Abstract
Rationale
Low sensitivity to alcohol is a well-established risk factor for alcohol use disorder (AUD). However, little is known about how the low sensitivity phenotype is expressed on a fine-grained, momentary level in drinkers’ daily experience.
Objectives
The objective of the study is to evaluate individual differences in subjective states and appraisals of alcoholic beverages during the ascending limb of real-world drinking episodes.
Methods
Social drinkers (N = 398) with varying degrees of alcohol sensitivity as indexed by the Self-Rating of the Effects of Alcohol form (SRE; Schuckit, Smith, and Tipp, 1997a) recorded diary entries over a 3-week monitoring period (2,576 drinking episodes containing 6,546 moments). Hierarchical linear modeling was used to evaluate whether individual differences in alcohol sensitivity predicted differing intra-episode estimated blood alcohol concentration (eBAC) trajectories, ratings of subjective states, and drink appraisals.
Results
Lower self-reported alcohol sensitivity was associated with consuming “too much, too fast,” as indicated by a steeper slope of ascending eBAC. In models adjusted for momentary eBAC level, participants reporting lower alcohol sensitivity at baseline showed blunted subjective intoxication and drink-contingent punishment.
Conclusions
The results suggest that low sensitivity to alcohol is associated with a blunting of some forms of subjective feedback (i.e., perceptions of intoxication and punishment) that might typically encourage drinking restraint. This may ‘tip the scales’ toward excess consumption and could help to explain why a low alcohol sensitivity forecasts AUD.
Keywords: alcohol use disorder, level of response to alcohol, alcohol sensitivity, ecological momentary assessment
Because alcohol consumption is a necessary prerequisite to developing an alcohol use disorder (AUD), investigators have been drawn to examining whether liability to the disorder might be explained by individual differences in response to alcohol. A germinal line of inquiry compared alcohol responses in groups with differing familial risk for alcoholism, culminating in the identification of individual differences in level of response to alcohol as an important AUD risk factor beyond family history alone (Heath et al., 1999; Pihl et al., 1990; Roche et al., 2014; Schuckit and Smith, 2000; Trim et al., 2009; Volavka et al., 1996).
Over the last several decades, a low level of response (or ‘low sensitivity’) to alcohol, measured either by experimental alcohol challenge or proxy questionnaire, has emerged as one of the most robust risk factors for alcohol abuse and alcoholism, even when analyzed in concert with other prominent risk factors such as family history of alcoholism, age at first drink, and heaviness of drinking (Trim et al., 2009). Sensitivity to alcohol can diminish as a consequence of heavy drinking, but low sensitivity is thought to reflect pre-drinking constitutional differences in addition to acquired alcohol tolerance. There is meaningful variation in alcohol response even among youth with very limited drinking experience (Schuckit et al., 2005a; Schuckit et al., 2005b), and self-reports of low sensitivity to initial lifetime drinking experiences are associated with heavy drinking even after estimates of acquired tolerance are statistically covaried (Corbin et al., 2013; Morean and Corbin, 2008). Twin and family studies indicate that approximately 40 – 60% of the phenotypic variance in alcohol sensitivity is attributable to genetic factors (Heath et al., 1999; Schuckit et al., 2005b; Viken et al., 2003).
Although its empirical status as a risk factor for AUD is now well established, a great deal remains to be learned concerning why and how a low sensitivity to alcohol fosters problematic drinking outcomes. Long-term longitudinal studies with widely-spaced assessments have indicated that associations between low alcohol sensitivity and AUD are partially mediated by changes in alcohol outcome expectancies, enhanced coping motives for drinking, and affiliation with heavy drinking peers (Schuckit et al., 2009; Schuckit et al., 2008a; Schuckit et al., 2008b). However, surprisingly little is known at a more granular level concerning the motivational and behavioral correlates of low-sensitivity risk status during ‘real world’ drinking episodes. Studying the natural expression of a low level of response to alcohol has the potential to provide additional clues about the intervening mechanisms between the diathesis and the disorder.
In a first step along these lines, we recently used data from an ecological momentary assessment (EMA) investigation to examine whether individual differences in sensitivity to alcohol were associated with alcohol hangover the morning after drinking (Piasecki et al., 2012a). The current article uses additional data from this EMA investigation to further characterize the natural expression of low alcohol sensitivity. Whereas the prior analyses focused on a morning-after adverse consequence of drinking, here we examine data collected during the drinking episode itself and focus on how self-reported individual differences in level of response to alcohol are associated with subjective states experienced during drinking and immediate appraisals of the rewarding and punishing effects of recently consumed drinks.
Two prominent theoretical accounts postulate distinct phenotypic expressions of alcoholism risk during drinking episodes. The Low Level of Response (LLR; Schuckit, 1980) model suggests that at-risk drinkers are less susceptible to all effects of alcohol, regardless of when those effects are measured. The LLR posits that low sensitivity drinkers must consume more alcohol to achieve desired effects, which in turn puts them at greater risk for heavy drinking, adverse consequences of alcohol use, and physical dependence (Schuckit and Smith, 2001). A rival account, the Differentiator Model (DM; Newlin and Thomson, 1990), suggests that at-risk drinkers experience blunted alcohol effects only while on the descending limb of the blood alcohol concentration (BAC) curve, when alcohol effects tend to be more sedating and hedonically unpleasant. The DM predicts that at-risk drinkers tend to experience heightened responses to those effects that are more hedonically pleasant and stimulating, which tend to predominate while BAC is rising. This account suggests the balance of subjective effects is tilted toward positive reinforcement in at-risk individuals. Escalated drinking may occur because drinks are experienced as more immediately stimulating or rewarding and are less likely to be followed by unpleasant, punishing effects. The bulk of research influenced by LLR and DM accounts has focused on how alcohol administration affects subjectively assessed stimulant and sedative states and objective indicators such as body sway or hormonal response in groups at high or low risk for AUD. A recent meta-analysis of the literature assessing subjective states found partial support for both models (Quinn and Fromme, 2011). Studies that grouped participants according to familial risk for alcoholism tended to support the LLR, while those utilizing typical consumption patterns showed greater support for the DM.
More recently, an independent line of research informed by various dual-process models (e.g., Wiers et al. 2007) and Incentive Sensitization Theory (Robinson and Berridge, 1993; Robinson and Berridge, 2003) has investigated whether low sensitivity drinkers display a unique pattern of motivational responses to alcohol cues relative to high sensitivity drinkers. This work has revealed that low sensitivity to alcohol is associated with larger P300 event-related potentials in response to alcohol stimuli (Bartholow et al., 2007; Bartholow et al,, 2010), attentional biases to alcohol cues (Shin et al., 2010), and a behavioral approach bias in the presence of alcohol cues (Fleming and Bartholow, 2014). Such findings suggest that alcohol cues may be imbued with exaggerated incentive salience for low sensitivity drinkers, perhaps making these individuals especially prone to experience subjective cravings for alcohol in the face of alcohol cues (Fleming and Bartholow, 2014).
In the current study, we examined data from repeated momentary assessments collected during drinking episodes to characterize the expression of low sensitivity risk in drinkers’ natural environments. Analyses are limited to reports made when the participant’s estimated blood alcohol concentration (eBAC) was rising because the bulk of our assessments were collected in this phase (Piasecki et al., 2012b). We examine subjective states and drink appraisals as a function of alcohol sensitivity, individuals’ momentary eBAC level, and their interaction. These analyses are roughly analogous to an alcohol challenge investigation, permitting tests probing whether self-reports of low sensitivity are associated with differential responses to a given level of alcohol exposure in the natural environment.
