Abstract
Background
Muscle wasting prevails in numerous diseases (e.g. diabetes, cardiovascular and kidney diseases, COPD,…) and increases healthcare costs. A major clinical issue is to devise new strategies preventing muscle wasting. We hypothesized that 8‐week docosahexaenoic acid (DHA) supplementation prior to fasting may preserve muscle mass in vivo.
Methods
Six‐week‐old C57BL/6 mice were fed a DHA‐enriched or a control diet for 8 weeks and then fasted for 48 h.
Results
Feeding mice a DHA‐enriched diet prior to fasting elevated muscle glycogen contents, reduced muscle wasting, blocked the 55% decrease in Akt phosphorylation, and reduced by 30–40% the activation of AMPK, ubiquitination, or autophagy. The DHA‐enriched diet fully abolished the fasting induced‐messenger RNA (mRNA) over‐expression of the endocannabinoid receptor‐1. Finally, DHA prevented or modulated the fasting‐dependent increase in muscle mRNA levels for Rab18, PLD1, and perilipins, which determine the formation and fate of lipid droplets, in parallel with muscle sparing.
Conclusions
These data suggest that 8‐week DHA supplementation increased energy stores that can be efficiently mobilized, and thus preserved muscle mass in response to fasting through the regulation of Akt‐ and AMPK‐dependent signalling pathways for reducing proteolysis activation. Whether a nutritional strategy aiming at increasing energy status may shorten recovery periods in clinical settings remains to be tested.
Keywords: Akt and AMPK signalling, Autophagy, Lipid droplets, Protein turnover, Ubiquitin–proteasome system
Introduction
A major goal to improve the health and quality of life of patients suffering from numerous diseases (i.e. cardiovascular diseases, cancer cachexia, chronic obstructive pulmonary diseases (COPD), chronic kidney diseases, diabetes, etc.) and/or stressful events is to develop strategies to preserve muscle protein mass. Skeletal muscle provides power and strength for locomotion and posture, but is also the major reservoir of amino acids mobilized during catabolic situations. An uncontrolled and sustained muscle wasting has detrimental metabolic consequences, reduces the efficiency of treatments, and increases lengths of hospitalization and mortality.1, 2 As mechanisms resulting in muscle wasting may differ from the nature of the catabolic situation, our study focused on the identification of a nutritional strategy that could limit muscle wasting during fasting. Nutritional strategies in humans aiming at increasing the intake of long chain n‐3 polyunsaturated fatty acids (n‐3 LCPUFAs), and particularly of the docosahexanoic acid (DHA), reduced inflammation and systemic risk factors in COPD3 and cardiovascular diseases,4 respectively. n‐3 LCPUFAs from fish oil or eicosapentanoic acid (EPA) alone reduced muscle proteolysis in several rodent models of wasting diseases5, 6, 7, 8, 9 sparing muscle mass in some conditions.6, 8, 9 However, the mechanisms responsible for this sparing effect still remained unclear.
In most catabolic conditions (including in humans), the ubiquitin–proteasome system (UPS)10, 11, 12 and autophagy, which both control muscle mass, are activated.13 Autophagy is not only involved in protein degradation, but also in the mobilization of intracellular lipids from lipid stores. This process, called lipophagy, is induced in rodent fasted hepatocytes, embryonic fibroblasts, endothelial cells, lymphoblasts, dendritic cells, glial cells, and neurons,14 but is not documented in muscle cells. In the latter, the transcription factor FoxO3 controls in part the expression of MAFbx/Atrogin‐1, MuRF1, and autophagy related genes in mice muscle cells,15, 16 resulting in ubiquitination of protein substrates for UPS degradation and in LC3 lipidation for autophagy induction.
FoxO3 can be phosphorylated by the protein kinase Akt, leading to its cytoplasmic sequestration and inactivation.17 Akt is at the crossroads of both protein synthesis and breakdown and is down‐regulated in muscle wasting conditions, such as fasting.17 Fasting situations are also characterized by changes in the adenosine monophosphate/triphosphate (AMP/ATP) ratio resulting in activation of the AMP‐activated protein kinase (AMPK), which subsequently induces genes involved in both UPS and autophagy,18, 19 but also in lipid metabolism20 in rodent and human cells.
Administration of EPA or fish oil blocks in vivo the induction of the muscle UPS in animal models of sepsis,5 cancer,6 immobilization,8 and fasting.7 In addition, fish oil administration has also been reported to improve muscle protein synthesis in rats21 and humans.22 However, fish oil preserved muscle mass in rodents,8, 9 while EPA has little6 if any5, 7 effect, suggesting a role of DHA in muscle mass maintenance. The effect of DHA alone has been little studied in vivo and did not affect proteasome subunit protein levels.7 In these studies DHA or EPA were administrated once by gavage of mice 24 h before sepsis induction5 or fasting.7 However, fish oil administration for 21 days in animals resulted in (i) increased EPA or DHA contents in skeletal muscle, and (ii) reduced activation of the UPS in muscle atrophy induced by lipopolysaccharides (LPS).9 The role of n‐3 PUFAs in muscle autophagy is however still unknown. The effect of DHA alone on signalling transduction pathways has been poorly studied. DHA was reported to activate the P70S6 kinase in C2C12 myotubes, without any effect on Akt, while EPA induced both kinases.23 Furthermore, it has been reported that DHA is incorporated into membrane phospholipids by substituting to arachidonic acid (AA). This may modulate the endocannabinoid system (ECS),24, 25 which can regulate the Akt‐dependent signalling pathway in muscle.26, 27 In addition, DHA countered the catabolic effects of palmitate in C2C12 cells by restoring Akt signalling and attenuated the activation of multiple proteolytic systems.28
We hypothesized that a supplementation of the diet with DHA for 8 weeks prior to fasting may (i) preserve muscle mass in vivo, (ii) prevent induction of the UPS and autophagy, (iii) regulate the Akt‐ and AMPK‐dependent signalling pathways, and (iv) modulate the ECS activity in fed and fasted mouse muscles.
Materials and methods
Animals, dietary treatments, and experimental design
Animal experiments have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments, and according to the European directive #2010/63/UE for the care and use of laboratory animals and to the institutional guidelines on animal experimentation in France. C57BL/6 mice (6 weeks old) were housed individually in controlled environmental conditions (room temperature 22°C; 12:12 h light–dark cycle, light period starting at 8 h), fed ad libitum, and given free access to water. After a 1 week adaptation period, the animals were fed during 8 week either a Control (CTL group, n = 20) or a DHA‐enriched (DHA group, n = 20) diet. Diets were formulated to maintain equal amounts of lipids, but to increase the proportion of DHA. For that purpose, CTL diets were made up with a safflower oil rich in linoleic fatty acid (n6/n3 ratio = 10) and the DHA diet with a DHA concentrated marine oil (n6/n3 ratio = 1) (Table 1). Fat and lean masses were determined at the beginning and at the end of nutritional intervention (QMR EchoMRI‐900TM, Houston, USA). This analyser provides body composition measurements of global body fat and lean masses in live animals. At the end of the experiment, the animals were slaughtered either at the fed state or after 48 h of fasting in both CTL and DHA groups (n = 10 mice/group). Hindlimb muscles were dissected and weighed. Fast‐twitch glycolytic muscles (e.g. the tibialis anterior (TA) muscle) atrophy to a greater extent in response to 48 h fasting compared with slow‐twitch oxidative muscles (i.e. soleus). Thus, all experiments were performed in TA muscles, which were frozen in liquid nitrogen, and stored at −80°C until analysis. Blood was recovered after cardiac puncture and serum were prepared using CryoPure Tubes (Sarstedt, Germany) following the manufacturer's instructions. During the experimentation, food intake and body weight were recorded every week.
Table 1.
Composition of the control and the docosahexaenoic acid‐enriched diets
| Control diet | Docosahexaenoic acid diet | |
|---|---|---|
| Ingredients (g/kg dry matter) | ||
| Casein | 200 | 200 |
| L cystine | 3 | 3 |
| Safflower oil | 45 | 25 |
| Oleic sunflower oil | 25 | 25 |
| Omegavie docosahexaenoic acid 70TG QSI (Polaris) | — | 20 |
| Cellulose | 50 | 50 |
| Mineral mix AIN 93 | 35 | 35 |
| Vitamin mix AIN 93 | 10 | 10 |
| Choline bitartrate (41% choline) | 2.5 | 2.5 |
| Saccharose | 100 | 100 |
| Lactose | 134 | 134 |
| Wheat flour | 400 | 400 |
Diets were provided by INRA (Unité de Préparation des Aliments Expérimentaux, Domaine de Vilvert, Jouy‐en‐Josas, France)
Measurement of muscle glycogen, and adenosine monophosphate and adenosine triphosphate levels
ATP and AMP levels were measured using the ATP/ADP/AMP Assay Kit (Biomedical Research Service Center USA, Buffalo, NY). Samples were prepared according to the manufacturer's instructions. Briefly, TA muscles (~30 mg) were homogenized in 10% TCA using a polytron, with the same tissue/TCA ratio for all samples.The homogenates were kept for 30 min on ice and then centrifuged at 15 000 g for 5 min (4°C). One third of the supernatant was treated with the same volume of saturated ether, mixed and briefly centrifuged. Ether was then removed from the top layer. These steps were repeated thrice. Tissue extracts were then stored at −80°C until use. AMP and ATP were assessed as described by the manufacturer using a luminometer (LB940 Multimode Reader Mithras, Berthold Technologies, Germany). The AMP/ATP ratio was calculated for each sample. Muscle glycogen was recovered after ethanol precipitation of the remaining supernatants. After centrifugation at 15 000 g for 15 min (4°C), the resulting pellet was resuspended in 100 μL of H2O and stored at −80°C until use. Fifty microlitres of glycogen samples and glycogen standards was incubated for 3 h at 37°C with 150 U/mL amyloglucosidase (AMG, Sigma) in acetate buffer (pH = 4.8) to transform glycogen into glucose units. A blank was performed without AMG for all samples or standards. Glucose was then measured with the Glucose RTU™ kit (Biomerieux) following the manufacturer's instructions. Glycogen contents in samples were calculated as the difference of glucose levels between the reactions with and without AMG. Data were expressed as µg/mg of muscle.
