Abstract
Purpose
Our previous retrospective analysis of clinically referred breast cancer survivors’ performance on learning and memory measures found a primary weakness in initial encoding of information into working memory with intact retention and recall of this same information at a delay. This suggests that survivors may misinterpret cognitive lapses as being due to forgetting when in actuality they were not able to properly encode this information at the time of initial exposure. Our objective in this study was to replicate and extend this pattern of performance to a research sample to increase the generalizability of this finding in a sample in which subjects were not clinically referred for cognitive issues.
Methods
We contrasted learning and memory performance between breast cancer survivors on endocrine therapy two to six years post-treatment with age- and education-matched healthy controls. We then stratified lower and higher performing breast cancer survivors to examine specific patterns of learning and memory performance. Contrasts were generated for four aggregate visual and verbal memory variables from the California Verbal Learning Test – 2 (CVLT-2) and the Brown Location Test (BLT): Single Trial Learning: Trial 1 performance; Multiple Trial Learning: Trial 5 performance; Delayed Recall: Long Delay Recall performance; and Memory Errors: False Positive errors.
Results
As predicted, breast cancer survivors’ performance as a whole was significantly lower on Single Trial Learning than the healthy control group but exhibited no significant difference in Delayed Memory. In the secondary analysis contrasting lower- and higher-performing survivors on cognitive measures, the same pattern of lower Single Trial Learning performance was exhibited in both groups, with the additional finding of significantly weaker Multiple Trial Learning performance in the lower-performing breast cancer group, and intact Delayed Recall performance in both groups.
Conclusions
As with our earlier finding of weaker initial encoding with intact recall in a cohort of clinically referred breast cancer survivors, our results indicate this same profile in a research sample of breast cancer survivors. Further, when the breast cancer group was stratified by lower and higher performance, both groups exhibited significantly lower performance on initial encoding, with more pronounced encoding weakness in the lower performing group. As in our previous research, survivors did not lose successfully encoded information over longer delays, either in the lower- or higher performing group, again arguing against memory decay in survivors. The finding of weaker initial encoding of information together with intact delayed recall in survivors points to specific treatment interventions in rehabilitation of cognitive dysfunction.
Implications for Cancer Survivors
The finding of weaker initial encoding of information together with intact delayed recall in survivors points to specific treatment interventions in rehabilitation of cognitive dysfunction and are discussed.
Keywords: Cancer, Memory, Attention, Cognition
Background
Previous research on cognitive dysfunction following cancer treatment has utilized both subjective and objective measures to assess cognitive function. Subjective measures typically involve asking the patient to complete a questionnaire and subjectively rate their own cognitive abilities, while objective measures test actual performance on cognitive tasks using measures adopted from clinical assessment methods. Early research on self-reported cognitive difficulties in mixed etiology cancer patients found that roughly half of patients self-reported difficulties in memory at some point in their treatment [1], with significant forgetfulness being reported by 52% of patients [2] at longer intervals. Similar results were also reported by Schagen et al. [3], who found persistent self-reported difficulties in memory and concentration in breast-cancer survivors, and by Ahles et al. [4] up to ten years post-treatment in breast and lymphoma survivors. While subjective reports following treatment suggest significant memory dysfunction, results of studies that objectively measure cognitive abilities using clinical, performance-based measures do not find a strong effect in actual, tested memory performance. A subset of studies find no observable cognitive effects following treatment [5], observable decline in only a subset of patients [6], or declines in only a subset of cognitive abilities with no overall decline in neuropsychological performance [7]. While these mixed findings may reflect limited sample size and/or relative sensitivity of clinical neuropsychological measures to detect subtle dysfunction [8, 9], results of meta-analyses that capitalize on increased power to detect dysfunction do find significant effects in a subset of cognitive domains, but not in memory performance [10]. Given this literature and clinical observations in our patients seen for neuropsychological evaluation following treatment, we have previously hypothesized that memory complaints are driven by encoding issues that are misidentified as actual forgetting by patients in daily activities. These difficulties in encoding lead to inefficient learning of information and, as a result, this same information is not available for delayed recall. Affected survivors experience this cognitive failure as “forgetfulness” when in fact they never properly learned or encoded this information when it was initially presented. Studies that utilize objective clinical measures may not identify this pattern because they focus on delayed recall performance after multiple learning trials are presented, and repeated exposure compensates for an initial encoding weakness that would be evident if information was available only in a single presentation. This is an important distinction for cancer survivors, and for research on cognition in survivorship, since a primary encoding weakness will implicate a different target of intervention in rehabilitation and suggests alternate methods for studies investigating learning and memory in cancer survivors.
