Abstract
Rationale
Stress exposure has been identified as one risk factor for alcohol abuse that may facilitate the transition from social or regulated alcohol use to the development of alcohol dependence. Additionally, stress is a common trigger for relapse and subsequent loss of control of drinking in alcohol-dependent individuals.
Objectives
The present study was designed to characterize effects of repeated forced swim stress (FSS) on ethanol consumption in three rodent drinking models that engender high levels of ethanol consumption.
Methods
Adult male C57BL/6J mice were exposed to 10-min FSS 4 hr prior to each drinking session in three different models of high ethanol consumption: chronic intermittent ethanol (CIE) drinking (a model of dependence-like drinking), drinking-in-the-dark (DID; a model of binge-like drinking), and intermittent vs. continuous access (a model of escalated drinking).
Results
In the CIE drinking paradigm, daily FSS facilitated the escalation of ethanol intake that is typically seen in CIE-exposed mice without altering ethanol consumption in control mice exposed to FSS. FSS prior to drinking sessions did not alter ethanol consumption in the DID or intermittent access paradigms, whereas stressed mice in the continuous access procedure consumed less ethanol than their non-stressed counterparts.
Conclusions
The CIE drinking paradigm may provide a helpful preclinical model of stress-induced transition to ethanol dependence that can be used to 1) identify underlying neural mechanisms that facilitate this transition and 2) evaluate the therapeutic potential of various pharmacological agents hypothesized to alleviate stress-induced drinking.
Keywords: Ethanol dependence, Stress, Mouse, Forced swim stress, Binge drinking
Alcohol use disorder remains a major public health concern with a large economic burden associated with health care costs, lost productivity, and crime. Stress exposure has been identified as one risk factor for alcohol abuse that may facilitate the transition from social drinking and regulated alcohol use to more excessive levels of consumption that can lead to the development of alcohol dependence (Uhart and Wand, 2009). Additionally, stress is a common trigger for relapse and subsequent loss of control of drinking in alcohol dependent individuals (Sinha, 2012).
Preclinical models of alcoholism have been instrumental for identifying neurobiological targets of ethanol action as well as developing strategies for therapeutic intervention (see Lovinger and Crabbe, 2005). However, characterization of an animal model that simulates stress enhancement of drinking has proven challenging. Recent reviews have highlighted the inconsistent effects of stress on drinking within the literature and the difficulty in modeling stress-induced increases in ethanol consumption (Becker et al., 2011). More recently, a meta-analysis of data collected in rats revealed a number of experimental factors that appear to influence the effect of stress exposure on ethanol consumption (Noori et al., 2014). Specifically, stress is more likely to increase home cage drinking than self-administration of the drug via operant conditioning procedures, with adult animals being more sensitive to stress than their adolescent counterparts and males more sensitive than females. This report also indicated that certain stressors such as forced swim and foot shock are more likely to result in elevated ethanol intake than other stress procedures (e.g., restraint) (Noori et al., 2014).
Several recent studies have also highlighted the importance of individual differences in stress reactivity, level of ethanol self-administration prior to stress, and the timing of stress exposure and termination in relation to ethanol access in contributing to stress-related changes in ethanol consumption. For example, exposure to predator odor was associated with higher operant self-administration and compulsive-like responding for ethanol in rats that demonstrated greater avoidance of the odor-paired context (Edwards et al., 2013). In another study, although three consecutive days of footshock stress did not alter acquisition of ethanol self-administration, rats that evidenced low baseline self-administration prior to stress exposure showed an increase in “relapse”-like intake following an extinction period (Logrip and Zorilla, 2012). Similarly, a history of repeated footshock stress was shown to increase later drinking in rats using a 24-hr intermittent access paradigm, an effect that was only observed when stressor exposure occurred before drinking was established (Meyer et al., 2013). Repeated exposure to social defeat stress was also shown to increase subsequent ethanol consumption, but only following a 10-day rest period following stress termination (Norman et al., 2015; Hwa et al., 2016). Finally, a number of studies have shown that stress exposure during early development can have long-lasting effects on ethanol consumption. For example, neonatal stress induced by periods of maternal separation and isolate housing during adolescence have been shown to elevate ethanol consumption in a variety of drinking paradigms in adulthood (Cruz et al., 2008; McCool and Chappell, 2009; Butler et al., 2014; Lopez et al., 2011). Thus, there are a number of studies that demonstrate stress-induced increases in ethanol consumption, but the majority of these models involve stress exposure occurring before ethanol availability, and often after a period following stress termination.
However, few studies have examined the effects of stress on ethanol drinking in the context of dependence. In one study, 24-hr ethanol consumption was elevated during and after three consecutive days of forced swim stress only in rats with a history of chronic ethanol exposure (Sommer et al., 2008). In a recent study from our laboratory, we also observed differential effects of stress on ethanol consumption in dependent compared to nondependent animals. More specifically, using our dependence and relapse drinking model involving chronic intermittent ethanol (CIE) exposure, daily forced swim stress exposure prior to drinking sessions further elevated home cage limited access ethanol consumption in dependent (CIE-exposed) mice but did not alter intake in nondependent mice (Lopez et al., 2016).
The present study was designed to replicate and more fully characterize the effects of repeated forced swim stress on ethanol consumption in the CIE drinking model, and to extend these findings to two other rodent models that engender high levels of consumption: drinking-in-the-dark and intermittent access procedures (Becker, 2013). The CIE drinking model is well established in the field and provides an ideal platform for evaluation of effects of stress on escalated versus stable levels of drinking displayed by dependent and nondependent mice, respectively. Drinking-in-the-dark (DID) is a relatively simple model that quickly promotes binge-like ethanol intake along with significantly elevated blood ethanol concentrations (Rhodes et al., 2005; Thiele and Navarro, 2014). The intermittent vs. continuous access drinking model has been previously established as a way to produce differential levels of ethanol consumption. Specifically, mice with intermittent access to ethanol (i.e., every other day) demonstrate elevated ethanol consumption relative to mice with continuous access to ethanol (i.e., every day) in the home cage (Hwa et al., 2011; Crabbe et al., 2012). In the present study, ethanol consumption in all three drinking paradigms was compared in mice exposed to daily forced swim stress and their non-stressed counterparts. Although our own previous data indicated that forced swim exposure did not alter moderate levels of ethanol consumption in nondependent subjects, we hypothesized that this stressor would increase ethanol intake in the DID and intermittent access models due to the high levels of consumption that occur using these procedures.