Serum and muscle fatty acid composition and muscle triglycerides
Total lipids were extracted with chloroform and methanol as described.29, 30 Lipids were evaporated to dryness under a gentle stream of N2 and dissolved in methanol and toluene (2:1, v/v) for methylation. Fatty acid methyl esters (FAME) were obtained after transesterification with boron trifluoride in methanol (14%; Sigma‐Aldrich) after heating at 90°C for 60 min as described.31 Gas chromatography (GC) analysis of FAME was performed using a GC Trace (Thermo Fischer Scientific, Courtaboeuf, France), equipped with a fused silica CP‐Sil 88 capillary column (100% cyanopropyl‐polysiloxane, Varian S.A, Les Ulis, France), a programmed temperature vaporization injector and a flame‐ionization detector. Samples were injected in the splitless mode. The oven temperature programme increased between 70 and 225°C in four separate steps. Helium gas was used as a carrier, with a constant pressure (264 kPa). Sample methyl esters were determined by comparing their relative retention times with well‐known external FAME standards (Supelco™ 37 Component Fatty Acid Methyl Esters Mix and Menhaden Oil; Sigma Aldrich, St Quentin Fallavier, France). Other standard FAME mixtures were obtained from Nu‐Chek‐Prep (Elysian, MN, USA). The relative content of each FAME was calculated from the area of each peak and expressed as a percentage of total FAME giving the relative fatty acid distribution in each group of mice. For muscle triglyceride (TG) contents, dried total lipid extracts were dissolved in chloroform containing 1% triton. TG were then immediately quantified using the triglycerides FS colorimetric kit (Diasys, Condom, France) using a commercial TG standard.
Muscle gene expression using real‐time reverse transcription‐polymerase chain reaction
Total RNA from TA muscle was extracted with Trizol reagent (Invitrogen), following by purification using RNeasy mini kit (Qiagen). RNA was quantified by measuring the absorbance at 260 nm on a NanoDrop ND‐1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA). RNA integrity was assessed on an Agilent 2100 Bioanalyzer (Agilent Technologies, CA, USA). For real‐time RT‐PCR, 1 µg of RNA was treated with DNase I (Invitrogen) prior cDNA synthesis. Treated RNA was then reverse transcribed using random primers and SuperScript II (Invitrogen) according to the manufacturer's instructions. Real‐time PCR was carried out using the CFX96 Real‐Time PCR detection system (Bio‐Rad). The PCR efficiency was tested for each pair of primers (sequences are provided in Table 2), using a standard curve of cDNA. PCR reactions were performed using the IQ SYBR Green Supermix (Bio‐Rad) according to manufacturer's instructions. The comparative threshold cycle (2ΔΔCT) method32 was used to compare the relative messenger RNA (mRNA) expression between each group, using 18S rRNA as a reference gene, because (i) all other housekeeping genes tested (Hprt, Ppia, Tbp, Znf207, Ywhaz) were up‐regulated after 48 h fasting, and (ii) 18S rRNA levels did not change in response to either DHA treatment or 48 h fasting. The relative mRNA abundance was arbitrarily set to 1 for the CTL fed group.
Table 2.
Primers used for reverse transcription‐quantitative polymerase chain reaction analysis
| Primer names | Accession no | Primer sens sequences | Primer antisens sequences |
|---|---|---|---|
| MAFbx | NM_026346 | 5′‐AGTGAGGACCGGCTACTGTG‐3′ | 5′‐GATCAAACGCTTGCGAATCT‐3′ |
| MuRF1 | NM_001039048 | 5′‐ATGGAGAACCTGGAGAAGCA‐3′ | 5′‐AACGACCTCCAGACATGGAC‐3′ |
| Cthl | NM_009984 | 5′‐CTGTTGCTATGGACGCAAGC‐3′ | 5′‐ACCAACAGAACCCCATGGTC‐3′ |
| Psmb1 | NM_011185 | 5′‐AATTGGCTGCAGTGGTTTCC‐3′ | 5′‐CCGTTGTCATGGCCTTGTTAT‐3′ |
| Psmb2 | NM_011970 | 5′‐AATTGTCTCCCACAGCAGCA‐3′ | 5′‐ATAGCCAGCCAGGAGGAGGT‐3′ |
| Psmb5 | NM_011186 | 5′‐CACCCTGGCCTTCAAGTTTC‐3′ | 5′‐TCACCGTCTGGGAAGCAATA‐3′ |
| Psmd2 | NM_134101 | 5′‐TCTTTGCTATGGGCATGGTG‐3′ | 5′‐GGGTCCTTGGCATGATATTGA‐3′ |
| Psmd3 | NM_009439 | 5′‐CACCCAAGCTGTTAGGACAGG‐3′ | 5′‐TCAGGGTGTAGGTCCCATCC‐3′ |
| Psmd5 | NM_080554 | 5′‐ATTGTTGGCGCAGATTCAGA‐3′ | 5′‐CTATCCATGACGGCCAGGTT‐3′ |
| USP19 | NM_027804 | 5′‐TCCTGTTCGGAACCTGGACT‐3′ | 5′‐TGCCTCCGTAGTGGTTGATG‐3′ |
| Atg5 | NM_053069 | 5′‐TCAACCGGAAACTCATGGAA‐3′ | 5′‐CGGAACAGCTTCTGGATGAA‐3′ |
| Atg12 | NM_026217 | 5′‐TAAACTGGTGGCCTCGGAAC‐3′ | 5′‐CCATCACTGCCAAAACACTCA‐3′ |
| Atg16 | NM_001205391 | 5′‐TCCCGTGATGACCTGCTAAA‐3′ | 5′‐CAGTCAGAGCCGCATTTGAA‐3′ |
| Rab7 | NM_009005 | 5′‐GGGAAACAAGATTGACCTGGA‐3′ | 5′‐CTCCTTGGCACTGGTCTCG‐3′ |
| Pld1 | NM_001164056 | 5′‐ATCGGTGATGGATGGAAAGG‐3′ | 5′‐CCCAGGACAAGTCTGAAGCA‐3′ |
| Bscl2 | NM_001136064 | 5′‐ACCGCTTCTCTCTGCAGGTT‐3′ | 5′‐CCGACTGCTGGGTAGATTCC‐3′ |
| Rab18 | NM_001278447 | 5′‐AGGACGTGCTGACCACTCTG‐3′ | 5′‐TGTGAACCTCAGGAGCAGGC‐3′ |
| Plin2 | NM_007408 | 5′‐GGGTGGAGTGGAAGAGAAGC‐3′ | 5′‐GAGCTGCTGGGTCAGGTTG‐3′ |
| Plin4 | NM_020568 | 5′‐GCTGCATGTGGGAAGCTGT‐3′ | 5′‐GTGCACAGCCTGTCCTGAG‐3′ |
| Plin5 | NM_025874 | 5′‐CCAGTTGGCCACAGTGAATG‐3′ | 5′‐GGCTGATGTCACCACCATGT‐3′ |
| Cnr1 | NM_007726 | 5′‐TCTACGTGGGCTCAAATGACA‐3′ | 5′‐TGGAAGGGACTACCCCTGAA‐3′ |
| 18S | NR_003278 | 5′‐AATCAGTTATGGTTCCTTTGTCG‐3′ | 5′‐GGTCTAGAATTACCACAGTTATCCAA‐3′ |
Atg, autophagy related gene; Bscl2, Berardinelli‐Seip congenital lipodystrophy 2 (seipin); Cnr1, Cannabinoid receptor 1; Cthl, Cathepin L; MAFbx, muscle atrophy F‐box/Atrogin‐1; MuRF1, muscle ring finger 1; plin, perilipin; Pld1, phospholipase D1; Psmb, proteasome subunit beta; Psmd, proteasome 26S subunit, non‐ATPase; Rab7, RAB7 member RAS oncogene family; Rab18, RAB18, member RAS oncogene family; USP, ubiquitin specific peptidase; 18S, 18S ribosomal RNA.
Western blot analyses
TA muscles (~30 mg) were homogenized using a polytron in 1 mL of an ice‐cold buffer (10 mM Tris pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X‐100, 0.5% Igepal CA630) containing protease and deubiquitinating enzyme inhibitors (Protease Inhibitor Cocktail, N‐EthylMaleimide (Sigma)) and phosphatase inhibitors (1 mM Na3VO3, 10 mM NaF). The homogenates were stirred for 1 h at 4°C and then centrifuged at 10 000 g for 15 min at 4°C. The resulting supernatants were then stored at −80°C until use. For ubiquitin–protein conjugate analysis, the residual pellets containing myofibrillar proteins were resuspended and sonicated in 50 mM Tris pH 7.5, 150 mM NaCl, 1 mM EDTA, 3 mM β‐Glycerophosphate, 10 mM MgCl2, 10% Glycerol, 1% Triton X‐100, 2% SDS, 0.5 mM DTT and protease, phosphatase, and deubiquitinase inhibitors as described above. Then, these extracts were briefly centrifuged for 5 min at 1000 g at 4°C. The resulting supernatants contain solubilized myofibrillar proteins. The concentration of proteins was determined using BCA Protein Assay Kit (Pierce). Proteins were then diluted in Laemmli sample buffer and stored at −80°C until use. Protein extracts were subjected to SDS‐PAGE electrophoresis, transferred onto a PVDF membrane (Hybond P, Amersham) and incubated overnight at 4°C with appropriated primary antibodies: against polyubiquitinylated proteins (FK1, Millipore), LC3 (# L7543, Sigma), and Akt (# 4091), Akt‐Ser473 (# 4060), rpS6 (# 2317), rpS6‐Ser240/244 (# 2215), AMPKα (# 2532), AMPKα‐Ser792 (# 2531), FoxO3A (# 2497), and FoxO1‐Thr24/FoxO3A‐Thr32 (#9464) (Cell Signaling Technology). Blots were then washed and incubated for 1 h with an appropriate secondary horseradish peroxidase conjugated antibody at room temperature. Signals were then detected after incubation with Luminata Crescendo Western HRP substrate (Millipore, MA, USA) and visualized using G:BOX ChemiXT4 (XL1) (Syngene, MD, USA). Signals were then quantified using the GeneTools software (Syngene, MD, USA) and normalized against the amount of proteins (determined by Ponceau red staining) to correct for uneven loading.