We expected that lower self-reported alcohol sensitivity would be associated with a steeper ascent in intra-episode eBAC. This finding would be consistent with, though not required by our earlier finding that less sensitive drinkers in this sample attained higher eBAC peaks (Piasecki et al., 2012a), and it would also be congruent with the theoretical assertion that low sensitivity drinkers would tend to consume greater quantities of alcohol in order to experience desired effects (Schuckit and Smith, 2001).
Predictions regarding subjective states in eBAC-adjusted analyses were less certain. According to the LLR, we might expect self-reported alcohol sensitivity to moderate all associations between subjective states and eBAC, indicating a domain-general blunted response to alcohol. The DM implies a more nuanced pattern of moderation, with less sensitive drinkers predicted to display exaggerated hedonically positive responses to alcohol but blunted negatively valenced responses. On the basis of prior cue exposure research (e.g., Fleming and Bartholow, 2014), we anticipated that low sensitivity drinkers might display elevated craving for alcohol during drinking episodes, which perforce involve exposure to exteroceptive and interoceptive alcohol stimuli. This hypothesis was tentative because the existing cue exposure research with low sensitivity drinkers has only investigated the effects of alcohol cues in the absence of alcohol administration, and the LLR and DM models do not explicitly address craving responses or incentive motivation per se.
Methods
Participants
Participants were frequent drinkers (self-report of drinking on 4 or more occasions in past month) recruited via mass email solicitation, posted flyers, and commercial circulars. Because the major aims of the larger project focused on co-use of alcohol and tobacco (Piasecki, et al, 2011), current cigarette smokers were deliberately oversampled. This sample has been the focus of previous reports and has been described in greater detail previously (Epler et al., 2014; Piasecki et al., 2011; Piasecki et al., 2012a; Piasecki et al., 2012b, Piasecki et al., 2014a; Robertson et al., 2012; Treloar et al., 2015). A total of 404 participants consented to participate and were issued a study diary. Of these, four were excluded from the current analyses because they did not report any drinking during the study, one was excluded because his weight was not recorded (preventing calculation of eBAC), and a final participant was excluded because she did not complete the SRE. Thus, the analyses reported here used data from 398 participants. On average, participants recorded 8.1 drinking episodes (SD = 5.2, range 1 [n = 12, 3.0%] to 28 [n = 1, 0.3%]). The research protocol was approved by the Institutional Review Boards at the University of Missouri and Washington University School of Medicine.
Procedure
All participants attended two laboratory sessions prior to beginning the diary phase of the study. At the initial session, participants completed a battery of questionnaire measures and were weighed using a physician’s scale. Participants were trained how to use their electronic diary device (ED) at a separate session lasting approximately 45 minutes. Training consisted of instructions on how to record the first drink of an episode, respond to follow-up prompts, and complete other reports not described in this article. Participants began the 3-week monitoring period immediately following the completion of the training session.
Diary protocol
The diary protocol is described in greater detail elsewhere (Epler et al., 2014; Piasecki et al., 2011; Piasecki et al., 2012a; Piasecki et al., 2012b, Piasecki et al., 2014a; Robertson et al., 2012; Treloar et al., 2015). Briefly, drinking events were recorded by participants immediately following the first drink of a drinking episode, triggering follow-up prompts at 30, 90, 150, and 210 minutes following the first drink record. Additional prompts at 60 minutes past the final scheduled prompt were added whenever an additional drink(s) was recorded (e.g., an additional drink between the first drink report and the 30 minute prompt added an additional prompt at 270 minutes). Thus, total follow-up time was flexibly extensible based on the drinking that occurred within a given episode. Participants could report that they were going to bed; in that event all remaining prompts were canceled. During the training session in the laboratory, participants were specifically instructed to initiate a bedtime report only when retiring for the evening. Participants could temporarily suspend prompts from the device for situations where responding would be contraindicated (e.g., while driving a car), but the suspend option was not available during ongoing drinking episodes and therefore could not be used to prevented delivery of drinking follow-up prompts. Participants were not prevented from recording multiple episodes in a single day. However any episodes after the first one recorded in a given day were not used in these analyses due to concern over whether secondary episodes would have started from a zero BAC1.
In addition to drinking reports, participants were prompted by the ED up to five times per day at random to make a report. These randomly prompted reports were identical to the userinitiated first drink reports and drinking follow-up reports with the exception that drink appraisal items (described below) were not administered. Random prompts were suspended while the drinking follow-up protocol was active.
Not all drinking episode assessments were triggered by a user-initiated first drink report2. As a precaution against potential under-reporting of alcohol use, the participants were asked in every type of assessment whether or not they had consumed alcohol since the last diary report. When this question was answered affirmatively, the sequence of drinking follow-up assessments was triggered3. This event acted as the first drink record for 1,032 episodes (31.6% of all episodes). The information collected in these reports was equivalent to that collected in userinitiated first drink reports excepting drink appraisal items. Drinking follow-up records were identical regardless of how the first drink was captured.
Measures
Participant-level variables
Participants completed the Self-rating of the Effects of Alcohol questionnaire (SRE; Schuckit et al., 1997a; Schuckit et al., 1997b) during the initial session in order to characterize their level of alcohol sensitivity. Previous investigations have found that a participant’s score on the SRE is significantly correlated with alcohol sensitivity measured during an alcohol challenge protocol (Fleming et al., 2016; Schuckit et al, 1997a; Schuckit et al., 1997b) making it an economical substitute measure of alcohol sensitivity. The SRE asks participants to record the number of drinks required to feel any effect of alcohol, to feel dizzy or begin slurring speech, to begin stumbling or walking in an uncoordinated manner, and to pass out. The number of drinks is recorded for each effect for three time periods: the first five times one drank, the most recent period of drinking at least once a month for three consecutive months, and the heaviest period of drinking. Participants were instructed to leave any effect they did not experience during the time period blank. Scores were computed using the method described by Lee and colleagues (2015) in order to account for the relationship between missing data and total score and to produce a less biased estimate of sensitivity. These were then standardized within-sex (zSRE) to avoid conflating conflating low sensitivity with male sex, thus, a 1-unit increment in zSRE reflects a 1-SD decrease in sensitivity relative to participants’ same-sex peers. Participant sex, weight, age, and score on the Alcohol Use Disorders Identification Test (AUDIT; Babor et al., 2001; Saunders et al., 1993) were also recorded during the initial session. Sex was dummy-coded such that females served as the reference category. Weight was measured in pounds. Participant age was also dummy-coded into categories of 18–20 years old, 21–30 years old, 31–40 years old, and 41+ years old (the 41+ years old group served as the reference group). The first two items of the AUDIT reflect typical frequency and quantity of drinking and were used to characterize each participant’s level of past month drinking.
Episode-level and momentary variables
All records made in the ED were date- and time-stamped. Those data were coded into either weekday (reference category; after 6 p.m. Sunday and before 6 p.m. Thursday) or weekend reports (6 p.m. Thursday to 6 p.m. Sunday). Time of day was coded into 4-hour blocks beginning with 12 a.m. to 4 a.m. (reference category). First-drink records asked participants to record their location (work or school, bar or restaurant, home, outside, in a vehicle, and other; work or school served as the reference category) and social companionship (alone, with a romantic partner, with friends, with coworkers, with a child, with parents, with other family members, or with other people; alone served as the reference category) using a binary checklist, marking all that applied. Reports of “with a child,” “with parents,” and “with other family members” were collapsed into a single “with family” variable due to low response (each no more than 6% of records). Location and social companionship were assessed only at the episode level (specifically at the outset of the episode) due to concerns over participant burden.