Recent work from our lab is supportive of this hypothesis [11]. We analyzed a retrospective sample of 64 clinically referred breast cancer patients in whom forgetfulness was the main presenting complaint (80%). We hypothesized that encoding in single trial learning performance would be a significant weakness, but that retention and recall of this information following a delay would be within the expected range. We focused on California Verbal Learning Test – Second Edition (CVLT-2) Trial-1 performance, a measure of single trial learning reflecting initial learning and encoding abilities, as well as a factor-analytically derived factor of Attention [12]. As predicted, results indicated significant weakness in initial learning of information after a single trial (Trial 1), less efficient attentional function across initial learning trials (Middle Region Recall), performance at or above normative expectations after repeated learning trials (Trial 5), and retention and recall of this information after a delay consistent with normative expectations (Short Delay Free Recall and Long Delay Free Recall). Importantly, analysis of true forgetting of information in the interval between learning trials and delayed recall trials indicates that the rate of forgetting in our breast cancer survivor group is indistinguishable from the original normative sample. These results suggest that initial encoding issues likely contribute to reported forgetfulness in our sample of breast cancer survivors and that repeated learning trials compensate for this weakness to allow for normal delayed memory performance.
Because our prior analysis relied on clinically referred breast cancer patients whose subjective cognitive dysfunction was severe enough to warrant a neuropsychological evaluation, the aim of the present study was to extend and replicate this analysis in a larger research sample of breast cancer survivors to improve generalizability of these findings. While clinical findings are suggestive, reliance on data from clinically referred survivors in whom dysfunction is severe enough to warrant neuropsychological evaluation runs the risk of overestimating objective cognitive dysfunction in survivors more generally. The replication of these findings in a research sample, specifically initial encoding deficits in the sample as a whole, as well as in both higher- and lower- performing survivor groups, improves the generalizability of this finding to the survivor population. We again focused on single trial learning performance using the previously established framework of single trial learning, multiple trial learning, and delayed memory [13] utilized in our previous clinical analysis [11]. The CVLT-2 and the Brown Location Test (BLT) both consist of five learning trials followed by a delayed memory trial and allow for decomposing learning and memory into initial encoding after a single trial, encoding after multiple trials, and retention and retrieval of information after a delay. We combined Trial 1 performance on the California Verbal Learning Test – Second Edition (CVLT-2), a serial verbal list learning task, with Trial 1 performance on the Brown Location Test (BLT), a serial visual learning task, and extended the analysis to combined Trial 5 performance, and Long Delay Recall performance to analyze discrepant learning and memory performance between breast cancer survivors and healthy controls. We further stratified the breast cancer group into higher and lower performing groups to further clarify learning and memory performance in more severely affected survivors. We hypothesized that breast cancer survivors as a whole would exhibit weaker initial encoding of information, that this weakness would be compensated for by multiple presentations, and that recall performance would be intact. For the lower and higher performing breast cancer groups, we again hypothesized weaker initial encoding and intact retention of information at a delay for both groups, with more pronounced weakness in later learning trials for the lower performing group.
Methods
Subjects
The breast cancer group ((BC) n=113) consisted of survivors currently treated with endocrine therapy two- to six-years post completion of treatment and a healthy control group ((HC) n=37) consisting of age- and education-matched individuals with no history of cancer or cancer treatment. Breast cancer survivors were included if they: 1) were between two- and six-years post-completion of surgery and/or chemotherapy treatment; 2) were currently treated with endocrine therapy; 3) were less than 70 years of age at the time of recruitment; 4) were post-menopausal prior to initial treatment; 5) had not experienced a recurrence since initial treatment; 6) had no history of neurobehavioral risk factors including history of neurological disorder, moderate to severe head trauma (loss of consciousness > 60 min or evidence of structural brain changes on imaging), or neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease, or multiple sclerosis; 7) did not self-report sleep disorders that could influence cognitive functioning including sleep apnea and chronic fatigue syndrome; 8) did not report major affective disorder (untreated), bipolar disorder, or schizophrenia (DSM-IV). The same inclusion criteria were used for healthy control (HC) recruitment with the exception of cancer criteria. All study methods, recruitment, data collection and analysis were approved by the MSKCC IRB prior to study initiation. Demographic information is presented in table 1.