Statistical analysis
All data are expressed as means ± SE. Food intake and body weight comparisons were assessed using repeated measures analysis of variance. Other measurements were analysed using a two‐way analysis of variance (ANOVA) (DHA and fasting effects). When significant differences were detected by ANOVA, post hoc comparisons between groups were made using the Fisher's PLSD test. For the LC3II/LC3I ratio, which is highly increased by fasting, we also analysed the effect of 8 weeks of DHA feeding at the fed state using the Student's t‐test. Level of significance was set at P < 0.05. All tests were performed by using XLSTAT (version 2012.4.01, AddinsoftTM).
Results
The tibialis anterior muscle mass is preserved in 48 h‐fasted mice previously fed the docosahexaenoic acid‐supplemented diet
Both control (CTL) and DHA groups exhibited similar body weight gain (CTL: 0.119 ± 0.003 g/day and DHA: 0.125 ± 0.003 g/day) and food intake (CTL: 3.25 ± 0.03 g/day and DHA: 3.27 ± 0.03 g/day) during the 8 week experimental protocol. This corresponds to a DHA intake of 2.5 g/kg/day during the 8 week experimental protocol and is similar to other studies.33, 34, 35 Blood and muscle lipid compositions changed in mice fed the DHA‐supplemented diet compared with the CTL group (see Supporting Information 1 and 2). Feeding mice with the DHA‐enriched diet increased the proportion of DHA by six‐fold and decreased the proportion of AA by seven‐fold in total muscle fat content compared with the CTL group (P < 0.05, Figure 1 A). In addition, fasting increased the proportion of DHA and AA in total muscle fat content by 3.8 and 5.9‐fold, respectively (P < 0.05) only in mice previously fed the CTL diet (Figure 1 A). Similar changes were also observed in blood fatty acids (Supporting Information 1). Fasted mice lost 19% of body weight (P < 0.05) in groups fed either the CTL or the DHA‐supplemented diet compared with fed mice after 8 weeks of the nutritional intervention (Figure 1 B). Lean mass increased similarly during the nutritional intervention in both mice fed the DHA or the CTL diet (Figure 1 C, left panel). In contrast, the increase in fat mass until the end of the experimental protocol was higher in mice fed the DHA diet compared with mice fed the CTL diet (+17.8% vs. 9.7%, respectively, P < 0.05) (Figure 1 C, right panel).
Figure 1.
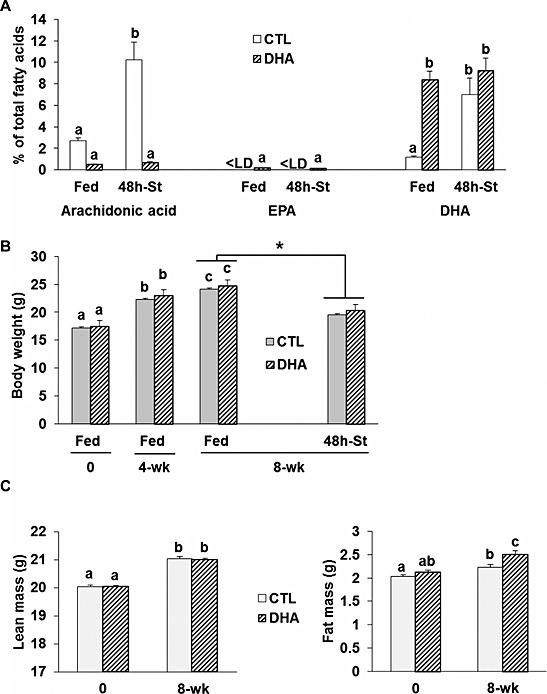
Animal characteristics after feeding a docosahexaenoic acid‐enriched diet. Animals were fed either the control or the docosahexaenoic acid‐enriched diet for 8 weeks (n = 20 mice/group). (A) Muscle lipid composition for arachidonic acid, eicosapentanoic acid, and docosahexaenoic acid in mice fed either the control or the docosahexaenoic acid‐enriched diet prior to 48 h of fasting (n = 7/group). Data are expressed as % of total fatty acids. The body weight (B) was recorded at the beginning (0) of the experiment, 4 and 8 weeks after feeding the control or the docosahexaenoic acid‐enriched diet, and after fasting the mice for 48 h (48 h‐St). Lean and fat masses were recorded at the beginning (0) of the experiment and 8 weeks after feeding the control or the docosahexaenoic acid‐enriched diet (C). Values are means ± SEM. Statistical differences were assessed by analysis of variance. Bars with different superscript letters are statistically different. *, P < 0.05 vs. Fed mice at 8 weeks after feeding the control or docosahexaenoic acid‐enriched diet. < LD stands for below detection limits.
Hindlimb muscle masses were identical between mice fed either the CTL or the DHA diet (data not shown). Fasting decreased tibialis anterior (TA) muscle mass by 13% (P < 0.05) in mice previously fed the CTL diet, but the TA mass was preserved in fasted mice previously fed the DHA‐supplemented diet (P < 0.05, Figure 2).
Figure 2.
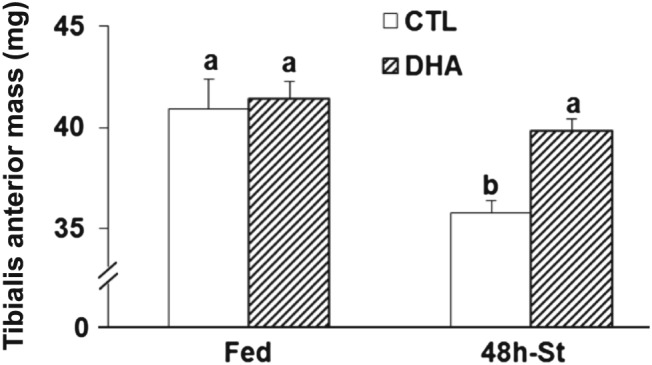
Feeding the mice a docosahexaenoic acid‐enriched diet prior to 48 h fasting preserves muscle mass. Tibialis anterior muscle mass was measured in each group (n = 10 mice/group). Values are means ± SEM. Statistical differences were assessed by analysis of variance. Bars with different superscript letters are statistically different.
Glycogen energy stores increased in muscle from mice fed the docosahexaenoic acid‐supplemented diet
We measured muscle glycogen and triglyceride levels, and the AMP/ATP ratio. Figure 3 A shows that the muscle glycogen content was four‐fold higher in muscles from mice fed the DHA‐enriched diet (P < 0.05 vs. mice previously fed the CTL diet). Glycogen content decreased during fasting in both mice previously fed either the CTL or the DHA‐enriched diet (~ − 90%, P < 0.05). Muscle triglyceride (TG) levels were not different between mice fed either the CTL or the DHA diet (Figure 3 B). However, TG levels decreased upon fasting by 63% (P < 0.05) in mice previously fed the CTL diet, and only by 38% (P < 0.05) in mice previously fed the DHA‐enriched diet (Figure 3 B). Accordingly, TG levels remained ~two‐fold higher in 48 h‐fasted muscles from mice previously fed the DHA diet compared with mice previously fed the CTL diet (Figure 3 B). As expected, the AMP/ATP ratio tended to increase (+89%) in fasted muscle from mice previously fed the CTL diet, but not in mice previously fed the DHA‐enriched diet (Figure 3 C). Altogether, these data suggest that the muscles from mice fed the DHA‐enriched diet exhibited higher glycogen stores and thus were more prone to preserve protein mass during fasting.
Figure 3.
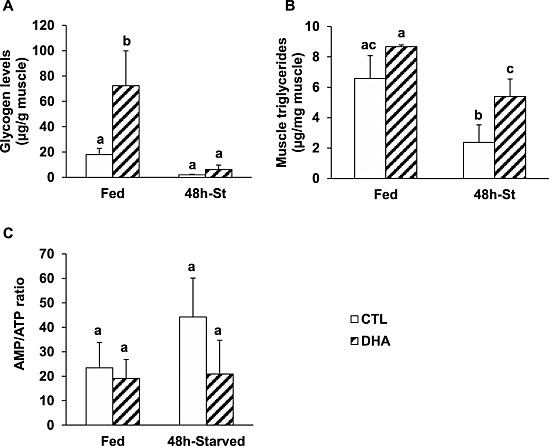
Feeding the mice with the docosahexaenoic acid‐enriched diet prior to 48 h fasting resulted in increased glycogen contents and an improved energy status in the fasted muscles. Levels of glycogen (A) and triglycerides (B), and the adenosine monophosphate/triphosphate ratio (C), were determined in muscles from control or docosahexaenoic acid groups. Data are means ± SEM (n = 4–6 mice/group). Statistical differences were assessed by analysis of variance. Bars with different superscript letters are statistically different.
