young rats (Baker and others 2007; Krajnak and others 2006).
Mechanosensitivity testing with von Frey filaments
Sensitivity to mechanical stimulation of the left TA was measured prior to iSSC exposure, and on days 2 and 9 after the exposure, using von Frey filaments. Rats were placed into a mesh wire container that limited their ability to walk, but still allowed them to move their limbs. Filaments of different tensile strengths were used to detect sensitivity of the exposed limb. A von Frey fiber was pushed against the lateral side of the injured limb and the fiber that induced a withdrawal response prior to the fiber bending was recorded as the level of pressure inducing a response. Animals (4/age/treatment) were tested three times on each day, with a 1 minute inter-test interval between tests. The test began using the filament with the lowest tensile strength (2 g), and fibers of increasing strength were tested until the rat responded. All rats responded to a tensile strength ≤ 26 g. The filament strength that induced a response was recorded. All rats responded to the same filament on at least 2 of the tests. The fiber strength that elicited the greatest number of responses was used for analyses. These time points were used for testing because the rats were awake and testing for pain would not interfere with post-exposure muscle function tests.
Muscle Processing
Immediately after the last force measurements (day 3 or 10), animals were anesthetized with pentobarbital (100 mg/kg), weighed, and euthanized by exsanguination. The left TA muscles were removed and weighed. The mid-belly of each TA muscle was then dissected, mounted onto cork board with OTC (VWR, West Chester, Pennsylvania), frozen in liquid nitrogen cooled isopentane, and stored at −80°C until sectioned for histology. Tibia lengths also were measured with calipers. The ipsilateral dorsal root ganglia (DRG) from the lumbar (L 4 or 5) region were also collected, placed in cryomolds with OTC, frozen on dry ice, and stored at −80°C.
Histology and immunohistochemistry (IHC)
Frozen sections (10 µm) from the mid-belly of the TA muscles were cut on a cryostat, thaw-mounted onto Super-Plus slides (Fisher Scienti?c, Pittsburgh, Pennsylvania) and stored at until processing. There were two muscle sections/slide. One slide from each rat was stained with hematoxylin and eosin using Harris’ procedure. Frozen sections (20 µm) from the DRG were also cut on a cryostat, thaw-mounted onto slides and stored at −80°C until processed. Each slide contained 4 sections of DRG and each section on a slide was 52 µm from the next section.
To assess the severity of muscle fiber injury, inflammation and edema, a 121-point, 11-line overlay graticule (0.04 mm2 with 100 divisions) at a magnification of 40X was used. The total number of points evaluated per section was 1210; or 121 points in 10 fields. Each point was identified as overlaying abnormal muscle fiber, degenerative muscle fiber, cellular interstitium or non-cellular interstitium. Three criteria were required for designation as a degenerative muscle fiber; 1) loss of contact with surrounding fibers, 2) inter-digitation of the sarcolemma by cellular infiltrates, and 3) internalization of cellular infiltrates. A detailed description of the stereological procedure and analyses used to analyze muscle injury is provide in (Baker and others 2006).
Another slide with muscle from each rat was used for CGRP IHC. IHC staining was performed using a protocol similar to that described (Krajnak and others 2006). Briefly, slides were fixed in 4% paraformaldehyde for 5 min, rinsed in 0.01 M phosphate buffered saline (PBS), and incubated in blocking buffer (5% normal donkey serum diluted in PBS 0.4 Triton X-100) at room temperature (RT) for 1 h. Slides were then incubated in rabbit anti-CGRP (Sigma-Aldrich Inc., St. Louis, MO, USA) diluted 1:4000 in blocking buffer) overnight at 4°C. The following day, slides were rinsed in PBS, incubated in Cy3 labeled donkey anti-rabbit IgG (1:1000) for 1hr at RT. Finally, rinsed in PBS, and then incubated in DAPI to stain nuclei (1:1,000, Sigma, St. Louis, MO, USA). After rinsing, slides were cover-slipped using Prolong Gold (Life Technologies; Brown Deer, WI), and allowed to dry in a cool, dark area. A subset of slides containing tissue from each condition was also processed in a similar manner, but the primary antibody incubation was not performed. These slides were used as controls for non-specific binding of the secondary antibody.
