Abstract
In humans, a considerable fraction of the retinoid pool in skin is derived from vitamin A2 (all-trans 3,4-dehydroretinal). Vitamin A2 may be locally generated by keratinocytes, which can convert vitamin A1 (all-trans retinol) into vitamin A2 in cell culture. We report that human cytochrome P450 (hP450) 27C1, a previously ‘orphan’ enzyme, can catalyze this reaction. Purified recombinant hP450 27C1 bound and desaturated all-trans retinol, retinal, and retinoic acid, as well as 11-cis retinal. Although the physiological role of 3,4-dehydroretinoids in humans is unclear, we have identified hP450 27C1 as an enzyme capable of efficiently mediating their formation.
Keywords: Cytochrome P450, retinoids, desaturation, spectroscopy, mass spectrometry
1. Introduction
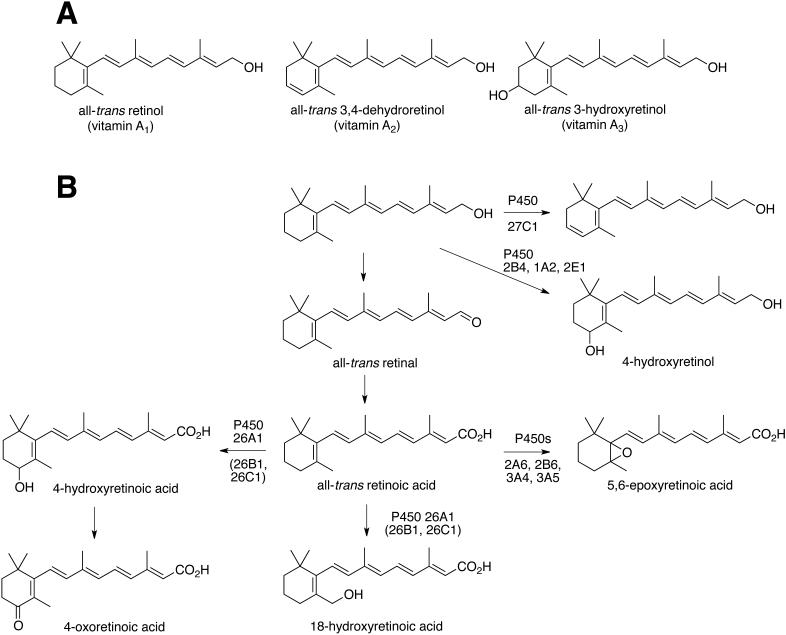
Retinoids, including derivatives of vitamin A1 (all-trans retinol), vitamin A2 (all-trans 3,4-dehydroretinol), and vitamin A3 (all-trans 3-hydroxyretinol), have numerous important biological functions [1-3] (Fig. 1A). Retinoic acid, an oxidation product of vitamin A1, interacts with nuclear receptor transcription factors to regulate gene expression during development and in the adult [4, 5]. Derivatives of vitamin A1 also play an important role in vision in humans because 11-cis retinal is the chromophore of most vertebrate visual pigments [6, 7]. However, the vitamin A2 derivative 3,4-dehydroretinal is also used as a visual chromophore in many cold-blooded vertebrates including lampreys, fish, amphibians, and some reptiles [8, 9]. The use of this alternate chromophore is associated with freshwater environments, and many animals that migrate between salt and fresh water dynamically switch from vitamin A1-based to A2-based chromophores [8]. Because vitamin A2 has a longer system of conjugated double bonds than A1, its light absorption spectrum is red-shifted relative to that of A1. This shift in sensitivity may enhance vision in murky freshwater environments in which longer-wavelength light predominates.
FIGURE 1.
A, Forms of vitamin A. B, Known transformations of vitamin A.
Vitamin A2 derivatives have also been found in other contexts, including in developing embryos in the form of dehydroretinoic acid [10, 11]. Reports on the occurrence and function of vitamin A2-based retinoids in mammals are more limited. Vahlquist and associates reported that vitamin A2 is found in humans and constitutes ~25% of the retinoid pool in skin [12-14], with the fraction increasing in psoriasis, atopic dermatitis, and lichen planus [15, 16]. However, the function of dehydroretinoids in human skin is currently unknown.
The metabolism of retinoids has been a matter of investigation for some time. Dehydrogenases convert retinol and retinaldehyde to retinoic acid [17]. Most of the studies of oxidation by cytochrome P450 (P450) enzymes have been with retinoic acid (Fig. 1B). Several rabbit P450s can catalyze the 4-hydroxylation of all-trans retinol, retinal, and retinoic acid [17]. Human P450s 2B6, 2C8, 3A4, 3A5, and 2A6 can form 4-hydroxy and 4-oxo retinoic acid, P450s 2B6, 2C8 and 2A6 can catalyze 18-hydroxylation, and P450s 2A6, 2B6, 3A4, and 3A5 have been reported to catalyze 5,6-epoxidation [18]. However, the main human liver P450 involved in retinoic acid oxidation is P450 26A1, which forms 4- and 18-hydroxy retinoic acid [19-22]. Human P450s 26B1 and 26C1 appear to carry out similar reactions, but 26B1 has extrahepatic localization and 26C1 is expressed during fetal development [23]. Retinoic acid metabolites also induce P450 26A1 [22]. Thus, a range of human P450s can act upon retinoid substrates.
