Abstract
Although microRNAs (miRNAs) have been widely studied as epigenetic regulation molecules, fewer studies focus on the gender difference at the miRNA and isomiR expression levels. In this study, we aim to understand the potential relationships between gender difference and miRNA/isomiR expression through a comprehensive analysis of small RNA-sequencing datasets based on different human diseases and tissues. Based on specific samples from males and females, we determined that some miRNAs may be diversely expressed between different tissues and genders. Thus, these miRNAs may exhibit inconsistent and even opposite expression between males and females. According to deregulated miRNA expression profiles, some dominantly expressed miRNA loci were selected to analyze isomiR expression patterns using rates of dominant isomiRs. In some miRNA loci, isomiRs showed statistical significance between tumor and normal samples and between males and females samples, suggesting that isomiR expression patterns are not always invariable but may vary between males and females, as well as among different tissues, tumors, and normal samples. The divergence implicates the fluctuation in the expression of miRNA and its detailed expression at the isomiR levels. The divergence also indicates that gender difference may be an important factor that affects the screening of disease-associated miRNAs and isomiRs. This study suggests that miRNA/isomiR expression and gender difference may be more complex than previously assumed and should be further studied according to specific samples from males or females.
Introduction
A class of small non-coding RNAs (ncRNAs), microRNAs (miRNAs), has been widely studied as endogenous negative regulatory molecules to control post-transcriptional gene expression [1]. miRNAs play a pivotal role in coding-non-coding RNA regulatory networks, and many abnormally expressed miRNAs were proven to be associated with human diseases, particularly in promoting carcinogenesis in some cancers [2, 3]. Although a single miRNA sequence is believed to come from a miRNA locus, a series of heterogeneous sequences have been widely detected and identified in both human and plant miRNAs by comprehensively analyzing high-throughput sequencing datasets [4–9]. Some isomiRs have been proven as functional small RNAs by associating with target mRNAs [6, 10–13] and influencing miRNA stability [14] or effectiveness [15, 16]. More studies focus on these multiple miRNA variants because of the versatile roles of isomiRs, particularly those dominantly expressed isomiRs, and isomiRs with 5’ variation and/or 3’ additions. Simultaneously, the analysis of miRNA-miRNA or isomiR-isomiR interaction combined with homologous and/or clustered miRNAs is necessary from the miRNA/isomiR levels [17], and miRNA may be thoroughly studied from the detailed isomiR expression.
Some relevant biological molecules, including proteomes, mRNA, and miRNA, were reported to be differently expressed or enriched between samples from males and females [18–21]. Specifically, some miRNAs were determined to have various expressions between genders [22, 23], and the promoter methylation of miR-137 was validated as a female-associated molecule [24]. Loher et al. [25] found that expressions of many isomiRs diverged between males and females. These findings demonstrate that miRNA or isomiR expression may be related with gender difference, and gender-associated miRNA or isomiR expression and function should be not ignored. Typical analyses and studies always disregard the relationship of miRNA or isomiR expression and gender difference, and some gender-related miRNAs may be ignored or balanced using the typical analysis. However, fewer systematic studies are carried out, particularly those that are based on the miRNA or isomiR biogenesis and the expression between males and females across different diseases and tissues.
In this study, based on the potential importance and relationships of miRNA/isomiR expression and gender difference, a comprehensive analysis was performed using specific and common diseases in males and females. The study aimed to explore the divergence of miRNA/isomiR expression profiles and gender difference at the miRNA and isomiR levels, respectively. Specifically, the potential expression divergence was analyzed between different tissues, tumor and normal groups, and isomiR expression patterns in different tissues and genders. According to miRNA/isomiR characteristics and gender difference, the study may provide more information and implications for studies on miRNA and isomiR, particularly specific miRNA and isomiR expression profiles in specific human diseases.
Materials and Methods
Flowchart
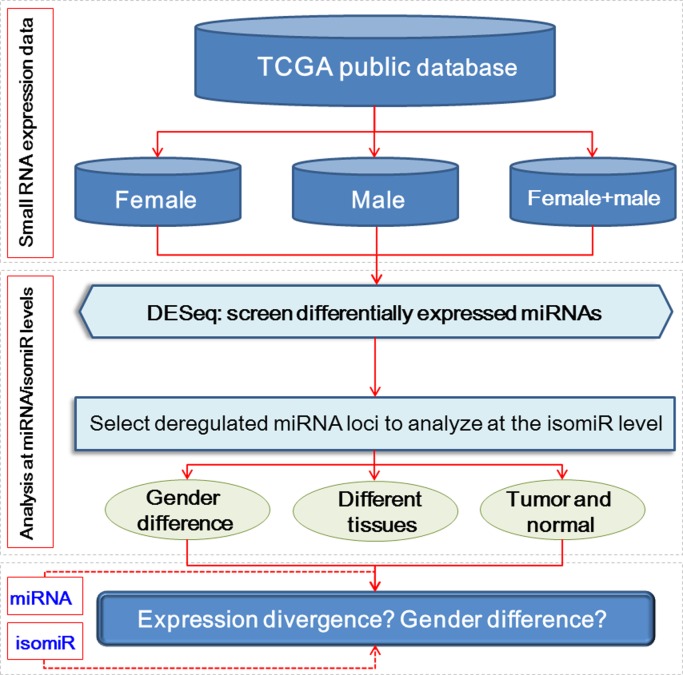
A flowchart of a comprehensive analysis of miRNA and isomiR expression was indicated in Fig 1, which would contribute in determining the relationship of miRNA/isomiR expression and gender difference.
Fig 1. The flowchart to analyze miRNA/isomiR in the study.