Participants were also asked during first-drink records whether they had smoked a cigarette in the last 15 minutes, and in drinking follow-ups to record the number of cigarettes they had smoked since the last report. Responses to these items were combined and recoded to form a single momentary level covariate indicating recent smoking (yes = 1, no = 0).
Estimated BAC
During follow-up records, participants recorded the number of drinks consumed since the last record. These data, in combination with weight and sex recorded at baseline, were used to calculate an eBAC according to a formula created by Matthews and Miller (1979). Estimates produced by this formula correlate with breath alcohol content and were found to perform best relative to estimates from other commonly used eBAC formulas (Hustad and Carey, 2005). Analyses were limited to records reported on the ascending limb of the eBAC curve, as determined by the report either being from the first-drink record in the episode or, for follow-up assessments, if the eBAC was greater than or equal to that of the immediate preceding record in the episode. The time spent consuming the first drink in the episode was not formally assessed and was instead assumed to be 20 minutes. This value was arbitrarily chosen, but we selected a non-zero value to reflect that alcohol was not absorbed instantaneously. This assumption is a constant across drinking episodes and affects the magnitude of the eBACs, but not the rank order or correlations with other measures. There were rare instances (< 0.5% of all records) where momentary eBAC exceeded 0.40 g/dl. Due to the rarity and uncertainty whether such high BAC were actually achieved (due to error in reporting or failure to absorb all drinks), those cases were winsorized to 0.404.
Subjective states
In all assessments, participants used 5-point scales (1 = not at all to 5 = extremely) to rate their experiences of a variety of subjective states over the prior 15 minutes. Three items assessed positive affect (‘enthusiastic,’ ‘excited,’ and ‘happy’), two items tapped negative affect (‘distressed’ and ‘sad’), two items indexed subjective intoxication (‘feel buzzed’ and ‘feel dizzy’), craving was assessed with a single item (‘crave a drink’), and a final three states (‘sluggish,’ ‘headache,’ and ‘nauseous’) reflected adverse effects. Because subjective states were assessed in both drinking records and in randomly prompted records it was possible to examine the average level of each state for the day outside of drinking episodes. The “baseline” level of each state was entered as a covariate into the model predicting that state (e.g., the daily average of ‘buzzed’ from nondrinking moments was a covariate in models predicting ‘buzzed’ during drinking episodes).
We considered forming composite measures by averaging ratings for the adjectives within each domain across conceptually related items. Simple coefficient alpha estimates of scale reliability suggested this was reasonable (e.g., positive affect α = .875; negative affect α = .727). However, more conservative estimates of reliability accounting for the multilevel nature of the data indicated caution was warranted. When reliability was calculated based off the generalizability theory approach described by Shrout and Lane (2012), lower-bound estimates of reliability were lower than is traditionally acceptable (e.g., positive affect RKN = 0.522; negative affect RKN = 0.261). As a result, we elected to analyze each subjective state item individually, but retained the general groupings for descriptive purposes in the results below.
Appraisals of the last drink
Participants rated drinks on a 5-point scale (1 = not at all to 5 = extremely) for positively reinforcing effects (“Was the last drink pleasurable?”), negatively reinforcing effects (“Did the last drink relieve unpleasant feelings or symptoms?”), and punishing effects (“Did the last drink make you feel worse?”). These appraisal items were administered for the first drink of a drinking episode and whenever new drinks were recorded during follow-up.
Analyses
General mixed linear models were computed using PROC MIXED in SAS software (SAS version 9.4, SAS Institute Inc., Cary, NC). All models contained covariates at the participant-level (sex, weight, age, and quantity-frequency of past-month drinking), episode- level (location, social companionship, and daily average of each subjective state across nondrinking records), and momentary-level (time of day, weekend status, and recent smoking) as described above.
An initial model examined whether individual differences in alcohol sensitivity were associated with distinct patterns of alcohol use. Estimated BAC served as the dependent variable and was predicted from linear and quadratic effects of time since the first drink, alcohol sensitivity level, and the interactions between sensitivity and the time trends in addition to the listed covariates. The units of time were hours since first drink, and sensitivity was measured using each participant’s zSRE score. The model featured a 3-level structure with moments nested within episodes, and episodes nested within participants. A random intercept was included at both the episode and participant levels.
Next, we fit a series of models aimed at characterizing whether individual differences in alcohol sensitivity are associated with variations in subjective experiences during drinking episodes. In each model, one of the subjective responses to alcohol or appraisal of drinks served as the dependent variable. The key predictors were momentary eBAC, zSRE, and their interaction. Random intercepts at the occasion and participant level were included, and eBAC was allowed to have a random slope. The covariates listed at the beginning of this section were also included in these models.
Due to the large number of tests conducted, a correction for a False Discovery Rate (FDR) was applied per Benjamini and Hochberg (1995) using PROC MULTITEST in SAS. Following the correction, the critical p-value for subjective state models shifted from the traditional p < 0.05 to p ≤ 0.011 (equivalent to p = 0.046).
Results
The 398 participants recorded 2,901 episodes (M = 7.3 episodes per participant, SD = 4.3) containing 7,846 drinking moments (6,559 also containing appraisals of the last drink). On average the final observation in an analyzed drinking episode occurred 1.8 hours after the triggering record (SD = 1.7 hours, Range = 0 [i.e. no follow-ups completed] - 11.5 hours). Participants reported consuming an average of 6.5 drinks per episode (SD = 5.6 drinks, Range = 1 – 36.). Participants were evenly split between males and females with an average age of 23.3 years old (SD = 7.1, Range = 18 – 70).
Although eligibility for the study only required that participants drink at least once per week, the final sample reported relatively heavy levels of drinking on average. Participants consumed 56.4 drinks per month on average (SD = 36.5, Range = 4.5 – 160) and had an average AUDIT total of 12.2 (SD = 5.5, Range = 2 – 29). In terms of the risk levels described by Babor and colleagues (2001), 21.7% of the sample fell into Zone I (Lowest risk; AUDIT total < 8), 51.6% were in Zone II (8 ≤ AUDIT total ≤ 15), 15.7% were in Zone III (16 ≤ AUDIT total ≤ 19), and the final 11.2% were in Zone IV (Highest risk; AUDIT total ≥ 20). Male participants had an average raw SRE score of 8.8 (SD = 3.0) and females had an average raw SRE score of 6.6 (SD = 2.2). A total of 254 (63.8%) participants described themselves as current smokers at baseline.
Drinking episodes lasting longer than 4 hours were rare (94% of all data was collected through 4 hours). Out of concern that reports made during lengthy episodes were not representative of typical drinking and might be influential outliers, data collected beyond that time point were excluded from the analyses. In addition, 305 drinking episodes occurred on days where no random prompts were completed thereby not allowing for the computation of average daily mood states. These episodes were also excluded from the final results presented below. The results below reflect the final sample of 2,596 drinking episodes containing 6,546 drinking moments (6,083 containing appraisals of the last drink). These results highlight effects involving the central predictors (i.e., momentary eBAC and alcohol sensitivity). Full model results including effects for all covariates are presented in supplemental tables S1 – S27.