Desaturation of retinol to 3,4-dehydroretinol (vitamin A2) (Fig. 1) has been reported in preparations of human breast skin [24] and keratinocytes in culture [25]. More recently UV light exposure was reported to increase the formation of 3,4-dehydroretinol in cultured human keratinocytes [26]. However, the identity of the enzyme that mediates 3,4-desaturation of retinoids in human skin has not been determined. Recently we reported that zebrafish P450 27C1 is an efficient retinol 3,4-desaturase and that this enzyme is expressed in the eye of fish and amphibians where it acts to red-shift the visual chromophore [27]. Although no bird or mammal has been shown to express P450 27C1 in the eye, an intact copy of the gene is present in the human genome. Here, we report that the human CYP27C1 gene encodes a retinoid 3,4-desaturase, hP450 27C1, with selectivity for all-trans retinol. We hypothesize that this enzyme may catalyze the production of 3,4-dehydroretinoids in human skin.
2. Materials and Methods
2.1. Chemicals
All-trans 3,4-dehydroretinol, 3,4-dehydroretinaldehyde, and 3,4-dehydroretinoic acid were synthesized as described elsewhere [27]. All-trans retinol, retinal, and retinoic acid were purchased from Sigma-Aldrich. 11-cis-Retinal and 11-cis-3,4-dehydroretinal were purchased from Toronto Research Laboratories. 4-Hydroxy and 4-oxo (all-trans) retinol were prepared by mild alkaline hydrolysis of the acetate esters, which were synthesized as described previously [27].
2.2. Enzymes
An N-terminal-modified human (h) P450 27C1 protein was expressed from an Escherichia coli codon-optimized cDNA as described previously (M3 construct) [28]. The protein was purified as described using Ni2+-nitrilotriacetic acid (NTA) chromatography [28]. Recombinant bovine adrenodoxin (Adx) [29] and NADPH-Adx reductase (ADR) [30] were expressed in E. coli and purified as described previously [27]. Recombinant rat NADPH-P450 reductase was expressed in E. coli and purified as described earlier [31]. [32]
2.3 Kd determinations
hP450 27C1 (2.0 μM in 50 mM potassium phosphate buffer, pH 7.4) was included in each of two 1.0-ml glass cuvettes, which were balanced against each other in an OLIS-Aminco DW-2 spectrophotometer (On-Line Instrument Systems, Bogart, GA). A baseline was recorded. Aliquots of retinoids, dissolved in ethanol, were added to the sample cuvette, with an equal volume of ethanol added to the reference cuvette after each addition (the maximum concentration of ethanol added was ≤ 2%, v/v). The contents of each cuvette were mixed (following each addition) using a plumper stick (NSG Precision Cells, Farmingdale, NY, catalog no. P68). A difference spectrum was measured following each addition. The data were fit to plots of Δ(A390-A418) (i.e. Amax vs. Amin) vs. retinoid concentration, using a quadratic equation of the form
, sometimes referred to as a Morrison equation, where ΔAmax is the extrapolated absorbance difference at infinite ligand concentration, ET is the total enzyme concentration, LT is the total ligand concentration, and Kd is the dissociation constant (Y=B+(A/2)*(1/E)*((Kd+E+X)sqrt((Kd+E+X)^2-(4*E*X))) in Prism 5.0 software, GraphPad, La Jolla, CA).
2.4 Retinoid desaturation assays
Assays for the desaturation of (all-trans) retinol, retinaldehyde, and retinoic acid were conducted as described [27]. Unless stated otherwise, typical assay conditions included 0.02-0.05 μM P450 27C1, 5 μM Adx, and 0.2 μM ADR in 50 mM potassium phosphate buffer (pH 7.4). An NADPH-generating system was used, with final concentrations of 10 mM glucose 6-phosphate, 0.5 mM NADP+, and 1 IU/ml yeast glucose 6-phosphate dehydrogenase [33]. All reactions and analyses were done in amber glass vials due to the light sensitivity of the retinoids. Extractions were done with ethyl acetate or tert-butyl methyl ether, as well as butylated hydroxytoluene (50 μg/ml) to prevent degradation of products due to radical damage [17].