Feeding mice with a docosahexaenoic acid‐supplemented diet prior to 48 h‐fasting preserved Akt activity and reduced adenosine monophosphate‐activated protein kinase activation in the tibialis anterior muscle
The higher AMP/ATP ratio in TA muscle from mice previously fed the DHA‐enriched diet may control signalling pathways involved in the regulation of muscle mass. Indeed, Akt and AMPK signalling pathways sense cellular protein‐energy status and control protein synthesis and proteolysis.17, 19 Total Akt, AMPK, ribosomal protein S6 (rpS6), and FoxO3A protein contents were similar in muscle from mice fed either the CTL or the DHA diets (Figure 4 A). The phosphorylations of Akt, AMPK, and the rpS6 on Ser 473, Thr172, and Ser240/244, respectively, were similar in muscle from mice fed either the CTL or the DHA diet (Figure 4 A–C, E). Akt phosphorylation on Ser473 was preserved in fasted TA from mice previously fed the DHA‐supplemented diet, whereas it decreased by 55% (P < 0.05) in muscle from animals previously fed the CTL diet (Figure 4 A and 4B). Fasting increased AMPK phosphorylation on Thr172 (+133%, P < 0.05) in the TA muscle from mice previously fed the CTL diet, but to a lower extent (+98%), and not significantly in mice previously fed the DHA‐enriched diet (Figure 4 A and 4C). As a target of Akt,17 we measured FoxO3A phosphorylation. The P‐Thr32 FoxO3A content tended to decrease in fasted TA from mice previously fed the CTL diet, but tended to increase in fasted TA from mice previously fed the DHA‐enriched diet (Figure 4 A). This effect was associated with increased total FoxO3A contents in fasted muscles from mice previously fed either the CTL or the DHA‐enriched diet (+140 or +180%, respectively, P < 0.05) (Figure 4 A). Consequently, the P‐Thr32FoxO3A/FoxO3A ratio decreased in fasted TA from mice previously fed the CTL diet (−63%, P < 0.05), but not significantly in mice previously fed the DHA‐enriched diet (−50%) (Figure 4 D). Reduced Akt phosphorylation also results in decreased protein synthesis through a signalling cascade involving the inactivation of rpS6. However, even though Akt phosphorylation was preserved in fasted muscles from mice previously fed the DHA‐supplemented diet, rpS6 phosphorylation on Ser240/244 was dramatically lower in fasted muscles from both CTL and DHA groups (−95% vs. fed mice, P < 0.05) (Figure 4 A and 4E). Overall, the preservation of Akt activity and the partial blockade of AMPK activation in fasted TA muscle from mice previously fed the DHA‐supplemented diet did not reverse the inactivation of rpS6.
Figure 4.
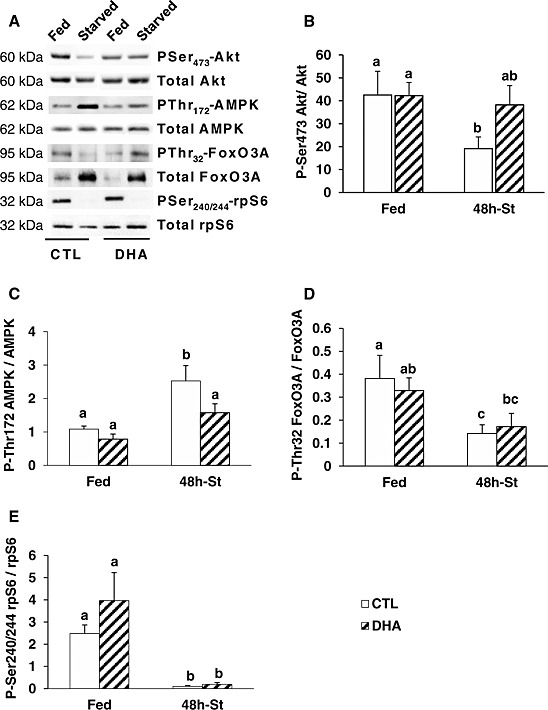
Feeding the mice with the docosahexaenoic acid‐enriched diet prior to 48 h fasting prevented decreased Akt phosphorylation and increased adenosine monophosphate‐activated protein kinase‐phosphorylation in the fasted tibialis anterior muscle. Representative Western blots using antibodies against total protein or specific phosphoprotein (i.e. PSer473‐Akt, PThr172‐adenosine monophosphate‐activated protein kinase, PThr32‐FoxO3, and PSer240/244‐rpS6) are shown in (A). After quantification, the P‐Ser473Akt/Akt (B), P‐Thr172AMPK/AMPK (C), P‐Thr32FoxO3A/Foxo3A (D), and P‐Ser240/244rpS6/rpS6 (E) ratios were calculated in mice fed either the control or the docosahexaenoic acid‐enriched diet. Data are means ± SEM (n = 6/group). Statistical differences were assessed by analysis of variance. Bars with different superscript letters are statistically different.
The endocannabinoid system activation is reduced in the 48 h‐fasted tibialis anterior muscle from mice previously fed the docosahexaenoic acid‐supplemented diet
Diets enriched with DHA reduced the production of major endocannabinoids (eCBs), such as anandamide,25 because AA is the precursor of eCB synthesis. In addition, antagonizing the ECS resulted in a sustained insulin‐dependent Akt activation in C2C12 cells.27 Therefore, we next investigated the role of the ECS in the maintenance of Akt phosphorylation by the DHA‐supplemented diet in the starved TA muscle. The mRNA levels for the cannabinoid receptor 1 (CB1) were unchanged between muscles from mice fed either the CTL or the DHA‐supplemented diet (Figure 5). However, fasting increased CB1 mRNA levels in TA muscle of mice previously fed the CTL diet (+140%, P < 0.05), but not in muscle from mice previously fed the DHA‐enriched diet (Figure 5). N‐Acyl phosphatidylethanolamine phospholipase D (NAPE‐PLD) and fatty acid amide hydrolase (FAAH) are involved in anandamide synthesis and degradation, respectively. Figure 5 also shows that NAPE‐PLD and FAAH mRNA levels were similar in muscle from mice fed the CTL or the DHA diet. NAPE‐PLD mRNA levels were 2.6‐fold higher in fasted TA muscle from mice previously fed the CTL diet, but not in fasted TA muscle from mice previously fed the DHA‐enriched diet. Conversely, fasting elevated FAAH mRNA levels by 1.7‐fold only in TA muscle from mice previously fed the DHA‐supplemented diet (Figure 5). Altogether, these data suggest that the DHA‐dependent preservation of Akt activity during fasting resulted from a reduced ECS activation, by limiting NAPE‐PLD‐dependent anandamide synthesis and by increasing FAAH‐dependent anandamide breakdown (Figure 5).
Figure 5.
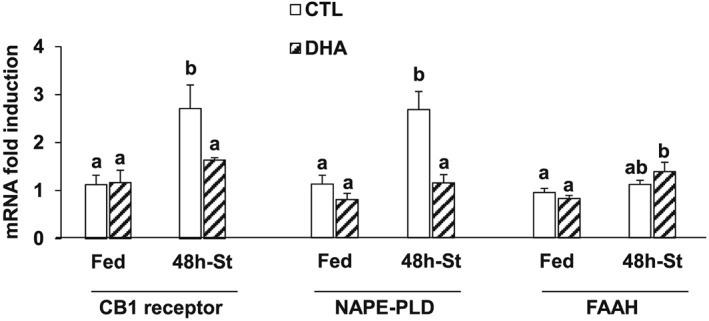
Feeding the mice with the docosahexaenoic acid‐enriched diet prior to 48 h fasting prevented adaptations in the endocannabinoid system in the fasted tibialis anterior muscle. Messenger RNA levels for the CB1 receptor, the N‐acyl phosphatidylethanolamine phospholipase D and the fatty acid amide hydrolase were measured by reverse transcription‐quantitative polymerase chain reaction in the tibialis anterior muscle from control and docosahexaenoic acid groups. Data (means ± SEM for n = 5–6 mice/group) are normalized against 18S and expressed as fold induction vs. control fed mice. Statistical differences were assessed by analysis of variance. Bars with different superscript letters are statistically different.
Increased ubiquitination in the 48 h‐fasted tibialis anterior muscle is partially prevented in mice previously fed a docosahexaenoic acid‐supplemented diet
Muscle mass maintenance is primarily driven by the UPS and autophagy.36 In the UPS, protein substrates are first tagged by a polyubiquitin chain built‐up by ubiquitin enzymes and then recognized by the 26S proteasome.36 The degradation step was assessed by measuring the expression of genes coding for subunits of the 20S proteasome. Feeding mice with the DHA‐supplemented diet did not change the regulation of any of the UPS components measured (Figure 6 and Supporting Information 3). mRNA levels of the β1, β2, and β5 catalytic subunits of the 20S proteasome increased by 2.5‐ to 4‐fold in TA muscle during fasting, with no effect of DHA (Supporting Information 3). Similarly, mRNA levels for some 19S proteasome subunits (S2, S3) were elevated in fasted TA muscle of mice previously fed either the CTL or the DHA‐supplemented diet, except for S5b (Supporting Information 3). As expected,36, 37 mRNA levels for the muscle‐specific E3 ligases MAFbx/atrogin‐1 and MuRF1, and the deubiquitinating enzyme USP19 increased respectively by 8‐, 44‐, and 2.8‐fold in fasted TA muscle from mice previously fed the CTL diet (P < 0.05 vs. fed mice) (Figure 6 A–C). Feeding mice with the DHA‐supplemented diet prior to fasting did not affect the mRNA induction of MAFbx/atrogin‐1 and USP 19 in fasted TA muscle (Figure 6 A and 6B). In contrast, MuRF1 mRNA levels were reduced by 40% in the fasted TA of mice previously fed the DHA‐enriched diet (P < 0.05, vs. CTL mice) (Figure 6 C). Polyubiquitin‐conjugates increased by 115% in fasted muscles from mice previously fed the CTL diet (P < 0.05 vs. fed mice), but not in muscle from mice previously fed the DHA diet (Figure 6 D).
Figure 6.
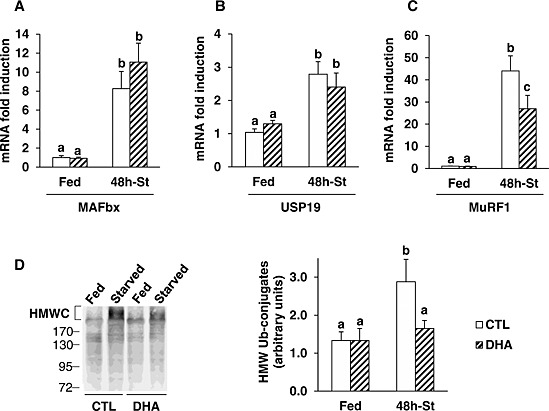
Feeding the mice with the docosahexaenoic acid‐enriched diet prior to 48 h fasting partially prevented the increased expression of MuRF1 and the accumulation of ubiquitin‐protein conjugates in the fasted tibialis anterior. Messenger RNA levels for MAFbx (A), USP19 (B), and MuRF1 (C) were assessed by reverse transcription‐quantitative polymerase chain reaction in the tibialis anterior from mice fed either the control or the docosahexaenoic acid enriched diet prior to fasting for 48 h. Data are normalized against 18S and are expressed as fold induction vs. control fed mice. (D) High molecular weight ubiquitin–protein conjugates were determined by Western blotting using an antibody that recognize polyubiquitin chains (left panel) in the tibialis anterior from control and docosahexaenoic acid groups. High molecular weight ubiquitin–protein conjugates were quantified (right panel). Data are means ± SEM (n = 5–6 mice/group). Statistical differences were assessed by analysis of variance. Bars with different superscript letters are statistically different.