Materials and Methods
Subjects
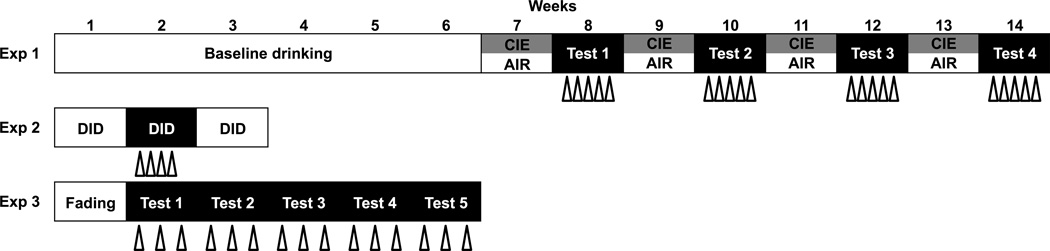
A total of 140 male C57BL/6J mice ordered from Jackson Laboratories (Bar Harbor, ME) were individually housed in standard polycarbonate cages with corncob bedding (Harlan Teklad 7092) in a temperature- and humidity-controlled vivarium within an AAALAC-accredited facility. Mice were 10–11 weeks of age at the start of each experiment. Subjects were maintained on a 12-hr modified reverse light/dark cycle with ad libitum access to food (Harlan Teklad Diet 2918) and water throughout experimentation. At all times, subjects were treated in accordance with the NIH Guide for the Care and Use of Laboratory Animals (8th edition, National Research Council, 2011) under protocols approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina. A procedural overview for each drinking model is depicted in Figure 1.
Fig. 1. Procedure overview.
Open triangles represent each 10-min exposure to forced swim stress (FSS) 4 hrs prior to ethanol access. In Experiment 1, once baseline drinking was established, alternating weeks of chronic intermittent ethanol (CIE) or air exposure and 5-day drinking tests occurred for a total of four cycles. FSS occurred prior to each daily drinking test session. In Experiment 2, the 4-day drinking-in-the-dark procedure was repeated for three cycles (baseline/stress/washout), with FSS prior to each drinking session during the second cycle only. In Experiment 3, ethanol concentration was gradually increased during a week of fading, followed by 6 weeks of either intermittent (24 hrs on Monday/Wednesday/Friday) or continuous access (24 hrs/7 days/week). FSS occurred prior to each Monday/Wednesday/Friday drinking session
Forced Swim Stress
In all experiments, stressed mice were subjected to a 10-min forced swim with an average delay of 4 hr between stress exposure and ethanol access. For swim stress exposure, each mouse was placed in a glass cylinder (20 cm diameter × 40 cm high) half-filled with 23–25° C tap water. Upon removal from the cylinders, subjects were hand-dried and allowed to recover on a heating pad for 5–10 min. Water in each cylinder was replaced between each subject.
Experiment 1: Ethanol Dependence (CIE)-Induced Drinking Model
Eighty mice were used in this experiment, with ethanol drinking occurring in the home cage under limited access conditions. Ethanol (15%, v/v) was presented in a free-choice situation (with tap water as the alternative fluid) for 2 hr starting 30 min before onset of the dark phase of the circadian cycle. After 6 weeks of baseline drinking, subjects were matched for average ethanol consumption during the final week of baseline and assigned to an ethanol exposure condition. Mice were subjected to repeated cycles of chronic intermittent ethanol (CIE) or air (CTL) exposure in inhalation chambers, with 5-day ethanol drinking test periods during intervening weeks. During test weeks, half of the CIE and CTL subjects were exposed to daily FSS treatment 4 hr prior to each drinking test session while the remaining groups were left undisturbed in their home cage. Body weights were recorded weekly during drinking weeks and daily during cycles of CIE exposure.
Chronic intermittent ethanol (CIE) exposure
Mice were exposed to ethanol vapor (CIE group) or air (CTL group) in Plexiglas inhalation chambers, as previously described (Griffin et al., 2009). Briefly, CIE (or air) exposure was delivered 16 hr/day for 4 consecutive days followed by a 3-day abstinence period, and this pattern of inhalation exposure was repeated for 4 weekly cycles with 5-day test drinking periods during intervening weeks (see Figure 1). Chamber ethanol concentrations were monitored daily using a breath alcohol tester (Lifeloc Technologies, Wheat Ridge, CO), with air flow adjusted as needed to maintain appropriate ethanol concentrations. Blood samples were collected once each week for analysis of blood ethanol concentration (BEC) as previously described (Griffin et al., 2009). Average BEC values from each cycle of CIE exposure are shown in Table 1.
Table 1.
Comparison of average blood ethanol concentrations (mg/dl) during cycles of chronic intermittent ethanol vapor exposure in non-stressed and stressed subjects.
| Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4 | |
|---|---|---|---|---|
| NS | 185 ± 9 | 213 ± 10 | 224 ± 9 | 229 ± 9 |
| FSS | 185 ± 7 | 204 ± 10 | 227 ± 12 | 224 ± 10 |
Lickometer circuitry
Each home cage was outfitted with a metal grid floor (resting over the bedding) that was connected to lickometer circuitry (Med Associates, St. Albans, VT). Water bottles were connected to the lickometer system at all times during drinking weeks. At the start of each 2-hr drinking session, ethanol bottles were connected to the lickometer as they were placed on each cage top. To reduce false lick counts due to extraneous contact with the metal sipper tubes, heat shrink tubing was used to coat spouts, leaving only the tip exposed. Licking responses were recorded by a computer located outside of the testing room and were expressed in 10-min bins across each 2-hr session and as total licks per session.