Trajectories of ascending eBAC
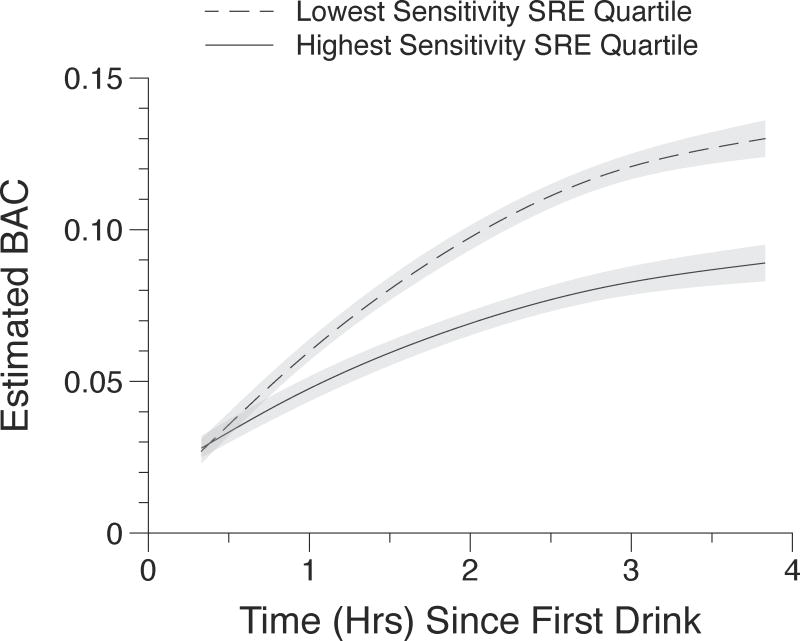
Results of the analysis predicting momentary eBAC are presented in Table 1. As expected, momentary eBAC increased as episodes extended and approached an asymptote. The trajectory of momentary eBAC differed significantly based on alcohol sensitivity. Lower sensitivity drinkers exhibited steeper ascending eBAC slopes over the course of the episode as evidenced by a significant time × zSRE interaction (b = 0.0127, p < 0.001). Lower sensitivity to alcohol was also associated with greater deceleration in in eBAC compared to higher sensitivity drinkers (time2 × zSRE interaction b = −0.0016, p < 0.001). Figure 1 illustrates these effects, plotting model-predicted eBACs and associated confidence intervals as a function of time since first drink at the mean of the highest- and lowest-sensitivity quartiles of the zSRE distribution.
Table 1.
Fixed effects from multilevel regression model predicting ascending momentary estimated blood alcohol concentration (eBAC).
| Predictor |
|
||
|---|---|---|---|
| Momentary eBAC
| |||
| b | SE | p | |
| Time | 0.0455 | 0.0016 | < 0.001 |
| Time2 | −0.0054 | 0.0004 | < 0.001 |
| zSRE | −0.0047 | 0.0019 | 0.011 |
| zSRE × Time | 0.0127 | 0.0020 | < 0.001 |
| zSRE × Time2 | −0.0016 | 0.0005 | 0.003 |
Note: Tabled effects are adjusted for person-level, episode-level, and momentary covariates described in the text. A full presentation of the model results, including coefficients for individual covariates, can be found in the Supplemental Materials (Table S1).
Fig. 1.
Model predicted eBAC values and associated 95% confidence intervals plotted against time since completion of the first drink. Lines are plotted at the mean zSRE scores for the top and bottom quartiles of the zSRE distribution to visualize the significant ZSRE x Time and ZSRE x Time2 interactions from Table 1.
Subjective States and Drink Appraisals5
Covariate-adjusted fixed effects from models predicting subjective states are presented in Table 2.
Table 2.
Fixed effects from multilevel regression models predicting subjective states and drink appraisals as a function of estimated blood alcohol concentration, alcohol sensitivity, and their interactions.
| Dependent Measure | eBAC
|
zSRE
|
zSRE × eBAC
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| b | SE | p | b | SE | p | b | SE | p | |
| Positive Affects | |||||||||
| Excited | 1.951 | 0.281 | <0.001 | −0.012 | 0.044 | 0.779 | −0.482 | 0.327 | 0.141 |
| Happy | 1.142 | 0.257 | <0.001 | −0.034 | 0.039 | 0.386 | 0.090 | 0.299 | 0.763 |
| Enthusiastic | 1.537 | 0.260 | <0.001 | −0.032 | 0.045 | 0.481 | −0.372 | 0.302 | 0.218 |
| Negative Affects | |||||||||
| Sad | 0.124 | 0.204 | 0.542 | −0.032 | 0.029 | 0.260 | 0.046 | 0.237 | 0.845 |
| Distressed | −0.389 | 0.228 | 0.087 | −0.021 | 0.037 | 0.570 | 0.138 | 0.264 | 0.602 |
| Craving | |||||||||
| Crave a drink | −0.022 | 0.351 | 0.950 | 0.006 | 0.066 | 0.929 | −0.192 | 0.411 | 0.640 |
| Intoxication | |||||||||
| Buzzed | 8.865 | 0.461 | <0.001 | −0.089 | 0.050 | 0.074 | −1.617 | 0.557 | 0.004 |
| Dizzy | 2.696 | 0.272 | <0.001 | −0.024 | 0.027 | 0.373 | −0.697 | 0.330 | 0.035 |
| Adverse Effects | |||||||||
| Sluggish | 0.562 | 0.272 | 0.039 | −0.059 | 0.040 | 0.140 | −0.222 | 0.320 | 0.488 |
| Headache | 0.437 | 0.172 | 0.011 | 0.031 | 0.028 | 0.273 | −0.448 | 0.201 | 0.026 |
| Nauseous | 0.453 | 0.170 | 0.008 | 0.008 | 0.022 | 0.721 | −0.055 | 0.202 | 0.786 |
| Drink Appraisals | |||||||||
| Pleasurable | 0.163 | 0.293 | 0.579 | 0.028 | 0.052 | 0.591 | −0.137 | 0.344 | 0.691 |
| Relieved | 0.112 | 0.348 | 0.749 | 0.006 | 0.077 | 0.943 | 0.017 | 0.406 | 0.967 |
| Felt worse | 1.147 | 0.191 | <0.001 | 0.016 | 0.023 | 0.500 | −0.646 | 0.223 | 0.004 |
Note: Tabled effects are adjusted for the person-level, episode-level, and momentary covariates described in the text. A full presentation of the model results, including coefficients for individual covariates, can be found in the Supplemental Materials (Tables S2 – S15).
Positive affects
Ratings of feeling excited (b = 1.951, p < 0.001), feeling happy (b = 1.142, p < 0.001), and feeling enthusiastic (b = 1.537, p < 0.001) all increased as a function of eBAC. Individual differences in alcohol sensitivity were not related to the average intensity of positive affects and did not moderate associations between positive affects and eBAC (ps ≥ 0.14).
Negative affects and craving revealed no significant effects of eBAC, alcohol sensitivity, or their interaction (ps ≥ 0.09).