Source data
The small RNA deep sequencing datasets used in this work were obtained from The Cancer Genome Atlas (TCGA) pilot project (https://tcga-data.nci.nih.gov/tcga/), and all the data were sequenced by the Illumina HiSeq sequencing platform. These datasets included small RNA sequencing of tumor and control samples from females (a specific disease in females, uterine corpus endometrial carcinoma (UCEC)), males (specific disease in males, prostate adenocarcinoma (PRAD)), and females and males (common diseases in males and females, lung squamous cell carcinoma (LUSC) and thyroid carcinoma (THCA)). Equal sample sizes were randomly obtained from the corresponding tumor samples based on the same and similar characteristics because of the divergence of sample sizes between tumor and normal samples (S1 Table).
Sequence and expression analysis
Small RNA expression profiles were first collected from TCGA (the original sequencing data were analyzed by mapping analysis), and these profiles were further analyzed at the miRNA and isomiR levels. Differentially expressed miRNAs were obtained using DESeq package [26] according to miRNA expression profiles if the profiles were statistically diverged and had relative abundant enrichment levels. Based on the obtained abnormal miRNA expression profiles, some abundantly expressed miRNA loci were selected to further screen the isomiR expression profiles (Fig 1).
According to all isomiRs from a specific miRNA locus, the relative expression level of each isomiR type was estimated using the percentage in each individual. The average percentages and standard deviations were used to describe isomiR expression according to the multiple individuals in a sample. The rates of the top four dominant isomiRs were selected to estimate the isomiR expression patterns based on several dominant isomiRs in the miRNA locus [4,9]. Specifically, the rates of dominant isomiRs were employed, including the rate of the most dominant and secondary dominant isomiRs, the rate of the most dominant and the third dominant isomiRs, and the rate of the most dominant and the fourth dominant isomiRs.
Statistical analysis
Venny’s distribution of deregulated expressed miRNAs was constructed using Venny web server 2.0 (http://bioinfogp.cnb.csic.es/tools/venny/index.html). The expression data at the miRNA/isomiR levels were described as mean ± standard deviation ( ± SD). Differentially expressed miRNA species were collected by utilizing a DESeq package that contained statistical analysis, including multiple comparison correction using false discovery rate (FDR). The rates of dominant isomiRs were adopted to estimate isomiR expression, and the distribution of each rate followed the normal distribution. A t test was used to estimate the difference between tumor and relevant normal tissues, and between samples from males and females in each disease. Furthermore, ANOVA analysis was employed to estimate the isomiR expression difference among different groups. If the P or Padj values (the associated FDR) were less than 0.05, the difference would be considered a statistical difference.
Results
Expression analysis at the miRNA level
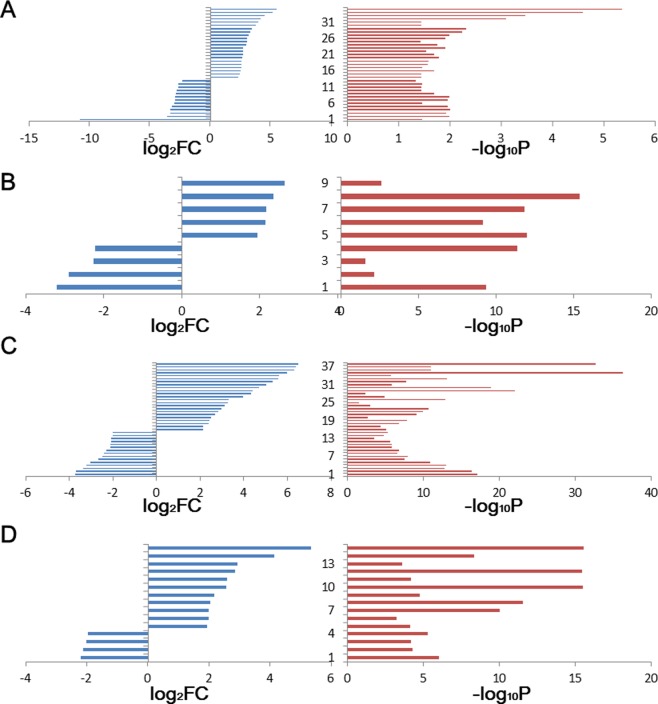
Based on relative expression levels at the miRNA level (the average sequence counts were more than 50 in tumor or normal tissues) (Fig 1 and S1 Table), a series of miRNAs were collected as significant abnormally expressed species in tumor tissues (|Log2(Fold-change)| ≥ 2.0 and Padj < 0.05), and the up-regulated miRNAs in these tumor samples were dominant (Fig 2). A total of 38 deregulated miRNAs were obtained in PRAD and followed by 35 miRNAs in UCEC, whereas less deregulated expressed miRNAs were detected in PRAD (9) and LUSC (15) according to the standard (S1A Fig). Among these deregulated miRNAs, less common species were detected across different tissues (S1B Fig).
Fig 2. Distributions of deregulated miRNAs in the 4 kinds of diseases.
A: UCEC; B: PRAD; C: LUSC; D: THCA.
Based on deregulated miRNAs and their relative expression levels (average sequence counts were more than 5000 in tumor or normal tissues, which could ensure further analysis at the isomiR levels), some typical miRNA loci were presented in S2 Table. Further analysis was performed according to the female and male samples in the same tissues (Table 1 and S2 Fig). A series of miRNAs were identified as deregulated species between different genders with significant difference. However, specific deregulated species were quite dominant (S2 Fig). Some specific miRNA, such as miR-375 in LUSC, exhibited opposite expression patterns in females (up-regulated, log2FC = 1.53) and males (down-regulated, log2FC = -2.73), whereas the miRNA was identified as normally expressed miRNA based on the mixed samples (log2FC = -0.35). Interestingly, the specific miRNA, miR-375, was identified as up-regulated miRNA in female-specific UCEC (log2FC = 2.61, P = 0.0262), whereas it was also determined as up-regulated miRNA without statistical significance in male-specific PRAD (log2FC = 2.15, P = 6.93E-10) (S2 Table).