Ultraperformance liquid chromatography (UPLC) separations were performed on an Acquity UPLC system (Waters Associates) coupled with a photodiode array (PDA) detector. Samples were separated on an Acquity UPLC BEH octadecylsilane (C18) column (1.7 μm, 2.1 mm × 50 mm) at a flow rate of 0.2 ml/min (for retinal) or a Thermo Scientific Hypersil Gold octadecilsilane (C18) column (3 μm, 2.1 mm × 150 mm) at a flow rate of 0.3 ml/min (retinoic acid). The column temperature was maintained at 40 °C. Solvent A contained 0.1% HCO2H in 95% H2O/4.9% CH3CN (v/v), and solvent B consisted of 0.1% HCO2H in in 95% CH3CN/4.9% H2O (v/v). A gradient program was run from 60-70% B (v/v) over 15 min, followed by a column wash at 100% B and re-equilibration back to 60% B (v/v).
LC-MS/MS analysis was performed on an Acquity UPLC system (Waters Associates) coupled to a Thermo-Finnigan LTQ mass spectrometer (Thermo Scientific, San Jose, CA) with an atmospheric pressure chemical ionization (APCI) source. Samples were separated on an Acquity UPLC BEH octadecylsilane (C18) column (1.7 μm, 2.1 mm × 50 mm) at a flow rate of 0.3 ml/min. The column temperature was maintained at 40 °C. Eluent A contained 0.1% HCO2H in 95% H2O/4.9% CH3CN (v/v), and eluent B consisted of 0.1% HCO2H in in 95% CH3CN/4.9% H2O (v/v). A gradient program was run as follows: 40-50% B over 10 min, then 60-70% B over 10 min, followed by a column wash at 100% B and re-equilibration back to 40% B (all v/v). MS data were acquired in the positive ion mode and controlled by Xcalibur 2.1 software (Thermo). Settings were as follows: capillary temperature 275 °C, APCI vaporizer temperature 300 °C, sheath gas flow 50, auxiliary gas flow 5, sweep gas flow 5, source voltage 6 kV, source current 5 μV, capillary voltage 47V, tube lens voltage 70V.
3. Results
3.1. Binding of retinoids to hP450 27C1
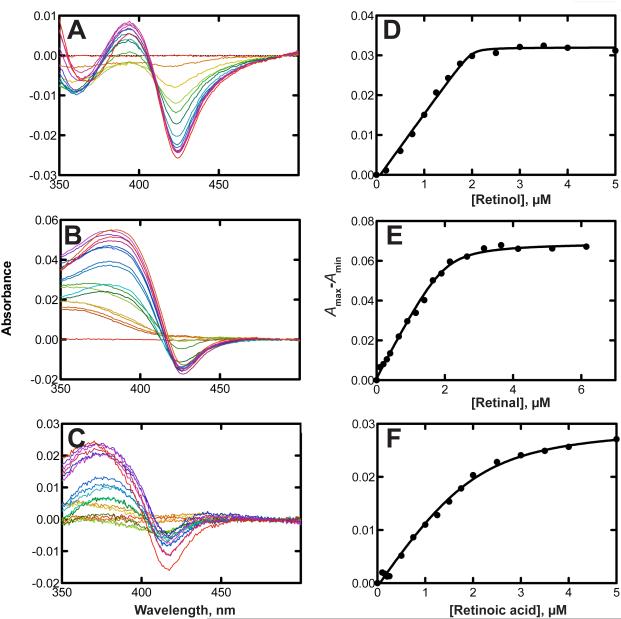
As reported with zebrafish P450 27C1 [27], we found that the binding of all-trans retinol to hP450 27C1 was tight, with a spectrally estimated Kd of 5.6 nM (Fig. 2A, 2D). All-trans retinal and retinoic acid were also tightly bound, with respective Kd values of 0.094 and 0.14 μM (Figs. 2B, 2C, 2E, 2F). Because of the nature of the assays and the enzyme concentrations needed, these low Kd values are considered to contain considerable error, even when quadratic analysis is applied, and may be overestimated. For instance, the titration with retinol shows a sharp inflection point upon saturation of the protein (at 2 μM, Fig. 2D).
FIGURE 2.
Binding of all-trans retinoids to P450 27C1. Spectral titrations were done as described under Experimental Procedures. Spectral changes are shown parts A-C. Kd values were estimated using a quadratic expression in GraphPad Prism (see Materials and Methods) and are presented ± SE ([P450] = 2.0 μM). No corrections were made for the retinoid absorbance at 390 nm. A and D, retinol (Kd 0.0056 ± 0.0072 μM); B and E, retinal (Kd = 0.094 ± 0.026 μM); C and F, retinoic acid (Kd 0.14 ± 0.08 μM).