A third set of sections was labeled with α-bungarotoxin (αBT) to identify the neuromuscular junction, and dystrophin to serve as a marker of the sarcolemma and make sure that the αBT was labeling synapses at the surface of the cell. αBT was used to assess synapse formation and methods similar to those used to perform the IHC for dystrophin were similar to those described above. The dystrophin (anti-mouse) was used at a final dilution of 1:30 in PBS. αBT staining was performed following the staining for dystrophin, using Alexa Fluor 488 (Life Technologies) diluted to a final concentration of 80 ng/µl. Slides were incubated for 1 hr at RT, rinsed in PBS and cover-slipped with Prolong Gold. CGRP immunostaining was also performed on these sections so that we could determine what structures CGRP was located in and if CGRP was localized to the synapse.
Cross-sections of DRG (20 μm) were cut with a cryostat microtome, thaw-mounted onto charged slides, and stored at −20°C until used for IHC. Consecutive sections on a slide were collected at 100 μm intervals. IHC for CGRP within the ganglia was performed using a protocol similar to that described above for the muscle tissue.
Nuclei in all of the sections were fluorescently stained with (4',6-diamidino-2-phenylindole) DAPI, coverslipped with Prolong Gold (Invitrogen), and digital images were taken using a Zeiss LSM510 laser scanning confocal microscope, with HeNe, Argon, and ultraviolet lasers, and integrated 2D and 3D image processing software. The top of the section was identified and then 10 (muscle) or 12 (DRG) images were collected at an intervals of 2 µm (in the Z-axis). The first and last three images were deleted, and the remaining images were re-combined for analysis. The re-combined images were imported into Scion Image (Scion Inc., Frederick MD), and density thresholds and brightness were standardized for all tissue samples processed with a single antibody. Changes in CGRP within the muscles and DRG were assessed by measuring the area with immunostaining above threshold and immunostaining density per unit area. For muscle, measurements were taken from two sections and from the DRG measurements were taken from 4-5 sections. Measurements were then averaged, and the average area or density of staining was used in the analysis. For the muscle sections stained for αBT and CGRP, a graticule overlay identical to the one described for immunohistochemistry was utilized and the number of synapses labels with αBT in each section was counted at a magnification of 20X. Higher magnification pictures (mag) were also taken and the area and density of αBT staining at individual synapses was measured. This was done because denervation or nerve injury that may have been associated with iSSC exposure can result in the dispersion of acetylcholine receptors, thereby altering both the shape, area and density of αBT at the neuromuscular junction (Rudolf and others 2014).
Statistical Analyses
All histological and immunohistological data were analyzed using 3-way ANOVAs (2 (young vs old) × 2 (control vs exposed) × 2 (day 3 or 10). Because none of the controls responded to stimulation with the von Frey filaments, their data were not included in the analyses. For exposed rats, the tensile strength of the filament they responded to was recorded and used in a 2-way ANOVA (age × test day). For all data, significant interactions were analyzed using appropriate post-hoc ANOVAS or Student’s t-tests. Significant differences were those with p < 0.05.
Results
Mechanosensitivity
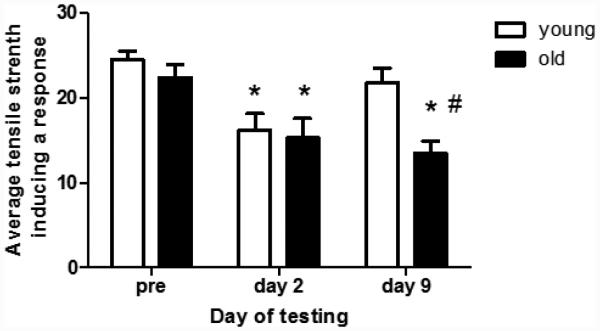
Figure 1 shows the average tensile strength needed to induce a response (i.e., withdrawal of the left hind limb) in rats exposed to iSSCs. Control rats responded at the same tensile strength on all days of the study (i.e., 26 g), and thus the data are not presented. In rats exposed to iSSCs, there were no age-related differences in sensitivity to the stimulus prior to the exposure (Figure 2). However, 2 days after iSSC exposure, both young and old rats responded to stimulation at a lower tensile strength (i.e., indicating increased sensitivity). By 9 days after the exposure, the response of young rats to stimulation returned to pre-injury levels. However, old rats responded to a lower tensile strength fiber indicating that they were still displaying an increased sensitivity to mechanical stimulation.