Table 1. Screened deregulated miRNAs in males (n = 12) and females groups (n = 12).
| Down-regulated | Up-regulated | |||||
|---|---|---|---|---|---|---|
| Disease sample | miRNA | Log2(FC) | Padj | miRNA | Log2(FC) | Padj |
| Female-LUSC | miR-144 | -5.05 | 0.0012 | miR-205 | 7.31 | 2.59E-06 |
| miR-451 | -5.06 | 0.0012 | miR-1269 | 8.94 | 3.92E-06 | |
| miR-486 | -4.75 | 0.0025 | miR-210 | 5.18 | 0.0005 | |
| miR-30a | -3.09 | 0.0427 | miR-183 | 3.74 | 0.0230 | |
| miR-338 | -3.16 | 0.0433 | miR-9 | 6.79 | 0.0300 | |
| Male-LUSC | miR-451 | -4.19 | 6.81E-07 | miR-205 | 7.68 | 2.59E-22 |
| miR-30a | -3.23 | 4.48E-05 | miR-210 | 5.15 | 1.45E-05 | |
| miR-101 | -3.11 | 0.0002 | miR-183 | 3.27 | 9.68E-05 | |
| miR-30d | -3.03 | 0.0009 | miR-9 | 5.62 | 0.0026 | |
| miR-375 | -2.73 | 0.0023 | miR-203 | 2.24 | 0.0046 | |
| miR-140 | -2.63 | 0.0047 | miR-182 | 2.38 | 0.0078 | |
| miR-29c | -2.58 | 0.0060 | miR-141 | 2.05 | 0.0324 | |
| miR-100 | -2.25 | 0.0107 | ||||
| miR-143 | -2.09 | 0.0175 | ||||
| miR-181a | -2.22 | 0.0465 | ||||
| Female-THCA | miR-451 | -2.51 | 0.0002 | miR-183 | 2.67 | 9.42E-05 |
| miR-486 | -2.40 | 0.0002 | miR-221 | 2.70 | 0.0006 | |
| miR-144 | -2.26 | 0.0005 | miR-182 | 2.35 | 0.0012 | |
| Male-THCA | miR-146b | 5.97 | 4.69E-05 | |||
| miR-221 | 3.29 | 0.0279 | ||||
Note: The sample size is selected according to the actual distribution of females and males in the normal sample, and the relevant selected individuals are collected according to the principle of homogeneous.
Expression analysis at the isomiR level in different diseases
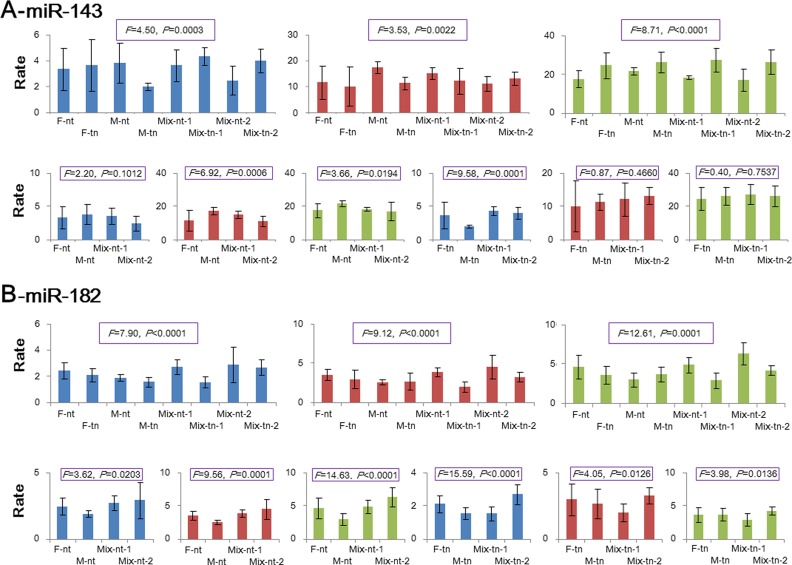
Deregulated miRNAs were analyzed based on the sum of all varied sequences, which were also called isomiR sequences in the corresponding miRNA gene loci, whereas the detailed sequences were not further analyzed. According to versatile biological roles and potential function, these isomiR sequences should be further involved in the in-depth analysis. Therefore, some common deregulated miRNA loci were selected to perform isomiR expression analysis based on gender-specific diseases and the common diseases, including down-regulated miR-143 and up-regulated miR-182 (S2 Table). The rates of dominant isomiRs from the two miRNA loci exhibited significant difference across the eight groups (Fig 3). Based on all the normal and tumor groups across different tissues, the rates of dominant isomiRs showed diverse expression except for miR-143 in normal groups. The rate of the most dominant and secondary dominant isomiRs showed a statistical difference among normal and tumor groups (Fig 3).
Fig 3. Expression distribution of rates of dominant isomiRs across different groups.
The F statistic and P values are also presented using ANOVA analysis among different groups. F-nt: female-UCEC-nt; F-tn: female-UCEC-tn; M-nt: male-PRAD-nt; M-tn: male-PRAD-tn; Mix-nt-1: mixed-LUSC-nt; Mix-tn-1: mixed-LUSC-tn; Mix-nt-2: mixed-THCA-nt; Mix-tn-2: mixed-THCA-tn. The blue bar indicates the rate of the most dominant isomiR and the secondary dominant isomiR, the red bar indicates the rate of the most dominant isomiR and the third dominant isomiR, and the green bar indicated the rate of the most dominant isomiR and the fourth dominant isomiR.