3.2 Desaturation of retinoids by hP450 27C1
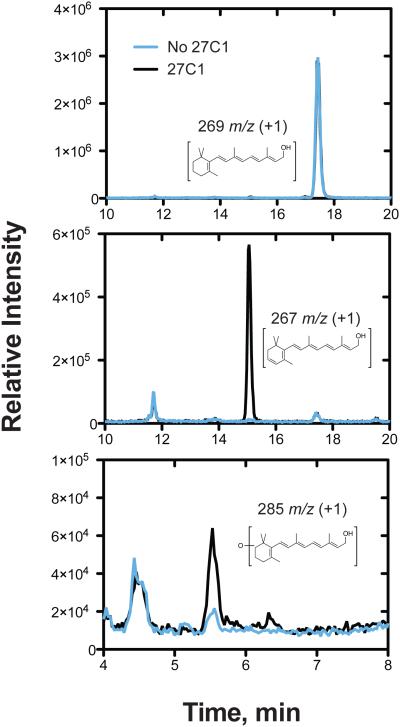
Our initial LC-UV studies demonstrated that all-trans retinol was desaturated by hP450 27C1 at the 3,4 position of the β-ionone ring. The results were confirmed by LC-MS analyses (Fig. 3). An oxygenated product was detected in the MH+ +16 window (m/z 285, corresponding to addition of one oxygen, i.e. hydroxylation). This product co-migrated with and had the same UV spectrum as standard 4-hydroxyretinol. Both the desaturation and the 4-hydroxylation activities were dependent upon the addition of the cofactor NADPH and (mitochondrial) ADR and Adx; no desaturation was detected when (microsomal) NADPH-P450 reductase was used instead (data not shown). The yield of the 4-hydroxy product was ~10% of the total products, based on the UV spectra and signal responses at wavelength maxima.
FIGURE 3.
Desaturation of all-trans retinol and 4-hydroxylation by P450 27C1. LC-MS/MS traces of all-trans retinol (50 μM) desaturation reactions performed in the presence (black) or absence (blue) of P450 27C1 (0.2 μM). A, detection of 269 m/z (retinol); B, detection of 267 m/z (3,4-dehydroretinol); and C, detection of 285 m/z (oxygenated retinol products). The tR 5.5 min peak seen in the m/z 285 chromatogram (part C) co-migrated with standard 4-hydroxyretinol.
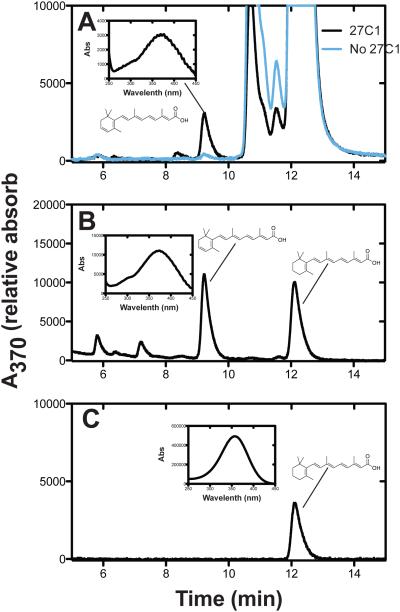
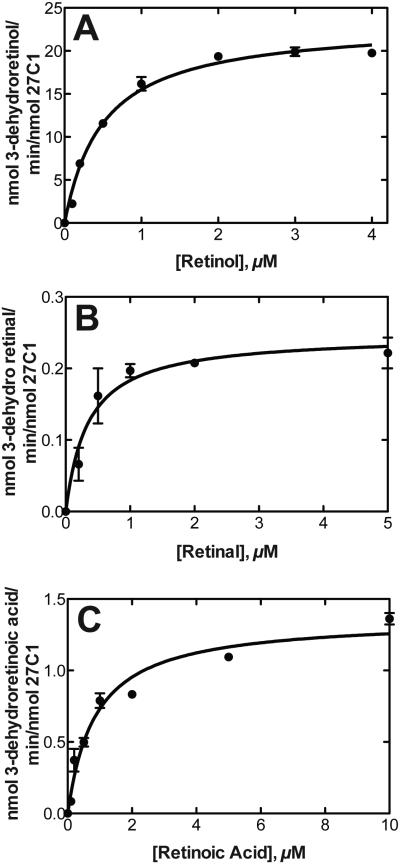
LC-UV analyses were used to obtain steady-state kinetic parameters for the desaturation of all-trans retinol, retinal, and retinoic acid (Fig. 4). Retinol was the most efficiently desaturated substrate (Fig. 5A-C), with an estimated catalytic efficiency of 7.7 × 105 M−1 s−1 under our conditions.
FIGURE 4.
Desaturation of retinoic acid by P450 27C1. UPLC-UV traces of A, all-trans retinoic acid (50 μM) reaction performed in the presence (black) or absence (blue) of P450 27C1 (0.2 μM); B, standard mixture of 3,4-dehydroretinoic acid and retinoic acid; C, standard retinoic acid.
FIGURE 5.
