Abstract
Purpose
Aldicarb and methomyl are carbamate pesticides commonly implicated in human poisonings. The primary toxic mechanism of action for carbamate poisoning is cholinesterase (ChE) inhibition. As such, it is logical to assume that the currently accepted therapies for organophosphate poisoning [muscarinic antagonist atropine and the oxime acetylcholinesterase reactivator pralidoxime chloride (2-PAM Cl),], could afford therapeutic protection. However, oximes have been shown to be contraindicated for poisoning by some carbamates.
Methods
A protective ratio study was conducted in guinea pigs to evaluate the efficacy of atropine and 2-PAM Cl. ChE activity was determined in both the blood and cerebral cortex..
Results
Co-administration of atropine free base (0.4 mg/kg) and 2-PAM Cl (25.7 mg/kg) demonstrated protective ratios of 2 and 3 against aldicarb and methomyl, respectively, relative to saline. The data reported here show that this protection was primarily mediated by the action of atropine. The reactivator 2-PAM Cl had neither positive nor negative effects on survival. Both blood acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) activities were significantly reduced at 15 minutes post-challenge but gradually returned to normal within 24 h. Analysis of cerebral cortex showed that BChE, but not AChE, activity was reduced in animals that succumbed prior to 24 h after challenge.
Conclusion
The results suggest that co-administration of atropine and 2-PAM Cl at the currently recommended human equivalent doses for use in the pre-hospital setting to treat organophosphorus nerve agent and pesticide poisoning would likely also be effective against aldicarb or methomyl poisoning.
Keywords: atropine, 2-PAM Cl, carbamates, pesticide
2.0 Introduction
Organophosphorus (OP) and carbamate compounds are widely used for pest control in agricultural, industrial, and household settings worldwide. Human exposure to either of these two classes of readily available pesticides may result from a deliberate act or accidental release and each class poses a significant acute health risk to humans (1, 2). As such, it is critical that an adequate medical response strategy be available.
Carbamate compounds inhibit cholinesterases (ChEs), specifically acetylcholinesterase (AChE) and butyrylcholinesterase (BChE), preventing them from performing normal physiological functions (3). At high levels of exposure, inhibition of ChEs by either carbamates or OPs rapidly leads to the accumulation of the neurotransmitter acetylcholine (ACh), the endogenous ligand for muscarinic and nicotinic receptors at synaptic junctions (3). If left uncontrolled, the sudden and rapid increase in ACh levels at the synapses results in hyperstimulation of the cholinergic receptors and symptoms of cholinergic crisis. Unfortunately, based on symptomology alone, it is difficult to discern whether the observed cholinergic crisis is a result of carbamate or OP intoxication.
The immediate medical response paradigm in the U.S. for the treatment of intoxication with a cholinesterase inhibitor is the administration of the muscarinic antagonist, atropine, and the oxime AChE reactivator, pralidoxime chloride (2-PAM Cl) (4). In pre-hospital first responder and military settings, this is typically accomplished using a Duodote® auto-injector, though no more than three such administrations are recommended in the absence of adequate supportive care (specifically, ventilator support) (5). It should be noted that the use of this FDA-approved medical countermeasure is primarily based on previous studies conducted using OP chemical warfare nerve agents (4, 6) and not carbamates. Additionally, previous studies have reported some evidence that 2-PAM Cl is contraindicated against certain carbamate pesticides, particularly propoxur and carbaryl (7–9). Therefore, it is unclear whether the standard U.S. countermeasure regimen of atropine and 2-PAM Cl should be administered following carbamate poisoning (6).
Aldicarb and methomyl are of concern because of the potential serious health effects following an acute exposure. Based on a similar mechanism of toxicity (i.e., ChE inhibition), an evaluation of the effectiveness of the current standard of care approved for OP intoxication against members of the carbamate class of pesticides is warranted. As such, whether atropine and 2-PAM Cl are useful therapies against aldicarb and methomyl is the subject of this research. Efficacy studies in both guinea pig in vivo (protective ratio study and blood brain barrier penetration) and in vitro models (ChE evaluation and reactivation determination) were conducted to address this subject.
3.0 Materials and Methods
3.1 Materials
The challenge materials (CMs) were aldicarb (Sigma-Aldrich, St Louis, MO), methomyl (ChemService, West Chester, PA), and sarin (U.S. Army Edgewood Chemical Biological Center, Edgewood, MD). Sarin was used as a positive control challenge material for the reactivation experiments. The therapeutics used were atropine (free base) at 1.64 mg/mL in an aqueous solution, pH 4.3, and pralidoxime chloride (2-PAM Cl) at 102.8 mg/mL in an aqueous solution, each procured from King Pharmaceuticals (St. Louis, MO). The challenge and test materials used in the study are summarized in Table 1. The carbamates were diluted in multisol (a biocompatible solution of 48.5% water, 40% propylene glycol, 10% ethanol, and 1.5% benzyl alcohol, v/v).
Table 1.
Identification of Challenge and Test Materials
| Challenge Material | CAS RN† | Synonym | Source | Cat. No. | Purity |
|---|---|---|---|---|---|
| aldicarb | 116-06-3 | 2-methyl-2-(methylthio)propanal O-(N-methylcarbamoyl)oxime | Sigma-Aldrich | 33386 | 99.9% |
| methomyl | 16752-77-5 | (E,Z)-methyl N-{[(methylamino)carbonyl] oxy}ethanimidothioate | ChemService | RPN-12399-1G | 99.5% |
| sarin | 107-44-8 | isopropyl methylphosphonofluoridate | U.S. Army Edgewood Chemical Biological Center | - | 96.2% |
| Therapy | CAS RN† | Synonym | Source | Lot No. | Conc. (mg/mL) |
| atropine free base | 51-55-8 | (3-endo)-8-Methyl-8-azabicyclo[3.2.1]oct-3-yl tropate | King Pharmaceuticals | RP-526-1 | 1.64 |
| 2-PAM Cl | 6735-59-7 | 2-formyl-1-methylpyridinium chloride | King Pharmaceuticals | RP-526-2 | 102.8 |
Chemical Abstracts Service Registry Number
3.2 Animals
A total of 292 male Dunkin-Hartley guinea pigs (Cavia porcellus) were procured from Charles River facilities (Raleigh, NC; Stone Ridge, NY). Each guinea pig had a vascular access port (VAP) surgically-implanted in the jugular vein by the vendor prior to arrival. Animals were singly housed due to VAP implantation, and food and water were available ad libitum. During the 3-day quarantine, the guinea pigs were weighed and randomized by body weight into test days and treatment groups. This study was conducted under an approved protocol from Battelle (2979-CG920832).