Increased autophagy in the 48 h‐fasted tibialis anterior muscle is partially prevented in mice previously fed a docosahexaenoic acid‐supplemented diet
We next studied the effect of DHA on the regulation of autophagy in the fasted TA muscle. Induction of autophagy involves Atg proteins with the fixation of the LC3 protein on a membrane cargo receptor that initiates the formation of autophagosomes, which then merge with lysosomes to form autophagic vacuoles.36 Feeding mice with the DHA‐supplemented diet did not change cathepsin L and Atg mRNA levels (Figure 7 A and 7B). mRNA levels for cathepsin L, which is strongly induced in several situations of muscle wasting,38, 39 were six‐fold higher in fasted TA (vs. fed TA, P < 0.05) from mice previously fed either the CTL or the DHA‐supplemented diet. (Figure 7 A). We next measured the expression of Atgs involved in the first steps of autophagosome formation (Atg5, Atg12, and Atg16). Atg5 mRNA levels were lower in fasted TA from mice previously fed the DHA diet (P < 0.05 vs. CTL diet). Atg12 and Atg16 mRNA levels were two‐fold higher in fasted TA from CTL mice (P < 0.05 vs. fed mice) (Figure 7 B). The conversion of the cytosolic form of LC3 (LC3‐I) to its lipidated membrane‐bound form (LC3‐II) was measured by Western blotting. The LC3‐II/LC3‐I ratio increased by two‐fold in the TA muscle from mice fed the DHA‐supplemented diet compared with TA muscle from mice fed the CTL diet (Figure 7 C). This difference, however, was not significant and may have been masked by the marked effect of fasting on this measurement. Indeed, and as expected, the LC3‐II/LC3‐I ratio increased by 45‐fold (P < 0.05) in the fasted TA of mice previously fed the CTL diet (Figure 7 C). The LC3‐II/LC3‐I ratio also increased in fasted TA muscle of mice previously fed the DHA‐supplemented diet, but to a lower extent (13‐fold) compared with mice previously fed the CTL diet (Figure 7 C). Finally, we next measured Rab7 expression, a marker of the fusion of autophagosomes with lysosomes.40 mRNA levels for Rab7 were similar in mice fed either the CTL or the DHA‐enriched diet. Rab7 mRNA levels were 2.7 fold higher (P < 0.05) in fasted TA of mice previously fed the CTL diet. This induction was partially prevented in the fasted TA from mice previously fed the DHA‐supplemented diet (Figure 7 D).
Figure 7.
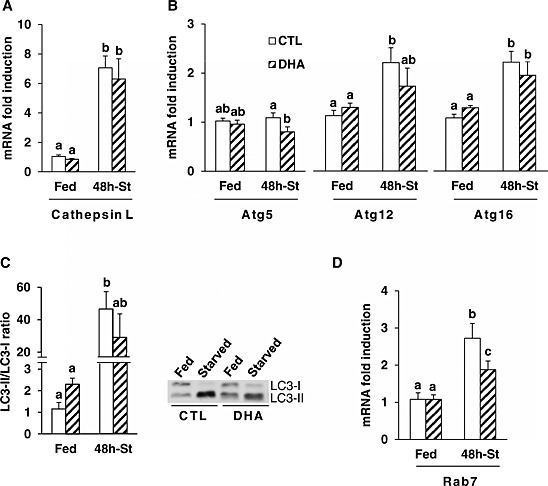
Feeding the mice with the docosahexaenoic acid‐enriched diet prior to 48 h fasting partially prevented the activation of the autophagic proteolytic pathway in the fasted tibialis anterior. Messenger RNA levels for cathepsin L (A), Atg5, Atg12, and Atg16 (B), and for Rab7 (D) were measured by reverse transcription‐quantitative polymerase chain reaction in the tibialis anterior from mice fed either the control or the docosahexaenoic acid‐enriched diet prior to 48 h of fasting. Messenger RNA levels are normalized against 18S and expressed as fold induction vs. control fed mice. LC3I and LC3II protein levels were determined by Western blotting in tibialis anterior from control and docosahexaenoic acid groups (C, right panel). Both bands were quantified and the ratio LC3II/LC3I was calculated (C, left panel). Data are means ± SEM (n = 5–6 mice/group). Statistical differences were assessed by analysis of variance. Bars with different superscript letters are statistically different.
Overall, the data suggest that the preservation of the fasted TA muscle mass in mice previously fed the DHA‐supplemented diet resulted mainly from a lower activation of both the UPS and autophagy, with little if any effect on protein synthesis.
Adaptations in lipid droplets in the 48 h‐fasted tibialis anterior muscle are modified in mice previously fed a docosahexaenoic acid‐supplemented diet
As mentioned above, our data indicate that DHA may have induced autophagy in the fed state (Figure 7 C). However, autophagy is not only involved in the degradation of muscle proteins but also in the regulation of other processes, including intracellular lipid mobilization. Lipid droplets (LDs) are intracellular organelles that function as lipid stores. Their metabolism plays crucial roles in the mobilization of intracellular lipids when energy supply is deficient. We thus first investigated the regulation of markers of LD formation, such as phospholipase D1 (PLD1),41 and Rab18.42, 43 Figure 8 A and 8B shows that PLD1 and Rab18 mRNA levels were similar in muscle from mice fed either the CTL or the DHA diet. PLD1 and Rab18 mRNA levels were higher in fasted TA muscle of mice previously fed the CTL diet (+90 and +60% respectively, P < 0.05), but not in mice fed previously the DHA‐supplemented diet (Figure 8 A and 8B). We next studied the regulation of seipin expression, a marker of LD morphology.44 There was no significant change in response to either fasting or the previously provided diet (Figure 8 C). Finally, perilipins (PLINs) are LD coated proteins that influence the distribution and the fate of LD.45, 46, 47, 48 PLIN mRNAs levels were similar in muscle from mice previously fed either the CTL or the DHA enriched diet (Figure 8 D). PLIN 2, 4, and 5 mRNAs levels were induced in the fasted TA of mice previously given both CTL and DHA diets (Figure 8 D). However, PLIN 4 mRNA levels increased to a lower extent and conversely PLIN 5 mRNA levels increased to a greater extent in the fasted TA muscle of mice previously fed the DHA‐supplemented diet. Overall, the data suggest that feeding mice with the DHA‐supplemented diet prior to fasting resulted in modifications in LD metabolism in the fasted TA muscle.
Figure 8.
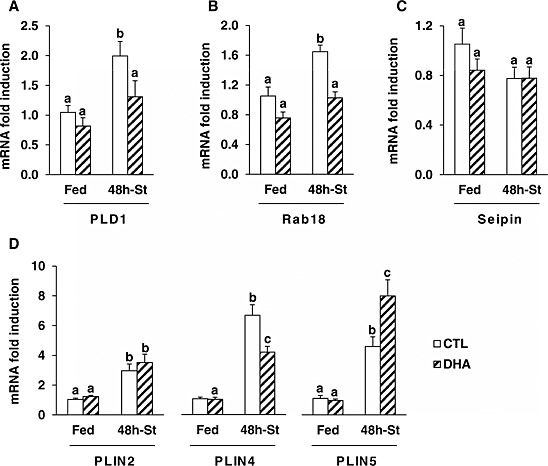
Feeding the mice with the docosahexaenoic acid‐enriched diet prior to 48 h fasting resulted in differential adaptations in markers of lipid droplet formation, size, and fate in the fasted tibialis anterior. Messenger RNA levels for PLD1 (A), Rab18 (B), seipin (C), and the perilipins (PLINs)‐2, ‐4, and ‐5 (D) were determined by reverse transcription‐quantitative polymerase chain reaction in the tibialis anterior from control or docosahexaenoic acid groups. Messenger RNA levels were normalized against 18S and expressed as fold induction vs. control fed mice. Data are means ± SEM (n = 5–6 mice/group). Statistical differences were assessed by analysis of variance. Bars with different superscript letters are statistically different.
Discussion
We report here that 8‐week administration of DHA elevated glycogen energy stores and prevented mouse muscle atrophy upon fasting. This was associated with a limited induction of ubiquitination and autophagy, with little if any effect on protein synthesis. The data suggest that this beneficial effect resulted from both a preservation of Akt activity and a lower induction of AMPK during fasting. They also suggest that DHA could maintain Akt activity by decreasing ECS induction during fasting. Finally, we suggest that changes in LD populations during fasting are sensitive to DHA.