For each pair of tumor, the corresponding normal samples, and male and female samples, a relevant t test was used to estimate the difference. As expected, isomiR expression may show a significant difference between tumor and control samples, particularly for miR-143 in male-specific PRAD disease and miR-182 in LUSC disease (S3 Table). The two miRNA loci showed diverse divergence between other pairwise samples, and they may also exhibit significant difference between various tissues, although the difference was more common between males and females. miRNA locus may generate inconsistent isomiR expression patterns between different gender-specific diseases, between different common diseases, and between males and females (S3 Table).
Expression analysis at the isomiR level between males and females
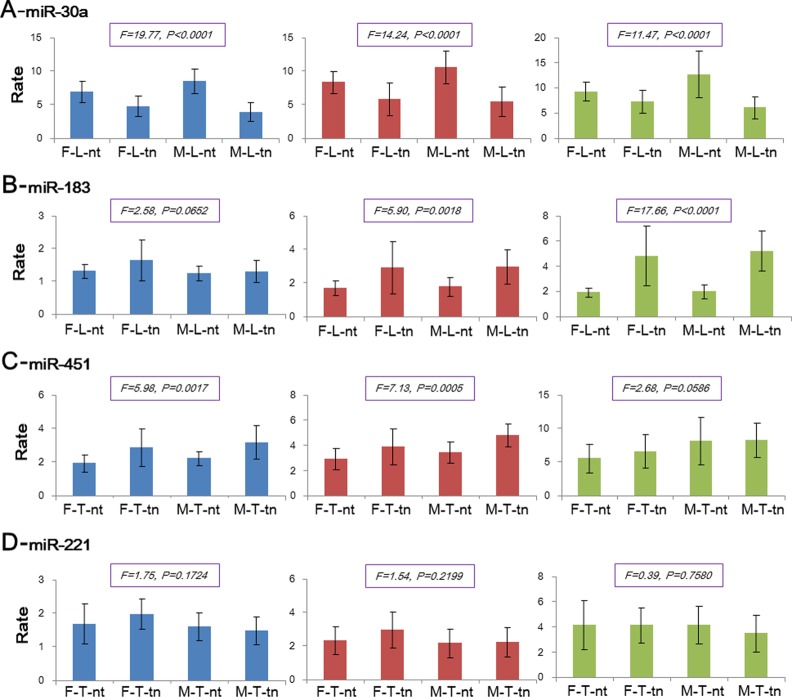
We further selected the common dominantly expressed miRNA loci to track isomiR expression patterns between different genders based on the same human diseases between males and females, including the diseases LUSC and THCA. Some miRNAs were collected to understand the isomiR expression in LUSC, including down-regulated miRNAs (miR-451 and miR-30a) and up-regulated miRNAs (miR-205, miR-210, miR-183, and miR-9). In THCA, only one up-regulated miR-221 was collected, and the further extension of down-regulated miR-451 was selected although it was identified as down-regulated miRNA without statistical significance in the male–THCA group (log2FC = -1.83, Padj = 0.6557).
Expression analysis showed that most of these deregulated miRNAs had similar isomiR expression patterns across the four groups with pairwise male and female groups. However, miR-30a loci exhibited statistical difference in all the most dominant isomiRs (P < 0.0001), and other miRNAs may show significant difference in some certain rates of dominant isomiR species (Fig 4). For example, in the miR-183 locus, the rate of the most dominant and secondary dominant isomiRs was similar across different groups, but other rates showed significant difference (P = 0.0018 and P < 0.0001, respectively, Fig 4). In the miR-451 locus, except for the rate of most dominant and fourth dominant isomiRs, other rates differed, which also demonstrated that the miRNA locus could generate different isomiR expression profiles. Further statistical analysis based on pairwise male and female samples and tumor and normal samples in specific disease were performed using an unpaired t test. The isomiR expression of the miR-30a locus showed statistical difference. The rate of the most dominant and secondary dominant isomiRs in miR-451 differed between normal and tumor samples in males and females, whereas other miRNA loci exhibited similar isomiR expression between normal and tumor samples (Table 2). For the expression between males and females, miR-30a showed significant expression divergence in normal tissues, whereas no significant difference was detected in tumor samples. The rate of the most dominant and secondary dominant isomiRs varied between tumors from males and females (t = 2.4642, P = 0.0254), whereas other miRNA loci showed similar isomiR expression (Table 2).
Fig 4. Expression distribution of dominant isomiRs across female and male groups in the specific common diseases.
F-L-nt: female LUSC control group; F-L-tn: female LUSC tumor group; M-L-nt: male LUSC control group; M-L-tn: male LUSC tumor group; F-T-nt: female THCA control group; F-T-tn: female THCA tumor group; M-T-nt: male THCA normal group; M-T-tn: male THCA tumor group. Other detailed annotations can be found in Fig 3.