Experiment 2: Drinking-in-the-Dark (DID) Model
Twenty mice were used in this experiment, with testing occurring four days a week (Monday-Thursday) for three weeks. Starting 3 hr into the dark phase of the circadian cycle, water bottles were removed from the home cage and replaced with a single bottle containing 20% ethanol (v/v). On the first three days of testing, ethanol was available for 2 hr, with access extended to 4 hr on the final test day each week. No manipulation occurred during the first week of drinking (baseline). Subjects were matched for average ethanol consumption during baseline and assigned to a stress condition. Mice in the stress group were subjected to FSS as described above during the second week of DID testing (stress challenge). To examine any potential lingering effects of FSS exposure, drinking was assessed in the DID model for another week in the absence of stress (washout) (see Figure 1).
Experiment 3: Intermittent vs. Continuous Ethanol Access Model
Forty mice were randomly assigned to receive either continuous access or intermittent access to ethanol in the home cage with tap water provided as an alternate solution at all times. Mice in the continuous access (CA) condition were given access to ethanol 20–24 hr every day, with bottles replaced every day at 1500 hr (3 hr into the dark cycle). Mice in the intermittent access (IA) condition received access to ethanol for 20 hr three times each week (Monday, Wednesday, Friday). The position of ethanol and water bottles was rotated every other day to prevent side preferences. During the first week of drinking, ethanol concentration was increased from 3% (Monday-Tuesday) to 6% (Wednesday-Thursday) to 10% (Friday-Sunday). Thereafter, all mice were given 20% ethanol (v/v). Stress (FSS) exposure was introduced prior to the first exposure to 20% ethanol and occurred every Monday, Wednesday, and Friday as described above (see Figure 1). On these days, ethanol bottles were removed from all CA mice prior to FSS exposure, with all subjects gaining access to ethanol at 1500 hr. Thus, ethanol access always resumed 4 hr after FSS.
Data analysis
In all experiments, volume of ethanol consumed (mls) was determined by reading graduated drinking tubes (accuracy = ±0.1 ml) and intake data were expressed as g/kg body weight (density adjusted depending on ethanol concentration for calculation). Data were analyzed by repeated measures ANOVAs, with Group as a between-subjects factor and Week and/or Day as repeated measures. Alpha was set at .05 and all significant interactions were further explored using Newman-Keuls post-hoc tests.
Results
Experiment 1: Effect of Forced Swim Stress in the Ethanol Dependence (CIE)-Induced Drinking Model
Average Weekly Ethanol Consumption
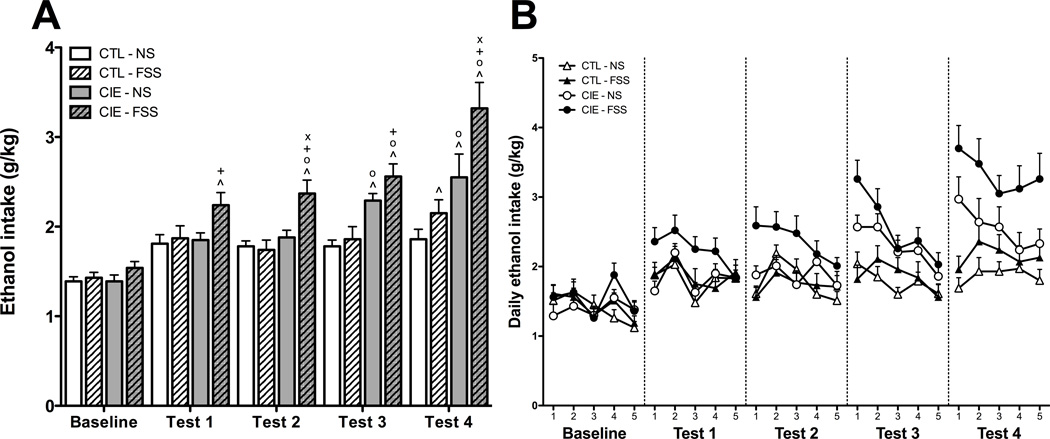
Average weekly ethanol consumption values from the final week of baseline drinking and the four test weeks were first analyzed using a 4 (Group) × 5 (Week) repeated measures ANOVA (see Figure 2A). This analysis revealed main effects of Group [F(3,73) = 15.635, p < 0.001] and Week [F(4,292) = 41.819, p < 0.001] in addition to a Group × Week interaction [F(12,292) = 3.865, p < 0.001]. Post-hoc analyses were used to compare each condition to their own baseline levels of ethanol intake. Ethanol consumption in the CTL-NS group did not change across the course of the experiment. For the CTL-FSS group, ethanol consumption was elevated relative to Baseline during Test 4. CIE-NS mice consumed more ethanol during Tests 3 and 4 relative to Baseline, whereas CIE-FSS mice demonstrated this increase across all four drinking test weeks.
Fig. 2.