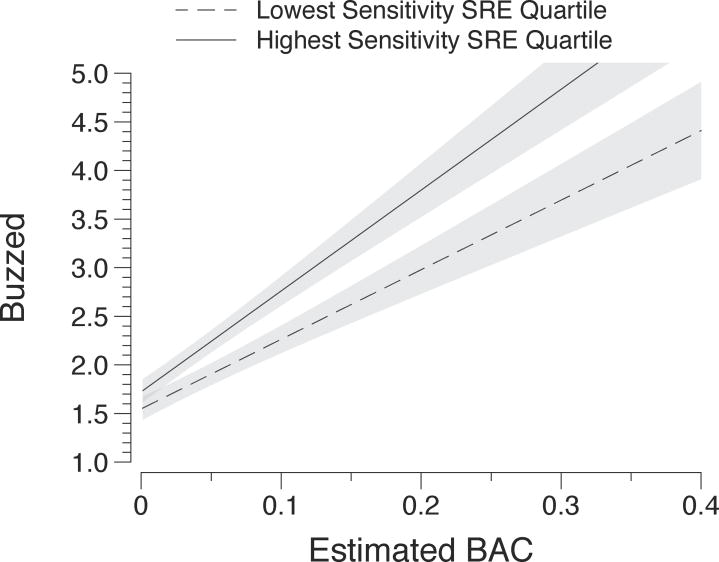
Subjective intoxication ratings were strongly related to concurrent eBAC level. Ratings of both feeling buzzed (b = 8.865, p < 0.001) and feeling dizzy (b = 2.696, p < 0.001) increased as a function of eBAC. The increases in ratings of both states were significantly moderated by sensitivity. Lower sensitivity drinkers reported feeling significantly less buzz relative to their same-sex higher sensitivity peers at the same level of alcohol exposure (eBAC × zSRE interaction b = −1.617, p < 0.01). A similar, though less strong interaction existed for reports of feeling dizzy, though this was only nominally significant (eBAC × zSRE interaction b = −0.697, p < 0.05). Figure 2 illustrates the effects of eBAC and alcohol sensitivity on feeling buzzed
Fig. 2.
Model predicted ratings and associated 95% confidence intervals for ‘buzzed’ plotted against momentary eBAC level. Lines are plotted at the mean zSRE scores of the top and bottom quartiles of the zSRE distribution to visualize the significant eBAC and eBAC x zSRE effects from Table 3. The y-axis was truncated at 5.0, as this was the maximum value of the response scale; predicted values exceeding this are eclipsed.
Adverse effects
Ratings of all three adverse effects states increased at higher eBAC levels, with the increases for feeling a headache (b = 0.437, p < 0.012) and feeling nauseous (b = 0.453, p < 0.01) surpassing the threshold of nominal significance. Ratings of feeling a headache displayed an interactive effect similar to the subjective intoxication ratings such that lower sensitivity drinkers reported feeling less headache relative to same-sex higher sensitivity peers, though this was only nominally significant (b = −0.448, p < 0.05).
Drink appraisals
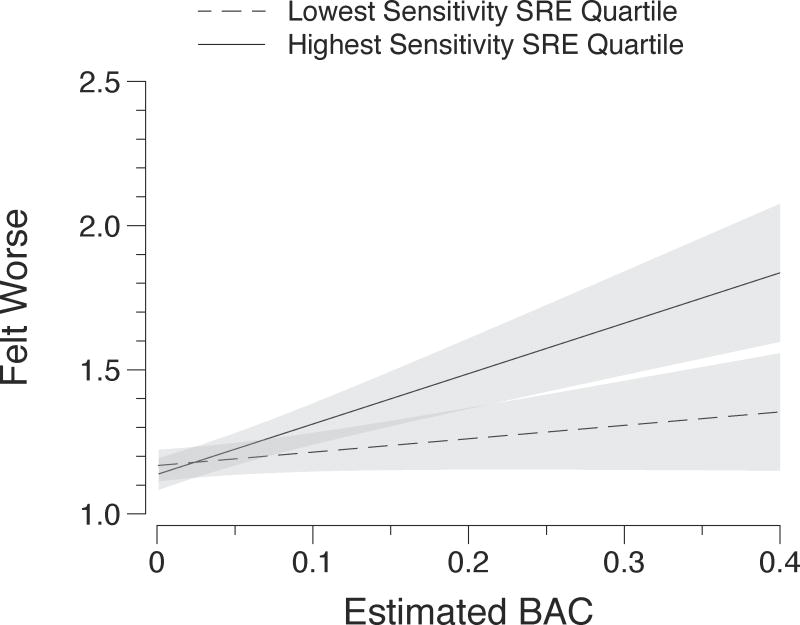
Ratings of the most recent drink being pleasurable or relieving displeasure did not significantly differ in these analyses (ps ≥ 0.58). Feeling worse as a result of the last drink was related to higher eBAC (b = 1.147, p < .001). This effect was qualified by a zSRE × eBAC interaction (b = −0.646, p < .01), indicating that experiencing punishing effects was less strongly related to eBAC among drinkers lower in alcohol sensitivity (Figure 3).
Fig. 3.
Model predicted ratings of ‘Felt Worse’ and associated 95% confidence intervals plotted against momentary eBAC level. Lines are plotted at the mean zSRE scores of the top and bottom quartiles of the zSRE distribution to visualize the significant eBAC and eBAC x zSRE effects from Table 3.
Discussion
The current study represents a unique approach to investigating the natural expression of an established risk factor for alcohol use disorder. Using ecological momentary assessment, we characterized trajectories of alcohol consumption and the subjective effects of alcohol on the ascending limb through the lens of individual differences in self-reported sensitivity to alcohol. Descriptive studies of this kind are needed to help flesh out our understanding of the intervening psychological mechanisms linking low sensitivity risk status to problematic drinking outcomes. At a behavioral level, the current findings revealed that less sensitive drinkers selected distinctive drinking patterns resulting in steeper ascending eBAC slopes. We had previously shown that low sensitivity was associated with higher peak eBAC in this sample (Piasecki et al., 2012a). The current findings add that this is not merely the result of extending drinking sessions. Rather, low sensitivity drinkers appear to drink ‘too much, too fast,’ a common conceptualization of binge drinking (e.g., Leeman et al., 2010; Li et al., 2007).
Models keyed to momentary eBAC revealed that a variety of positive and negative experiences were directly associated with level of alcohol exposure, with effects most pronounced for subjective intoxication. Individual differences in alcohol sensitivity moderated the effects of eBAC on subjective intoxication and appraised punishing effects of the last drink. Specifically, buzz and punishment responses to alcohol were blunted among drinkers lower in alcohol sensitivity, with a similar, but weaker effect for dizzy responses.
Contrary to what might be expected based upon an extrapolation of the DM (Newlin and Thomson, 1990), the eBAC-adjusted analyses did not identify any positively-valenced states or appraisals that were exaggerated in low-sensitivity drinkers after correcting for multiple comparisons. The findings were more consistent with the Low Level of Response Model, although they did not show the domain-general blunting of alcohol responses anticipated by the strong form of the model. In some cases, the absence of zSRE × eBAC interactions might be attributable to the use of inappropriate dependent measures. For example, the negative affects and appraisals of positive and negative reinforcing effects were not directly related to eBAC. If such experiences do not represent valid or strong alcohol responses, then the absence of moderation effects may not directly address the predictions of the LLR model. However, this cannot explain all of the results because the positive affects were clearly related to momentary eBAC but these responses did not vary by sensitivity level.
Craving is an important feature of problematic drinking, and has recently been incorporated into the diagnostic criteria for AUD in DSM-5 (American Psychiatric Association, 2013; Agrawal et al., 2011). Contrary to our hypotheses, less-sensitive drinkers did not report higher levels of alcohol craving across drinking sessions. We anticipated that lower sensitivity drinkers might show elevated alcohol craving on the basis of electrophysiological, cognitive, and behavioral evidence (e.g., approach motivation) from prior laboratory-based studies of alcohol cue exposure (Bartholow et al., 2007; Bartholow et al., 2010; Fleming and Bartholow, 2014; Shin et al., 2010). Notably, cue reactivity was measured in the absence of alcohol in all of these prior studies. It is possible that sensitivity-craving associations may be more evident in the sober state between drinking episodes; investigating this was beyond the scope of the current study.