Steady-state kinetics of (all-trans) retinoid desaturation by human P450 27C1. Parameters were estimated using hyperbolic (non-linear regression) in GraphPad Prism (see Materials and Methods). A, Retinol. kcat 23 ± 1 min−1, Km 0.50 ± 0.05 μM, kcat/Km 46 μM min−1 (7.7 (± 0.8) × 105 M−1 s−1). B, Retinaldehyde. kcat 0.25 ± 0.02 min−1, Km 0.35 ± 0.11 μM, kcat/Km 0.71 μM−1 min−1 (1.1 ± 0.1 × 104 M−1 s−1). C, Retinoic acid. kcat 1.4 ± 0.1 min−1, Km 0.87 ± 0.15 μM, kcat/Km 1.6 μM−1 min−1 (2.7 ± 0.8 × 104 M−1 s−1).
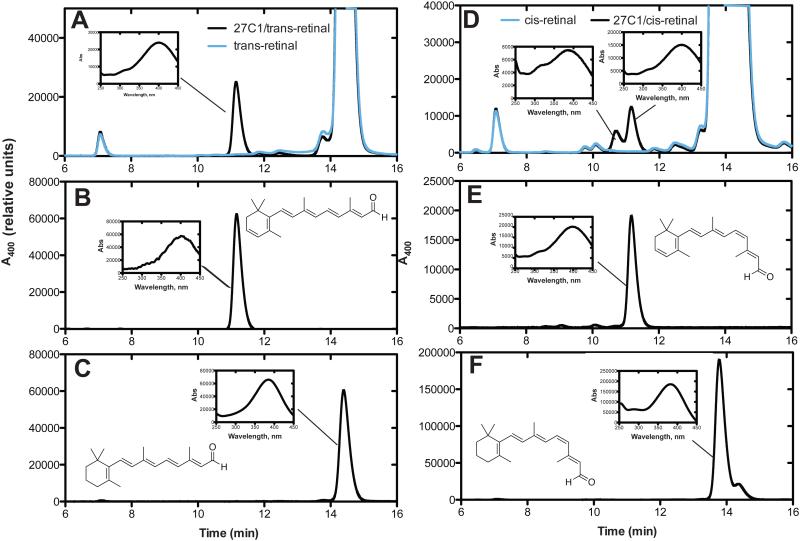
11-cis retinal was also a substrate for hP450 27C1 and is compared to all-trans retinaldehyde in Fig. 6). Two peaks were repeatedly seen in the analyses of the 11-cis products (Fig. 6D), with the second eluting component migrating at the position of an authentic commercial standard of 11-cis dehydroretinal. The earlier eluting component had very similar UV and mass spectra. On the basis of the literature [3], we believe this to be an isomer of 3,4-dehydroretinal, different from 3,4-dehydro 11-cis retinal.
FIGURE 6.
A, HPLC of reactions of all-trans retinal (50 μM) done with P450 27C1 (black line); B, standard all-trans 3,4-dehydroretinal; C, standard all-trans retinal. D, HPLC of reactions of 11-cis retinal (50 μM) done with P450 27C1 (black line) and without; E, standard 11-cis 3,4-dehydroretinal; F, standard 11-cis retinal.
4. Discussion
We provide evidence that the 'orphan' hP450 27C1 protein [28] is a retinoid 3,4-desaturase. The catalytic activities are similar to the zebrafish P450 27C1 enzyme, although somewhat less efficient [27]. The low Km and Kd values with retinoids, along with the overall catalytic efficiency, argue that retinoids are the endogenous substrates. The catalytic efficiency of hP450 27C1 in all-trans retinol desaturation (7.7 × 105 M−1 s−1 under our conditions, Fig. 5) is 200-fold higher than the best efficiency of a rabbit P450 (2B4) for any retinoid oxidation (~ 3 × 103 M−1 s−1) [17]. The activity of hP450 27C1 is surprising in that P450s 27A1 and 27B1, the most closely related proteins, are recognized for their abilities to oxidize unrelated vitamin D compounds (as well as cholesterol in the case of P450 27A1) [23]. In other experiments not presented here, we prepared hP450 27A1 (38% sequence identity) and were able to detect a low rate of desaturation of all-trans retinol. The rate was 0.23 nmol dehydroretinol formed/min/nmol P450 27A1 at a substrate concentration of 10 μM, compared with a kcat of 23 nmol dehydroretinol formed/min/nmol for P450 27C1, i.e. 100-fold higher, Fig. 5. Thus, some degree of retinoid desaturase activity may be a common feature of this P450 subfamily.
We had reported that zebrafish P450 27C1 was only a desaturase, devoid of other activities [27]. In our preliminary studies with hP450 27C1, we only observed the desaturated products. However, with a shallower HPLC gradient we could detect all-trans retinol 4-hydroxylation activity with the human enzyme, although the extent was only ~ 1/10 of the desaturation. No 4-oxo retinol was detected (based on use of a commercial standard), but we have not extended the search (longer incubation times, etc.). Some P450 desaturases are known in plants [34, 35] and yeast [36, 37], but in mammals [23, 38-40] P450-mediated desaturation reactions have always minor in comparison to the associated oxygenations (alcohol products) [23, 38-40]. The biochemical basis for why P450 27C1 strongly favors desaturation is currently unknown. However, studies with non-heme iron oxygenases have revealed that a few key amino acid residues can control a delicate balance between desaturation and hydroxylation pathways [41, 42].