(A) Average weekly ethanol consumption. Increases relative to baseline ethanol consumption (indicated by ^) were observed during Test 4 for CTL-FSS mice (hatched white bars), Tests 3–4 for CIE-NS mice (gray bars), and Tests 1–4 for CIE-FSS mice (hatched gray bars). Elevated ethanol consumption relative to CTL-NS mice (white bars) was observed during Tests 3–4 for CIE-NS mice and Tests 2–4 for CIE-FSS mice (indicated by o). CIE-FSS also demonstrated greater ethanol intake than CTL-FSS mice during Tests 1–4 (indicated by +) and greater intake than CIE-NS mice during Tests 2 and 4 (indicated by x). Values shown are Means ± SEMs
(B) Average daily ethanol consumption. During Test 2, CIE-FSS mice (black circles) drank more than all other groups. During Test 3, both CIE-NS (white circles) and CIE-FSS groups consumed more ethanol than CTL-NS (white triangles) and CTL-FSS (black triangles) mice. CIE-FSS mice consumed more ethanol than all other groups during Test 4. Additionally, CIE-NS mice consumed more ethanol than mice in the CTL-NS condition. No interactions of Group and Day were observed during any week of testing. Values shown are Means ± SEMs
Separate follow-up analyses were conducted for each week of drinking to further explore group differences in ethanol consumption. As expected, no differences in ethanol intake were detected between groups during the Baseline phase of the study. During Test 1, a marginally significant effect of Group [F(3,73) = 2.662, p = 0.05] was revealed, with post hoc analysis indicating that the CIE-FSS mice consumed more ethanol than the CTL-FSS mice (p < 0.05), and tended to drink more than the CTL-NS and CIE-NS groups as well (ps < 0.07). Analysis of Test 2 data revealed a significant main effect of Group [F(3,73) = 8.056, p < 0.001], with CIE-FSS mice consuming more ethanol than all other groups. During Test 3, a main effect of Group [F(3,73) = 10.407, p < 0.001] emerged, with post hoc tests indicating that the CIE-FSS group consumed more ethanol than the CTL-NS and CTL-FSS groups, and that CIE-NS mice consumed more ethanol than CTL-NS mice. Finally, during Test 4, post hoc analysis of the main effect of Group [F(3,73) = 9.7669, p < 0.001] revealed that the CIE-FSS mice consumed more ethanol than all other groups (ps < 0.05) and CIE-NS mice consumed more ethanol than CTL-NS mice (p = 0.05).
Average Daily Ethanol Consumption
To determine whether the group differences reported above varied across drinking day within each test week, daily ethanol consumption values were analyzed using a 4 (Group) × 5 (Week) × 5 (Day) repeated measures ANOVA (see Figure 2B). This analysis revealed a significant Group × Week × Day interaction [F(48,1168) = 1.423, p < 0.05], and follow-up 2-way ANOVAs (Group × Day) were conducted during each week of testing. During Baseline, a main effect of Day emerged [F(4,292) = 4.224, p < 0.01], with post hoc tests indicating that consumption was lower on Day 5 relative to Days 1–2 and 4. Analysis of Test 1 daily intake revealed a main effect of Day [F(4,292) = 6.465, p < 0.001], with elevated drinking on Day 2 relative to all other days of testing. During Test 2, main effects of Group [F(3,73) = 8.031, p < 0.01] and Day [F(4,292) = 4.258, p < 0.01] were observed, with post-hoc tests indicating that CIE-FSS mice consumed more ethanol than all other groups, and that overall drinking was higher on Day 2 relative to Days 1 and 4–5. During Test 3, main effects of Group [F(3,73) = 10.411, p < 0.001] and Day [F(4,292) = 11.136, p < 0.001] emerged. Post hoc tests revealed that both CIE-NS and CIE-FSS groups consumed more ethanol than CTL-NS and CTL-FSS groups, with greater overall consumption observed on Days 1–2 relative to all other days of the test week. Analysis of Test 4 drinking data revealed only a main effect of Group [F(3,73) = 9.7784, p < 0.001]. Post hoc analysis indicated that CIE-FSS mice consumed more ethanol than all other groups. Additionally, CIE-NS mice consumed more ethanol than mice in the CTL-NS condition. No interactions of Group and Day were observed during any week of testing.
Ethanol Lick Responses
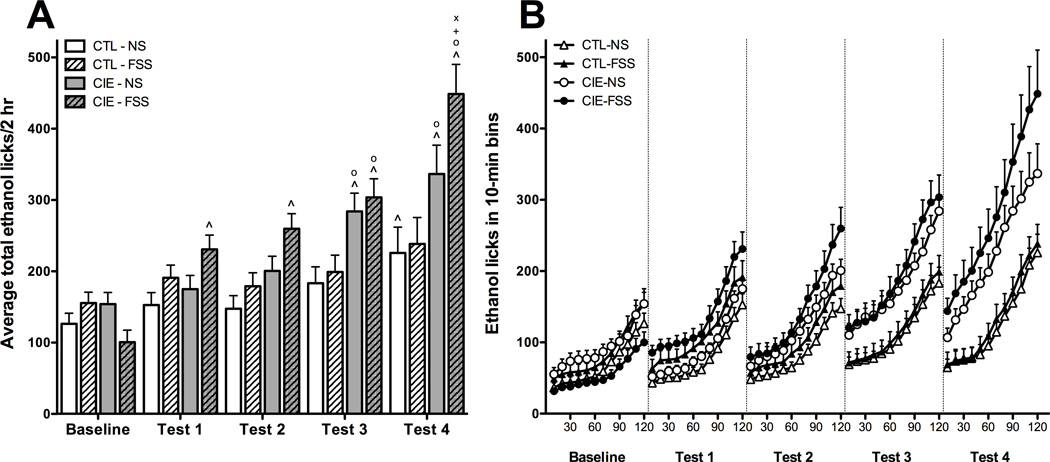
Average total licks on the ethanol bottle during the 2-hr drinking sessions within each test week were analyzed using a 4 (Group) × 5 (Week) repeated measures ANOVA (Figure 3A). Analysis revealed main effects of Group [F(3,70) = 6.775, p < 0.001] and Week [F(4,280) = 42.517, p < 0.001] in addition to a significant Group × Week interaction [F(12,280) = 4.827, p < 0.001]. Post hoc analysis indicated that, relative to Baseline, CIE-NS mice evidenced greater lick responses during Tests 3 and 4 whereas mice in the CIE-FSS condition showed greater ethanol licks during all test weeks (Tests 1–4). CTL-NS mice made more ethanol lick responses than Baseline during Test 4. Both CIE-NS and CIE-FSS groups had higher ethanol lick responses than CTL-NS mice during Tests 3 and 4. CIE-FSS mice made more ethanol lick responses than CIE-NS and CTL-FSS groups during Test 4. Data expressed as average licks across 10-min bins revealed a similar profile of results (Figure 3B).