Figure 1.
The data presented in this graph represent the average tensile strength (± SEM) needed to induce a response in the hindlimb of rats pre-exposure toiSSC, and 2 and 10 days after injury. Two days after exposure to iSSCs, both the young and old rats showed an increase in sensitivity to mechanical stimulation (i.e., they responded to a fiber with a lower tensile strength). By day 10 sensitivity had returned to pre-exposure levels in young but not old rats (p <0.05; * < pre-exposure values and # < young rats).
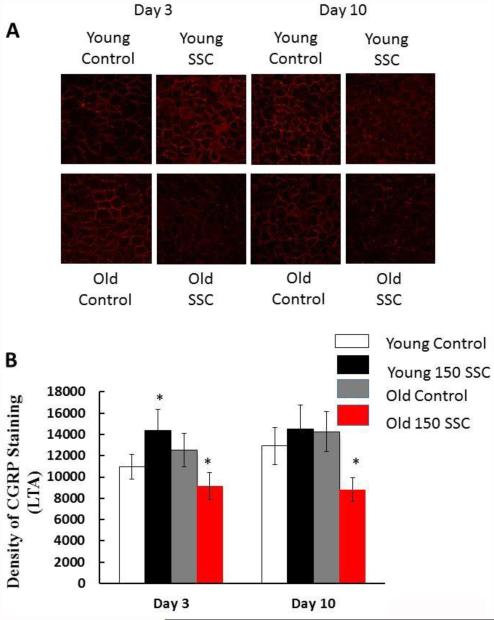
Figure 2.
The photomicrographs in (A) demonstrate the density of CGRP staining in the LTA of young and old rats. Three days after iSSC exposure, young rats show an increase in CGRP staining, but old rats show a decrease. Ten days after iSSC exposure, CGRP density was back to control levels in the LTA of young rats, but it were still reduced in the LTA of old rats (*different than time matched controls, p < 0.05).
Dorsiflexor injury: Morphological and performance changes
Changes in muscle morphology assessed in hematoxylin and eosin stained slides, along with changes in performance, are published in a companion paper (Rader and others 2015).
CGRP and αBT staining
The photomicrographs in Figure 2A show CGRP staining in the LTA of young and old control animals and animals exposed to iSSCs. In the LTA, CGRP staining in the LTA was significantly increased in young iSSC-exposed as compared to young control rats 3 days after the exposure (Figure 2B). However, in old rats, there was a reduction in the density of CGRP staining. Ten days following the injury, CGRP density had returned to control levels in the LTA of young rats exposed to iSSCs. However, in old rats, CGRP staining was significantly reduced in the LTA of iSSC-exposed vs control rats.
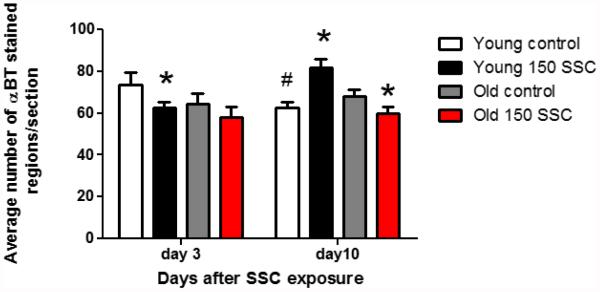
The average number of synapses (αBT-stained regions/section) is presented in Figure 3. In young rats, there was a significant reduction in the number of synapses (p < 0.05) 3 days after exposure to iSSCs. However there was no exposure-induced change in staining in old rats 3 days after exposure. Although synapse number was lower in the LTA of old as compared to young rats on day 3, this difference was not significant. After 10 days of recovery, synapse number in the LTA of young control rats was reduced as compared to control rats on day 3. However, young rats exposed to iSSCs displayed an increase in staining 10 days following injury. In old rats, αBT was reduced in the LTA 10 days after exposure.