Table 1.
Sample Demographic Characteristics
| Breast Cancer (n=113) | Healthy Control (n=37) | |
|---|---|---|
| Age at baseline (years) | 62.7 (3.9) | 62.5 (5.2) |
| Estimated premorbid IQ (WRAT-4 SS) | 112.5 (15.3) | 118.6 (18.6) |
| Handedness | ||
| Right | 99 | 33 |
| Left or ambidexterous | 14 | 4 |
| Cancer stage | ||
| I | 65 | |
| III | 37 | |
| IV | 9 | |
| Endocrine Therapy | ||
| Tamoxifen | 12 | |
| Arimidex | 64 | |
| Aromasin | 18 | |
| Femara | 19 | |
| Surgery | 113 | |
| Mastectomy | 36 | |
| Lumpectomy | 85 | |
| Sentinel node biopsy | 101 | |
| Axillary node biopsy | 40 | |
| Time since surgery | 4.2 (1.2) | |
| Received radiotherapy | 88 | |
| Years since radiotherapy | 3.8 (1.3) | |
| Received chemotherapy | 59 | |
| AC-T | 38 | |
| CMF | 14 | |
| EC-T | 3 | |
| FEC | 1 | |
| CAF | 2 | |
| Taxol | 1 | |
| Years since chemotherapy | 4.2 (1.1) | |
| STAI Anxiety | 32.4 (8.6) | 33.1 (1.4) |
| CESD Depression | 8.6 (8.2) | 7.8 (6.5) |
| FACT-COG | ||
| Memory | 20.4 (5.9) | 23.5 (3.2) |
| Verbal | 18.5 (4.8) | 19.2 (3.6) |
| Concentration | 12.4 (3.2) | 13.6 (2.4) |
| Mental Acuity | 12 (3.4) | 13.4 (2) |
| PCI | 56.5 (12.7) | 59.4 (8.3) |
| QOL Impact | 13.7 (3) | 14.3 (2.4) |
| PCA | 19.5 (6.3) | 22.7 (4.5) |
Measures and Procedure
In addition to primary serial learning and memory measures (CVLT-2; BLT), all subjects were also administered the following objective and self-report measures in a single session: Wide Range Achievement Test-Reading subtest (WRAT-4); FAS Controlled Oral Word Association Test (FAS-COWAT); Paced Auditory Serial Addition Test (PASAT): Trail Making Tests A and B: Grooved Pegboard: Digit Symbol (WAIS-III); Continuous Performance Test (CPT); Center for Epidemiological Study - Depression (CES-D); Spielberger State Anxiety Inventory (STAI); Fatigue Symptom Inventory (FSI); Functional Assessment of Cancer Therapy-Cognition (FACT-Cog).
Analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) and the Microsoft Excel package was used for data visualization.
All subjects’ raw scores were transformed to z-scores by normalizing performance to the healthy control group collected as part of this study. BC and healthy control groups were matched for age and education during subject recruitment. For the primary analysis contrasting learning and memory performance between Breast Cancer (BC) and Healthy Control (HC) groups, four variables were calculated:1) Single Trial Learning (Trial 1 performance for the CVLT-2 and BLT); 2) Multiple Trial Learning: Trial 5 performance for the CVLT-2 and BLT); 3) Delayed Recall (Long Delay Recall performance for the CVLT-2 and BLT); and4) Memory Errors: (False Positive errors on the Recognition Trial for the CVLT-2 and BLT). Resulting averaged z-scores were entered into a two-group (BC versus HC) between-group analysis of covariance (ANCOVA) with WRAT-Reading performance as a covariate.