Fig. 3.
(A) Average total ethanol licks. Increases relative to baseline ethanol licks (indicated by ^) were observed during Test 4 for CTL-NS mice (white bars), Tests 3–4 for CIE-NS mice (gray bars), and Tests 1–4 for CIE-FSS mice (hatched gray bars). During Tests 3–4, both CIE-NS and CIE-FSS groups demonstrated greater ethanol lick responses than CTL-NS mice (indicated by o). The CIE-FSS group also had greater ethanol licks than both CTL-FSS mice (indicated by +) and CIE-NS mice (indicated by x) during Test 4. Values shown are Means ± SEMs
(B) Average temporal distribution of ethanol licks in 10-min bins. Average weekly ethanol licks across the 2-hr drinking sessions are shown for CTL-NS (white triangles), CTL-FSS (black triangles), CIE-NS (white circles), and CIE-FSS (black circles) mice.
Experiment 2: Effect of Forced Swim Stress on Binge-Like Drinking in the DID Model
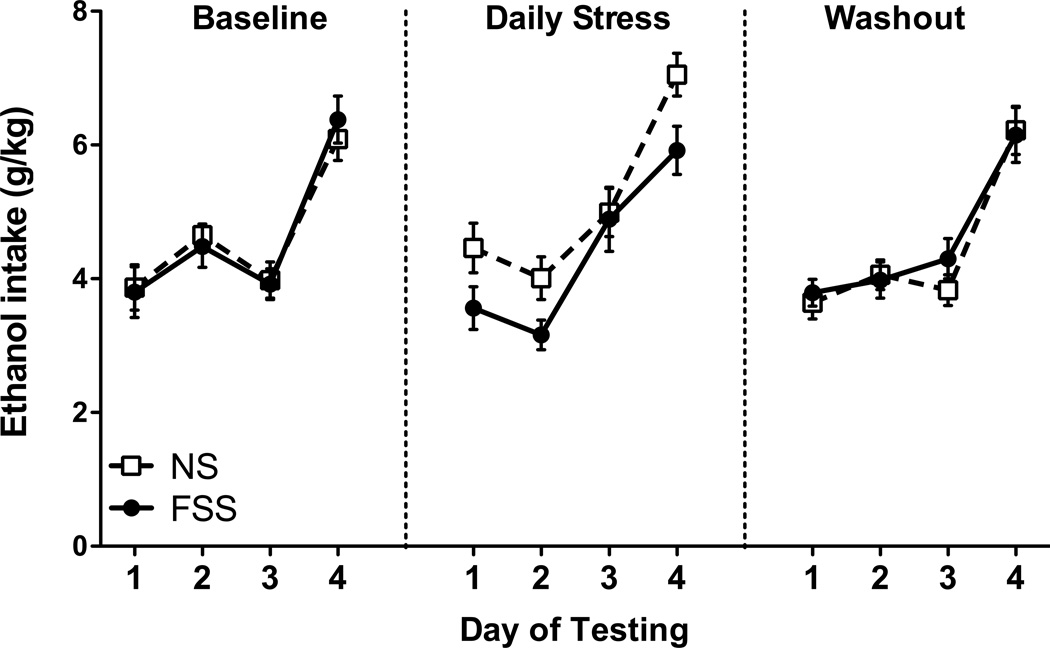
Drinking data from the 2-hr sessions each week (expressed in g/kg) were subjected to a 2 (Group) × 3 (Day) repeated measures ANOVA. Data from the 4-hr tests were analyzed separately each week using a t-test. ANOVAs did not reveal a significant effect of FSS exposure on ethanol intake at any time during the study (see Figure 4).
Fig. 4.
Average daily ethanol consumption. Forced swim stress (FSS; white squares) did not influence ethanol consumption relative to non-stressed mice (NS; black circles) during 2-hr (Days 1–3) or 4-hr (Day 4) drinking sessions in the drinking-in-the-dark paradigm. Values shown are Means ± SEMs
Experiment 3: Effect of Forced Swim Stress on Drinking in the Intermittent vs. Continuous Ethanol Access Model
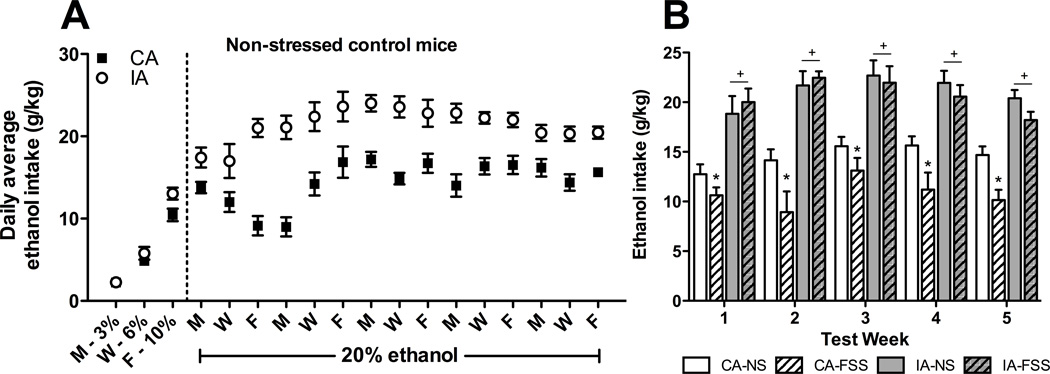
After the acclimation/fading period, daily average intake was greater in non-stressed mice with intermittent access to ethanol compared to non-stressed mice given continuous access to ethanol, and this effect persisted over the 5 weeks of the study (Figure 5A). Weekly average ethanol intake calculated from Monday-Wednesday-Friday drinking data was analyzed using a 4 (Group) × 5 (Week) repeated measures ANOVA. This analysis indicated significant main effects of Group [F(3,35) = 40.451, p < 0.001] and Week [F(4,140) = 6.504, p < 0.001]. Post hoc tests revealed that, regardless of stress condition, both intermittent access groups consumed more ethanol than both continuous access groups during all test weeks. Among mice with continuous access to ethanol, FSS exposure reduced consumption each week. In contrast, FSS did not significantly alter drinking in mice with intermittent ethanol access (Figure 5B). Overall, ethanol consumption was higher during Weeks 3 and 4 than Week 1.