Figure 3.
This graph shows the average number of αBT-stained regions ( ± SEM) per section. On day three, there is a reduction in αBT staining in the young rats exposed to iSSCs. However, 10 days after injury, control rats displayed a reduction in αBT staining as compared to day 3 control rats (# p <0.05). In the LTA of young rats αBT staining was increased, but decreased in the LTA of old rats (*p <0.05, different than control rats).
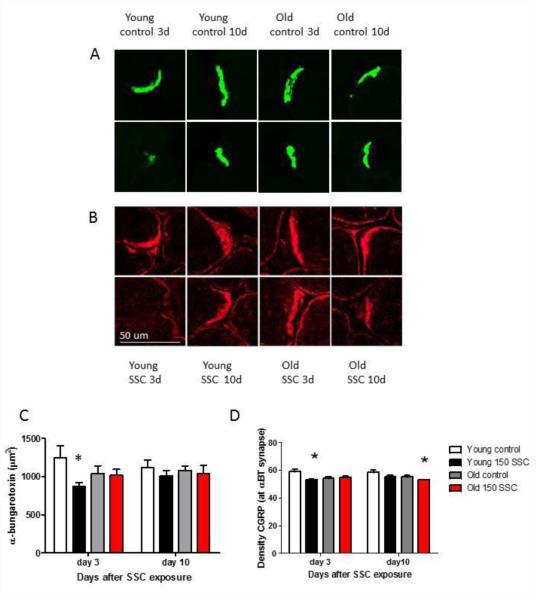
The photomicrographs in Figure 4A shows the density of αBT staining in individual synapses in the LTA of rats, and Figure 4C shows the average density/synapse. In young rats, the density of staining/synapse was reduced 3 days after iSSC exposure (* p < 0.05). There were no other age, or vibration-related changes in αBT-staining in the muscle. CGRP colocalized in the region of the synapse can be seen in Figures 4B, and 4D. The density of CGRP staining was reduced 3 days after iSSC exposure in young rats. However, CGRP staining at the synapse was back to control levels 10 days after iSSC exposure. In old rats, there was a reduction in CGRP staining at the synapse 10 days after iSSC exposure.
Figure 4.
Photomicrographs showing αBT staining (A) and CGRP staining (B) at the neuromuscular juction. The top row of each panel are photomicrographs from control rats and the bottom panels are photos from iSSC exposed rats. The area stained with αBT in samples of the NMJ are presented in C. Young rats showed a reduction in the area stained with αBT 3 days following iSSC exposure (*p < 0.05, less than age-matched controls). There were no other significant changes in αBT staining. The density of CGRP staining that was co-localized with αBT and the NMJ is presented in Figure D. CGRP staining was reduced at the NMJ of youg rats 3 days following iSSC exposure. By 10 days CGRP was back to control levels at the NMJ of young rats. However in old rats exposed to iSSCs, CGRP at the synpase was reduced (*p < 0.05, less than age-matched controls, Bar in Figure B is 50 µm).