For the secondary analysis contrasting learning and memory performance of higher (hpBC n=76) and lower performing (lpBC n=37) breast cancer groups with healthy controls (HC), the breast cancer group was stratified by performance criteria of two measures >1.5 sd or one measure >2 sd below the mean on individual measures exclusive of performance on learning and memory measures. Performance on Single Trial Learning, Multiple Trial Learning, Long Delay Recall, and Memory Errors variables was contrasted between each of the BC groups (lpBC; hpBC) and the HC group using a two-group (lpBC or hpBC versus HC) analysis of covariance (ANCOVA) with WRAT-Reading performance as a covariate.
Results
Single Trial, Multiple Trial, Delayed Recall and Memory Errors Performance
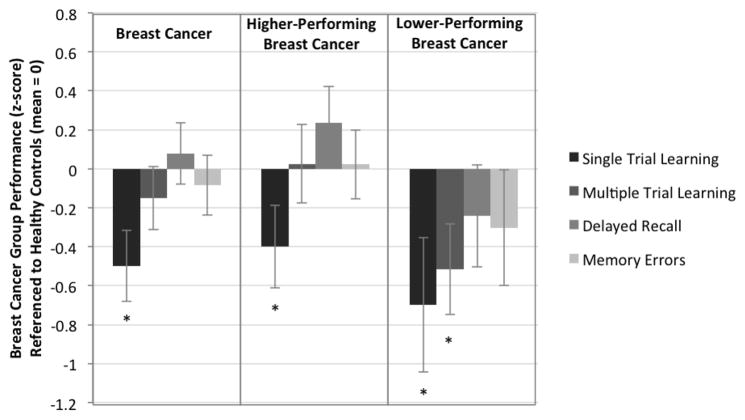
The BC group exhibited significantly lower performance on Single Trial Learning than the HC group, F (2, 146) = 5.685, p = 0.018, confirming predicted lower performance in initial encoding of information. In contrast, performance discrepancies between the BC and HC groups on all other learning and memory variables were not significant: Multiple Trial Learning – F (2,146) = .552, p = 0.459; Delayed Recall – F (2, 146) = .556, p = 0.453; Memory Errors – F (2,146) = .054, p = 0.816 (Table 2; Figure 1).
Table 2.
Results of ANCOVA models on Single Trial Learning, Multiple Trial Learning, Delayed Memory and Memory Errors variables.
| Breast Cancer | Higher Performing Breast Cancer | Lower Performing Breast Cancer | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| N | Mean (sd) | N | Mean (sd) | N | Mean (sd) | |
|
| ||||||
| Single Trial Learning | 113 | −0.50 (0.97)* | 76 | −0.40 (0.92)* | 37 | −0.70 (1.05)* |
| Multiple Trial Learning | 113 | −0.15 (0.86) | 76 | 0.03 (0.87) | 37 | −0.51 (0.71)* |
| Delayed Memory | 113 | 0.08 (0.84) | 76 | 0.23 (0.81) | 37 | −0.24 (0.80) |
| Memory Errors | 113 | −0.08 (0.83) | 76 | 0.02 (0.77) | 37 | −0.30 (0.91) |
p<=0.05
Figure 1.
Single Trial Learning (CVLT-2 Trial 1 and BLT Trial 1), Multiple Trial Learning (CVLT-2 Trial 5 and BLT Trial 5), Delayed Recall (CVLT-2 Long Delay Recall and BLT Long Delay Recall) and Memory Errors (CVLT-2 False Positives and BLT False Positives) performance for Breast Cancer (BC), lower performing Breast Cancer (lpBC), and higher performing Breast Cancer (hpBC) groups referenced to the Health Control (HC) group (mean = 0). * p<=0.05.
When the BC group was stratified into lower- (lpBC) and higher-performing (hpBC) groups on measures exclusive of learning and memory and compared to the HC group, a similar, but more pronounced pattern emerged in the lpBC group. Single Trial Learning performance was again significantly weaker compared to HC, F (2, 70) = 4.375, p = 0.040, together with weaker Multiple Trial Learning performance, F (2,70) = 5.253, p = 0.025 but no difference between lpBC and HC groups for Delayed Recall performance, F (2, 70) = .518, p = 0.474, or for Memory Errors, F (2,70) = .518, p = 0.474. For the contrast of the hpBC versus HC groups, Single Trial Learning performance was again significantly lower in the hpBC group compared to HC, F (2, 110) = 4.066, p = 0.046, while remaining learning and memory variable performance was not significantly different: Multiple Trial Learning – F (2, 110) = .012, p = 0.912; Delayed Recall – F (2, 110) = 1.958, p = 0.165; Memory Errors – F (2, 110) = .004, p = 0.952 (Table 2; Figure 1).