Fig. 5.
(A) Average daily ethanol consumption among non-stressed controls. Data are shown only for days during which both continuous (CA; black squares) and intermittent (IA; white circles) groups had access to ethanol. (B) Average weekly ethanol consumption during 24-hr intervals. Mice in the CA-FSS group (hatched white bars) had reduced ethanol consumption relative to CA-NS (white bars) during Tests 1–5 (indicated by *). IA-NS (gray bars) and IA-FSS (hatched gray bars) groups did not differ during any week of testing, although both groups consumed more ethanol than the CA groups during all 5 test weeks (indicated by +). Values shown are Means ± SEMs
Discussion
The present series of experiments applied repeated FSS exposure 4 hr prior to drinking sessions in three different models that engender high levels of ethanol consumption in C57BL/6J mice. In the CIE drinking paradigm, daily FSS facilitated escalation of ethanol intake that is typically seen in dependent (CIE-exposed) mice without altering ethanol consumption in nondependent (air-exposed) mice. In contrast, FSS exposure did not alter binge-like ethanol consumption in the DID model. FSS also did not alter intake in mice given intermittent access to ethanol, whereas this stressor reduced consumption in mice with continuous access to ethanol. Taken together, these results suggest that FSS experience interacts uniquely with ethanol dependence to further enhance voluntary drinking.
Results from Experiment 1 are consistent with previous work our laboratory that demonstrated daily FSS selectively augmented ethanol consumption in CIE-exposed mice, but not control mice (Lopez et al., 2016). In the current study, FSS facilitated the emergence of escalated drinking in CIE-exposed mice. That is, whereas non-stressed CIE-exposed mice evidenced greater ethanol consumption above baseline levels of intake during the third and fourth week of testing, FSS exposure resulted in higher ethanol consumption above baseline intake in CIE-exposed mice across all four test weeks of the study. Moreover, FSS further elevated drinking beyond intake levels of non-stressed CIE mice. As in our previous report, FSS did not significantly alter ethanol consumption in nondependent mice. Analysis of licking responses on the ethanol bottle indicated a similar profile of results, providing further support for the pattern of acceleration and augmentation of dependence-related drinking in this model. Although the daily drinking data appear to suggest more robust effects of FSS exposure on drinking in the CIE-exposed mice during test sessions early in the week, these interactions did not reach statistical significance. Overall, these results suggest that FSS both accelerated the transition to dependence-like drinking and augmented the escalation of ethanol consumption in CIE-exposed mice.
In contrast, FSS exposure did not alter drinking in the DID model. The DID paradigm provides a model of binge-like ethanol consumption, but not dependence-related drinking. Thus, these results are consistent with the lack of an effect of FSS exposure on drinking in the air-exposed CTL mice in Experiment 1, despite the fact that intake was significantly higher in the DID model compared to intake of nondependent mice in the CIE model. Although repeated weekly cycles of DID have been shown to consistently produce high levels of ethanol consumption, this model does not produce other features of an ethanol dependence-like phenotype such as anxiety, motor impairment, or increased sensitivity to handling-induced convulsions (Cox et al., 2013). The DID procedure in Experiment 2 was repeated for a total of three cycles, a relatively brief exposure regimen, and the effect of FSS on drinking was only assessed during a single DID cycle. It is possible that drinking in an extended DID exposure model (i.e., six or more weekly cycles) may have yielded a different outcome (significant stress-induced changes in intake).
As shown by others using rats (e.g., Simms et al., 2008) and mice (Hwa et al., 2011), ethanol consumption was significantly elevated when the drug was made available on an intermittent rather than continuous schedule. However, in the present study, FSS exposure did not significantly alter the elevated drinking induced by the intermittent scheduled access to ethanol. In contrast, FSS exposure reduced ethanol consumption when it was continuously available to an independent group of mice. At present, it is unclear why FSS exposure decreased ethanol consumption in mice with continuous, but not intermittent, access to ethanol. Recent studies have demonstrated that 10 consecutive days of social stress induced by moderate/severe (but not mild) social defeat increased ethanol consumption when intermittent 24-hr access to ethanol was initiated 10 days following stress termination (Norman et al., 2015; Hwa et al., 2016). Thus, while the intermittent access drinking paradigm has been shown to be sensitive to effects of stress, the nature and timing of the stress exposure appear to be critical factors that influence these effects. In the present study, forced swim stress exposure administered 4 hr prior to the drinking sessions did not alter intake. However, whether FSS exposure prior to the induction of the intermittent access drinking paradigm might also result in further elevation of ethanol consumption remains to be determined.
