Abstract
Background
Despite the well‐known health benefits of physical activity, it is a great challenge to stay physically active for frail–older adults with mobility limitations. The aim of this study was to test the (cost‐) effectiveness of a patient‐centred physical therapy strategy (Coach2Move) in which individualized treatment (motivational interviewing, physical examination, individualized goal setting, coaching and advice on self management, and physical training) is combined to increase physical activity level and physical fitness and, thereby, to decrease the level of frailty.
Methods
A randomized controlled trial was performed in 13 physical therapy practices with measurements at 3 and 6 months. Eligible patients were aged 70 years or over and had mobility problems (i.e. difficulties with walking, moving, getting up and changing position from bed or chair to standing, or stair climbing). The primary outcome was physical activity (total and moderate intensity) in minutes per day. Secondary outcomes were as follows: frailty, walking speed and distance, mobility, and quality of life. Data were analysed using linear mixed models for repeated measurements. Healthcare costs and quality‐adjusted life years (QALYs) were computed and combined using net monetary benefit (NMB) for different willingness to pay thresholds. Data on costs, QALYs, and NMBs were analysed using linear mixed models.
Results
One hundred and thirty patients participated in this study. At 6 months, the between‐group difference was significant for moderate‐intensity physical activity in favour of the Coach2Move group [mean difference: 17.9 min per day; 95% confidence interval (CI) 4.0 to 34.9; P = 0.012]. The between‐group difference for total physical activity was 14.1 min per day (95% CI −6.6 to 34.9; P = 0.182). Frailty decreased more in the Coach2Move group compared with usual care [mean difference: −0.03 (95% CI: −0.06 to −0.00; P = 0.027)]. Compared with usual treatment, the Coach2Move strategy resulted in cost savings (€849.8; 95% CI: 1607 to 90; P = 0.028), an improvement in QALYs, (0.02; 95% CI: 0.00 to 0.03; P = 0.03), and a higher NMB at every willingness to pay threshold.
Conclusions
Older adults with mobility problems are able to safely increase physical activity in their own environment and reduce frailty. This study emphasizes both the potential cost‐effectiveness of a patient‐centred approach in the frail elderly and the importance of physical activity promotion in older adults with mobility limitations.
Keywords: Physical activity, Frailty, Mobility limitations, Physical therapy
Introduction
Physical activity (PA) is known to be of substantial value in the prevention and treatment of many chronic diseases1 and disease‐related status such as decreased physical functioning,2 mobility limitations,3 sarcopenia,4, 5 anxiety, and depression.6 In addition, PA has a positive influence on quality of life7 and is thought to be the most promising intervention to prevent and reduce frailty status in older adults.8, 9 Frailty refers to a reduced reserve capacity leading to an increased risk of adverse health outcomes such as disability, falls, hospital admission, and death.10 In fact, reduced PA is often the result of sarcopenia, fatigue, a period of bed rest, fear of falling, and so on. However, at the same time, reduced PA causes reduced walking speed, muscle strength, endurance, and loss of co‐ordination, which in turn increases the risk of negative health outcomes.11 Moreover, psychological and social factors are also involved in this vicious cycle: mobility limitations are associated with, for example, depression and increasing loneliness.12, 13
Although the health benefits of PA are widely known and recognized, it is a great challenge to increase PA in older adults with already existing limitations in physical functioning.2 Many older adults remain or become sedentary after acute illnesses (e.g. stroke, fractures, and infections with a long period of bed rest) or life events (social isolation), despite the advice to stay physically active by, for instance, participating in exercise programmes.14 Globally, 31% of the population does not meet the PA standards of 30 min of moderate intensity PA on 5 to 7 days per week.1 In the UK, only 9% of older adults of 75 years and over adhered to the PA standard in 2008,13 whilst in the Netherlands, this percentage was 49% (in older adults aged 65 years or over) in 2011.15 Even though the adherence rates vary in different countries, it is clear that globally, a substantial proportion of older adults is sedentary and that inactivity increases with increasing age and mobility difficulties.14 A more patient‐centred intervention seems to be necessary, especially if mobility is threatened by sarcopenia, chronic conditions, or acute physical or social events.16, 17, 18
Patient centredness is considered to be one of the means to improve healthcare systems by achieving better health outcomes, greater patient satisfaction, and reduced health costs.19 Activities identified as being relevant for patient‐centred care are as follows: patient information, patient involvement (shared decision‐making), involvement of family and friends, patient empowerment (self‐management), physical support, and emotional support. We developed a physical therapy strategy to improve PA in older adults with mobility problems that includes these patient‐centred activities. The patient‐centred strategy under study was designed using the Medical Research Council20, 21 recommendations for designing complex interventions and was based on both extensive literature studies and expert consultation including consultation with older adults with mobility problems. Our hypothesis underlying the intervention was to focus on training of physical disabilities as detected in the physical examination and to increase the PA level leading to a decreased level of frailty. At the same time, we tried to increase adherence to PA and training and self‐efficacy by detecting the individual social needs, by taking away contextual barriers and, by giving concrete feedback during the training on goal attainment (e.g. gait velocity). The potential effectiveness of the Coach2Move strategy on PA, physical fitness and mobility, and thereby frailty and quality of life was shown in a pilot study, which justified a larger randomized controlled trial (RCT).22
The aim of the present RCT was to test the Coach2Move strategy on (cost‐) effectiveness. We expected the Coach2Move strategy to be more effective than usual care physical therapy in improving PA and, as a consequence, frailty and quality of life. The Coach2Move strategy and usual care physical therapy were both expected to improve mobility. In addition, we expected the Coach2move strategy to be more cost effective than usual care physical therapy because of the more patient‐centred focus, increased adherence, self‐efficacy, and expected reduction in frailty.