BC and HC performance on remaining measures was analyzed in a two group (HC vs BC) between subjects design with WRAT-Reading included as a covariate (Table 2). Only WMS-III Logical Memory II performance was significantly lower in the BC group, F (146) = 4.232, p = 0.041.
Conclusions
This study sought to replicate and extend previous findings [11] that suggest a prominent initial encoding deficit in breast cancer survivors that may explain frequently reported forgetfulness in survivorship. Consistent with our previously reported findings, breast cancer survivors treated with endocrine therapy two- to six-years post-treatment exhibited significantly decreased initial encoding of both visual and verbal information. Repetition of this information in multiple learning trials compensated for this initial encoding weakness, and information that was successfully learned was retained and recalled at longer intervals. Underscoring the encoding weakness in breast cancer survivors as a whole, both lower- and higher-performing breast cancer survivors exhibited the same pattern of decreased initial encoding, with the lower-performing group continuing to exhibit weaker encoding even after multiple learning trials, followed by intact retention and recall of successfully learned information by both groups following a delay. Significantly, neither disease stage or treatment was associated with memory performance or membership in lower and higher performing groups. These results extend our previously published findings to both visual and verbal encoding in a non-clinical sample not selected for subjective or objective cognitive dysfunction. The findings of initial encoding deficits with intact delayed recall following repeated learning trials lead to observations about survivors’ experience of their own cognitive lapses, to suggestions in designing research protocols aimed at clarifying objective dysfunction in survivors, and to potential interventions in rehabilitation.
First, from our clinical experience, patients ascribe the majority of their cognitive errors and difficulties in adjustment to “forgetfulness,” and indeed memory dysfunction in our original clinical dataset was reported by 80% of patients. Detailed review of history, however, reveals cognitive errors that may be more consistent with initial encoding weaknesses, found here. In our previous research, we attributed these encoding weaknesses to inattention and distractibility. Initial learning performance after a single trial has previously been found to be highly correlated with performance on the Attention/Concentration Index and Digits Forward of the Weschler Memory Scale – Revised [14] and the CVLT-2 technical manual interprets Trial 1 performance as being an indicator of auditory attention [15]. However, performance on a measure of sustained attention (CPT) included in our research battery was not significantly different between cancer and healthy control groups. It is possible that the CPT is tapping different aspects of attentional function separate from brief auditory attention, e.g., sustained attention, and that this aspect of attention is not affected in survivors, but this seems unlikely. One potential explanation is that our analysis of CPT data is limited to mean reaction times, rather than trial level performance, since trial-level performance data was not available in our dataset. Previous work using the CPT in survivors has found differences in performance at the intra-individual level, with survivors exhibiting significantly more variable reaction times from trial to trial under specific conditions, with this finding interpreted as potentially variable attention across task presentation [16].
With regard to research design, our results emphasize the importance of including serial list learning and memory measures in research batteries as well as analysis of early versus late learning trials and resulting delayed recall performance due to the potential confounding effect of single trial learning on later recall performance. Differences in learning and memory performance should be expected depending on the measures included in a given study. In this regard, significantly decreased delayed memory performance on the WMS Logical Memory II (LM II) subtest is suggestive, since this is the only measure that exhibited intact immediate recall performance and weaker delayed memory performance in our survivor group. This may be suggestive of true forgetting of information under specific conditions in which encoding opportunities are limited. In contrast to the serial learning and memory measures such as the CVLT-2 and BLT, this version of the LM II task (WMS-III) allows only two presentations for the first narrative, and one presentation for the second narrative. Survivor subjects may have benefitted from context and substance of the narrative material to improve immediate recall performance but limited access to encoding trials may have weakened the memory trace leading to poorer delayed recall performance.