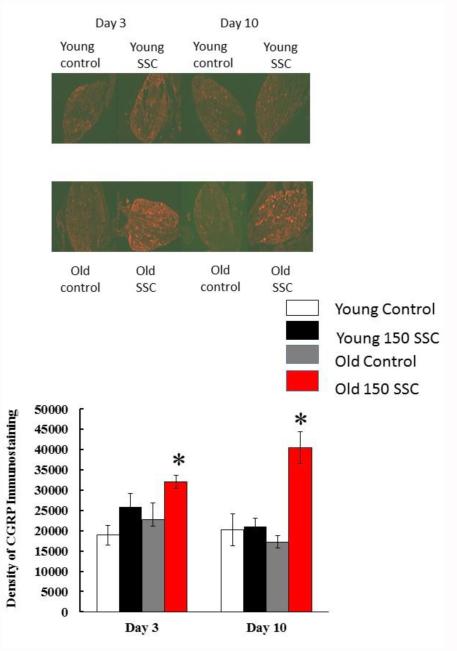
Figure 5 shows CGRP immunostaining in the DRG of young and old iSSC and control rats. In the DRG, iSSC-exposure resulted in an increase in CGRP staining density in both the young and old rats 3 days after exposure. However, the increase was only statistically significant in the old rats (Figure 5B). Ten days after SSC-exposure, DRG staining returned to baseline levels in young animals, but remained elevated in old rats.
Figure 5.
The photomicrographs in (A) display the average density of CGRP staining in the ipsilateral DRG of young and old rats. Three days after iSSC exposure, staining density appeared to be higher in the DRG of injured as compared to young rats. However, this difference was only significant in old rats. Ten days after iSSC exposure,CGRP staining was still increased in the LTA of old rats exposed to SSCs. (*p < 0.05, different that time matched controls).
Discussion
The goal of this study was to use an established model of repetitive motion-induced muscle injury in young and old rats to determine if this exposure resulted in pain (i.e. hyperalgesia in response to a tactile stimulus). To do this, iSSC exposure was used to generate an injury and functional and morphological indices of muscle injury were examined (Rader and others 2015). Interestingly, the morphological changes in young rats were similar to those previously reported in Sprague Dawley rats (Baker and others 2007; Cutlip and others 2007b; Cutlip and others 2009). There was an increase in edema (as measured by an increase in the non-cellular interstitium), an increase in extracellular infiltrates (cellular interstitium), and an increase in degenerative myofibers 3 days following the exposure. By 10 days after the exposure, the injury had been resolved in the young animals, and dynamic force generation returned to pre-exposure levels (Rader and others 2015). In contrast old Fisher hybrid rats displayed a reduction in dynamic force that was similar to that seen in young rats 3 days after iSSCs, but these changes in force were not accompanied by significant changes in muscle morphology. There was some edema, but there was not an overt inflammatory response as seen in the young animals, or as those previously described in old Sprague Dawley rats (Baker and others 2007; Cutlip and others 2007a; Cutlip and others 2007b). There also was not an increase in degenerative myofibers (Rader and others 2015). After 10 days of recovery, dynamic force was still reduced in old rats, suggesting that old rats had not healed. In addition, the genomic and protein responses were age-dependent following the iSSCs, as well as prior to injury exposure. Collectively, these data suggest that the microenvironment of the muscle is age-dependent and may affect the integrated biological response needed for competent healing following soft-tissue injury.(Rader and others 2015).
The effects of iSSCs on mechanosensitivity, CGRP, and αBT staining also showed age-dependent differences. Two days following iSSC exposure, both young and old rats displayed an increased sensitivity, or hyperalgesia, in response to hind limb stimulation with the von Frey filaments. In young rats, this increased sensitivity was associated with an increase in CGRP-immunostaining in the LTA, and an insignificant increase in immunostaining in the DRG. This is consistent with findings that have been reported in other models examining mechanisms regulating pain and peripheral nerve injury/regeneration. For example, increases in CGRP release in response to muscle puncture result in an increase in local blood flow and edema (Shinbara and others 2013), which can induce hyperalgesia. In humans, pain associated with osteoarthritis has been associated with an increase in CGRP within joints and bones (Reid and others 2015). Other studies have demonstrated that CGRP released from motor neurons, or at the neuromuscular junction, can act by phosphorylating the acetylcholine receptor (AChR) and rapidly desensitizing the receptor (Swope and others 1999). Desensitization of the AChR at the neuromuscular junction may act to prevent additional stress and injury to the muscle by reducing the force of contractions stimulated by the release of acetylcholine. CGRP may also play a role in muscle regeneration after injury and during development; in cultured myotubes, CGRP stimulates the synthesis and release of glial-derived neurotrophic factor (GDNF). GDNF is involved in stimulating the regeneration of damaged nerve fibers (Rose and others 2015). Thus, the changes in CGRP may not only have effects on sensory perception, but they may also play a role in regulating changes in muscle force production.