The role of LC‐PUFAs has mainly be investigated using fish oil,8, 9 which contains both EPA and DHA, or EPA alone.5, 6, 7 A single study7 investigated the effect of DHA alone on muscle atrophy and proteolysis in fasting, but without positive effect. However, in the latter study, DHA was administrated once by gavage one day before fasting. Here, we report that an 8 week administration of DHA increased whole body fat mass as observed in mice fed a DHA enriched diet for several months.49 However, this effect has not been consistently observed, because another study reported that fat mass was either unchanged or decreased in mice fed a DHA‐enriched diet for 8 or 16 weeks, respectively.50 Nevertheless, we also show that feeding mice with a DHA‐enriched for 8 weeks also increased epididymal fat mass (+15%, P < 0.05 vs. CTL, data not shown). The increased fat mass reported in our study was not associated with changes in muscle triglyceride contents, suggesting that there was minor if any DHA effect on muscle lipid deposition. In addition, we show that the LC3‐II/LC3‐I ratio, which is related to autophagosome formation, slightly increased in muscle from mice fed the DHA‐supplemented diet (Figure 7 C). Accordingly, besides protein breakdown, autophagy contributes to the breakdown of any macromolecules, including intracellular lipids.14 Thus, the moderate increase of autophagy observed in fed TA muscle from the DHA group may have contributed to prevent muscle lipid accumulation in mice fed the DHA‐supplemented diet. We also report that an 8‐week administration of DHA elevated muscle glycogen stores, which were efficiently mobilized upon fasting (Figure 3 A). Indeed, n3‐PUFAs improved insulin sensitivity and stimulated glycogen synthesis in muscle.51, 52 We also report that TG contents were reduced only in fasted muscles from CTL mice. This suggests that TA of CTL mice run out of glycogen earlier and thus rapidly mobilized their TG stores in response to fasting. In contrast, DHA fed animals have higher glycogen stores to mobilize during fasting resulting in the maintenance of higher TG levels. Accordingly, the AMP/ATP ratio in fasted muscle from these mice was similar to the ratio observed in fed animals. Thus, the ability of fasted muscle to provide cells with energy was higher in mice previously fed the DHA‐enriched diet.
The preservation of muscle mass in 48 h‐fasted mice correlated with a lower activation of the UPS, according to previous reports showing that acute EPA or fish oil administration limited muscle atrophy and UPS activation in several catabolic conditions,5, 6, 9 including fasting.7 In agreement with our findings, DHA only reduced, but did not fully suppress, enhanced MuRF1 mRNA levels in LPS‐treated animals.9 The preservation of muscle mass and the lower induction of MuRF1 mRNA expression upon fasting is in accordance with a major role for MuRF1 in targeting major contractile proteins (i.e. myosin heavy chains and α‐actin) for breakdown by the 26S proteasome.53, 54 In addition, the present findings indicate that the effect of DHA was restricted to the ubiquitination step, no effect being detected on proteasome subunit mRNA expressions. We further show that markers of autophagosome formation (i.e. the LC3‐II/LC3‐I ratio) and of endosome‐autophagosome fusion (i.e. Rab 7) were induced to a lower extent in fasted muscle when mice were previously fed the DHA‐supplemented diet. We and others already established the importance of autophagy during nutrient deprivation,15, 55, 56, 57 but we report here for the first time that using a DHA‐supplemented diet prior to fasting may regulate increased autophagy in fasted muscle. Altogether, the data suggest that administration of DHA for 8 weeks contributes to preserve muscle mass by limiting the induction of both ubiquitination and autophagy in fasted muscles.
The Akt‐dependent pathway induces muscle anabolism by both stimulating protein synthesis and inhibiting proteolysis.58 When anabolic factors are reduced, including in starvation, Akt phosphorylation decreases, leading to the transcription of genes involved in both ubiquitination and autophagy.58 We report here that DHA prevents the decreased Akt phosphorylation in 48 h‐fasted muscle. FoxO3A is a target of Akt17 and induces the transcription of atrogenes.15, 16 We report here that feeding mice with a DHA‐enriched diet reduced the dephosphorylation of FoxO3A in fasted muscle. Overall, this suggests that the effect of DHA on Akt and FoxO3A phosphorylations resulted in a lesser activation of autophagy and ubiquitination. This is in agreement with a recent study showing that DHA counteracted the palmitate‐induced myotube atrophy by restoring the Akt/FoxO3 signalling and limiting activation of proteolysis.28 n‐3 LC‐PUFAs were previously reported to modulate the activity of the ECS,24, 25 which can regulate Akt‐dependent signalling in muscle.26, 27 DHA was efficiently taken up by muscle as feeding mice the DHA‐enriched diet increased DHA contents in blood and muscle and, as expected, decreased the content in AA, which is a precursor of endocannabinoid (eCB) biosynthesis. We report a reduction in the expression of the eCB receptor 1 (i.e. CB1) and of anandamide biosynthesis enzyme (NAPE‐PLD) in 48 h‐fasted muscle from mice previously fed the DHA‐supplemented diet (Figure 5), concomitantly with the maintenance of Akt phosphorylation. The decrease in NAPE‐PLD mRNA expression that was observed in fasted muscle from mice previously fed the DHA diet may affect anandamide concentrations for reasons other than diminished AA content. Changes in mRNA expression do not always reflect similar changes in protein levels. However, our data suggest that the effect of DHA on Akt phosphorylation in fasting could be mediated by a regulation of ECS activation.
Fasting also activates the muscle AMPK by increasing the AMP/ATP ratio, resulting in the induction of UPS and autophagy.18, 19 Accordingly, AMPK was activated during 48 h fasting in muscle, concomitantly with a trend in increased AMP/ATP ratio. However, we report that feeding mice with a DHA‐supplemented diet prior to fasting reduced AMPK activation in fasted muscle mice, as well as ubiquitination and autophagy. Therefore, the lower induction of proteolysis in fasted muscle from mice previously fed the DHA‐supplemented diet may also result from the partial blockade of AMPK activation. However, further investigations should be designed to ascertain the causal relationship between the regulation of the Akt‐ and AMPK‐dependent signalling pathways by DHA and the limitation of ubiquitination and autophagy during 48 h‐fasting. Akt activation induces protein translation through a signalling cascade involving the mammalian target of rapamycin complex 1(mTORC1) and ribosomal protein S6 (rpS6). Although Akt phosphorylation was preserved in 48 h‐fasted muscle from mice previously fed the DHA‐supplemented diet, rpS6 phosphorylation was similarly abolished during fasting in both groups of animals (Figure 4). This lack of effect of DHA on rpS6 phosphorylation presumably resulted from the lack of nutrient intake. Indeed, the activation of mTORC1 particularly depends on amino acid levels58 and AMPK inhibits mTORC1 activation.59 Furthermore, the DHA‐supplemented diet did not reduce the elevated mRNA levels for both MAFbx/atrogin‐1 and USP19 in 48 h‐fasted muscle (Figure 4). So far, the targets of MAFbx/atrogin‐1 (MyoD60, 61 and eiF3f62, 63) seem more related to depressed protein synthesis than to enhanced proteolysis in skeletal muscle.64 In addition, enhanced expression of USP19 was also reported to depress the synthesis of contractile proteins.65 Altogether, our data suggest that the partial maintenance of AMPK activation and the enhanced expression of both MAFbx/atrogin‐1 and USP19 in fasted muscle from mice previously fed the DHA‐supplemented diet contributed to maintain depressed protein synthesis.
Our observations suggest that DHA also regulated the metabolism of intracellular lipid stores during fasting. The increased expression of PLD1 and Rab18 was prevented in fasted muscles when mice were previously fed the DHA‐supplemented diet. Both proteins are involved in LD formation,41, 42 but the precise role of PLD1 remains elusive. In contrast, Rab18 mainly localizes on lipolytically active LD43 and regulates the LD‐associated membrane formation, which is likely to be involved in mobilizing lipid ester stores.42 DHA modulated the fasting‐induced overexpression of PLINs suggesting changes in protein composition at the LD surface. Such changes in LD associated protein composition are suspected to regulate lipolysis.48 PLIN 5, which is overexpressed in fasted muscle from mice previously fed the DHA‐supplemented diet, has been reported to localize to muscle mitochondria,46 regulate oxidative LD hydrolysis, and facilitate the release of fatty acids from LDs as substrates for mitochondrial oxidation.47, 48 Altogether, these data suggest that DHA may improve skeletal muscle intracellular lipid mobilization during fasting and may contribute to maintain the energetic status. This mobilization may involve the autophagy pathway (i.e. lipophagy). Indeed, the degradation of LDs by lipophagy increases in fasted hepatocytes, thus providing cells with energetic nutrients.14 Thus, an activation of skeletal muscle lipid mobilization through lipophagy may limit the activation of fuel sensing molecules such as AMPK and proteolysis resulting in a better adaptation to nutrient deprivation (Figure 9). Altogether our data suggest that in addition to the only well described role of autophagy in muscle, i.e. protein breakdown and elimination of abnormal/damaged mitochondria,66 this system is possibly also involved in the control of energy production and utilization.
Figure 9.
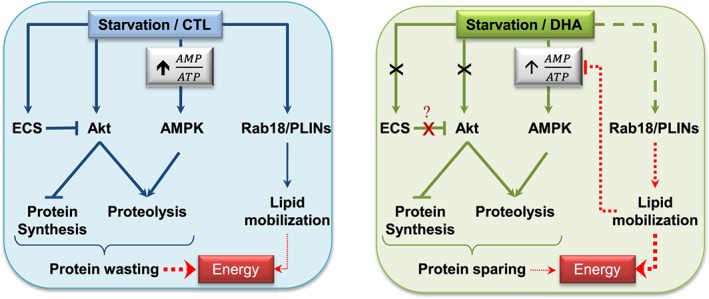
Schematic model of the effects of docosahexaenoic acid on muscle protein turnover and lipid mobilization during 48 h fasting. Left panel: Akt‐ and adenosine monophosphate‐activated protein kinase‐dependent signalling pathways are down and up‐regulated by fasting, respectively, resulting in reduced protein synthesis and enhanced proteolysis. The endocannabinoid system can inactivate the Akt‐dependent signalling pathway (22, 23), and is also induced in fasting. Finally, fasting also induced lipid droplet markers (Rab18 and PLINs expression), providing cells with energy through lipid mobilization. Right panel: docosahexaenoic acid (i) preserved Akt activity and blocked the activation of the endocannabinoid system during fasting. Whether the effect of docosahexaenoic acid on Akt activity during fasting is direct or indirect via the endocannabinoid system remains to be elucidated. Furthermore, docosahexaenoic acid had (ii) no effect on markers of protein synthesis, but (iii) partially prevented adenosine monophosphate‐activated protein kinase and proteolysis activation, and iv) modulated the overexpression of lipid droplet markers. The latter changes may modify the subpopulations of lipid droplets and therefore influence lipid mobilization (34, 38–40). This scheme suggests that docosahexaenoic acid changed either the nature of intracellular lipids and/or improve lipid mobilization efficiency, resulting in subsequent muscle protein sparing. Arrows indicate positive inputs whereas lines ending with a cross bar indicate inhibitory inputs. The docosahexaenoic acid effect is indicated in green lines vs. standard conditions in blue lines. Crosses indicate that docosahexaenoic acid blocked the fasting effect. Finally, dashed green line indicates that docosahexaenoic acid modified the fasting effect. Thick or thin lines denote strong or slight effects, respectively. Effects represented by dotted lines remain to be demonstrated.