Our previous work using mRNA blots indicated that hCYP27C1 transcripts are detectable in human liver, kidney, and several other tissues [28]. In contrast, LC-MS assays of liver have failed to demonstrate the presence of the protein (data not shown) and we have been unable to detect retinol desaturation activity in human liver homogenates (data not shown). Further studies on the cellular localization of hP450 27C1 are in progress.
The exact physiological function of retinoid desaturation in mammals is currently unclear. Desaturated retinoids are not thought to be involved in human vision, as the characteristic red-shift associated with the use of the vitamin A2-derived chromophore [27] has never been reported in man. In contrast, vitamin A2 (3,4-dehydroretinol) has been reported to constitute about 25% of retinoids in human skin [12-14] and skin cells had been shown to catalyze this reaction [24, 25]. It has been reported that ultraviolet light exposure increases the biosynthesis of dehydroretinoids in human keratinocytes in culture [26]. The latter finding raises the intriguing possibility that 3,4-dehydroretinoids might play a role in UV light protection in human skin.
Acknowledgements
This work was supported by United States National Institutes of Health grants R37 CA090426 (F.P.G.), EY026672 (J.C.C.), EY024958 (J.C.C.), T32 ES007028 (V.M.K.), T32 CA009582 (K.M.J.) T32 EY013360 (J.M.E., M.B.T), and F31 NS083170 (J.M.E.), the National Science Foundation DBI1202776 (M.B.T.), and the Human Frontier Science Program (J.C.C.). We thank K. Trisler for her assistance in preparation of the manuscript.
Footnotes
Disclosures
No conflicts of interest, financial or otherwise, are declared by the authors.
References
- 1.Wald G. The porphyropsin visual system. J. Gen. Physiol. 1939;22:775–794. doi: 10.1085/jgp.22.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rando RR. The chemistry of vitamin A and vision. Angew. Chem. Int. Ed. 1990;29:461–480. [Google Scholar]
- 3.Kiser PD, Golczak M, Palczewski K. Chemistry of the retinoid (visual) cycle. Chem. Rev. 2014;114:194–232. doi: 10.1021/cr400107q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gudas LJ. Retinoids and vertebrate development. J. Biol. Chem. 1994;269:15399–15402. [PubMed] [Google Scholar]
- 5.Love JM, Gudas LJ. Vitamin A, differentiation and cancer. Curr. Opin. Cell Biol. 1994;6:825–831. doi: 10.1016/0955-0674(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 6.Wald G. Molecular basis of visual excitation. Science. 1968;162:230–239. doi: 10.1126/science.162.3850.230. [DOI] [PubMed] [Google Scholar]
- 7.Palczewski K. Chemistry and biology of vision. J. Biol. Chem. 2012;287:1612–1619. doi: 10.1074/jbc.R111.301150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beatty DD. A study on the succession of visual pigments in Pacific salmon (Oncorhynchus) Canad. J. Zool. 1966;44:429–455. doi: 10.1139/z66-045. [DOI] [PubMed] [Google Scholar]
- 9.Toyama M, Hironaka M, Yamahama Y, Horiguchi H, Tsukada O, Uto N, Ueno Y, Tokunaga F, Seno K, Hariyama T. Presence of rhodopsin and porphyropsin in the eyes of 164 fishes, representing marine, diadromous, coastal and freshwater species—a qualitative and comparative study. Photochem. Photobiol. 2008;84:996–1002. doi: 10.1111/j.1751-1097.2008.00344.x. [DOI] [PubMed] [Google Scholar]
- 10.Thaller C, Eichele G. Isolation of 3,4-didehydroretinoic acid, a novel morphogenetic signal in the chick wing bud. Nature. 1990;345:815–819. doi: 10.1038/345815a0. [DOI] [PubMed] [Google Scholar]
- 11.Maden M, Sonneveld E, van der Saag PT, Gale E. The distribution of endogenous retinoic acid in the chick embryo: implications for developmental mechanisms. Development. 1998;125:4133–4144. doi: 10.1242/dev.125.21.4133. [DOI] [PubMed] [Google Scholar]
- 12.Vahlquist A. The identification of dehydroretinol (vitamin A2) in human skin. Experientia. 1980;36:317–318. doi: 10.1007/BF01952299. [DOI] [PubMed] [Google Scholar]