Table 2. Statistical analysis between paired tumor and normal samples or male and female samples using t test in Fig 4.
| miRNA | Tissues | The first/second | The first/third | The first/fourth |
|---|---|---|---|---|
| miR-30a | Female: LUSC-nt & LUSC-tn | t = 3.3228, P = 0.0031 | t = 2.9598, P = 0.0072 | t = 2.2636, P = 0.0338 |
| Male: LUSC-nt & LUSC-tn | t = 6.7896, P<0.0001 | t = 5.4469, P<0.0001 | t = 4.4999, P = 0.0002 | |
| LUSC-nt: Female & male | t = -2.2102, P = 0.0378 | t = -2.6317, P = 0.0152 | t = -2.3787, P = 0.0265 | |
| LUSC-tn: Female & male | t = 1.4378, P = 0.1646 | t = 0.3988, P = 0.6939 | t = 1.3850, P = 0.1799 | |
| miR-183 | Female: LUSC-nt & LUSC-tn | t = -1.6891, P = 0.1053 | t = -2.6513, P = 0.0146 | t = -4.1722, P = 0.0004 |
| Male: LUSC-nt & LUSC-tn | t = -0.4891, P = 0.6296 | t = -3.4908, P = 0.0021 | t = -6.7018, P = 0.0001 | |
| LUSC-nt: Female & male | t = 0.8350, P = 0.4127 | t = -0.3776, P = 0.7093 | t = -0.2330, P = 0.8179 | |
| LUSC-tn: Female & male | t = 1.6558, P = 0.1119 | t = -0.0634, P = 0.9500 | t = -0.4613, P = 0.6491 | |
| miR-451 | Female: THCA-nt & THCA-tn | t = -2.6401, P = 0.0153 | t = -2.0416, P = 0.0540 | t = -1.0727, P = 0.2956 |
| Male: THCA-nt & THCA-tn | t = -3.0656, P = 0.0057 | t = -3.7824, P = 0.0010 | t = -0.0637, P = 0.9498 | |
| THCA-nt: Female & male | t = -1.4825, P = 0.1524 | t = -1.4711, P = 0.1554 | t = -2.1874, P = 0.0396 | |
| THCA-tn: Female & male | t = -0.7010, P = 0.4910 | t = -1.7565, P = 0.0936 | t = -1.5505, P = 0.1360 | |
| miR-221 | Female: THCA-nt & THCA-tn | t = -1.2174, P = 0.2384 | t = -1.4958, P = 0.1511 | t = 0.0682, P = 0.9463 |
| Male THCA-nt & THCA-tn | t = 0.7464, P = 0.4646 | t = -0.1590, P = 0.8753 | t = 1.0025, P = 0.3287 | |
| THCA-nt: Female & male | t = 0.3472, P = 0.7317 | t = 0.5005, P = 0.6217 | t = 0.0353, P = 0.9722 | |
| THCA-tn: Female & male | t = 2.4642, P = 0.0254 | t = 1.5584, P = 0.1387 | t = 0.9358, P = 0.3633 |
Other deregulated miRNAs were only analyzed at the isomiR levels in tumor or normal tissues of males and females because these miRNAs were rarely detected in the corresponding normal or tumor tissues. All of these miRNAs showed similar expression using the three kinds of rates, although some of them tended to involve in a larger tendency of dispersion (S3 Fig), particularly for the rate of the most dominant and the fourth dominant isomiRs.
The results shown here are in whole based upon data generated by the TCGA Research Network: http://cancergenome.nih.gov/.
Discussion
The crucial roles of miRNAs in various diseases have attracted many researches, and disease-related miRNAs, including their target mRNAs, can be predicted based on biological interaction network [27–35]. Systematic analysis and study of roles and interactions of miRNAs in specific biological process, such as cell death [36–38], cell proliferation [39, 40], have been performed in diverse human diseases. The phenomenon of multiple isomiRs further enriches the miRNA/isomiR study, and disease-related isomiRs may be next crucial markers to study occurrence and development of diseases. Although the phenomenon of isomiRs was first detected by analyzing deep-sequencing small RNA datasets, these miRNA variants with various sequences and expressions gained the attention of researchers based on their versatile biological functions [6, 10–16]. In the specific miRNA locus, different isomiRs may demonstrate diverse deregulated expression patterns and adverse expression, although these sequence-related isomiRs may have close functional relationships, including co-regulating target mRNAs. The typical analysis of miRNAs without considering the isomiR level may be a partial solution, and the comprehensive analysis from the isomiR level is quite important, particularly because some deregulated miRNAs are prone to detect abnormal isomiR expression patterns [41]. Loher et al. reported that isomiR expression profiles may be population-dependent and gender-dependent [25], particular for the diversion of gene expression and protein between males and females [18–21]. For small non-coding RNAs, more studies focused on the gender-specific miRNAs or miRNA expression with gender difference [42–45], but systematic analysis of isomiR expression based on miRNA loci and studies based on deregulated miRNA loci are rare. Is the gender-dependent isomiR expression profile highly significant? This study may provide more implications for miRNA and isomiR studies in gender-relevant samples. Based on these results and our previous studies, we aimed to explore the potential isomiR expression in different human diseases and genders, including male-specific and female-specific diseases and diseases for both genders. Further analysis was performed between males and females using the common tissues and diseases.
Diversely deregulated miRNA and isomiR expression profiles can be found in specific diseases from males and females (Fig 2, S2 Table, and S1 Fig). Although some miRNAs are identified as common deregulated species in gender-specific diseases, such as deregulated miR-182 and miR-183, these miRNAs always exhibit different levels of up- or down-regulated expression (S2 Table). Most of these miRNAs were studied as crucial miRNAs in the occurrence and development of some diseases. For example, miR-182 and miR-183 are identified as oncogenic miRNAs and contribute to early breast cancer development [46]. Specific miRNAs may show opposite expression patterns between females and males, whereas they may be identified as normally expressed miRNAs using the mixed samples. Thus, some abnormally expressed miRNAs may be disregarded or balanced in typical analysis using mixed genders where gender may be an important factor in examining miRNA. Disease-associated miRNAs are always selected and identified from these deregulated miRNAs, and inconsistent and abnormal expression profiles reveal that the small RNA expression may be influenced by gender difference. Diverse miRNA expression profiles lead to diverse isomiR profiles, which complicate and interrupt further experimental validation of disease-associated miRNAs.