One limitation of this work is the fact that learning and memory performance on the serial learning measures reported here cannot be further decomposed to examine more fine-grained attention and encoding processes that may explain performance discrepancies. Analysis of single trial learning can only assess immediate recall after the list is recited, but not stimulus processing steps throughout list monitoring that may be influencing performance. While analysis of specific learning variables on the CVLT-II can be assessed, for example, semantic and serial clustering, primacy, recency, and middle region recall, these failed to find any significant differences that would have further explained encoding differences between groups, and do not capture earlier attentional or other sub-processes. These sub-processes lie on a continuum of relatively automatic, involuntary processes to more effortful, voluntary processes under conscious control, including sensory gating, alerting, selective orienting and attending to relevant information, reconciling conflict and competing demands for attention, and maintaining vigilance over longer intervals. As such, the point at which initial encoding is assessed in traditional clinical measures combines the contributions of each sub-process to ultimate performance. Plans for future research include a more specific and accurate assessment of attention and encoding processes that is able to sample behavior at earlier stages. This can be achieved by including psychophysiological measures, such as startle electromyography [17], saccade and anti-saccade eye tracking methodology [18], and cognitive-experimental paradigms (Attention Network Test (ANT) [19, 20]) that are specifically designed for assessing individual subprocesses on a continuum of automatic to controlled processing. Work in our lab is ongoing to develop these methods, with a focus on electromyography, anti-saccade eye-tracking methods, and electroencephalogram recording with cognitive-experimental attention tasks
To the extent that our results are consistent with an initial encoding weakness, recommendations to survivors and cognitive rehabilitation strategies should be tailored to address remediation of this phase of learning and memory. Examples of compensatory strategies include limiting distractions and multi-tasking at the time of learning, mnemonic strategies that emphasize deeper levels of processing at the time of learning (semantic or visualization strategies), reducing environmental cognitive load, and increasing self-monitoring for inattention and distraction. Direct, restorative brain-training approaches [21] still have little data to prove their efficacy, although physical exercise, mindfulness-based practice, and, transcranial stimulation technologies (TMS; TDCS) may be a key focus in future research, as well as continued research of stimulant and non-stimulant medications that aim to improve attention and focus that may support more efficient encoding of information.
Table 3.
Results of ANCOVA models for neurocognitive battery.
| Breast Cancer
|
||
|---|---|---|
| N | Mean (sd) | |
|
| ||
| PASAT 3″ | 110 | −0.25 (1.18) |
| PASAT 2′ | 108 | −0.12 (1.25) |
| WAISIII Digit Symbol | 113 | −0.37 (0.97) |
| Grooved Pegboard DH | 113 | −0.10 (1.42) |
| Grooved Pegboard NH | 113 | −0.32 (1.70) |
| Trails A | 113 | 0.06 (0.75) |
| Trails B | 113 | −0.36 (2.00) |
| WMSIII Logical Memory I | 113 | −0.19 (0.91) |
| WMSIII Logical Memory II | 113 | −0.45 (1.03)* |
| FAS-COWAT | 113 | −0.09 (1.12) |
| WRAT-Reading | 112 | −0.17 (0.83) |
| Gordon CPT RT | 113 | 0.32 (0.98) |
p<=0.05
Acknowledgments
Funding: This study was funded by the T.J. Martell Foundation, the Chanel Foundation and T32 Training Grant #T32 CA 009461.
Footnotes
Conflict of Interest: James Root declares that he has no conflict of interest. Charissa Andreotti declares that she has no conflict of interest. Loretta Tsu declares that she has no conflict of interest. Timothy Ellmore declares that he has no conflict of interest. Tim Ahles declares that he has no conflict of interest.
Compliance with Ethical Standards
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Contributor Information
James C. Root, Assistant Attending Neuropsychologist, Neurocognitive Research Laboratory, Department of Psychiatry and Behavioral Sciences, Memorial Sloan Kettering Cancer Center, Assistant Professor, Weill Cornell Medical College.
Charissa Andreotti, Postdoctoral Fellow, Neurocognitive Research Laboratory, Department of Psychiatry and Behavioral Sciences, Memorial Sloan Kettering Cancer Center.
Timothy M. Ellmore, Associate Professor, Department of Psychology, Program in Behavioral and Cognitive Neuroscience, The Graduate Center, City University of New York, The City College of the City University of New York.
Tim A. Ahles, Attending Psychologist, Neurocognitive Research Laboratory, Department of Psychiatry and Behavioral Sciences, Memorial Sloan Kettering Cancer Center, Professor, Weill Cornell Medical College.