- 13.Vahlquist A. Vitamin A in human skin: I. detection and identification of retinoids in normal epidermis. J. Invest. Dermatol. 1982;79:89–93. doi: 10.1111/1523-1747.ep12500032. [DOI] [PubMed] [Google Scholar]
- 14.Vahlquist A, Lee JB, Michaelsson G, Rollman O. Vitamin A in human skin: II. Concentrations of carotene, retinol and dehydroretinol in various components of normal skin. J. Invest. Dermatol. 1982;79:94–97. doi: 10.1111/1523-1747.ep12500033. [DOI] [PubMed] [Google Scholar]
- 15.Rollman O, Vahlquist A. Psoriasis and vitamin A. Plasma transport and skin content of retinol, dehydroretinol and carotenoids in adult patients versus healthy controls. Arch. Dermatol. Res. 1985;278:17–24. doi: 10.1007/BF00412490. [DOI] [PubMed] [Google Scholar]
- 16.Rollman O, Vahlquist A. Vitamin A in skin and serum—studies of acne vulgaris, atopic dermatitis, ichthyosis vulgaris and lichen planus. Br. J. Dermatol. 1985;113:405–413. doi: 10.1111/j.1365-2133.1985.tb02354.x. [DOI] [PubMed] [Google Scholar]
- 17.Roberts ES, Vaz AD, Coon MJ. Role of isozymes of rabbit microsomal cytochrome P-450 in the metabolism of retinoic acid, retinol, and retinal. Mol. Pharmacol. 1992;41:427–433. [PubMed] [Google Scholar]
- 18.Marill J, Cresteil T, Lanotte M, Chabot GG. Identification of human cytochrome P450s involved in the formation of all-trans-retinoic acid principal metabolites. Mol. Pharmacol. 2000;58:1341–1348. doi: 10.1124/mol.58.6.1341. [DOI] [PubMed] [Google Scholar]
- 19.Lutz JD, Dixit V, Yeung CK, Dickmann LJ, Zelter A, Thatcher JE, Nelson WL, Isoherranen N. Expression and functional characterization of cytochrome P450 26A1, a retinoic acid hydroxylase. Biochem. Pharmacol. 2009;77:258–268. doi: 10.1016/j.bcp.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimshoni JA, Roberts AG, Scian M, Topletz AR, Blankert SA, Halpert JR, Nelson WL, Isoherranen N. Stereoselective formation and metabolism of 4-hydroxy-retinoic acid enantiomers by cytochrome P450 enzymes. J. Biol. Chem. 2012;287:42223–42232. doi: 10.1074/jbc.M112.404475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chithalen JV, Luu L, Petkovich M, Jones G. HPLC-MS/MS analysis of the products generated from all-trans-retinoic acid using recombinant human CYP26A. J. Lipid Res. 2002;43:1133–1142. doi: 10.1194/jlr.m100343-jlr200. [DOI] [PubMed] [Google Scholar]
- 22.Topletz AR, Tripathy S, Foti RS, Shimshoni JA, Nelson WL, Isoherranen N. Induction of CYP26A1 by metabolites of retinoic acid: evidence that CYP26A1 is an important enzyme in the elimination of active retinoids. Mol. Pharmacol. 2015;87:430–441. doi: 10.1124/mol.114.096784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guengerich FP. In: Human cytochrome P450 enzymes, in Cytochrome P450: Structure, Mechanism, and Biochemistry. 4th Ortiz de Montellano PR, editor. Springer; New York: 2015. pp. 523–785. [Google Scholar]
- 24.Torma H, Vahlquist A. Biosynthesis of 3-dehydroretinol (vitamin A2) from all-trans-retinol (vitamin A1) in human epidermis. J. Invest. Dermatol. 1985;85:498–500. doi: 10.1111/1523-1747.ep12277290. [DOI] [PubMed] [Google Scholar]
- 25.Rollman O, Wood EJ, Olsson MJ, Cunliffe WJ. Biosynthesis of 3,4-didehydroretinol from retinol by human skin keratinocytes in culture. Biochem. J. 1993;293 ( Pt 3):675–682. doi: 10.1042/bj2930675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tafrova JI, Pinkas-Sarafova A, Stolarzewicz E, Parker KA, Simon M. UVA/B exposure promotes the biosynthesis of dehydroretinol in cultured human keratinocytes. Mol. Cell. Biochem. 2012;364:351–361. doi: 10.1007/s11010-012-1237-7. [DOI] [PubMed] [Google Scholar]