3.3 In vitro, 2-PAM Cl reactivation of gp-AChE-R
Assay buffer (1X phosphate buffered saline, pH 7.4 + 0.01% bovine serum albumin + 0.01% glycerol) was added to each well of a 96-well microtiter plate and pre-warmed at 37°C for ≥ 60 min. Vehicle or 2-PAM Cl was added to the pre-warmed plate for a final concentration of 10 NM, 100 NM, or 500 NM per the test conditions being evaluated. Concurrently, 0.8 mL of assay buffer was added to microtiter tubes and pre-warmed at 37°C for ≥60 min. To each pre-warmed microtiter tube, 0.1 mL of diluted recombinant guinea pig acetylcholinesterase (gpAChE-R; Chesapeake PERL, Savage, MD: read-through transcript, AA976) was added. The target enzyme activity was 0.1 units/mL. Subsequently, 0.1 mL of varying concentrations of aldicarb, methomyl, or sarin was added at the experimentally determined the concentration of the challenge material to result in 95% inhibition of the enzyme (IC95). The targeted IC95 for each inhibitor was the inhibition concentration that was determined to yield a final relative activity of ~5% activity of the unchallenged control samples on the plate. Samples were incubated at 37°C for approximately 1 min in order to mimic the in vivo study. Twenty NL of the challenged gpAChE-R was added to each of the appropriate wells on the plate. At t = 0, 0.5, 1, 2, 4, and 24 h, 20 NL of a 1:1 mixture of acetylthiocholine iodide (ATC; Sigma Aldrich, St. Louis, MO: A5751) and Ellman’s reagent (DTNB; 5,5′-Dithiobis(2-nitrobenzoic acid); Sigma Aldrich: D8130) was added to each well. The final concentrations of ATC and DTNB were 1.00E-03 M and 5.00E-04 M, respectively. For all 37°C incubations, the plates and/or microtiter tube boxes were covered with lids to minimize evaporation. At this point, each well of the test plate contained a final volume of 0.2 mL. Each plate was sealed and kinetically analyzed by spectrophotometric readings at a wavelength of 412 nm, obtained using a SpectraMax® Plus384 microplate reader (Molecular Devices, Sunnyvale, CA) programmed to incubate the plate at 37°C for the duration of the experiment. Readings were taken every 15 seconds for a period of 10 min. For analysis purposes, plates were normalized to a 1 cm path length, and the extinction coefficient of DTNB used was 13,600 M−1cm−1. Furthermore, wells containing identical 2-PAM Cl concentrations but without gpAChE-R were evaluated. These wells served as oximolysis controls, and the values obtained were subtracted during data analysis to determine the effect of 2-PAM Cl reactivation on challenged gpAChE-R. As described by Willie and colleagues (9), to calculate the reactivation rate constants, relative activity (determined by reference to identically treated control samples) was plotted versus time using GraphPad Prism® 5 (GraphPad Software, Inc. La Jolla, CA), and plots were fit to a one-phase exponential, nonlinear regression model.
3.4 Protective Ratio Experimental Study Design
Probit analysis of 24 h lethality rate as a function of challenge dose was used to calculate the median lethal dose (MLD) for each carbamate with and without treatment. The treatments were one of four different combinations designated as groups in Table 2. The protective ratio for a particular treatment was defined as the MLD for that treatment divided by the MLD for the saline/saline control group.
Table 2.
Treatment Combinations
| Group | N (per dose) | Right, Hind Limb | Left, Hind Limb |
|---|---|---|---|
| 1 | 2 | Saline | Saline |
| 2 | 2 | Atropine | Saline |
| 3 | 2 | Saline | 2-PAM Cl |
| 4 | 2 | Atropine | 2-PAM Cl |
On the day prior to challenge, each animal was weighed to ensure that it was within the designated weight range (350 to 500 grams) and to provide the basis for calculating the challenge and treatment doses. A blood sample was collected for a baseline ChE analysis, and the animals were clipped of hair on the lateral aspects of the right and left thighs (treatment injection sites) and on the dorsum between the scapulae (challenge injection site).
On each of five challenge days, three sets of two guinea pigs each were administered varying doses of either aldicarb (0.05 to 25 mg/kg) or methomyl (0.9 to 450 mg/kg) by subcutaneous injection at the challenge site. Challenge doses were selected based on statistical analysis in order to provide the points needed to complete a dose/lethality curve for each challenge agent/treatment combination while minimizing animal use. At 1 min post challenge, one of four treatment combinations was administered by intramuscular (IM) injection into the hind limbs of the animal as detailed in Table 2. In addition to the carbamate challenged animals, two control animals per challenge day were exposed to the vehicle only.
The standard therapy for OP poisoning a first responder can administer is three DuoDote® autoinjectors which contain both atropine free base and 2-PAM Cl. The atropine free base level of 0.4 mg/kg in the guinea pig was selected for this study based on the body surface area-corrected equivalent dose given to a human (the human equivalent dose) victim of OP poisoning in a pre-hospital setting or given by first responder, three DuoDote® autoinjectors (5). The 2-PAM Cl dose administered to the guinea pig was 25.7 mg/kg (146 Nmol/kg) – equivalent to the available dosage in three DuoDote® autoinjectors given to a 70 kg human (the human-relevant dose - HRD).
The primary endpoint, 24 h survival, was used to determine the protective ratio of the treatment groups. Secondary endpoints included clinical observations and cholinesterase activity measurements in serial blood and terminal brain cortex samples. Clinical observations were recorded pre-challenge and at 5, 15, 30, 60 min, and 2, 4, 6, and 24 h post-challenge. If a guinea pig was found dead, the time was recorded upon observation. Using the VAP, blood samples were collected prior to challenge and at 15 and 60 min; 2, 4 and 24 h post-challenge. In addition, animals that succumbed prior to the 6 h post-challenge time point were necropsied immediately, and the cerebral cortex was collected for analysis. After the final observation, each surviving animal was euthanized by catheter injection of euthanasia solution and the cerebral cortex tissue was harvested. The cerebral cortex was rinsed of residual blood; however, it was not perfused.
3.5 Blood processing and cholinesterase activity assay
Whole blood samples were processed and analyzed as described in McGarry et.al. (10). Briefly, whole blood samples were treated with HemogloBind™ to remove hemoglobin – which interferes with the ChE activity assay due to spectral overlap. To prepare the HemogloBind™ treated blood samples for ChE activity analysis, samples were diluted 2-fold in assay buffer (1X Phosphate Buffered Saline (PBS)). Subsequently, samples were diluted an additional 2-fold into the test plate by adding 100 NL of sample to a total volume of 200 μL in each well of a 96-well plate. Cholinesterase activity was assessed using a spectrophotometric assay conducted in a manner similar to Ellman et.al. (11) as described in the in vitro reactivation section above. The relative AChE activity level for each animal (RAAChE) was defined as the acetylthiocholine (ATC) turnover rate in the terminal blood sample divided by that in the same animal’s baseline blood sample. A similar calculation was performed using butyrylthiocholine (BTC) turnover rates to determine RABChE.
3.6 Tissue processing and cholinesterase activity assay
After brain extraction and resection of the perfused cerebral cortex, the tissue was immediately rinsed to remove any residual blood. The sample was then flash frozen in liquid nitrogen and stored at ≤−70°C until processing. Each tissue sample was later homogenized using one of two methods. In the first method, cerebral cortex samples that were used for ex vivo ChE activity analysis and blood brain barrier (BBB) permeability determination were pulverized using a Covaris™ CryoPrep™ system. Briefly, ~1g of frozen tissue was placed into a Kapton® tissue tube (Covaris™), placed into liquid nitrogen and pulverized. The resulting coarse powder was then added to appropriately sized homogenization vials and weighed. Tissue homogenization buffer (1X PBS, pH 7.4, Sigma Aldrich, + 1% TritonX-100, Sigma Aldrich: X100. + 1% protease inhibitor, Sigma Aldrich) was added to the pulverized cerebral cortex to create a 100 mg/mL solution. The samples were then homogenized for approximately 2 min with a PowerGen™ High Throughput Homogenizer. Samples requiring analysis for both cholinesterase activity and blood brain barrier penetration studies (described below) were homogenized using the Covaris™ CryoPrep™ system as this allowed an equal division of the samples between the two analysis teams. In the second method, cerebral cortex samples collected from animals used for the efficacy study were added to appropriately sized homogenization vials and weighed. Tissue homogenization buffer, composed of the same components described for the first method, was added such that the final tissue concentration was 100 mg/mL. The samples were then homogenized for 1–2 min using an Omni tissue homogenizer. Upon completion of either of the two aforementioned homogenization methods, the homogenized samples were placed on a rocker at 2–8°C and allowed to rock for ≥ 1 h. Samples were then centrifuged at 10,000 x g for 2 min. The supernatant was removed and retained at ≤ −70°C for cholinesterase activity analysis. Prior to cholinesterase activity analysis, the homogenate was thawed and diluted 5-fold in assay buffer (1X PBS). Cholinesterase activity was then determined as described previously.