In contrast, SSC-induced hyperalgesia in old rats was associated with a reduction in the density of CGRP staining in the LTA, and an increase in CGRP staining in the DRG. When peptides are released from nerve terminals, it is often difficult to detect them by IHC unless certain fixatives are used, and thus an increase in the release of CGRP from nerve terminals in old rats may be the reason there is a reduction in the amount of the peptide in nerve terminals and a reduced ability to detect it by IHC. The rate of axonal transport of CGRP from the DRG to the nerve terminal might also be slower in aged rats, or there may be a change in nerve conduction and release a of peptides from sensory neurons (Bergman and Ulfhake 2002). In a model of vibration-induced injury, the myelin sheath surrounding sensory nerves show signs of injury and changes in 2,3-cyclic nucleotide phosphatase (CNPase) suggesting that injury may affect myelin-nerve communication (Kiedrowski and others 2015).
Nine days following iSSC exposure, responses to mechanical stimulation returned to pre-exposure levels in young rats. CGRP staining in the LTA and DRG also returned to pre-baseline levels in these rats. However, αBT staining was significantly higher in young iSSC-exposed than young controls. The slight reduction in the control rats may have been a delayed response to the isometric force test. The increase in αBT staining in iSSC-exposed rats may have been due to recovery and regeneration of synapses. Old rats were still more sensitive to mechanical stimulation 9 days after iSSC exposure than their control counterparts. They also displayed a reduction in CGRP staining density in the LTA and an increase in staining in the DRG. Thus, in old rats, the effects of iSSC exposure appear to take longer to recover.
Our observations are consistent with findings from studies performed in humans showing that older individuals take longer to recover from muscle injury after work or exercise (BLS 2014; Bugajska and Sagan 2014). Additional studies have shown that altering work schedules or making modifications in the workplace to reduce physical stress and strain, along with encouraging healthy behaviors, may help reduce the incidence, and pain associated with MSDs (Phillips and Miltner 2014). Animal studies also support these findings (Cutlip and others 2007b; Cutlip and others 2009). Thus, as the workforce ages, employers may need to alter the workplace environment, or add organizational strategies, that prevent the development of injuries, the development of chronic pain, and time out of work.
Highlights.
Aging results in a reduction in muscle force and extended recovery after injury.
Injury-inducing contractions result in prolonged hyperalgesia in older rats.
Cholinergic synapse number at the neuromuscular junction (NMJ) changes with injury.
Changes in CGRP in the muscle and DRG may contribute to the maintenance of pain.