There were numerous limitations to this study that bear mentioning. Estimated BAC calculations involve several assumptions and idealizations, and thus are not as precise as objective breath alcohol concentration measurements (Hustad and Carey, 2005). Potential sources of error include variations in drink volumes and ethanol concentrations, topping off, fluctuations in participants’ body weight throughout the study period and individual differences in the rate of ethanol absorption and elimination. Though these caveats are important, the current eBAC estimates are likely to be improvements over cruder alternative, such a count of drinks consumed, that do not take into account participant sex, body weight, or the time over which the drinks were consumed. Future work might take advantage of recent advancements in ambulatory assessment of drinking, such as using smartphone cameras to photograph beverages and transdermal ethanol sensors (Luczak and Rosen, 2014; Luczak et al., 2015). The sample used in this study was recruited to include social drinkers, with oversampling for current cigarette smoking. The final sample consisted of heavy drinkers, with a large proportion achieving AUDIT scores indicative of hazardous drinking. It is unclear exactly how these results would generalize to samples with fewer smokers or less risky drinking patterns, and additional studies are needed to explore the consistency of the results.
In order to reduce response burden, the diary incorporated brief assessments and tapped only a handful of phenomenological domains. It would clearly be valuable to extend this research using alternate measures to more fully catalogue the natural correlates of self-reported low alcohol sensitivity (e.g., Morean et al., 2013; Rueger and King, 2013). We set out to characterize the real-world expression of a low sensitivity to alcohol as indexed by responses on the SRE. It is important to acknowledge that the low level of response construct has complex roots in alcohol research. The LLR and DM were initially formulated to describe mechanisms that might explain genetic risk for AUD, not self-reported sensitivity differences per se. Identification of the importance of low sensitivity to alcohol emerged from this line of work on familial risk, and increasingly has been established as an independent risk factor (Trim et al., 2009). We found partial support for the LLR and no clear support for the DM, though divergent results may have emerged if other risk factors were considered (e.g., King et al., 2011; Newlin and Renton, 2010; Quinn and Fromme, 2010; Ray et al., 2010) or other metrics for alcohol sensitivity were used. The SRE focuses on predominantly negative alcohol effects (e.g., passing out); our findings might differ if alcohol sensitivity had been measured with an instrument tapping both positive and negative effects of alcohol (e.g., Fleming et al., 2016). Additionally, it is important to bear in mind that our study utilized data exclusively from the ascending limb of drinking episodes. This was done out of necessity given the smaller number of data points on the descending limb (relative to the ascending limb). A more comprehensive comparison of the two models would require data from both ascending and descending limbs.
Although we were able to detect associations between SRE scores and a variety of theoretically relevant alcohol responses, these effects were sometimes modest in magnitude and often occurred against a backdrop of substantial inter- and intra-individual variability in subjectively reported states. Contextual factors may influence the profile of cravings and other experienced alcohol effects through mechanisms such as expectancy activation, alcohol myopia, or situational tolerance (e.g., Corbin et al., 2015; Siegel 2008; Steele and Josephs, 1990). We assessed contextual factors at the outset of each episode, but did not repeatedly assess them during the episode (out of concern for participant burden). We also did not assess some potentially critical contextual features (e.g., distractions, ambient stimulation, density of alcohol cues, alcohol availability, and peer drinking). It is possible that self-selection of different drinking environments is a cause or consequence of drinkers’ recent subjective experiences. Unfortunately, the current data do not allow an investigation of how frequently intra-episode shifts occurred, or whether they explain significant variations in subjective states.
In sum, the current findings suggest that low sensitivity drinkers and their higher sensitivity peers have relatively similar experiences of drinking episodes. However, low sensitivity drinkers differ in their experience of certain subjective signals of intoxication and punishment - sensations that might serve as negative feedback or satiety signals for typical drinkers. Additional research is necessary to further probe the potential relationship between low sensitivity to alcohol and craving, and to better understand the causes, correlates, and consequences of low sensitivity. The current study indicates that ecological momentary assessment represents a promising and viable tool for advancing this important research agenda.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health grants P50AA011998 (Heath), K05AA017688 (Heath), and K05AA017242 (Sher) and T32AA01352 (Sher).
Footnotes
There were 369 secondary episodes containing 829 moments. The likelihood of reporting a secondary episode did not relate to alcohol sensitivity (OR = 1.01; 95% CI: 0.85 – 1.20). Further, the pattern of results did not differ substantially whether these episodes were included or not.
The likelihood of this occurring was not significantly related to alcohol sensitivity, though there was a trend level association (OR = 1.09, 95% CI: 0.99 – 1.21, p = 0.08).
The fact that a drinking episode assessment was triggered by this “precautionary” question concerning alcohol use since last report does not necessarily indicate non-compliance with the diary recording instructions. This could occur, for example, in instances of alcohol-tobacco couse if a participant logged a smoking event prior to finishing the first drink.
The pattern of results presented did not differ when un-winsorized eBAC was used.
In order to explore raw (vs. dose-adjusted) experience during self-paced drinking sessions, we estimated a series of models in which subjective states and drink appraisals were examined as a function of time since completion of the first drink rather than momentary eBAC. The findings (Supplemental Tables S16 – S29) did not reveal any Time x zSRE interactions that would survive correction for multiple testing.