3.7 Blood Brain Barrier (BBB) penetration determination
Five animals per group were challenged at multiples of the determined MLD: 0.5, 1, and 2 x MLD. A pre-challenge and a 30-min post-challenge blood sample were collected for ChE analysis and determination of carbamate concentration. Following the 30-min post-challenge blood collection, the brain was perfused with 0.9% saline solution to remove any blood from the tissue for analysis of the challenge material (CM). Similar experimental design to assess the BBB permeability to an administered compound has been previously described (12). Briefly, following perfusion, the cerebral cortex was removed and the tissue was prepared using a Covaris™ CryoPrep™ System for extraction and analysis. After preparing the tissue samples, acetonitrile was added, the samples were vortexed with a 0.375 inch stainless steel homogenization ball for 30 sec, and then centrifuged at 1,300 × g for 10 min. The resultant clear supernatant was added to approximately 0.2 g of 4:1 magnesium sulfate:sodium acetate. The samples were vortexed and then centrifuged at 1,300 × g for 1 min at 4°C. If necessary, the supernatant was further processed by transferring the supernatant to approximately 0.05 g dispersive solid-phase extraction sorbent (3:1:1 magnesium sulfate: primary and secondary amine exchange material/C18). The samples were again vortexed and centrifuged at 1,300 × g for 1 min at 4°C. The supernatant was diluted two-fold with Millipore water, and the samples were directly analyzed for the CM using liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS). The aldicarb or methomyl was identified by retention times and specific multiple reaction monitoring (MRM) transitions. Quantification was performed using the isotopically labeled carbamates as internal standards.
3.8 Statistical Analysis
For each compound and treatment, a probit dose-response model was fit to lethality data using the method of maximum likelihood (13). Estimated parameters of probit dose-response models were used to compute each MLD. The Fieller’s method or the delta method was used to compute a 95 percent confidence interval for each MLD. STATA® 11.0 (StataCorp, College Station, TX) was used to analyze the lethality data.
The calculated MLD for the treated groups, when divided by values for the non-treated groups (saline/saline) MLD, provided an estimated protective ratio (PR) for that particular treatment against each carbamate. The PR is the expected protection that a particular treatment provides against a particular poisoning. The PRs for treatment groups were calculated as
PRs and 95 percent confidence intervals were calculated for each pair of treatment groups for each compound. The analysis used pairwise tests to compare treatment group means and significance was adjusted for multiple comparisons using the Bonferroni correction.
4.0 Results
4.1 Reactivation of AChE inhibited with Aldicarb or Methomyl
To determine 2-PAM Cl-assisted reactivation of AChE inhibited by aldicarb or methomyl, the IC95 of each was experimentally determined to ensure near complete inhibition of the recombinant guinea pig AChE (gpAChE-R) without an excess of the carbamate in solution (Table 3). The capacity of 2-PAM Cl to reactivate AChE inhibited by sarin has been well characterized (14, 15). Therefore; sarin was used as a positive control for the reactivation experiments.
Table 3.
Determination of Aldicarb and Methomyl IC50 and IC95 with gpAChE-R
| Challenge agent | IN VITRO IC50 | IN VITRO IC95† |
|---|---|---|
| Aldicarb | 67 μM | 509 μM |
| Methomyl | 15 μM | 174 μM |
| Sarin | 0.04 μM | 0.472 μM |
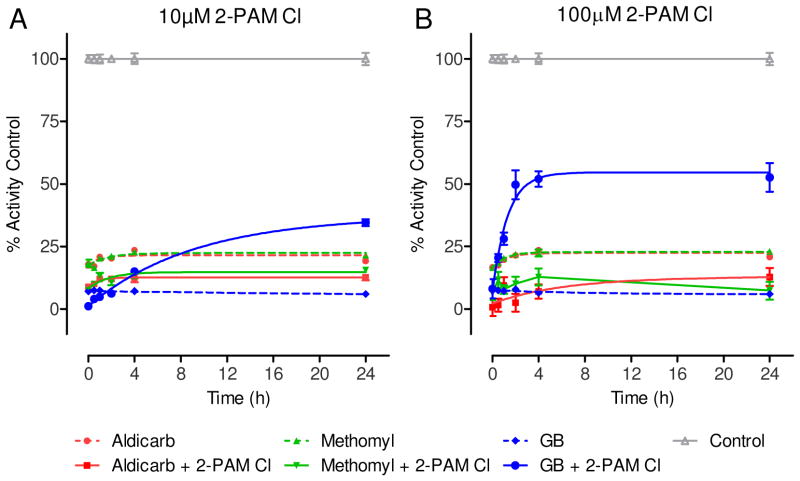
The IC95 was used to determine the ability of 2-PAM Cl in reactivating gpAChE-R when inhibited by carbamate, Figure 1.
The gpAChE-R was challenged at the previously determined IC95 of aldicarb, methomyl, or sarin. One min post-challenge, 2-PAM Cl was added at either 10 or 100 NM, and samples were analyzed for enzyme activity at specific time points. The 10 and 100 NM 2-PAM Cl levels were selected based on results from a clinical study investigating the effectiveness of 2-PAM Cl at different administered doses against OP pesticide poisoning cases (16). Notably, 500 NM 2-PAM Cl produced a signal to noise ratio that was too low to distinguish specific reactivation effects due to oximolysis, and the data are not reported herein.
As shown in Figure 1, aldicarb, methomyl, and sarin elicited inhibition of AChE activity of the gpAChE-R relative to the unchallenged control samples. While the experimentally determined IC95 was targeted, approximately 80–85% inhibition of gpAChE-R was attained in this set of experiments. The reduction in AChE activity due to aldicarb or methomyl was not reversed by the addition of 2-PAM Cl at either 10 NM (Figure 1A) or 100 NM (Figure 1B). In fact, a decrease of ~10 to 15% percent activity was observed between the 2-PAM Cl-treated and untreated aldicarb and methomyl challenged gpAChE-R. This decrease in relative activity, which was presumably due to the oxime reversibly inhibiting the gpAChE-R (17), was observed to be ~20% for 10 NM 2-PAM Cl alone and ~35% for 100 NM 2-PAM Cl alone (data not shown). This reduction could account for the difference observed between the carbamate only and carbamate plus 2-PAM Cl. This decrease in activity as a result of addition of the oxime was not observed for the sarin control, presumably because the increase in activity due to reactivation of sarin-inhibited gpAChE-R greatly exceeded the relatively weak inhibition of gpAChE-R by 2-PAM Cl.
Figure 1. In vitro, 2-PAM Cl does not reactivate gpAChE-R when inhibited by a carbamate.
Using the Ellman’s based assay, the reactivation of gpAChE-R by 2-PAM Cl, against the experimentally determined IC95 of aldicarb (509 μM), methomyl (174 μM), or sarin (GB) (0.475 μM) was evaluated. Unchallenged gpAChE-R was used to determine maximum activity. To more closely mimic the in vivo study, at one minute post-challenge 2-PAM Cl was added at a final concentration of (A) 10 μM or (B) 100 μM. The indicator and substrate were added at 0, 0.5, 1, 2, 4, or 24 h and read immediately to determine activity. Activity is represented as a percentage of the unchallenged control. Data shown are mean ± SEM, representing n ≥ 5. For both carbamates but not GB, both treatment levels of 2-PAM Cl significantly contributed to additional enzyme inhibition. For GB, both treatment levels of 2-PAM Cl significantly ameliorated enzyme inhibition. gpAChE-R remained relatively stable for the duration of the 24 hour incubation as ≤ 15% decrease in activity was observed as compared to T=0 hours (data not shown).
Reactivation rate constants (kreact) are presented in Table 4. Neither concentration of 2-PAM Cl had a significant effect on the kreact of gpAChE-R inhibited with aldicarb or methomyl (Table 4). In order to establish that 2-PAM Cl could reactivate AChE in this in vitro model, the OP nerve agent sarin was tested concurrently, under similar conditions. Previously reported data have shown that 2-PAM Cl is an effective reactivator of sarin-inhibited AChE (14, 18). In the present study, sarin inhibited gpAChE-R activity and the addition of 2-PAM Cl resulted, as expected, in reactivation in a dose-dependent manner (Figure 1A, B).
Table 4.