- 27.Enright JM, Toomey MB, Sato SY, Temple SE, Allen JR, Fujiwara R, Kramlinger VM, Nagy LD, Johnson KM, Xiao Y, How MJ, Johnson SL, Roberts NW, Kefalov VJ, Guengerich FP, Corbo JC. Cyp27c1 red-shifts the spectral sensitivity of photoreceptors by converting vitamin A1 into A2. Curr. Biol. 2015;25:3048–3057. doi: 10.1016/j.cub.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu ZL, Bartleson CJ, Ham AJ, Guengerich FP. Heterologous expression, purification, and properties of human cytochrome P450 27C1. Arch. Biochem. Biophys. 2006;445:138–146. doi: 10.1016/j.abb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Palin MF, Berthiaume L, Lehoux JG, Waterman MR, Sygusch J. Direct expression of mature bovine adrenodoxin in Escherichia coli. Arch. Biochem. Biophys. 1992;295:126–131. doi: 10.1016/0003-9861(92)90497-k. [DOI] [PubMed] [Google Scholar]
- 30.Sagara Y, Wada A, Takata Y, Waterman MR, Sekimizu K, Horiuchi T. Direct expression of adrenodoxin reductase in Escherichia coli and the functional characterization. Biol. Pharmaceut. Bull. 1993;16:627–630. doi: 10.1248/bpb.16.627. [DOI] [PubMed] [Google Scholar]
- 31.Hanna IH, Teiber JF, Kokones KL, Hollenberg PF. Role of the alanine at position 363 of cytochrome P450 2B2 in influencing the NADPH- and hydroperoxide-supported activities. Arch. Biochem. Biophys. 1998;350:324–332. doi: 10.1006/abbi.1997.0534. [DOI] [PubMed] [Google Scholar]
- 32.Salamanca-Pinzon SG, Guengerich FP. A tricistronic human adrenodoxin reductase-adrenodoxin-cytochrome P450 27A1 vector system for substrate hydroxylation in Escherichia coli. Prot. Express. Purif. 2011;79:231–236. doi: 10.1016/j.pep.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guengerich FP. In: Analysis and characterization of enzymes and nucleic acids relevant to toxicology, in Hayes' Principles and Methods of Toxicology. Hayes AW, Kruger CL, editors. CRC Press-Taylor & Francis Boca Raton; FL: 2014. pp. 1905–1964. [Google Scholar]
- 34.Morikawa T, Mizutani M, Aoki N, Watanabe B, Saga H, Saito S, Oikawa A, Suzuki H, Sakurai N, Shibata D, Wadano A, Sakata K, Ohta D. Cytochrome P450 CYP710A encodes the sterol C-22 desaturase in Arabidopsis and tomato. Plant Cell. 2006;18:1008–22. doi: 10.1105/tpc.105.036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arnqvist L, Persson M, Jonsson L, Dutta PC, Sitbon F. Overexpression of CYP710A1 and CYP710A4 in transgenic Arabidopsis plants increases the level of stigmasterol at the expense of sitosterol. Planta. 2008;227:309–317. doi: 10.1007/s00425-007-0618-8. [DOI] [PubMed] [Google Scholar]
- 36.Skaggs BA, Alexander JF, Pierson CA, Schweitzer KS, Chun KT, Koegel C, Barbuch R, Bard M. Cloning and characterization of the Saccharomyces cerevisiae C-22 sterol desaturase gene, encoding a second cytochrome P-450 involved in ergosterol biosynthesis. Gene. 1996;169:105–109. doi: 10.1016/0378-1119(95)00770-9. [DOI] [PubMed] [Google Scholar]
- 37.Kelly SL, Lamb DC, Baldwin BC, Corran AJ, Kelly DE. Characterization of Saccharomyces cerevisiae CYP61, sterol Δ22-desaturase, and inhibition by azole antifungal agents. J. Biol. Chem. 1997;272:9986–9988. doi: 10.1074/jbc.272.15.9986. [DOI] [PubMed] [Google Scholar]
- 38.Rettie AE, Rettenmeier AW, Howald WN, Baillie TA. Cytochrome P-450 catalyzed formation of δ4-VPA, a toxic metabolite of valproic acid. Science. 1987;235:890–893. doi: 10.1126/science.3101178. [DOI] [PubMed] [Google Scholar]
- 39.Guengerich FP. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity. Chem. Res. Toxicol. 2001;14:611–650. doi: 10.1021/tx0002583. [DOI] [PubMed] [Google Scholar]
- 40.Ortiz de Montellano PR. In: Substrate oxidation, in Cytochrome P450: Structure, Mechanism, and Biochemistry. 4th Ortiz de Montellano PR, editor. Springer; New York: 2015. pp. 111–176. [Google Scholar]
- 41.Broun P, Shanklin J, Whittle E, Somerville C. Catalytic plasticity of fatty acid modification enzymes underlying chemical diversity of plant lipids. Science. 1998;282:1315–7. doi: 10.1126/science.282.5392.1315. [DOI] [PubMed] [Google Scholar]
- 42.Broadwater JA, Whittle E, Shanklin J. Desaturation and hydroxylation. Residues 148 and 324 of Arabidopsis FAD2, in addition to substrate chain length, exert a major influence in partitioning of catalytic specificity. J. Biol. Chem. 2002;277:15613–20. doi: 10.1074/jbc.M200231200. [DOI] [PubMed] [Google Scholar]