In summary, we report here for the first time that an 8‐week DHA nutritional supplementation elevated muscle glycogen stores and thus improved the ability of fasted muscle to provide cells with energy, resulting in preservation of muscle mass in response to food deprivation in mice. The mechanisms responsible for this adaptation include the regulation of Akt and AMPK signalling pathways, leading to reduced activation of UPS and autophagy. Furthermore, our data strongly suggest that providing a DHA‐enriched diet contributed to shift muscle metabolism and presumably energy production towards a better use of intracellular energy stores resulting in a better resilience of muscle protein mass to nutritional stress. On a clinical perspective, we suggest that nutritional or pharmacological strategies aiming at increasing skeletal muscle energy stores may be used prior to planned hospitalization periods, with long‐term immobilization or bed rest, which result in muscle wasting. Indeed, this may shorten recovery periods and significantly reduce health care costs and improve the patients' quality of life.
Conflict of interest
None declared.
Supporting information
ONLINE RESOURCE 1. Effect of the DHA‐enriched diet on blood fatty acid composition
ONLINE RESOURCE 2. Effect of the DHA‐enriched diet on muscle fatty acid composition
ONLINE RESOURCE 3. Feeding the mice with the DHA‐enriched diet prior to starvation had no effect on the expression of 20S (A) and 26S (B) proteasome subunits, but S5b in the starved TA. mRNA levels were determined by RT‐qPCR in the TA from CTL or DHA groups and normalized against 18S. Data are expressed as fold induction vs. CTL fed mice and are means ± SEM (n = 5‐6 mice/group). Statistical differences were assessed by ANOVA. Bars with different superscript letters are statistically different.
Supporting info item
Supporting info item
Supporting info item
Acknowledgements
We thank Arlette Cissoire, Benoît Cohade, Medhi Dejelloul, and Philippe Lhoste from the Unité d'Expérimentation en Nutrition (INRA Clermont‐Ferrand‐Theix, France) for excellent assistance with animal care. We thank Chrystele Jouve and Sarah De Saint‐Vincent for their help in biochemical experiments. We also thank Sergio Polakov and Jérémie David for help in muscle glycogen measurements. This study was supported by funding from the Institut National de la Recherche Agronomique (INRA, France) and the Société Française de Nutrition. The authors certify that they comply with the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015.67
Deval, C. , Capel, F. , Laillet, B. , Polge, C. , Béchet, D. , Taillandier, D. , Attaix, D. , and Combaret, L. (2016) Docosahexaenoic acid‐supplementation prior to fasting prevents muscle atrophy in mice. Journal of Cachexia, Sarcopenia and Muscle, 7: 587–603. doi: 10.1002/jcsm.12103.
References
- 1. Cui Z, Schoenfeld MJ, Bush EN, Chen Y, Burge R. Characteristics of hip fracture patients with and without muscle atrophy/weakness: predictors of negative economic outcomes. J Med Econ 2015; 18:1–11. [DOI] [PubMed] [Google Scholar]
- 2. Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc 2004;52:80–85. [DOI] [PubMed] [Google Scholar]
- 3. Shahar E, Boland LL, Folsom AR, Tockman MS, McGovern PG, Eckfeldt JH. Docosahexaenoic acid and smoking‐related chronic obstructive pulmonary disease. The Atherosclerosis Risk in Communities Study Investigators. Am J Resp Crit Care Med 1999;159:1780–1785. [DOI] [PubMed] [Google Scholar]
- 4. Cottin SC, Sanders TA, Hall WL. The differential effects of EPA and DHA on cardiovascular risk factors. Proc Nutr Soc 2011;70: 215–231. [DOI] [PubMed] [Google Scholar]
- 5. Khal J, Tisdale MJ. Downregulation of muscle protein degradation in sepsis by eicosapentaenoic acid (EPA). Biochem Biophys Res Commun 2008;375:238–240. [DOI] [PubMed] [Google Scholar]
- 6. Whitehouse AS, Smith HJ, Drake JL, Tisdale MJ. Mechanism of attenuation of skeletal muscle protein catabolism in cancer cachexia by eicosapentaenoic acid. Cancer Res 2001;61:3604–3609. [PubMed] [Google Scholar]
- 7. Whitehouse AS, Tisdale MJ. Downregulation of ubiquitin‐dependent proteolysis by eicosapentaenoic acid in acute starvation. Biochem Biophys Res Commun 2001;285: 598–602. [DOI] [PubMed] [Google Scholar]
- 8. You JS, Park MN, Song W, Lee YS. Dietary fish oil alleviates soleus atrophy during immobilization in association with Akt signaling to p70s6k and E3 ubiquitin ligases in rats. Appl Physiol Nutr Metab 2010; 35:310–318. [DOI] [PubMed] [Google Scholar]
- 9. Liu Y, Chen F, Odle J, Lin X, Zhu H, Shi H, et al Fish oil increases muscle protein mass and modulates Akt/FOXO, TLR4, and NOD signaling in weanling piglets after lipopolysaccharide challenge. J Nutr 2013; 143:1331–1339. [DOI] [PubMed] [Google Scholar]
- 10. Dardevet D, Sornet C, Taillandier D, Savary I, Attaix D, Grizard J. Sensitivity and protein turnover response to glucocorticoids are different in skeletal muscle from adult and old rats. Lack of regulation of the ubiquitin–proteasome proteolytic pathway in aging. J Clin Invest 1995;96:2113–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Voisin L, Breuille D, Combaret L, Pouyet C, Taillandier D, Aurousseau E, et al Muscle wasting in a rat model of long‐lasting sepsis results from the activation of lysosomal, Ca2+‐activated, and ubiquitin–proteasome proteolytic pathways. J Clin Invest 1996;97:1610–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mansoor O, Beaufrere B, Boirie Y, Ralliere C, Taillandier D, Aurousseau E, et al Increased mRNA levels for components of the lysosomal, Ca2+‐activated, and ATP‐ubiquitin‐dependent proteolytic pathways in skeletal muscle from head trauma patients. Proc Natl Acad Sci U S A 1996;93: 2714–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Attaix D, Ventadour S, Codran A, Bechet D, Taillandier D, Combaret L. The ubiquitin–proteasome system and skeletal muscle wasting. Essays Biochem 2005;41:173–186. [DOI] [PubMed] [Google Scholar]
- 14. Singh R, Cuervo AM. Autophagy in the cellular energetic balance. Cell Metab 2011;13:495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, et al Foxo3 controls autophagy in skeletal muscle in vivo. Cell Metab 2007;6:458–471. [DOI] [PubMed] [Google Scholar]
- 16. Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, et al Foxo3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab 2007;6:472–483. [DOI] [PubMed] [Google Scholar]
- 17. Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol 2005;37:1974–1984. [DOI] [PubMed] [Google Scholar]
- 18. Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, et al The energy sensor AMP‐activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem 2007;282: 30107–30119. [DOI] [PubMed] [Google Scholar]
- 19. Polge C, Heng AE, Combaret L, Bechet D, Taillandier D, Attaix D. Recent progress in elucidating signalling proteolytic pathways in muscle wasting: potential clinical implications. Nutr Metab Cardiovasc Dis 2013;23:S1–S5. [DOI] [PubMed] [Google Scholar]
- 20. Canto C, Gerhart‐Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, et al AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009;458:1056–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kamolrat T, Gray SR, Thivierge MC. Fish oil positively regulates anabolic signalling alongside an increase in whole‐body gluconeogenesis in ageing skeletal muscle. Eur J Nutr 2013;52:647–657. [DOI] [PubMed] [Google Scholar]
- 22. Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, Rennie MJ, et al Dietary omega‐3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr 2011;93: 402–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kamolrat T, Gray SR. The effect of eicosapentaenoic and docosahexaenoic acid on protein synthesis and breakdown in murine C2C12 myotubes. Biochem Biophys Res Commun 2013;432:593–598. [DOI] [PubMed] [Google Scholar]
- 24. Rossmeisl M, Jilkova ZM, Kuda O, Jelenik T, Medrikova D, Stankova B, et al Metabolic effects of n‐3 PUFA as phospholipids are superior to triglycerides in mice fed a high‐fat diet: possible role of endocannabinoids. PLoS One 2012;7:e38834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wood JT, Williams JS, Pandarinathan L, Janero DR, Lammi‐Keefe CJ, Makriyannis A. Dietary docosahexaenoic acid supplementation alters select physiological endocannabinoid‐system metabolites in brain and plasma. J Lipid Res 2010;51: 1416–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heyman E, Gamelin FX, Aucouturier J, Di Marzo V. The role of the endocannabinoid system in skeletal muscle and metabolic adaptations to exercise: potential implications for the treatment of obesity. Obes Rev 2012;13:1110–1124. [DOI] [PubMed] [Google Scholar]
- 27. Watkins BA, Hutchins H, Li Y, Seifert MF. The endocannabinoid signaling system: a marriage of PUFA and musculoskeletal health. J Nutr Biochem 2010;21:1141–1152. [DOI] [PubMed] [Google Scholar]
- 28. Woodworth‐Hobbs ME, Hudson MB, Rahnert JA, Zheng B, Franch HA, Price SR. Docosahexaenoic acid prevents palmitate‐induced activation of proteolytic systems in C2C12 myotubes. J Nutr Biochem 2014;25:868–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226:497–509. [PubMed] [Google Scholar]