References Cited
- 1.Cull A, Stewart M, Altman DG. Assessment of and intervention for psychosocial problems in routine oncology practice. Br J Cancer. 1995;72(1):229–35. doi: 10.1038/bjc.1995.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cull A, et al. What do cancer patients mean when they complain of concentration and memory problems? Br J Cancer. 1996;74(10):1674–9. doi: 10.1038/bjc.1996.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schagen SB, et al. Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer. 1999;85(3):640–50. doi: 10.1002/(sici)1097-0142(19990201)85:3<640::aid-cncr14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 4.Ahles TA, et al. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol. 2002;20(2):485–93. doi: 10.1200/JCO.2002.20.2.485. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins V, et al. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. British Journal of Cancer. 2006;94(6):828–834. doi: 10.1038/sj.bjc.6603029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schagen SB, et al. Change in cognitive function after chemotherapy: a prospective longitudinal study in breast cancer patients. J Natl Cancer Inst. 2006;98(23):1742–5. doi: 10.1093/jnci/djj470. [DOI] [PubMed] [Google Scholar]
- 7.Wefel JS, et al. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer. 2004;100(11):2292–9. doi: 10.1002/cncr.20272. [DOI] [PubMed] [Google Scholar]
- 8.Jansen CE, et al. A meta-analysis of the sensitivity of various neuropsychological tests used to detect chemotherapy-induced cognitive impairment in patients with breast cancer. Oncol Nurs Forum. 2007;34(5):997–1005. doi: 10.1188/07.ONF.997-1005. [DOI] [PubMed] [Google Scholar]
- 9.Andreotti C, et al. Reliable change in neuropsychological assessment of breast cancer survivors. Psychooncology. 2015 doi: 10.1002/pon.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jim HS, et al. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J Clin Oncol. 2012;30(29):3578–87. doi: 10.1200/JCO.2011.39.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Root JC, et al. Learning and memory performance in a cohort of clinically referred breast cancer survivors: the role of attention versus forgetting in patient-reported memory complaints. Psycho-Oncology. 2014 doi: 10.1002/pon.3615. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donders J. A confirmatory factor analysis of the California Verbal Learning Test--Second Edition (CVLT-II) in the standardization sample. Assessment. 2008;15(2):123–31. doi: 10.1177/1073191107310926. [DOI] [PubMed] [Google Scholar]
- 13.Donders J. Subtypes of learning and memory on the California Verbal Learning Test-Second Edition (CVLT-II) in the standardization sample. J Clin Exp Neuropsychol. 2008;30(7):741–8. doi: 10.1080/13803390701689595. [DOI] [PubMed] [Google Scholar]
- 14.Delis DC, et al. Wechsler memory scale-revised and california verbal learning test: Convergence and divergence. The Clinical Neuropsychologist. 1988;2:188–196. [Google Scholar]
- 15.Delis DC, et al. California Verbal Learning Test-Second Edition. The Psychological Corporation; San Antonio, TX: 2000. [Google Scholar]
- 16.Bernstein LJ, Catton PA, Tannock IF. Intra-individual variability in women with breast cancer. J Int Neuropsychol Soc. 2014;20(4):380–90. doi: 10.1017/S1355617714000125. [DOI] [PubMed] [Google Scholar]
- 17.Gandal MJ, et al. A novel electrophysiological model of chemotherapy-induced cognitive impairments in mice. Neuroscience. 2008;157(1):95–104. doi: 10.1016/j.neuroscience.2008.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edelman JA, Xu KZ. Inhibition of voluntary saccadic eye movement commands by abrupt visual onsets. J Neurophysiol. 2009;101(3):1222–34. doi: 10.1152/jn.90708.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan J, et al. Testing the behavioral interaction and integration of attentional networks. Brain Cogn. 2009;70(2):209–20. doi: 10.1016/j.bandc.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan J, et al. The activation of attentional networks. Neuroimage. 2005;26(2):471–9. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Kesler S, et al. Cognitive training for improving executive function in chemotherapy-treated breast cancer survivors. Clin Breast Cancer. 2013;13(4):299–306. doi: 10.1016/j.clbc.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]