While all three drinking models used in the present study produce high levels of ethanol consumption, forced swim stress exposure only increased intake in CIE-exposed mice. One distinguishing feature of the CIE drinking model compared to the DID and intermittent access models is that elevated drinking in the CIE model involves periods of high and sustained blood ethanol levels produced via inhalation exposure. Increased drinking was not observed when ethanol exposure produced high, but transient blood ethanol levels (Griffin et al., 2009). Thus, results from this study suggest that FSS increases ethanol intake in the CIE model due to a distinct interaction of the stress exposure and chronic ethanol exposure that renders subjects dependent. At present, the nature and underlying mechanism of this interaction is not fully understood.
It is possible that the addition of stress exposure to the CIE drinking paradigm may promote elevated drinking by increasing the reinforcing properties of ethanol, accentuating withdrawal effects (thereby enhancing the drive to consume ethanol to relieve these effects), or both. A previous study supporting the former possibility found that FSS, in addition to producing a transient increase in ethanol consumption, potentiated ethanol conditioned place preference when the stress exposure occurred shortly before each conditioning trial (Sperling et al., 2010). The authors speculated that dysphoria elicited by repeated stress might have enhanced the relative rewarding value of ethanol. Enhanced ethanol-induced conditioned place preference has also been reported following psychosocial stress (Bahi, 2013). However, if FSS exposure enhances the reinforcing effects of ethanol, then this stressor would be expected to increase ethanol consumption not only in CIE-exposed mice but in nondependent mice, as well as drinking in the DID and intermittent access models. Given the apparent selective effects of FSS in facilitating and augmenting ethanol drinking in CIE-exposed mice, this effect is not likely due to a simple enhancement of ethanol reward.
Alternatively, it is possible that stress may enhance the negative reinforcing capacity of ethanol. That is, stress experience in the context of repeated cycles of chronic ethanol exposure and withdrawal may magnify withdrawal-related dysphoria, thereby promoting greater ethanol consumption. Supportive of this idea, studies have observed elevated anxiety during protracted ethanol withdrawal in stressed rats relative to their non-stressed counterparts (Breese et al., 2004; Knapp et al., 2007; Valdez et al., 2003, Gillett et al., 2013). In the present study, FSS accelerated the emergence of escalated drinking in CIE-exposed mice, and this may be due to stress-induced facilitation of the negative reinforcing effects of ethanol in the model. Future studies involving operant conditioning procedures will enable more detailed examination of the interaction of stress with the motivational effects of ethanol in the context of dependence.
From a mechanistic standpoint, the progression from regulated ethanol consumption to excessive and uncontrolled drinking associated with dependence is hypothesized to involve recruitment of brain stress systems (Becker, 2012; Koob, 2013). For example, adaptations in CRF (Heilig and Koob, 2007; Zorilla et al., 2014), dynorphin (Kissler et al., 2014; Walker et al., 2011), and glucocorticoid (Vendruscolo et al., 2012) signaling have been linked to escalated drinking associated with dependence. Because stress exposure engages these same neural systems that have been implicated in the transition to ethanol dependence, such neuroadaptations resulting from CIE procedure may become exaggerated by stress exposure, ultimately leading to further escalation of ethanol consumption. Future studies will focus on elucidating the contribution of these and other adaptations that promote stress enhancement of drinking, especially in the context of dependence.
In summary, the current series of experiments examined the effects of repeated FSS exposure on ethanol consumption in three different rodent drinking paradigms. In the CIE drinking model, FSS exposure facilitated the emergence of and enhanced the magnitude of escalated ethanol intake in dependent, but not nondependent mice. In contrast, FSS exposure did not significantly alter ethanol consumption in two other drinking models (DID and intermittent access paradigms) that promote high levels of ethanol consumption. These data suggest that stress exposure (particularly forced swim stress) may interact in a unique manner with ethanol dependence to further promote excessive levels of drinking. Thus, the FSS-CIE model may serve as a useful preclinical model to (1) identify neural mechanisms underlying the ability of stress to facilitate transition to heavy drinking associated with dependence; and (2) evaluate the therapeutic potential of various pharmacological agents hypothesized to alleviate stress-induced drinking.
Acknowledgments
This work was funded by NIAAA grants P50 AA 10761 (HCB), U01 AA01095 (HCB), and U01 AA020929 (MFL). RIA was supported by T32 AA007474 and F32 AA023700. The authors wish to thank Chelsea Johnson, Joshua Palmer, and India Robbins for technical assistance.
Footnotes
All authors declare that they have no conflicts of interest to disclose.