Reactivation rate constant (kreact)
| Inhibitor | 2-PAM Cl (μM) | Kreact(min−1) |
|---|---|---|
| Aldicarb | none | 0.0142 ± 0.0055 |
| 10 | 0.0200 ± 0.0144 | |
| 100 | 0.0026 ± 0.0031 | |
| Methomyl | none | 0.0116 ± 0.0040 |
| 10 | 0.0095 ± 0.0050 | |
| 100 | 0.0293 ± 0.1374 | |
| Sarin | none | 0.0014 ± 0.0036 |
| 10 | 0.0018 ± 0.0002 | |
| 100 | 0.0127 ± 0.0030 |
4.2 Protective ratios study (PRs)
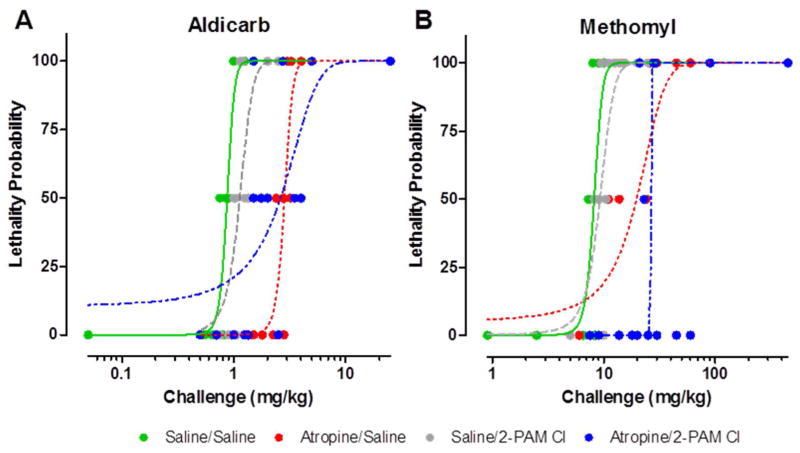
The probit dose-response analysis was calculated from the lethality data from each treatment combination (Table 2). Each probit analysis involved 30 guinea pigs to characterize the curves and establish the MLD. Once the probit curves (Figure 2) were established and the MLD was calculated for each treatment group, PRs relative to saline only controls (i.e., negative controls) were calculated by dividing the MLD for a treatment group by that of the saline/saline treated control group (Table 5).
Figure 2. Atropine treatment alone produces a two to three fold protection over saline treated when challenged with carbamates. 2-PAM Cl afforded no additional protection.
Probit analysis was performed based the lethality data using Dunkin-Hartley guinea pigs results from the protective ratio study. Varying doses were used in this in vivo study to determine a probit curve for each treatment group. These probit analyses were then compared to calculate the protective ratios. For each data point, n = 2; for each probit curve, n = 30 per curve. See table 6 for statistical analysis results.
Table 5.
MLD and Protective Ratios for Treatment Groups
| Challenge Compound | Therapy | n | MLD (mg/kg) | 95% Confidence Interval | Probit Slope | PR1 | p value1 |
|---|---|---|---|---|---|---|---|
| Aldicarb | Saline/Saline | 36 | 0.86 | (0.74, 1.51) | 18 | N/A | N/A |
| Atropine/Saline | 30 | 2.82 | (2.43, 3.46) | 15 | 3.30 | <0.0001 | |
| 2-PAM/Saline | 30 | 1.15 | (0.96, 1.39) | 10 | 1.34 | 0.003 | |
| Atropine/2-PAM | 30 | 2.31 | (1.45, 5.74) | 3.0 | 2.70 | <0.0001 | |
| Methomyl | Saline/Saline | 36 | 8.06 | (7.07, 8.97) | 18 | N/A | N/A |
| Atropine/Saline | 30 | 15.20 | (9.49, 23.94) | 4.0 | 1.89 | 0.0003 | |
| 2-PAM/Saline | 30 | 9.22 | (6.68, 10.79) | 12 | 1.14 | 0.0683 | |
| Atropine/2-PAM | 30 | 22.95 | (17.62, 27.87) | 13 | 2.85 | <0.0001 |
Protective ratio, the shift in the MLD between treatment groups, compared to Saline/Saline treated animals
A comparison of the calculated PRs based on inter-component comparison is presented in Table 6. Atropine provided a significant level of protection (p < 0.0083) to animals challenged with aldicarb or methomyl, while 2-PAM Cl alone did not have any effect on the protective ratio (p < 0.0083) (Table 6).
Table 6.
Protective Ratio Comparison
| Compound | Numerator Treatment | Denominator Treatment | Protective Ratio | 95% Confidence Interval | P-value |
|---|---|---|---|---|---|
| Aldicarb | Atropine/Saline | Saline/Saline | 3.3* | (2.86, 3.81) | <0.0001 |
| Saline/2-PAM Cl | Saline/Saline | 1.3 | (1.14, 1.57) | 0.003 | |
| Atropine/2-PAM Cl | Saline/Saline | 2.7* | (1.77, 4.14) | <0.0001 | |
| Atropine/2-PAM Cl | Atropine/Saline | 0.82 | (0.54, 1.25) | 0.3570 | |
| Atropine/2-PAM Cl | Saline/2-PAM Cl | 2.0* | (1.31, 3.13) | 0.0014 | |
| Atropine/Saline | Saline/2-PAM Cl | 2.4* | (2.13, 2.86) | <0.0001 | |
| Methomyl | Atropine/Saline | Saline/Saline | 1.9* | (1.33, 2.67) | 0.0003 |
| Saline/2-PAM Cl | Saline/Saline | 1.1 | (0.99, 1.32) | 0.0683 | |
| Atropine/2-PAM Cl | Saline/Saline | 2.9* | (2.45, 3.29) | <0.0001 | |
| Atropine/2-PAM Cl | Atropine/Saline | 1.5 | (1.05, 2.17) | 0.0251 | |
| Atropine/2-PAM Cl | Saline/2-PAM Cl | 2.5* | (2.08, 2.94) | <0.0001 | |
| Atropine/Saline | Saline/2-PAM Cl | 1.6* | (1.15, 2.38) | 0.0065 |
Indicates a significant difference based on the Bonferroni correction, p<0.0083. The Protective ratio is the shift in the MLD between treatment groups.
Additionally, clinical observations were recorded throughout the 24 h post-challenge observation period. At least 80% of the animals that showed signs of cholinergic overstimulation had increased salivation, lacrimation, hyperpnoea, and tremors; the severity and time to onset of these signs depend on dose of CM and the presences of treatment (data not shown). More than 90% of those animals that survived for the 24 h observation period were normal within 6 h post-challenge (data not shown).