IsomiR expressions in selected common deregulated miRNA loci, including miR-143 and miR-182, differ across the four groups with different diseases (P < 0.01, Fig 3). Analyses of isomiRs in normal and tumor samples showed that rates of dominant isomiRs may be similar or diverged among different genders and tissues (from different diseases), thus suggesting that isomiR expressions may diverge between genders and tissues (Fig 3). The analysis also focuses gender difference and tissue difference, which may contribute to various isomiR expressions. After further pairwise comparisons of isomiRs between normal and tumor samples, two miRNAs may be significantly diverged between some tumor and normal samples (P < 0.05) or have similar expression between tumor and normal samples (P > 0.05, S3 Table). The three rates of dominant isomiRs also have an inconsistent expression. However, the rate of the top-dominant isomiRs may be most important mark to estimate the isomiR expression (the top two dominant isomiRs may possess nearly 80% expression in certain miRNA loci). These findings indicate that the selected deregulated miRNA loci may generate inconsistent isomiR expression between genders, normal and tumor samples, and different tissues. Although analysis at the miRNA level indicates that these miRNAs are abnormally expressed in tumor samples, the detailed and real expression at the isomiR levels may vary. The divergence of isomiR expression patterns implies that isomiRs may be diverged between different tissues and genders, which may affect the selection of deregulated miRNAs for further experimental validation. More importantly, in these multiple isomiRs, some isomiRs with varied or shifted “seed sequences” may also have higher enrichment levels. However, the changed functional regions may result in new target mRNAs or binding sites with target mRNAs. These isomiRs may enrich the function in the miRNA locus, and analysis at the isomiR levels may be particularly considered in studies on miRNAs.
To further understand the potential expression divergence between males and females in the same tissues, two common diseases in males and females (LUSC and THCA) were selected to analyze isomiRs in some miRNA loci between males and females. These selected miRNAs were identified as deregulated species. However, not all of these miRNA loci showed diverged isomiR expression across different groups (such as miR-183 and miR-221, Fig 4). Similarly, pairwise comparisons of isomiRs demonstrate that miR-30a and miR-221 diverged between genders; and miR-30a and miR-451 are diverged between normal and tumor samples (Table 2). These findings suggest that isomiRs from a specific miRNA locus may vary between males and females and between normal and tumor samples in the specific samples. However, typical analysis at the miRNA level could disregard this divergence, particularly when mixed samples from females and males are adopted. The final screening of deregulated miRNAs would not reflect the real expression and change at the isomiR levels. Analysis at the isomiR level can lead to inconsistent expression patterns although these isomiRs are generated from the specific miRNA locus. These multiple isomiRs always have close functional relationships (the same seed sequences or shifted seed sequence) [41]. A comprehensive analysis at the miRNA and isomiR levels is necessary to further reveal the complex small RNAs.
Taken together, this study determined that miRNA and isomiR expressions may diverge between normal and tumor samples and between females and males. These expression divergences suggest that gender may be an important factor in miRNA and isomiR expression. A canonical analysis of miRNA/isomiR without considering gender difference may disregard some deregulated miRNAs, and isomiRs in miRNA loci, which would increase the false positive rate. Candidate disease-associated miRNAs should be screened and examined better based on gender-difference at the isomiR levels.
Supporting Information
(TIF)
(TIF)
These deregulated miRNA loci are only abundantly expressed in tumor or control groups. The detailed annotations can be found in Figs 3 and 4.
(TIF)
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work has been supported by the National Natural Science Foundation of China (Nos. 61301251, 31401009 and 61370010), the Research Fund for the Doctoral Program of Higher Education of China (No. 20133234120009), the National Natural Science Foundation of Jiangsu (No. BK20130885), the Natural Science Foundation of the Jiangsu Higher Education Institutions (No. 13KJB330003), the State Key Laboratory of Medicinal Chemical Biology, Shandong Provincial Key Laboratory of Functional Macromolecular Biophysics, the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and sponsored by NUPTSF (No. NY215068). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–33. 10.1016/j.cell.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benderska N, Dittrich AL, Knaup S, Rau TT, Neufert C, Wach S, et al. miRNA-26b Overexpression in Ulcerative Colitis-associated Carcinogenesis. Inflamm Bowel Dis. 2015;21(9):2039–51. Epub 2015/06/18. 10.1097/MIB.0000000000000453 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li X, Wu J, Zheng J, Li Y, Yang T, Hu G, et al. Altered miRNA expression profiles and miR-1a associated with urethane-induced pulmonary carcinogenesis. Toxicol Sci. 2013;135(1):63–71. Epub 2013/06/14. 10.1093/toxsci/kft131 [pii]. . [DOI] [PubMed] [Google Scholar]