References
- Agrawal A, Heath AC, Lynskey MT. DSM-IV to DSM-5: the impact of proposed revisions on diagnosis of alcohol use disorders. Addiction. 2011;106(11):1935–1943. doi: 10.1111/j.1360-0443.2011.03517.x. http://dx.doi.org/10.1111/j.1360-0443.2011.03517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. American Psychiatric Association; Washington, DC: 2013. [Google Scholar]
- Babor TF, Biddle-Higgins JC, Saunders JB, Monteiro MG. AUDIT: The alcohol use disorders identification test: guidelines for use in primary health care. World Health Organization; Geneva, Switzerland: 2001. [Google Scholar]
- Baker TB, Morse E, Sherman JE. The motivation to use drugs: a psychobiological analysis of urges. Nebr Symp Motiv. 1986;34:257–323. [PubMed] [Google Scholar]
- Bartholow BD, Henry EA, Lust SA. Effects of alcohol sensitivity on P3 event-related potential reactivity to alcohol cues. Psychol Addict Behav. 2007;21(4):555–563. doi: 10.1037/0893-164X.21.4.555. http://dx.doi.org/10.1037/0893-164X.21.4.555. [DOI] [PubMed] [Google Scholar]
- Bartholow BD, Lust SA, Tragesser SL. Specificity of P3 event-related potential reactivity to alcohol cues in individuals low in alcohol sensitivity. Psychol Addict Behav. 2010;24(2):220–228. doi: 10.1037/a0017705. http://dx.doi.org/10.1037/a0017705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar D, Berg CJ, Rapoport JL, et al. Behavioral and physiological effects of ethanol in high-risk and control children: A pilot study. Alcohol Clin Exp Res. 1983;7:404–410. doi: 10.1111/j.1530-0277.1983.tb05495.x. http://dx.doi.org/10.1111/j.1530-0277.1983.tb05495.x. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57(1):289–300. [Google Scholar]
- Corbin WR, Scott C, Boyd SJ, Menary KR, Enders CK. Contextual influences on subjective and behavioral responses to alcohol. Exp Clin Psychopharm. 2015;23(1):59–70. doi: 10.1037/a0038760. http://dx.doi.org/10.1037/a0038760. [DOI] [PubMed] [Google Scholar]
- Corbin WR, Scott C, Leeman RF, Fucito LM, Toll BA, O’Malley S. Early subjective response and acquired tolerance as predictors of alcohol use and related problems in a clinical sample. Alcohol Clin Exp Res. 2013;37(3):490–497. doi: 10.1111/j.1530-0277.2012.01956.x. http://dx.doi.org/10.1111/j.1530-0277.2012.01956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epler AJ, Tomko RL, Piasecki TM, Wood PK, Sher KJ, Shiffman S, Heath AC. Does hangover influence the time to next drink? An investigation using ecological momentary assessment. Alcohol Clin Exp Res. 2014;38(5):1461–1469. doi: 10.1111/acer.12386. http://dx.doi.org/10.1111/acer.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming KA, Bartholow BD. Alcohol cues, approach bias, and inhibitory control: applying a dual process model of addiction to alcohol sensitivity. Psychol Addict Behav. 2014;28(1):85–96. doi: 10.1037/a0031565. http://dx.doi.org/10.1037/a0031565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming KA, Bartholow BD, Hilgard JB, McCarthy DM, O’Neill SE, Steinley D, Sher KJ. The Alcohol Sensitivity Questionnaire: Evidence for construct validity. Alcohol Clin Exp Res. 2016;40(4):880–888. doi: 10.1111/acer.13015. http://dx.doi.org/10.1111/acer.13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Madden PAF, Bucholz KK, et al. Genetic differences in alcohol sensitivity and the inheritance of alcoholism risk. Psychol Medicine. 1999;29:1069–1081. doi: 10.1017/s0033291799008909. http://dx.doi.org/10.1017/S0033291799008909. [DOI] [PubMed] [Google Scholar]
- Hustad JT, Carey KB. Using calculations to estimate blood alcohol concentrations for naturally occurring drinking episodes: a validity study. J Stud Alcohol. 2005;66(1):130–138. doi: 10.15288/jsa.2005.66.130. http://dx.doi.org/10.15288/jsa.2005.66.130. [DOI] [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiat. 2011;68(4):389–399. doi: 10.1001/archgenpsychiatry.2011.26. http://dx.doi.org/10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Bartholow BD, McCarthy DM, Pedersen SL, Sher KJ. Two alternative approaches to conventional person-mean imputation scoring of the Self-Rating of the Effects of Alcohol Scale (SRE) Psychol Addict Behav. 2015;29:231–236. doi: 10.1037/adb0000015. http://dx.doi.org/10.1037/adb0000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Heilig M, Cunningham CL, Stephens DN, Duka T, O’Malley SS. Ethanol consumption: how should we measure it? Achieving consilience between human and animal phenotypes. Addict Biol. 2010;15:109–124. doi: 10.1111/j.1369-1600.2009.00192.x. http://dx.doi.org/10.1111/j.1369-1600.2009.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TK, Hewitt BG, Grant BF. Is there a future for quantifying drinking in the diagnosis, treatment, and prevention of alcohol use disorders? Alcohol Alcoholism. 2007;42(2):57–63. doi: 10.1093/alcalc/agl125. http://dx.doi.org/10.1093/alcalc/agl125. [DOI] [PubMed] [Google Scholar]
- Luczak SE, Rosen IG. Estimating BrAC from transdermal alcohol consentration data using the BrAC estimator software program. Alcohol Clin Exp Res. 2014;38(9):2243–2252. doi: 10.1111/acer.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczak SE, Rosen IG, Wall TL. Development of a real-time repeated-measures assessment protocol to capture change over the course of a drinking episode. Alcohol Alcoholism. 2015;50(2):180–187. doi: 10.1093/alcalc/agu100. http://dx.doi.org/10.1093/alcalc/agu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DB, Miller WR. Estimating blood alcohol concentration: two computer programs nd their applications in therapy and research. Addict Behav. 1979;4(1):55–60. doi: 10.1016/0306-4603(79)90021-2. http://dx.doi.org/10.1016/0306-4603(79)90021-2. [DOI] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, O’Malley SS, et al. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiat. 2009;66:185–190. doi: 10.1016/j.biopsych.2009.01.029. http://dx.doi.org/10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, Corbin WR. Subjective alcohol effects and drinking behavior: the relative influence of early response and acquired tolerance. Addict Behav. 2008;33:1306–1313. doi: 10.1016/j.addbeh.2008.06.007. http://dx.doi.org/10.1016/j.addbeh.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Morean ME, Corbin WR, Treat TA. The Subjective Effects of Alcohol Scale: Development and psychometric evaluation of a novel assessment tool for measuring subjective response to alcohol. Psychol Assessment. 2013;25:780–795. doi: 10.1037/a0032542. http://dx.doi.org/10.1037/a0032542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlin DB, Renton RM. High risk groups often have higher levels of alcohol response than low risk: the other side of the coin. Alcohol Clin Exp Res. 2010;34(2):199–205. doi: 10.1111/j.1530-0277.2009.01081.x. http://dx.doi.org/10.1111/j.1530-0277.2009.01081.x. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Strubler KA. The habitual brain: an “adapted habit” theory of substance use disorder. Subst Use and Misuse. 2007;42(2–3):503–526. doi: 10.1080/10826080601144606. http://dx.doi.org/10.1080/10826080601144606. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychol Bull. 1990;108(3):383–402. doi: 10.1037/0033-2909.108.3.383. http://dx.doi.org/10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Alley KJ, Slutske WS, et al. Low sensitivity to alcohol: Relations with hangover occurrence and susceptibility in an Ecological Momentary Assessment investigation. J Stud Alcohol Drugs. 2012a;73:925–932. doi: 10.15288/jsad.2012.73.925. http://dx.doi.org/10.15288/jsad.2012.73.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Cooper ML, Wood PK, Sher KJ, Shiffman S, Heath AC. Dispositional drinking motives: associations with appraised alcohol effects and alcohol consumption in an ecological momentary assessment investigation. Psychol Assessment. 2014a;26(2):363–369. doi: 10.1037/a0035153. http://dx.doi.org/10.1037/a0035153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Jahng S, Wood PK, et al. The subjective effects of alcohol-tobacco co-use: An ecological momentary assessment investigation. J Abnorm Psychol. 2011;120:557–571. doi: 10.1037/a0023033. http://dx.doi.org/10.1037/a0023033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Wood PK, Shiffman S, Sher KJ, Heath AC. Responses to alcohol and cigarette use during ecologically assessed drinking episodes. Psychopharmacology. 2012b;223:331–344. doi: 10.1007/s00213-012-2721-1. http://dx.doi.org/10.1007/s00213-012-2721-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihl RO, Peterson J, Finn P. Inherited predisposition to alcoholism: characteristics of sons of male alcoholics. J Abnorm Psychol. 1990;99(3):291–301. doi: 10.1037//0021-843x.99.3.291. http://dx.doi.org/10.1037//0021-843x.99.3.291. [DOI] [PubMed] [Google Scholar]