Acknowledgments
Disclaimer
This research was funded by the National Institute for Occupational Safety and Health. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: C. Johnson: Tissue collection and preparation, immunohistochemistry, preparation of manuscript
G.R. Miller: Immunohistochemistry, data collection and analyses, tissue preparation, critical comments on manuscript
B.A. Baker: Design of muscle exposure and animal model, histology, tissue collection and preparation, critical comments on manuscript
M. Hollander: Animal exposures, tissue collection, review of manuscript
M.L. Kashon: Statistics and review of manuscript
S. Waugh: Tissue collection and review of manuscript
K. Krajnak: design of hyperalgesia portion of the study, tissue collection, data analyses, preparation of the manuscript
References
- Baker BA, Mercer RR, Geronilla KB, Kashon ML, Miller GR, Cutlip RG. Stereological analysis of muscle morphology following exposure to repetitive stretch-shortening cycles in a rat model. Applied Physiology Nutriti Metab. 2006;31:167–179. doi: 10.1139/h05-009. [DOI] [PubMed] [Google Scholar]
- Baker BA, Mercer RR, Geronilla KB, Kashon ML, Miller GR, Cutlip RG. Impact of repetition number on muscle performance and histological response. Med Sci Sports Exerc. 2007;39:1275–1281. doi: 10.1249/mss.0b013e3180686dc7. [DOI] [PubMed] [Google Scholar]
- Barry CM, Kestell G, Gillan M, Haberberger RV, Gibbins IL. Sensory nerve fibers containing calcitonin gene-realted peptide in gastrocnemius, latissimus dorsi and erector spinae muscle and thoracolumbar fascia in mice. Neuroscience. 2015;291:106–117. doi: 10.1016/j.neuroscience.2015.01.062. [DOI] [PubMed] [Google Scholar]
- Bergman E, Ulfhake B. Evidence for loss of myelinated input to the spinal cord in senescent rats. Neurobiol Aging. 2002;23:271–286. doi: 10.1016/s0197-4580(01)00274-3. [DOI] [PubMed] [Google Scholar]
- BLS . Nonfatal occupational injuries and illnesses requiring days away from work, 2013. BLS; Washington DC: 2014. [Google Scholar]
- Brooks SV, Faulkner JA. Contraction-induced injury: recovery of skeletal muscles in young and old mice. Am J Physiol. 1990;258:C436–C442. doi: 10.1152/ajpcell.1990.258.3.C436. [DOI] [PubMed] [Google Scholar]
- Brooks SV, Faulkner JA. The magnitude of the initial injury induced by stretches of maximally activated muscle fibres of mice and rats increases in old age. J Physiol. 1996;497(Pt 2):573–580. doi: 10.1113/jphysiol.1996.sp021790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugajska J, Sagan A. Chronic musculoskeletal disorders as risk factors for recued work ability in younger and aging workers. International Journal of Occupatioonal Safety and Ergonomics. 2014;2014:4. doi: 10.1080/10803548.2014.11077069. [DOI] [PubMed] [Google Scholar]
- Bulling DG, Kelly D, Bond S, McQueen DS, Seck JR. Adjuvant-induced joint inflammation causes very rapid transcription of beta-preprotachykinin and alpha-CGRP genes in innervating sensory ganglia. J Neurochemistry. 2001;77:372–382. doi: 10.1046/j.1471-4159.2001.00175.x. [DOI] [PubMed] [Google Scholar]
- Cutlip RG, Baker BA, Geronilla KB, Kashon ML, Wu JZ. The influence of velocity of stretch-shortening contractions on muscle performance during chronic exposure: age effects. Appl Physiol Nutr Metab. 2007a;32:443–453. doi: 10.1139/H07-014. [DOI] [PubMed] [Google Scholar]
- Cutlip RG, Baker BA, Geronilla KB, Kashon ML, Wu JZ. The influence of velocity of stretch-shortening contractions on muscle performance during chronic exposure: age effects. Appl Physiol Nutr Metab. 2007b doi: 10.1139/H07-014. [DOI] [PubMed] [Google Scholar]
- Cutlip RG, Baker BA, Hollander M, Ensey J. Injury and adaptive mechanisms in skeletal muscle. J Electromyogr Kinesiol. 2009;2009:3. doi: 10.1016/j.jelekin.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Cutlip RG, Geronilla KB, Baker BA, Kashon ML, Miller GR, Schopper AW. Impact of muscle length during stretchshortening contractions on real-time and temporal muscle performance measures in rats in vivo. J Appl Physiol. 2004;96:507–516. doi: 10.1152/japplphysiol.00046.2003. [DOI] [PubMed] [Google Scholar]