- 4.Guo L, Yang Q, Lu J, Li H, Ge Q, Gu W, et al. A Comprehensive Survey of miRNA Repertoire and 3' Addition Events in the Placentas of Patients with Pre-eclampsia from High-throughput Sequencing. PloS one. 2011;6(6):e21072 10.1371/journal.pone.0021072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129(7):1401–14. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee LW, Zhang S, Etheridge A, Ma L, Martin D, Galas D, et al. Complexity of the microRNA repertoire revealed by next generation sequencing. Rna-a Publication of the Rna Society. 2010;16:2170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morin RD, Aksay G, Dolgosheina E, Ebhardt HA, Magrini V, Mardis ER, et al. Comparative analysis of the small RNA transcriptomes of Pinus contorta and Oryza sativa. Genome Research. 2008;18(4):571–84. 10.1101/gr.6897308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neilsen CT, Goodall GJ, Bracken CP. IsomiRs—the overlooked repertoire in the dynamic microRNAome. Trends Genet. 2012;28(11):544–9. 10.1016/j.tig.2012.07.005 [DOI] [PubMed] [Google Scholar]
- 9.Guo L, Chen F. A Challenge for miRNA: Multiple IsomiRs in miRNAomics. Gene. 2014;544:1–7. 10.1016/j.gene.2014.04.039 [DOI] [PubMed] [Google Scholar]
- 10.Cloonan N, Wani S, Xu Q, Gu J, Lea K, Heater S, et al. MicroRNAs and their isomiRs function cooperatively to target common biological pathways. Genome Biol. 2011;12(12):R126 10.1186/gb-2011-12-12-r126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llorens F, Banez-Coronel M, Pantano L, del Rio JA, Ferrer I, Estivill X, et al. A highly expressed miR-101 isomiR is a functional silencing small RNA. BMC Genomics. 2014;14:104 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babapoor S, Fleming E, Wu R, Dadras SS. A novel miR-451a isomiR, associated with amelanotypic phenotype, acts as a tumor suppressor in melanoma by retarding cell migration and invasion. PloS one. 2014;9(9):e107502 10.1371/journal.pone.0107502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan GC, Chan E, Molnar A, Sarkar R, Alexieva D, Isa IM, et al. 5' isomiR variation is of functional and evolutionary importance. Nucleic Acids Res. 2014;42(14):9424–35. 10.1093/nar/gku656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez-Valverde SL, Taft RJ, Mattick JS. Dynamic isomiR regulation in Drosophila development. Rna-a Publication of the Rna Society. 2010;16(10):1881–8. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burroughs AM, Ando Y, de Hoon MJL, Tomaru Y, Nishibu T, Ukekawa R, et al. A comprehensive survey of 3 ' animal miRNA modification events and a possible role for 3 ' adenylation in modulating miRNA targeting effectiveness. Genome Research. 2010;20(10):1398–410. 10.1101/gr.106054.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu SF, Sun YH, Chiang VL. Adenylation of plant miRNAs. Nucleic Acids Research. 2009;37(6):1878–85. 10.1093/nar/gkp031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo L, Zhang H, Zhao Y, Yang S, Chen F. In-depth exploration of miRNA: a new approach to study miRNA at the miRNA/isomiR levels. Current Bioinformatics. 2014;9:522–30. [Google Scholar]
- 18.Eidelman O, Jozwik C, Huang W, Srivastava M, Rothwell SW, Jacobowitz DM, et al. Gender dependence for a subset of the low-abundance signaling proteome in human platelets. Hum Genomics Proteomics. 2010;2010:164906 10.4061/2010/164906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miike K, Aoki M, Yamashita R, Takegawa Y, Saya H, Miike T, et al. Proteome profiling reveals gender differences in the composition of human serum. Proteomics. 2010;10(14):2678–91. 10.1002/pmic.200900496 [DOI] [PubMed] [Google Scholar]
- 20.Dai R, Ahmed SA. Sexual dimorphism of miRNA expression: a new perspective in understanding the sex bias of autoimmune diseases. Ther Clin Risk Manag. 2014;10:151–63. 10.2147/TCRM.S33517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan H, Dong G, Zhao G, Liu F, Yao G, Zhu Y, et al. Gender differences of B cell signature in healthy subjects underlie disparities in incidence and course of SLE related to estrogen. J Immunol Res. 2014;2014:814598 10.1155/2014/814598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duttagupta R, Jiang R, Gollub J, Getts RC, Jones KW. Impact of cellular miRNAs on circulating miRNA biomarker signatures. PloS one. 2011;6(6):e20769 10.1371/journal.pone.0020769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang K, Yuan Y, Cho JH, McClarty S, Baxter D, Galas DJ. Comparing the MicroRNA spectrum between serum and plasma. PloS one. 2012;7(7):e41561 10.1371/journal.pone.0041561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langevin SM, Stone RA, Bunker CH, Grandis JR, Sobol RW, Taioli E. MicroRNA-137 promoter methylation in oral rinses from patients with squamous cell carcinoma of the head and neck is associated with gender and body mass index. Carcinogenesis. 2010;31(5):864–70. 10.1093/carcin/bgq051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loher P, Londin ER, Rigoutsos I. IsomiR Expression Profiles in Human Lymphoblastoid Cell Lines Exhibit Population and Gender Dependencies. Oncotarget. 2014;5(18):8790–802. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11(10):R106 10.1186/gb-2010-11-10-r106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng X, Zhang X, Zou Q. Integrative approaches for predicting microRNA function and prioritizing disease-related microRNA using biological interaction networks. Brief Bioinform. 2016;17(2):193–203. Epub 2015/06/11. 10.1093/bib/bbv033 [pii]. . [DOI] [PubMed] [Google Scholar]