- 30. Moilanen T, Nikkari T. The effect of storage on the fatty acid composition of human serum. Clin Chim Acta 1981;114: 111–116. [DOI] [PubMed] [Google Scholar]
- 31. Morrison WR, Smith LM. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride‐methanol. J Lipid Res 1964;5:600–608. [PubMed] [Google Scholar]
- 32. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 33. Bando JM, Fournier M, Da X, Lewis MI. Effects of malnutrition with or without eicosapentaenoic acid on proteolytic pathways in diaphragm. Respir Physiol Neurobiol 2012;180:14–24. [DOI] [PubMed] [Google Scholar]
- 34. Castillero E, Martin AI, Lopez‐Menduina M, Villanua MA, Lopez‐Calderon A. Eicosapentaenoic acid attenuates arthritis‐induced muscle wasting acting on atrogin‐1 and on myogenic regulatory factors. Am J Physiol Regul Integr Comp Physiol 2009;297:R1322–R1331. [DOI] [PubMed] [Google Scholar]
- 35. Hutchins‐Wiese HL, Li Y, Hannon K, Watkins BA. Hind limb suspension and long‐chain omega‐3 PUFA increase mRNA endocannabinoid system levels in skeletal muscle. J Nutr Biochem 2012;23:986–993. [DOI] [PubMed] [Google Scholar]
- 36. Bonaldo P, Sandri M. Cellular and molecular mechanisms of muscle atrophy. Dis Model Mech 2013;6:25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Combaret L, Dardevet D, Rieu I, Pouch MN, Bechet D, Taillandier D, et al A leucine‐supplemented diet restores the defective postprandial inhibition of proteasome‐dependent proteolysis in aged rat skeletal muscle. J Physiol 2005;569:489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Deval C, Mordier S, Obled C, Bechet D, Combaret L, Attaix D, et al Identification of cathepsin L as a differentially expressed message associated with skeletal muscle wasting. Biochem J 2001;360:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, et al Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J 2004;18:39–51. [DOI] [PubMed] [Google Scholar]
- 40. Hyttinen JM, Niittykoski M, Salminen A, Kaarniranta K. Maturation of autophgosomes and endosomes: a key role for Rab7. Biochim Biophys Acta 1833;2013: 503–510. [DOI] [PubMed] [Google Scholar]
- 41. Andersson L, Bostrom P, Ericson J, Rutberg M, Magnusson B, Marchesan D, et al PLD1 and ERK2 regulate cytosolic lipid droplet formation. J Cell Sci 2006; 119:2246–2257. [DOI] [PubMed] [Google Scholar]
- 42. Ozeki S, Cheng J, Tauchi‐Sato K, Hatano N, Taniguchi H, Fujimoto T. Rab18 localizes to lipid droplets and induces their close apposition to the endoplasmic reticulum‐derived membrane. J Cell Sci 2005;118: 2601–2611. [DOI] [PubMed] [Google Scholar]
- 43. Martin S, Parton RG. Lipid droplets: a unified view of a dynamic organelle. Nature Rev 2006;7:373–378. [DOI] [PubMed] [Google Scholar]
- 44. Yang H, Galea A, Sytnyk V, Crossley M. Controlling the size of lipid droplets: lipid and protein factors. Curr Opin Cell Biol 2012;24:509–516. [DOI] [PubMed] [Google Scholar]
- 45. Gjelstad IM, Haugen F, Gulseth HL, Norheim F, Jans A, Bakke SS, et al Expression of perilipins in human skeletal muscle in vitro and in vivo in relation to diet, exercise and energy balance. Arch Physiol Biochem 2012;118:22–30. [DOI] [PubMed] [Google Scholar]
- 46. Bosma M, Minnaard R, Sparks LM, Schaart G, Losen M, de Baets MH, et al The lipid droplet coat protein perilipin 5 also localizes to muscle mitochondria. Histochem Cell Biol 2012;137:205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bosma M, Sparks LM, Hooiveld GJ, Jorgensen JA, Houten SM, Schrauwen P, et al Overexpression of PLIN5 in skeletal muscle promotes oxidative gene expression and intramyocellular lipid content without compromising insulin sensitivity. Biochim Biophys Acta 1831;2013:844–852. [DOI] [PubMed] [Google Scholar]
- 48. Wang H, Sreenivasan U, Hu H, Saladino A, Polster BM, Lund LM, et al Perilipin 5, a lipid droplet‐associated protein, provides physical and metabolic linkage to mitochondria. J Lipid Res 2011;52:2159–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bonnet N, Ferrari SL. Effects of long‐term supplementation with omega‐3 fatty acids on longitudinal changes in bone mass and microstructure in mice. J Nutr Biochem 2011;22:665–672. [DOI] [PubMed] [Google Scholar]
- 50. Kim J, Carlson ME, Kuchel GA, Newman JW, Watkins BA. Dietary DHA reduces downstream endocannabinoid and inflammatory gene expression and epididymal fat mass while improving aspects of glucose use in muscle in C57BL/6 J mice. Int J Obes 2015;doi:10.1038/ijo.2015.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hirabara SM, Folador A, Fiamoncini J, Lambertucci RH, Rodrigues CF Jr, Rocha MS, et al Fish oil supplementation for two generations increases insulin sensitivity in rats. J Nutr Biochem 2013;24:1136–1145. [DOI] [PubMed] [Google Scholar]
- 52. Figueras M, Olivan M, Busquets S, Lopez‐Soriano FJ, Argiles JM. Effects of eicosapentaenoic acid (EPA) treatment on insulin sensitivity in an animal model of diabetes: improvement of the inflammatory status. Obesity 2011;19:362–369. [DOI] [PubMed] [Google Scholar]
- 53. Polge C, Heng AE, Jarzaguet M, Ventadour S, Claustre A, Combaret L, et al Muscle actin is polyubiquitinylated in vitro and in vivo and targeted for breakdown by the E3 ligase MuRF1. FASEB J 2011; 25:3790–3802. [DOI] [PubMed] [Google Scholar]
- 54. Clarke BA, Drujan D, Willis MS, Murphy LO, Corpina RA, Burova E, et al The E3 Ligase MuRF1 degrades myosin heavy chain protein in dexamethasone‐treated skeletal muscle. Cell Metab 2007;6: 376–385. [DOI] [PubMed] [Google Scholar]
- 55. Mofarrahi M, Guo Y, Haspel JA, Choi AM, Davis EC, Gouspillou G, et al Autophagic flux and oxidative capacity of skeletal muscles during acute starvation. Autophagy 2013;9:1604–1620. [DOI] [PubMed] [Google Scholar]
- 56. Mordier S, Deval C, Béchet D, Tassa A, Ferrara M. Leucine limitation induces autophagy and activation of lysosome‐dependent proteolysis in C2C12 myotubes through a mammalian target of rapamycin‐independent signaling pathway. J Biol Chem 2000;275:29900–29906. [DOI] [PubMed] [Google Scholar]
- 57. Tassa A, Roux MP, Attaix D, Bechet DM. Class III phosphoinositide 3‐kinase–Beclin1 complex mediates the amino acid‐dependent regulation of autophagy in C2C12 myotubes. Biochem J 2003;376:577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J 2013;280:4294–4314. [DOI] [PubMed] [Google Scholar]
- 59. Sanchez AM, Candau RB, Csibi A, Pagano AF, Raibon A, Bernardi H. The role of AMP‐activated protein kinase in the coordination of skeletal muscle turnover and energy homeostasis. Am J PhysiolCell Physiol 2012;303:C475–C485. [DOI] [PubMed] [Google Scholar]
- 60. Lagirand‐Cantaloube J, Cornille K, Csibi A, Batonnet‐Pichon S, Leibovitch MP, Leibovitch SA. Inhibition of atrogin‐1/MAFbx mediated MyoD proteolysis prevents skeletal muscle atrophy in vivo. PLoS One 2009;4:e4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tintignac LA, Lagirand J, Batonnet S, Sirri V, Leibovitch MP, Leibovitch SA. Degradation of MyoD mediated by the SCF (MAFbx) ubiquitin ligase. J Biol Chem 2005; 280:2847–2856. [DOI] [PubMed] [Google Scholar]
- 62. Lagirand‐Cantaloube J, Offner N, Csibi A, Leibovitch MP, Batonnet‐Pichon S, Tintignac LA, et al The initiation factor eIF3‐f is a major target for atrogin1/MAFbx function in skeletal muscle atrophy. EMBO J 2008;27:1266–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sanchez AM, Csibi A, Raibon A, Docquier A, Lagirand‐Cantaloube J, Leibovitch MP, et al eIF3f: a central regulator of the antagonism atrophy/hypertrophy in skeletal muscle. Int J Biochem Cell Biol 2013;45:2158–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Attaix D, Baracos VE. MAFbx/Atrogin‐1 expression is a poor index of muscle proteolysis. Curr Opin Clin Nutr Metab Care 2010;13:223–224. [DOI] [PubMed] [Google Scholar]
- 65. Sundaram P, Pang Z, Miao M, Yu L, Wing SS. USP19‐deubiquitinating enzyme regulates levels of major myofibrillar proteins in L6 muscle cells. Am J Physiol Endocrinol Metab 2009;297:E1283–E1290. [DOI] [PubMed] [Google Scholar]
- 66. Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, et al Autophagy is required to maintain muscle mass. Cell Metab 2009;10:507–515. [DOI] [PubMed] [Google Scholar]
- 67. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ONLINE RESOURCE 1. Effect of the DHA‐enriched diet on blood fatty acid composition
ONLINE RESOURCE 2. Effect of the DHA‐enriched diet on muscle fatty acid composition
ONLINE RESOURCE 3. Feeding the mice with the DHA‐enriched diet prior to starvation had no effect on the expression of 20S (A) and 26S (B) proteasome subunits, but S5b in the starved TA. mRNA levels were determined by RT‐qPCR in the TA from CTL or DHA groups and normalized against 18S. Data are expressed as fold induction vs. CTL fed mice and are means ± SEM (n = 5‐6 mice/group). Statistical differences were assessed by ANOVA. Bars with different superscript letters are statistically different.
Supporting info item
Supporting info item
Supporting info item