4.3 AChE and BChE activity in the blood and cerebral cortex following carbamate challenge
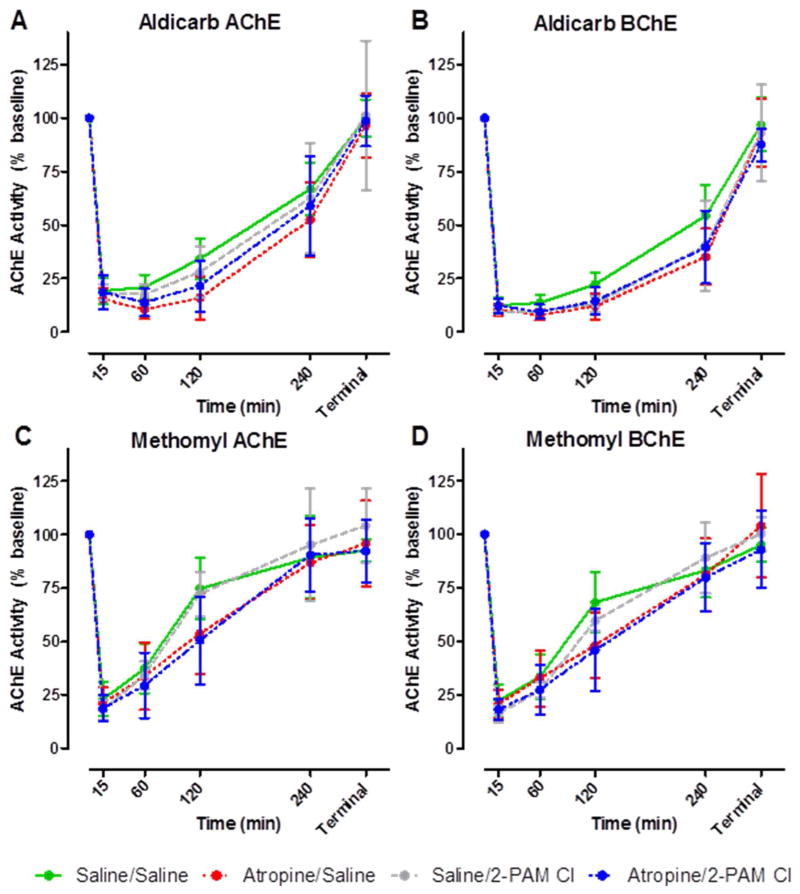
For aldicarb challenges, blood enzyme assays indicate that both AChE and BChE (evaluated by ATC and BTC hydrolysis, respectively) were inhibited rapidly (Figure 3A,B). ChE activity returned to baseline within the 24 h observation period. There was no difference between the treatment groups with regard to ChE activity trends. ChE activity in methomyl challenged animals was very similar to the aldicarb-treated animals (Figure 3C, D). The only noticeable difference was that the rate of recovery to baseline was faster for methomyl than for aldicarb-challenged animals. No statistical analysis was able to be conducted between specific data points due to the nature of the varying challenge doses in the development of the probit analysis. All data based on treatment group were pooled at each specific time point and only trends in ChE activity were observed.
Figure 3. Serial blood samples were taken and AChE and BChE activity was determined following challenge with aldicarb (A and B) or methomyl (C and D). Within 15mins following challenge, ChE activity was reduced by ~75% but returned to pre-challenged levels within 24hrs regardless of treatment.
ChE analysis was performed using an Ellman’s based assay. Animals were challenged with varying concentrations of aldicarb or methomyl and blood was collected at specific time points post challenge: 0, 0.25, 4, 2, 4, 24h. Each time point is the mean ± SEM of all samples collected for that treatment and CM group (n ≥ 6) including varying CM concentrations. Due to the varying challenge doses with each treatment group, this data only reflects trends in ChE activity and no statistical analysis was able to be conducted.
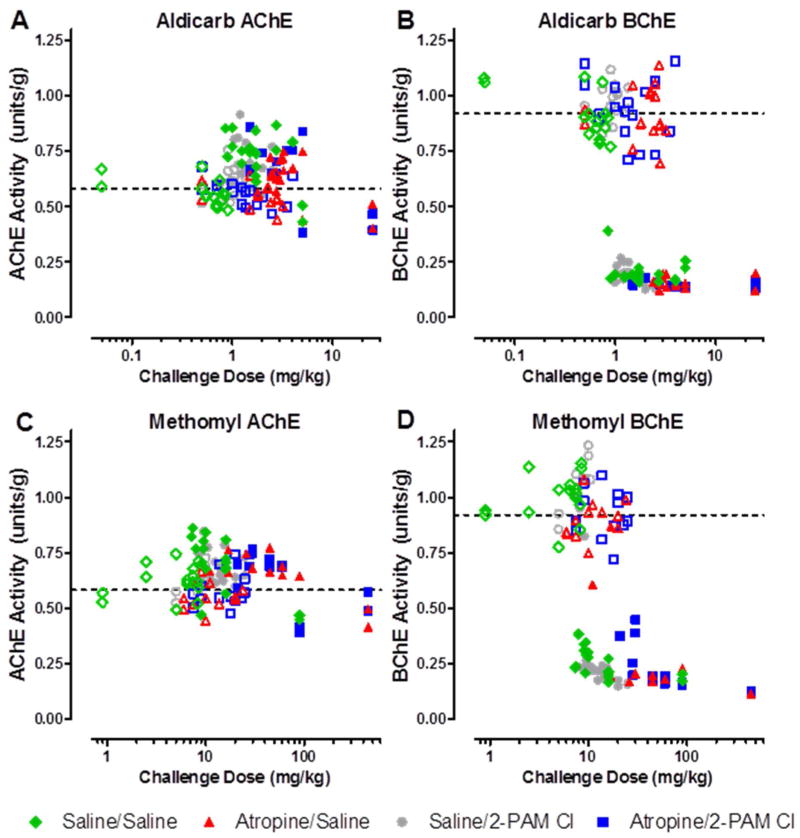
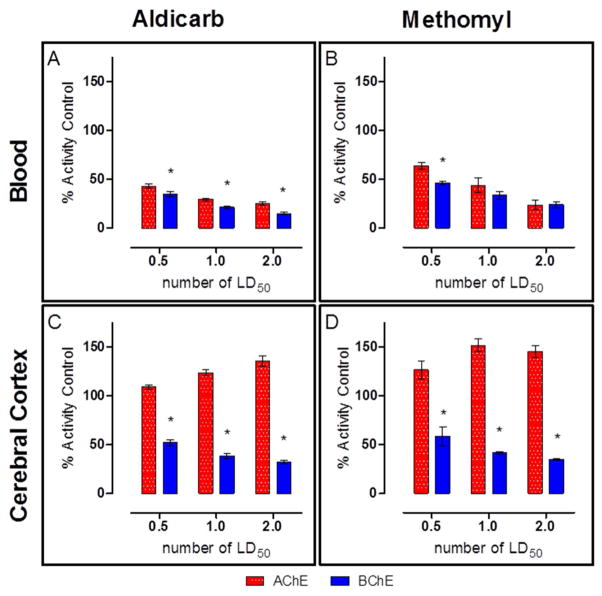
Cortex AChE and BChE activities are presented in Figure 4. There were no observable differences in ChE activity between the treatment groups regardless of challenge material. However, there appears to be a distinct preference of the carbamate for cortical BChE (Figure 4B, D) over AChE (Figure 4A, C). AChE activity in the cerebral cortex was not affected by carbamate challenge. Interestingly, in the animals that died during the observation period (i.e., prior to 24 h post-challenge), an elevation (~15%) of AChE activity from the baseline control animals was observed. The observed elevation in activity could be due to stress-induced read-through AChE in response to the carbamate challenge since it is known that a stress-induced read-through isoform of AChE (AChE-R) can be produced (19) and can be observed within 30 min (20). On the other hand, BChE activity was affected based on time to death. If an animal survived the full 24 h observation period, its BChE activity levels were observed to be near baseline. However, if the animal died due to challenge, the level of BChE activity was reduced nearly 75%. This difference between the BChE activity based on survivability was statistically significant (p<0.0001).
Figure 4. Terminal AChE and BChE activity in the cerebral cortex. AChE is unaffected by either aldicarb (A) or methomyl (C), however BChE activity is significantly reduced by both aldicarb (B) and methomyl (D).
Upon death due to challenge or at the completion of the 24hr observation period, the cortex was removed and ChE analysis was performed using an Ellman’s based assay. Closed circles represent animals that succumbed to challenge prior to 24 hours post-challenge. Open circles represent those animals that were euthanized following the 24h observation period. The dashed line is the average of all untreated control animals, n = 36. AChE/BChE activity is recorded in enzyme units per gram of tissue.