- Quinn PD, Fromme K. Self-regulation as a protective factor against risky drinking and sexual behavior. Psychol Addict Behav. 2010;24(3):376–385. doi: 10.1037/a0018547. http://dx.doi.org/10.1037/a0018547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn PD, Fromme K. Subjective response to alcohol challenge: A quantitative review. Alcohol Clin Exp Res. 2011;35(10):1–12. doi: 10.1111/j.1530-0277.2011.01521.x. http://dx.doi.org/10.1111/j.1530-0277.2011.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Miranda R, Jr, Tidey JW, et al. Polymorphisms of the μ-opioid receptor and dopamine D receptor genes and subjective responses to alcohol in the natural environment. J Abnorm Psychol. 2010;119(1):115–125. doi: 10.1037/a0017550. http://dx.doi.org/10.1037/a0017550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson BM, Piasecki TM, Slutske WS, Wood PK, Sher KJ, Shiffman S, Heath AC. Validity of the Hangover Symptoms Scale: Evidence from an electronic diary study. Alcohol Clin Exp Res. 2012;36(1):171–177. doi: 10.1111/j.1530-0277.2011.01592.x. http://dx.doi.org/10.1111/j.1530-0277.2011.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. http://dx.doi.org/10.1016/0165-0173(93)90013-P. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. http://dx.doi.org/10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Roche DJO, Palmeri MD, King AC. Acute alcohol response phenotype in heavy social drinkers is robust and reproducible. Alcohol Clin Exp Res. 2014;38(3):844–852. doi: 10.1111/acer.12280. http://dx.doi.org/10.1111/acer.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueger SY, King AC. Validation of the Brief Biphasic Alcohol Effects Scale (B-BAES) Alcohol Clin Exp Res. 2013;37(3):470–476. doi: 10.1111/j.1530-0277.2012.01941.x. http://dx.doi.org/10.1111/j.1530-0277.2012.01941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT). WHO collaborative project on early detection of persons with harmful alcohol consumption. II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. http://dx.doi.org/10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Self-rating of alcohol intoxication by young men with and without family histories of alcoholism. J Stud Alcohol. 1980;41(3):242–249. doi: 10.15288/jsa.1980.41.242. http://dx.doi.org/10.15288/jsa.1980.41.242. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. The relationship of a family history of alcohol dependence, a low level of response to alcohol and six domains of life functioning to the development of alcohol use disorders. J Stud Alcohol. 2000;61:827–835. doi: 10.15288/jsa.2000.61.827. http://dx.doi.org/10.15288/jsa.2000.61.827. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. The clinical course of alcohol dependence associated with a low level of response to alcohol. Addiction. 2001;96:903–910. doi: 10.1046/j.1360-0443.2001.96690311.x. http://dx.doi.org/10.1046/j.1360-0443.2001.96690311.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Danko GP, et al. Evaluation of a level of response to alcohol-based structural equation model in adolescents. J Stud Alcohol. 2005a;66(2):174–184. doi: 10.15288/jsa.2005.66.174. http://dx.doi.org/10.15288/jsa.2005.66.174. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Danko GP, Kuperman S, Bierut LJ, Hesselbrock V. Correlations among first-degree relatives for responses on the self-rating of the effects of alcohol questionnaire in teenagers. J Stud Alcohol. 2005b;66(1):62–65. doi: 10.15288/jsa.2005.66.62. http://dx.doi.org/10.15288/jsa.2005.66.62. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Danko GP, et al. An evaluation of the full level of response to alcohol model of heavy drinking and problems in COGA offspring. J Stud Alcohol Drugs. 2009;70(3):436–445. doi: 10.15288/jsad.2009.70.436. http://dx.doi.org/10.15288/jsad.2009.70.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Tipp JE. The self-rating of the effects of alcohol (SRE) form as a retrospective measure of the risk for alcoholism. Addiction. 1997a;92(8):979–988. http://dx.doi.org/10.1111/j.1360-0443.1997.tb02977.x. [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Trim R, Heron J, Horwood J, Davis JM, Hibbein JR. The performance of elements of a “level of response to alcohol”-based model of drinking behaviors in 13-year-olds in Bristol, England. Addiction. 2008a;103(11):1786–1792. doi: 10.1111/j.1360-0443.2008.02325.x. http://dx.doi.org/10.1111/j.1360-0443.2008.02325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Trim R, Kreikebaum S, Hinga B, Allen R. Testing the level of response to alcohol-based model of heavy drinking and alcohol problems in offspring from the San Diego Prospective Study. J Stud Alcohol Drugs. 2008b;69(4):571–579. doi: 10.15288/jsad.2008.69.571. http://dx.doi.org/10.15288/jsad.2008.69.571. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Tipp JE, Smith TL, Wiesbeck GA, Kalmijn J. The relationship between self-rating of the effects of alcohol and alcohol challenge results in ninety-eight young men. J Stud Alcohol. 1997b;58(4):397–404. doi: 10.15288/jsa.1997.58.397. http://dx.doi.org/10.15288/jsa.1997.58.397. [DOI] [PubMed] [Google Scholar]
- Shin E, Hopfinger JB, Lust SA, Henry EA, Bartholow BD. Electrophysiological evidence of alcohol-related attentional bias in social drinkers low in alcohol sensitivity. Psychol Addict Behav. 2010;24(3):508–515. doi: 10.1037/a0019663. http://dx.doi.org/10.1037/a0019663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Lane SP. Psychometrics. In: Mehl MR, Conner TS, editors. Handbook of research methods for studying daily life. Guilford; New York: 2012. pp. 302–320. [Google Scholar]
- Siegel S. Learning and the wisdom of the body. Learn Behav. 2008;36(3):242–252. doi: 10.3758/lb.36.3.242. http://dx.doi.org/10.3758/LB36.3.242. [DOI] [PubMed] [Google Scholar]
- Steele CM, Josephs RA. Alcohol myopia: Its prized and dangerous effects. Am Psychol. 1990;45(8):921. doi: 10.1037//0003-066x.45.8.921. http://dx.doi.org/10.1037/0003-066X.45.8.921. [DOI] [PubMed] [Google Scholar]
- Treloar HR, Piasecki TM, McCarthy DM, Sher KJ, Heath AC. Ecological evidence that affect and perceptions of drink effects depend on alcohol expectancies. Addiction. 2015;110:1432–1442. doi: 10.1111/add.12982. http://dx.doi.org/10.1111/add.12982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trim RS, Schuckit MA, Smith TL. The relationship of the level of response to alcohol and additional characteristics to alcohol use disorders across adulthood: A discrete-time survival analysis. Alcohol Clin Exp Res. 2009;33(9):1562–1570. doi: 10.1111/j.1530-0277.2009.00984.x. http://dx.doi.org/10.1111/j.1530-0277.2009.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viken RJ, Rose RJ, Morzorati SL, Christian JC, Li TK. Subjective intoxication in response to alcohol challenge: heritability and covariation with personality, breath alcohol level, and drinking history. Alcohol Clin Exp Res. 2003;27(5):795–803. doi: 10.1097/01.ALC.0000067974.41160.95. http://dx.doi.org/10.1097/01.alc.0000067974.41160.95. [DOI] [PubMed] [Google Scholar]
- Volavka J, Czobor P, Goodwin DW, et al. The electroencephalogram after alcohol administration in high-risk mean and the development of alcohol use disorders 10 years later. Arch Gen Psychiat. 1996;53:258–263. doi: 10.1001/archpsyc.1996.01830030080012. http://dx.doi.org/10.1001/archpsyc.1996.01830030080012. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Bartholow BD, Wildenberg EVD, et al. Automatic and controlled processes and the development of addictive behaviors in adolescents: a review and model. Pharmacol Biochem Be. 2007;86:263–283. doi: 10.1016/j.pbb.2006.09.021. http://dx.doi.org/10.1016/j.pbb.2006.09.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.