- Cutlip RG, Stauber WT, Willison RH, Mcintosh TA, Means KH. Dynamometer for rat plantar flexor muscles in vivo. Med Biol Eng Comput. 1997;35:540–543. doi: 10.1007/BF02525537. [DOI] [PubMed] [Google Scholar]
- Dessem D, Ambalavanar M, Evancho A, Moutanni C, Yallampalli C, Bai G. Eccentric muscle contraction and stretching evoke mechanical hyperalgesia and modulate CGRP and P2X3 expression in a functionally relevant manner. Pain. 2010;149:284–295. doi: 10.1016/j.pain.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander M, Baker B, Ensey J, Kashon M, Cutlip RG. Effects of age and glutathione levels on oxidative stress in rats after chronic exposure to stretch-shortening contractions. Euro J of Appl Physiol. 2010;108:588–597. doi: 10.1007/s00421-009-1246-8. [DOI] [PubMed] [Google Scholar]
- Kehl LJ, Trempe TM, Hargreaves KM. A new animal model for assessing mechanisms and management of muscle hyperplasia. Pain. 2000;85:333–343. doi: 10.1016/S0304-3959(99)00282-1. [DOI] [PubMed] [Google Scholar]
- Kiedrowski M, Waugh S, Miller R, Johnson C, Krajnak K. The effects of repetitive vibration on sensorineural function: biomarkers of sensorineural injury in an animmals model of metabolic syndrome. Brain research. 2015;1627:216–224. doi: 10.1016/j.brainres.2015.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajnak K, Waugh S, Miller R, Baker B, Geronilla K, Alway SE, Cutlip RG. Proapoptic factor Bax is increased in satellite cells in the tibialis anterior muscles of old rats. Muscle & Nerve. 2006;36:720–730. doi: 10.1002/mus.20656. [DOI] [PubMed] [Google Scholar]
- Lilje SC, Skillgate E, Anderberg P, Berglund J. Negative psychosocial and heavy physical workloads associated with musculoskeletal pain interfering with normal life in older adults: cross-sectional analysis. Scandinavian journal of public health. 2015;43:453–459. doi: 10.1177/1403494815580876. [DOI] [PubMed] [Google Scholar]
- Phillips JA, Miltner R. Work hazards for an aging nursing workforce. J Nurs Manag. 2014 doi: 10.1111/jonm.12217. in press. [DOI] [PubMed] [Google Scholar]
- Rader EP, Layner KN, Triscuit AM, Kashon ML, Gu JK, Ensey J, Baker BA. Desensitized morphological and cytokine response after stretch-shortening muscle contractions as a feature of aging in rats. Experimental gerontology. 2015;72:138–149. doi: 10.1016/j.exger.2015.09.020. [DOI] [PubMed] [Google Scholar]
- Reid KF, Price LL, Harvey WF, Driban JB, Hau C, Fielding RA, Wang C. Muscle power is an independent determinant of pain and quality of life in knee osteoarthritis. Arthritis & rheumatology (Hoboken, NJ) 2015 doi: 10.1002/art.39336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert A, Kaske A, Mense S. Inflammation-induced increase in the density of neuropeptide-immunoreactive nerve endings in rat skeletal muscle. Experimental brain research. 1998;121:174–180. doi: 10.1007/s002210050449. [DOI] [PubMed] [Google Scholar]
- Rose E, Cha J, Bain JR, Fahnestock M. Calcitonin gene-related peptide regulation of glial cell-line derived neurotrophic factor in differentiated rat myotubes. J Neurosci Res. 2015;93:514–520. doi: 10.1002/jnr.23512. [DOI] [PubMed] [Google Scholar]
- Rudolf R, Khan MM, Labeit S, Deschenes MR. Degeneration of neuromuscular junction in age and dystrophy. Front Aging Neurosci. 2014:6. doi: 10.3389/fnagi.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinbara H, Okubo M, KKimura K, Mizunuma K, Sumiya E. Participation of calcitonin gene related peptide released via reflex in the local increasse in muscle blood flow following manual accupuncture. Acupunture Medicine. 2013;31:81–87. doi: 10.1136/acupmed-2012-010253. [DOI] [PubMed] [Google Scholar]
- Swope SL, Moss SJ, Raymond LA, Huganir RL. Regulation of ligand-gated ion channels by protein phosphorylation. Advances in Second Messenger and Phosphoprotein Research. 1999;33:49–78. doi: 10.1016/s1040-7952(99)80005-6. [DOI] [PubMed] [Google Scholar]