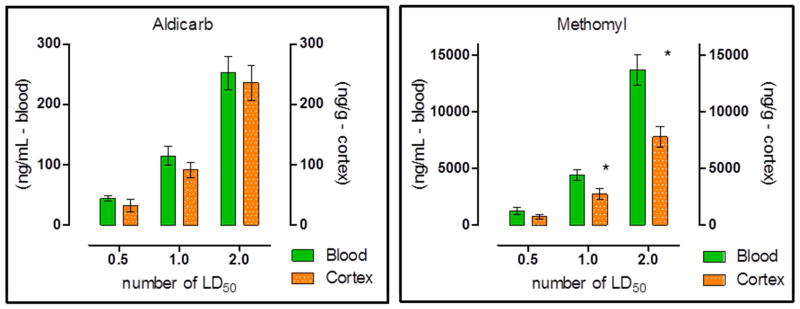
4.4 Blood-brain barrier (BBB) permeability of aldicarb and methomyl
There were no significant differences between aldicarb levels in the blood and cerebral cortex within 30 min of challenge (Figure 5). Methomyl, on the other hand, produced an approximate 40% difference in blood and cerebral cortex levels 30 min post-challenge following administration of 1X and 2 x MLD (Figure 5). These data indicate that aldicarb and methomyl crossed the BBB within 30 min of challenge, and aldicarb crossed to a greater extent than methomyl.
Figure 5. Blood and cerebral cortex concentration of Aldicarb (A) or Methomyl (B) 30 min post exposure.
Cerebral cortex was perfused prior to removal to prevent potential cross contamination between the blood and cortex. (Control non-perfused cortexes were collected to determine the impact of this potential cross contamination by the blood in the tissue found that no significate impact was present.) Animals were challenged with multiples of the previously determined MLD: 0.5, 1 or 2. Data shown are mean ± SEM, representing n = 5, (*) p<0.05, significance reflects difference between levels found in the blood and brain at a particular challenge level. Blood concentration is recorded in nanograms of carbamate per milliliter of blood, and cerebral cortex concentration is recorded in nanograms of carbamate per gram of tissue.
Determination of ChE activity in the blood and the cerebral cortex collected from animals that were sacrificed 30 min after challenge (Figure 6) confirmed the results from the PR Study (Figure 4), in which AChE activity in the cerebral cortex significantly increased by as much as 50% above baseline, possibly due to stress-mediated production of read-through AChE (20); BChE activity was significantly reduced in the brain. In addition, ChE results in the blood indicated a slight, but statistically significant, preference for BChE over AChE for both aldicarb and methomyl at all challenge levels at 30 min post exposure. On the other hand, both carbamates appear to have a preference for BChE over AChE in the cerebral cortex at 30 min post exposure.
Figure 6. Blood and Cerebral cortex ChE activity 30min post exposure to Aldicarb (A and C) or Methomyl (B and D). Blood AChE and BChE activities were similar but a statistically significant difference was observed between AChE and BChE activity. Additionally, the cerebral cortex also had a statistically significant difference between AChE and BChE activities but the difference was greater than in the blood.
Cerebral cortex was perfused prior to removal. Animals were challenged with multiples of the previously determined MLD: 0.5, 1 or 2. Data shown are mean ± SEM, representing n = 5, (*) p<0.05.
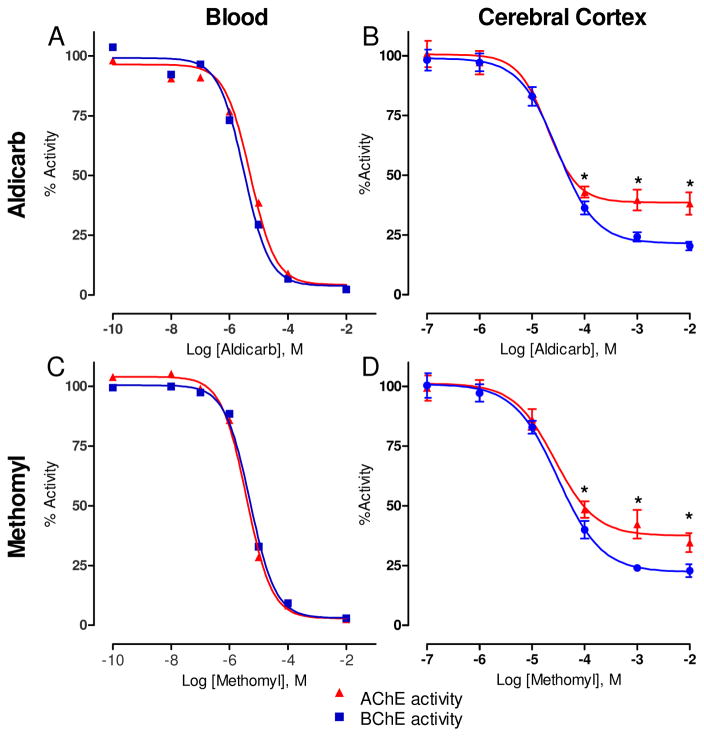
4.5 Carbamate preference for AChE or BChE in the blood or cerebral cortex
Naïve blood and cerebral cortex samples were collected and dosed with multiple concentrations of either aldicarb or methomyl and analyzed for ChE activity to confirm whether a preference for AChE or BChE existed. For both aldicarb and methomyl, no preference was detected in the inhibition of AChE or BChE in the blood (Figure 7A, C). However, in the cerebral cortex homogenate, a significant preference for BChE was observed for both compounds at the three highest concentrations tested (Figure 7B, D). The concentrations of challenge agent required to produce this BChE preferential response in the cerebral cortex tissue equated to approximately one MLD calculated from the protective ratio study (Figure 2). This comparison takes into account the observed carbamate concentration in the cerebral cortex from the BBB assay (Figure 5) following a 30 min exposure of one MLD. These data verified that a preference does exist for BChE over AChE in the cortex, and it also clarifies that this preference is not due to an inability for the carbamates to inhibit synaptic AChE as both cerebral cortex AChE and BChE were inhibited by both carbamates.
Figure 7. Ex vivo challenge of blood and cerebral cortex homogenate revealed that Aldicarb (A and B) and Methomyl (C and D) have a preference for cortex BChE.
Naive blood and homogenized cerebral cortex samples were challenged with varying concentrations of the challenge material for 30 min. ChE activity was determined using an Ellman’s based assay. Data shown are mean ± SEM, representing n = 6, (*) p<0.05.
5.0 Discussion
The ultimate goal of this work was to determine if the current medical countermeasures approved for use in the U.S. to protect against OP intoxication, namely atropine (free base) and 2-PAM Cl (6) via the DuoDote®, are efficacious against the carbamates aldicarb and methomyl in a guinea pig model. Previous studies have shown that 2-PAM Cl was contraindicated with the carbamate pesticide carbaryl (7, 8). This has led to questions regarding the safe and effective use of oxime AChE reactivators for carbamate poisonings in general (7). It has been theorized that the contraindication is a unique response of carbaryl to the oxime due to structural variations inherent to N-methylcarbamates (7). However, physostigmine which has a similar N-methylcarbamate structure does not share the adverse effects observed in the presence of 2-PAM Cl (21). Similarly, a recent report also contradicted the notion that oximes are contraindicated against carbamate poisonings, including carbaryl (22). In light of these conflicting reports regarding the effectiveness of oximes against carbamate ChE intoxication, further investigation is necessary to evaluate the safety and efficacy of atropine and 2-PAM Cl for carbamate (i.e., aldicarb and methomyl) poisoning.
When administered at the maximum recommended pre-hospital human equivalent dose for OP poisoning, concurrent administration of atropine and 2-PAM Cl provided 2- to 3-fold greater protection than vehicle (saline) treatment in guinea pigs challenged with aldicarb or methomyl. The results also indicated that protection was primarily due to the action of atropine, as the addition of 2-PAM Cl did not improve the protection provided by atropine alone. Interestingly, this result did not align with results previously reported in male rats (23). In that study, which utilized substantially higher levels of the antidotal compounds, administration of 17.4 mg/kg atropine sulfate offered 5-fold protection following an aldicarb challenge and the addition of 50 mg/kg 2-PAM methane sulfonate increased overall protection to 6-fold. In that same report, atropine sulfate offered a 1.6-fold protection against methomyl, while the addition of 2-PAM methane sulfonate also elevated overall protection to 1.9-fold (23). The differences in the results reported by Natoff et al. and those of the current study may be due to the bioavailability/distribution of the different salts of 2-PAM, differences in dose levels, interspecies variability between rats and guinea pigs, or some combination thereof.