Methods
Design
The design of this study was an RCT in 13 physical therapy practices. All older adults (≥70 years old) who, because of mobility problems, had signed up for physical therapy in a participating practice were invited to participate before they were seen by a physical therapist. When willing to participate, potential eligibility was determined by the research team via a telephone call, and, in cases of eligibility, an appointment was made for the baseline measurement. All measurements took place either in the patient's home or at the physical therapy practice where the patient would receive treatment. Written informed consent was signed at the start of the baseline measurement. A detailed description of the trial protocol has been outlined elsewhere.23
Setting and participants
Participants were older adults aged 70 years or over who had signed up for physical therapy because of mobility problems. Mobility problems in our subjects were problems related to walking either inside or outside of the house, stair climbing, getting into or out of a bed or a chair, standing up from the floor, and so on.24, 25 In addition, potential participants had a sedentary lifestyle or were at risk of losing an active lifestyle in the near future (as rated by the participant, relatives, or a referring physician). At‐risk patients were patients who had recently fallen and showed fear avoidance behaviour, or patients who showed reduced co‐ordination and unstable gait patterns or decreased gait velocity. These problems can lead to a downward vicious cycle of inactivity and related physical problems such as sarcopenia, mobility limitations, and disability leading to social isolation. Exclusion criteria were as follows: (i) unable to walk 5 m (walking aid allowed); (ii) unable to follow verbal or written instructions because of cognitive problems (minimal mental state examination score < 21) or unable to understand the Dutch language; (iii) palliative phase of illness; (iv) acute illness with hospital indication; (v) living in a nursing home; (vi) severe degenerative neurological disease; (vii) having a contraindication to be physically active; and (viii) had physical therapy for a period longer than 4 weeks during the last 6 months.
Randomization
Randomization was prepared at patient level for each physical therapy practice using a computer‐generated random‐sequence table by a researcher not involved in patient inclusion. After the baseline measurement, a sealed envelope securing randomization concealment, containing a sheet of paper indicating one of the two interventions, was delivered to the practice secretary or physical therapist. Patients were informed about their treating therapist after the research assistant involved in the baseline measurement had left. Physical therapy was given according to the Coach2Move strategy by an educated geriatric physical therapist and usual care physical therapy by a physical therapist without this additional education in geriatrics. All participating physical therapists had a comparable amount of clinical experience. Patients were not informed of whether they were in the ‘experimental’ or ‘control’ group. Follow‐up measurement at 3 and 6 months was performed by research assistants who were not aware of group allocation.