- 28.Zou Q, Li J, Zeng X, Wang G. Similarity computation strategies in the microRNA-disease network: a survey. Brief Funct Genomics. 2016;15(1):55–64. 10.1093/bfgp/elv024 [DOI] [PubMed] [Google Scholar]
- 29.Zou Q, Li J, Wang C, Zeng X. Approaches for recognizing disease genes based on network. Biomed Res Int. 2014;2014:416323 Epub 2014/04/08. 10.1155/2014/416323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng X, Liao Y, Liu Y, Zou Q. Prediction and validation of disease genes using HeteSim Scores. IEEE/ACM Trans Comput Biol Bioinform. 2016. Epub 2016/02/19. 10.1109/TCBB.2016.2520947 . [DOI] [PubMed] [Google Scholar]
- 31.Mork S, Pletscher-Frankild S, Palleja Caro A, Gorodkin J, Jensen LJ. Protein-driven inference of miRNA-disease associations. Bioinformatics. 2014;30(3):392–7. Epub 2013/11/26. 10.1093/bioinformatics/btt677 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nalluri JJ, Kamapantula BK, Barh D, Jain N, Bhattacharya A, Almeida SS, et al. DISMIRA: Prioritization of disease candidates in miRNA-disease associations based on maximum weighted matching inference model and motif-based analysis. BMC Genomics. 2015;16 Suppl 5:S12 Epub 2015/06/05. 10.1186/1471-2164-16-S5-S12 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X, Wu D, Chen L, Li X, Yang J, Fan D, et al. RAID: a comprehensive resource for human RNA-associated (RNA-RNA/RNA-protein) interaction. RNA. 2014;20(7):989–93. Epub 2014/05/08. 10.1261/rna.044776.114 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, Wang C, Miao Z, Bi X, Wu D, Jin N, et al. ViRBase: a resource for virus-host ncRNA-associated interactions. Nucleic Acids Res. 2015;43(Database issue):D578–82. Epub 2014/10/03. 10.1093/nar/gku903 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Chen L, Chen B, Li X, Kang J, Fan K, et al. Mammalian ncRNA-disease repository: a global view of ncRNA-mediated disease network. Cell Death Dis. 2013;4:e765 Epub 2013/08/10. 10.1038/cddis.2013.292 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu D, Huang Y, Kang J, Li K, Bi X, Zhang T, et al. ncRDeathDB: A comprehensive bioinformatics resource for deciphering network organization of the ncRNA-mediated cell death system. Autophagy. 2015;11(10):1917–26. Epub 2015/10/03. 10.1080/15548627.2015.1089375 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Zhuang L, Wang Y, Hu Y, Wu Y, Wang D, et al. Connect the dots: a systems level approach for analyzing the miRNA-mediated cell death network. Autophagy. 2013;9(3):436–9. Epub 2013/01/17. 10.4161/auto.23096 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prajapati P, Sripada L, Singh K, Bhatelia K, Singh R. TNF-alpha regulates miRNA targeting mitochondrial complex-I and induces cell death in dopaminergic cells. Biochimica et biophysica acta. 2015;1852(3):451–61. Epub 2014/12/08. 10.1016/j.bbadis.2014.11.019 [pii]. . [DOI] [PubMed] [Google Scholar]
- 39.Fan SJ, Li HB, Cui G, Kong XL, Sun LL, Zhao YQ, et al. miRNA-149* promotes cell proliferation and suppresses apoptosis by mediating JunB in T-cell acute lymphoblastic leukemia. Leuk Res. 2016;41:62–70. Epub 2016/01/05. 10.1016/j.leukres.2015.11.016 [pii]. . [DOI] [PubMed] [Google Scholar]
- 40.Lu K, Wang J, Song Y, Zhao S, Liu H, Tang D, et al. miRNA-24-3p promotes cell proliferation and inhibits apoptosis in human breast cancer by targeting p27Kip1. Oncol Rep. 2015;34(2):995–1002. Epub 2015/06/06. 10.3892/or.2015.4025 . [DOI] [PubMed] [Google Scholar]
- 41.Guo L, Zhao Y, Yang S, Zhang H, Chen F. A genome-wide screen for non-template nucleotides and isomiR repertoires in miRNAs indicates dynamic and versatile microRNAome. Mol Biol Rep. 2014;41(10):6649–58. 10.1007/s11033-014-3548-0 [DOI] [PubMed] [Google Scholar]
- 42.Freitak D, Knorr E, Vogel H, Vilcinskas A. Gender- and stressor-specific microRNA expression in Tribolium castaneum. Biol Lett. 2012;8(5):860–3. Epub 2012/05/26. 10.1098/rsbl.2012.0273 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang FY, Wong DK, Seto WK, Lai CL, Yuen MF. Estradiol induces apoptosis via activation of miRNA-23a and p53: implication for gender difference in liver cancer development. Oncotarget. 2015;6(33):34941–52. Epub 2015/10/07. 10.18632/oncotarget.5472 [pii]. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simon LM, Edelstein LC, Nagalla S, Woodley AB, Chen ES, Kong X, et al. Human platelet microRNA-mRNA networks associated with age and gender revealed by integrated plateletomics. Blood. 2014;123(16):e37–45. Epub 2014/02/14. 10.1182/blood-2013-12-544692 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou X, Zhu J, Zhang H, Zhou G, Huang Y, Liu R. Is the microRNA-146a (rs2910164) polymorphism associated with rheumatoid arthritis? Association of microRNA-146a (rs2910164) polymorphism and rheumatoid arthritis could depend on gender. Joint Bone Spine. 2015;82(3):166–71. Epub 2015/01/30. 10.1016/j.jbspin.2014.12.009 [pii]. . [DOI] [PubMed] [Google Scholar]
- 46.Hannafon BN, Sebastiani P, de las Morenas A, Lu J, Rosenberg CL. Expression of microRNA and their gene targets are dysregulated in preinvasive breast cancer. Breast Cancer Res. 2011;13(2):R24 Epub 2011/03/08. 10.1186/bcr2839bcr2839 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
These deregulated miRNA loci are only abundantly expressed in tumor or control groups. The detailed annotations can be found in Figs 3 and 4.
(TIF)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.