The general aim of oxime AChE reactivators is to reactivate inhibited AChE. The results of this study indicated that 2-PAM Cl did not restore the activity of gpAChE-R inhibited with aldicarb or methomyl. Additionally, the rate of observed ChE activity recovery in the blood was not altered by any of the treatments tested. This contrasts previous research investigating the effect of 2-PAM on carbamylation and decarbamylation rate constants in membrane-bound bovine erythrocyte AChE (24). In that report, the authors showed slight, but statistically significant, reductions in both carbamylation and decarbamylation rate constants attributable to the presence of the oxime (24). It is possible that the observed reduction in both carbamylation and decarbamylation rates by 2-PAM Cl determined by Dawson and colleagues may offset each other, thus explaining the lack of an effect in both the gpAChE-R and blood samples observed in the current study. It is also plausible that inter-species variability could at least in part account for the observed difference, since differences in the kinetics of AChE derived from different species are well documented (14, 25, 26). The different results are not likely to be due to differences in erythrocyte AChE vs. read-through AChE, since the core of each variant of AChE is identical (27) and previous studies have reported similar kinetics between erythrocyte AChE and synaptic AChE (25, 28).
The observed recovery of the ChE activities in the serial blood samples were expected based on the combination of (a) short half-lives of carbamates (30 to 40 min for aldicarb (29) and 2 to 3 h for methomyl (30)) and (b) minimal effect of oximes (22). However, the preferential inhibition of BChE over ACHE by both carbamates in the cerebral cortex was not expected based on blood ChE inhibition where no such preference was observed. In fact, AChE and BChE maintain ~50% amino acid homology within species, and share a conserved active site catalytic triad (31, 32). While there is only one presumed isoform for BChE (although multiple oligomeric forms exist), various AChE isoforms (erythrocytic [-E] and synaptic [-S]) variants exist – all retaining a conserved active site gorge. This conservation between AChE isoforms, as well as the identification of only a single isoform of BChE, should, in theory, maintain continuity in terms of enzyme-substrate affinity for carbamate regardless of the source tissue, i.e., blood or cerebral cortex (27). That said, it is still worthwhile to note that even though the enzymes share a great deal of homology, differences with the active site gorge do exist. For example, BChE only has six aromatic amino acids within its gorge compared to 14 in AChE (33). Fewer aromatic amino acids lining the active site gorge results in a more open gorge configuration which allows for BChE to accommodate a much larger array of substrates than AChE.
Another parameter that could influence the different BChE and AChE inhibition levels observed in the CNS is the BBB penetrability of the carbamates. Previous reports have shown that both aldicarb and methomyl do readily cross the BBB and enter the CNS (34, 35). In this study, both aldicarb and methomyl readily crossed guinea pig BBB as they were detectable in the cortex at 30 min after a subcutaneous challenge at levels that were at least half of those detected in the blood. In these experiments, the cortex tissue was perfused prior to collection to prevent any cross contamination of these results with the carbamate found in the blood. Additionally, the finding that CNS BChE was inhibited to a greater extent than AChE was also observed with the perfused cerebral cortex samples, further demonstrating that this observation is not an artifact of ChEs in the blood.
Both aldicarb and methomyl were observed to preferentially inhibit brain BChE over AChE in ex vivo samples (Figure 7). It is important to note that the concentration of carbamate needed to produce this preference is similar to the calculated MLD found in the protective ratio study. Furthermore, this preference for BChE had previously been reported with aldicarb in starlings (36).
Preferential inhibition of BChE by the carbamates in the brain suggests the enzyme can sequester or scavenge the challenge agent, thus protecting AChE. Furthermore, clinical observations from carbamate-poisoned individuals found minimal CNS depression (34, 37), whereas peripheral effects, such as lacrimation, hyperpnoea, and tremors, predominated. (38).
6.0 Conclusion
In conclusion, the data presented herein suggest that the current U.S. pre-hospital therapeutic regimen for OP chemical warfare nerve agent and pesticide exposures, namely atropine and 2-PAM Cl in the form of DuoDote® kits, is also effective against two important carbamate insecticides, aldicarb and methomyl, that have been commonly implicated in human poisonings. However, protection against these two carbamate compounds is primarily due to the effects of atropine and 2-PAM Cl provides neither beneficial nor harmful contributions. Further research is needed to elucidate the interactions of 2-PAM Cl and other oximes with other carbamate pesticides.
Acknowledgments
We would like to acknowledge Dr. Jill Harvilchuck and Thomas Snider for their scientific input and technical review of this manuscript. The authors, also, wish to thank all technical staff here at Battelle that contributed to this research, especially Julie Lucas, Laura Hines, and Tyson Winters as the lead technicians, Dr. Patrick DeArmond as the lead Chemist, and Benjamin Carper and Nancy Niemuth for statistical support.
Footnotes
8.0 Declaration of Interest
The authors have no known conflicts of interest. This work was supported by the NIH Office of the Director through an interagency agreement between the NIAID and Department of Defense (DoD) and prepared under the auspices of both the NIH and the DoD Defense Technical Information Center (DTIC) under the Chemical, Biological, Radiological & Nuclear Defense Information Analysis Center (CBRNIAC) program, Contract No. SP0700-00-D-3180, Delivery Order Number 0687, CBRNIAC Task 832/CB-IO-OOI2.
The views expressed in this article are those of the authors and do not reflect the official policy of the NIH, Department of Health and Human Services, or the U.S. Government. No official support or endorsement of this article by the NIAID, NINDS, NIH, or DoD is intended or should be inferred. The experimental protocol was approved by the Institutional Animal Care and Use Committee at Battelle. All procedures were conducted in accordance with the principles stated in the Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act of 1966 (P.L. 89-544), as amended.
Declaration of Interest: The authors have no known conflicts of interest.