References
- Bahi A. Increased anxiety, voluntary alcohol consumption and ethanol-induced place preference in mice following chronic psychosocial stress. Stress. 2013;16:441–451. doi: 10.3109/10253890.2012.754419. [DOI] [PubMed] [Google Scholar]
- Becker HC. Effects of alcohol dependence and withdrawal on stress responsiveness and alcohol consumption. Alcohol Res. 2012;34:448–458. doi: 10.35946/arcr.v34.4.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC. Animal models of excessive alcohol consumption in rodents. Current topics in behavioral neurosciences. 2013;13:355–377. doi: 10.1007/7854_2012_203. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, Doremus-Fitzwater TL. Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology (Berl) 2011;218:131–156. doi: 10.1007/s00213-011-2443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH. Stress sensitization of ethanol withdrawal-induced reduction in social interaction: inhibition by CRF-1 and benzodiazepine receptor antagonists and a 5-HT1A-receptor agonist. Neuropsychopharmacology. 2004;29:470–482. doi: 10.1038/sj.npp.1300282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler TR, Ariwodola OJ, Weiner JL. The impact of social isolation on HPA axis function, anxiety-like behaviors, and ethanol drinking. Frontiers in integrative neuroscience. 2014;7:102. doi: 10.3389/fnint.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BR, Olney JJ, Lowery-Gionta EG, Sprow GM, Rinker JA, Navarro M, Kash TL, Thiele TE. Repeated cycles of binge-like ethanol (EtOH)-drinking in male C57BL/6J mice augments subsequent voluntary EtOH intake but not other dependence-like phenotypes. Alcohol Clin Exp Res. 2013;37:1688–1695. doi: 10.1111/acer.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Harkness JH, Spence SE, Huang LC, Metten P. Intermittent availability of ethanol does not always lead to elevated drinking in mice. Alcohol Alcohol. 2012;47:509–517. doi: 10.1093/alcalc/ags067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, Quadros IM, Planeta Cda S, Miczek KA. Maternal separation stress in male mice: long-term increases in alcohol intake. Psychopharmacology. 2008;201:459–468. doi: 10.1007/s00213-008-1307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Baynes BB, Carmichael CY, Zamora-Martinez ER, Barrus M, Koob GF, Gilpin NW. Traumatic stress reactivity promotes excessive alcohol drinking and alters the balance of prefrontal cortex-amygdala activity. Translational psychiatry. 2013;3:e296. doi: 10.1038/tp.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillett K, Harshberger E, Valdez GR. Protracted withdrawal from ethanol and enhanced responsiveness stress: regulation via the dynorphin/kappa opioid receptor system. Alcohol. 2013;47:359–365. doi: 10.1016/j.alcohol.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Becker HC. Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcohol Clin Exp Res. 2009;33:1893–1900. doi: 10.1111/j.1530-0277.2009.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA. Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res. 2011;35:1938–1947. doi: 10.1111/j.1530-0277.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Holly EN, DeBold JF, Miczek KA. Social stress-escalated intermittent alcohol drinking: modulation by CRF-R1 in the ventral tegmental area and accumbal dopamine in mice. Psychopharmacology. 2016;233:681–690. doi: 10.1007/s00213-015-4144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissler JL, Sirohi S, Reis DJ, Jansen HT, Quock RM, Smith DG, Walker BM. The one-two punch of alcoholism: role of central amygdala dynorphins/kappa-opioid receptors. Biol Psychiatry. 2014;75:774–782. doi: 10.1016/j.biopsych.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Breese GR. Baclofen blocks expression and sensitization of anxiety-like behavior in an animal model of repeated stress and ethanol withdrawal. Alcohol Clin Exp Res. 2007;31:582–595. doi: 10.1111/j.1530-0277.2007.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Addiction is a Reward Deficit and Stress Surfeit Disorder. Frontiers in psychiatry. 2013;4:72. doi: 10.3389/fpsyt.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Zorrilla EP. Stress history increases alcohol intake in relapse: relation to phosphodiesterase 10A. Addict Biol. 2012;17:920–933. doi: 10.1111/j.1369-1600.2012.00460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Anderson RI, Becker HC. Effect of different stressors on voluntary ethanol intake in ethanol-dependent and nondependent C57BL/6J mice. Alcohol. 2016;51:17–23. doi: 10.1016/j.alcohol.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Doremus-Fitzwater TL, Becker HC. Chronic social isolation and chronic variable stress during early development induce later elevated ethanol intake in adult C57BL/6J mice. Alcohol. 2011;45:355–364. doi: 10.1016/j.alcohol.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, Crabbe JC. Laboratory models of alcoholism: treatment target identification and insight into mechanisms. Nat Neurosci. 2005;8:1471–1480. doi: 10.1038/nn1581. [DOI] [PubMed] [Google Scholar]
- McCool BA, Chappell AM. Early social isolation in male Long-Evans rats alters both appetitive and consummatory behaviors expressed during operant ethanol self-administration. Alcoholism, clinical and experimental research. 2009;33:273–282. doi: 10.1111/j.1530-0277.2008.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer EM, Long V, Fanselow MS, Spigelman I. Stress increases voluntary alcohol intake, but does not alter established drinking habits in a rat model of posttraumatic stress disorder. Alcoholism, clinical and experimental research. 2013;37:566–574. doi: 10.1111/acer.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noori HR, Helinski S, Spanagel R. Cluster and meta-analyses on factors influencing stress-induced alcohol drinking and relapse in rodents. Addict Biol. 2014;19:225–232. doi: 10.1111/adb.12125. [DOI] [PubMed] [Google Scholar]
- Norman KJ, Seiden JA, Klickstein JA, Han X, Hwa LS, DeBold JF, Miczek KA. Social stress and escalated drug self-administration in mice I. Alcohol and corticosterone. Psychopharmacology. 2015;232:991–1001. doi: 10.1007/s00213-014-3733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. How does stress lead to risk of alcohol relapse? Alcohol research : current reviews. 2012;34:432–440. doi: 10.35946/arcr.v34.4.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol Psychiatry. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Sperling RE, Gomes SM, Sypek EI, Carey AN, McLaughlin JP. Endogenous kappa-opioid mediation of stress-induced potentiation of ethanol-conditioned place preference and self-administration. Psychopharmacology (Berl) 2010;210:199–209. doi: 10.1007/s00213-010-1844-5. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Navarro M. "Drinking in the dark" (DID) procedures: a model of binge-like ethanol drinking in non-dependent mice. Alcohol. 2014;48:235–241. doi: 10.1016/j.alcohol.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhart M, Wand GS. Stress, alcohol and drug interaction: an update of human research. Addict Biol. 2009;14:43–64. doi: 10.1111/j.1369-1600.2008.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez GR, Zorrilla EP, Roberts AJ, Koob GF. Antagonism of corticotropin-releasing factor attenuates the enhanced responsiveness to stress observed during protracted ethanol abstinence. Alcohol. 2003;29:55–60. doi: 10.1016/s0741-8329(03)00020-x. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Jr, Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Zorrilla EP, Koob GF. Systemic kappa-opioid receptor antagonism by nor-binaltorphimine reduces dependence-induced excessive alcohol self-administration in rats. Addict Biol. 2011;16:116–119. doi: 10.1111/j.1369-1600.2010.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]