9.0 References
- 1.Burklow TR, Yu CE, Madsen JM. Industrial chemicals: terrorist weapons of opportunity. Pediatric annals. 2003;32:230–234. doi: 10.3928/0090-4481-20030401-06. [DOI] [PubMed] [Google Scholar]
- 2.Grube ADD, Kiely T, Wu L. Pesticides Industry Sales and Usage, 2006–2007 Market Estimates. US Environmental Protection Agency; Feb, 2011. [Google Scholar]
- 3.Rotenberg M, Shefi M, Dany S, Dore I, Tirosh M, Almog S. Differentiation between organophosphate and carbamate poisoning. Clinica Chimica Acta. 1995;234:11–21. doi: 10.1016/0009-8981(94)05969-y. [DOI] [PubMed] [Google Scholar]
- 4.Lee EC. Clinical manifestations of sarin nerve gas exposure. JAMA : the journal of the American Medical Association. 2003;290:659–662. doi: 10.1001/jama.290.5.659. [DOI] [PubMed] [Google Scholar]
- 5.Toxicology, P. a, editor Guidance for Industry Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Jul, 2005. [Google Scholar]
- 6.Ma J, Cline D, Tintinalli J, Kelen G, Stapczynski S. Toxicology and Pharmacology: Insecticides, Herbicides, and Rodenticides. Emergency Medicine Manual. 2006:533–537. [Google Scholar]
- 7.Harris LW, Talbot BG, Lennox WJ, Anderson DR. The relationship between oxime-induced reactivation of carbamylated acetylcholinesterase and antidotal efficacy against carbamate intoxication. Toxicology and applied pharmacology. 1989;98:128–133. doi: 10.1016/0041-008x(89)90140-3. [DOI] [PubMed] [Google Scholar]
- 8.Lieske CN, Clark JH, Maxwell DM, Zoeffel LD, Sultan WE. Studies of the amplification of carbaryl toxicity by various oximes. Toxicology letters. 1992;62:127–137. doi: 10.1016/0378-4274(92)90016-d. [DOI] [PubMed] [Google Scholar]
- 9.Wille T, Kaltenbach L, Thiermann H, Worek F. Investigation of kinetic interactions between approved oximes and human acetylcholinesterase inhibited by pesticide carbamates. Chemico-biological interactions. 2013;206:569–572. doi: 10.1016/j.cbi.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 10.McGarry KG, Bartlett RA, Machesky NJ, Snider TH, Moyer RA, Yeung DT, Brittain MK. Evaluation of HemogloBind treatment for preparation of samples for cholinesterase analysis. Adv Biosci Biotechnol. 2013;4:1020–1023. doi: 10.4236/abb.2013.412136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical pharmacology. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 12.Boje KM. In vivo measurement of blood-brain barrier permeability. In: Crawley Jacqueline N, et al., editors. Current protocols in neuroscience/editorial board. Unit 7. Chapter 7. 2001. p. 19. [DOI] [PubMed] [Google Scholar]
- 13.Finney D. Probit Analysis. 3. Cambridge: Cambridge Univ. Press; 1971. [Google Scholar]
- 14.Worek F, Reiter G, Eyer P, Szinicz L. Reactivation kinetics of acetylcholinesterase from different species inhibited by highly toxic organophosphates. Archives of toxicology. 2002;76:523–529. doi: 10.1007/s00204-002-0375-1. [DOI] [PubMed] [Google Scholar]
- 15.Worek F, Thiermann H, Szinicz L, Eyer P. Kinetic analysis of interactions between human acetylcholinesterase, structurally different organophosphorus compounds and oximes. Biochemical pharmacology. 2004;68:2237–2248. doi: 10.1016/j.bcp.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 16.Eddleston M, Eyer P, Worek F, Juszczak E, Alder N, Mohamed F, Senarathna L, Hittarage A, Azher S, Jeganathan K, Jayamanne S, von Meyer L, Dawson AH, Sheriff MH, Buckley NA. Pralidoxime in acute organophosphorus insecticide poisoning--a randomised controlled trial. PLoS medicine. 2009;6:e1000104. doi: 10.1371/journal.pmed.1000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jokanovic M, Stojiljkovic MP. Current understanding of the application of pyridinium oximes as cholinesterase reactivators in treatment of organophosphate poisoning. European journal of pharmacology. 2006;553:10–17. doi: 10.1016/j.ejphar.2006.09.054. [DOI] [PubMed] [Google Scholar]
- 18.Koplovitz I, Harris LW, Anderson DR, Lennox WJ, Stewart JR. Reduction by pyridostigmine pretreatment of the efficacy of atropine and 2-PAM treatment of sarin and VX poisoning in rodents. Fundamental and applied toxicology : official journal of the Society of Toxicology. 1992;18:102–106. doi: 10.1016/0272-0590(92)90201-r. [DOI] [PubMed] [Google Scholar]
- 19.Sternfeld M, Shoham S, Klein O, Flores-Flores C, Evron T, Idelson GH, Kitsberg D, Patrick JW, Soreq H. Excess “read-through” acetylcholinesterase attenuates but the “synaptic” variant intensifies neurodeterioration correlates. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:8647–8652. doi: 10.1073/pnas.140004597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaufer D, Friedman A, Seidman S, Soreq H. Acute stress facilitates long-lasting changes in cholinergic gene expression. Nature. 1998;393:373–377. doi: 10.1038/30741. [DOI] [PubMed] [Google Scholar]
- 21.Kurtz PH. Pralidoxime in the treatment of carbamate intoxication. The American journal of emergency medicine. 1990;8:68–70. doi: 10.1016/0735-6757(90)90299-f. [DOI] [PubMed] [Google Scholar]
- 22.Mercurio-Zappala M, Hack JB, Salvador A, Hoffman RS. Pralidoxime in carbaryl poisoning: an animal model. Human & experimental toxicology. 2007;26:125–129. doi: 10.1177/0960327107070849. [DOI] [PubMed] [Google Scholar]
- 23.Natoff IL, Reiff B. Effect of oximes on the acute toxicity of anticholinesterase carbamates. Toxicology and applied pharmacology. 1973;25:569–575. doi: 10.1016/0041-008x(73)90026-4. [DOI] [PubMed] [Google Scholar]
- 24.Dawson RM. Oxime effects on the rate constants of carbamylation and decarbamylation of acetylcholinesterase for pyridostigmine, physostigmine and insecticidal carbamates. Neurochemistry international. 1995;26:643–654. doi: 10.1016/0197-0186(94)00161-m. [DOI] [PubMed] [Google Scholar]
- 25.Herkert NM, Thiermann H, Worek F. In vitro kinetic interactions of pyridostigmine, physostigmine and soman with erythrocyte and muscle acetylcholinesterase from different species. Toxicology letters. 2011;206:41–46. doi: 10.1016/j.toxlet.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Luo C, Tong M, Chilukuri N, Brecht K, Maxwell DM, Saxena A. An in vitro comparative study on the reactivation of nerve agent-inhibited guinea pig and human acetylcholinesterases by oximes. Biochemistry. 2007;46:11771–11779. doi: 10.1021/bi701002f. [DOI] [PubMed] [Google Scholar]
- 27.Grisaru D, Sternfeld M, Eldor A, Glick D, Soreq H. Structural roles of acetylcholinesterase variants in biology and pathology. European journal of biochemistry/FEBS. 1999;264:672–686. doi: 10.1046/j.1432-1327.1999.00693.x. [DOI] [PubMed] [Google Scholar]
- 28.Herkert NM, Eckert S, Eyer P, Bumm R, Weber G, Thiermann H, Worek F. Identical kinetics of human erythrocyte and muscle acetylcholinesterase with respect to carbamate pre-treatment, residual activity upon soman challenge and spontaneous reactivation after withdrawal of the inhibitors. Toxicology. 2008;246:188–192. doi: 10.1016/j.tox.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Programme, U. N. E, editor Aldicarb, Health and Safety Guide. 1991. [Google Scholar]
- 30.Programme, U. N. E, editor Methomyl, Health and Safety Guide. 1995. [Google Scholar]
- 31.Chatonnet A, Lockridge O. Comparison of butyrylcholinesterase and acetylcholinesterase. The Biochemical journal. 1989;260:625–634. doi: 10.1042/bj2600625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Darvesh S, Darvesh KV, McDonald RS, Mataija D, Walsh R, Mothana S, Lockridge O, Martin E. Carbamates with differential mechanism of inhibition toward acetylcholinesterase and butyrylcholinesterase. Journal of medicinal chemistry. 2008;51:4200–4212. doi: 10.1021/jm8002075. [DOI] [PubMed] [Google Scholar]
- 33.Darvesh S, Hopkins DA, Geula C. Neurobiology of butyrylcholinesterase. Nature reviews Neuroscience. 2003;4:131–138. doi: 10.1038/nrn1035. [DOI] [PubMed] [Google Scholar]
- 34.Saadeh AM, al-Ali MK, Farsakh NA, Ghani MA. Clinical and sociodemographic features of acute carbamate and organophosphate poisoning: a study of 70 adult patients in north Jordan. Journal of toxicology Clinical toxicology. 1996;34:45–51. doi: 10.3109/15563659609020232. [DOI] [PubMed] [Google Scholar]
- 35.Rosman Y, Makarovsky I, Bentur Y, Shrot S, Dushnistky T, Krivoy A. Carbamate poisoning: treatment recommendations in the setting of a mass casualties event. The American journal of emergency medicine. 2009;27:1117–1124. doi: 10.1016/j.ajem.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 36.Parker ML, Goldstein MI. Differential toxicities of organophosphate and carbamate insecticides in the nestling European starling (Sturnus vulgaris) Archives of environmental contamination and toxicology. 2000;39:233–242. doi: 10.1007/s002440010100. [DOI] [PubMed] [Google Scholar]
- 37.Lima JS, Reis CA. Poisoning due to illegal use of carbamates as a rodenticide in Rio de Janeiro. Journal of toxicology Clinical toxicology. 1995;33:687–690. doi: 10.3109/15563659509010629. [DOI] [PubMed] [Google Scholar]
- 38.Services, D. o. H. a. H, editor Nerve Agents and Organophosphates Pesticides. 2005. [Google Scholar]