Intervention
The Coach2Move strategy is developed in several phases conforming to the phases of the Medical Research Council framework.20, 21 Using the theoretical construct of Fried and Rockwood,11, 26 the hypothesis‐oriented algorithm for clinicians27, 28 and the results of two systematic reviews2, 29 are combined with an analysis of daily practice and the perceptions of patients and healthcare providers. Coach2Move helps geriatric physical therapists by providing a patient‐centred approach of supporting clinical reasoning to detect needs, limitations, and strength in both the patient and the physical and social environment and choosing evidence‐based interventions taking into account co‐morbidity. Clinical reasoning is stimulated by using an extensive, pre‐structured, systematically organized, hypothesis‐oriented diagnostic protocol supported by an electronic health record. Based on this comprehensive intake, a stratified, goal‐oriented treatment plan that fits the preferences, needs, and barriers of the patient and his or her environment is designed. The patient is categorized according to one of three intervention profiles with a pre‐defined number of consults based on expected recovery. Switching between profiles was allowed and, despite the advice given on a maximum number of consults, the geriatric physical therapist could deviate when appropriate to reach the determined goals. The geriatric physical therapist coaches and motivates the patient to increase adherence to PA using feedback on goal attainment in all intervention profiles. Participating geriatric physical therapists were educated in the Coach2Move strategy during a 2 days training (Table 1). From the patient perspective, treatment according to the Coach2Move strategy means a more detailed examination, active involvement in decisions on (meaningful) treatment goals, appropriate evidence‐based intervention (shared decision‐making), and active involvement in and feedback on reaching these goals in which family, friends, or (informal) caregivers are involved.
Table 1.
Coach2Move strategy, implementation strategy, and similarities and contrasts between studied interventions
| Intervention profile | Population | Intervention |
|---|---|---|
| Profile 1 (maximum of four sessions) | Physically inactive older adults without physical constraints to become (more) physically active. | Coaching on self‐management to become more physically active. |
| Profile 2 (maximum of nine sessions) | Older adults with minor or acute mobility problems. | (1) Temporary physical therapy intervention to overcome barriers to become (more) physically active (e.g. training of strength, endurance, flexibility or balance, fear reduction, involving social environment, adaptation to personal factors, advising walking aids, etc.). (2) Coaching on self‐management to become more physically active. |
| Profile 3 (maximum of 18 sessions) | Older adults with moderate‐to‐severe mobility problems and specific problems in activities and participation. | (1) Physical therapy intervention aimed at decreasing mobility problems and problems in activity and participation. (2) Temporary physical therapy intervention to overcome barriers to become (more) physically active (e.g. training of strength, endurance or balance, fear reduction, involving social environment, advising walking aids, etc.). (3) Coaching on self‐management to become more physically active. |
| General | Older adults (≥70 years) with mobility problems with or without a physically inactive lifestyle who are at risk to lose mobility or an active lifestyle in the near future. | Key elements of this six‐step Coach2Move strategy: (1) exploring the question for help and the barriers and facilitators (physical, social, and environmental) in relation to physical activity by using motivational interviewing techniques in an extensive intake; (2) setting priorities in physiotherapy diagnosis and treatment by using an algorithm that emphasizes clinical reasoning; (3) shared decision‐making on meaningful treatment goals focused on abrogating barriers and increasing physical activity; (4) coaching on self‐management and self‐efficacy to increase long‐term results; (5) focus on meaningful activities at home with help from family, friends, and/or professionals; and (6) stratified intervention by using three patient‐tailored intervention profiles with a pre‐defined number of intervention sessions. |
| Implementation strategy | (1) Two‐day training in Coach2Move strategy; (2) three follow‐up meetings in which problems encountered were discussed; (3) use of Coach2Move supportive electronic patient file; (4) coaching in the execution of the Coach2Move strategy by researcher (N. d. V.) during the RCT: N. d. V. checked all health records and contacted GPTs to give instructions and advice, when necessary; and (5) possibility to consult researcher (N. d. V.) with questions considering the execution of Coach2Move. | |
| Similarities and contrasts with usual care physiotherapy | Similarities: individual intervention and use of physical therapy modalities (such as training of strength, endurance, balance, flexibility, functional training, etc.). | |
| Contrast: using an extensive intake based on a decision algorithm (clinical reasoning), using motivational interviewing, setting meaningful goals on increasing (adherence for) PA, enhancing self‐efficacy and self‐management, giving feedback on progress, using personal and environmental factors, using intervention profiles with a pre‐defined number of consults (based on expected recovery), and intervention given by a PT with additional education in geriatrics and Coach2Move. | ||
GPT, geriatric physical therapist; PA, physical activity; PT, physical therapist; RCT, randomized controlled trial.
To facilitate the intake and achieve a detailed examination, finances were given to the participants to prolong the intake session from 30 to 90 min. The duration of intervention sessions was 30 min, as is usual for a physical therapy session in the Netherlands. A detailed description of (the development of) the Coach2Move strategy has been provided in an earlier publication.22
The control intervention consisted of usual care physical therapy.30 No instructions were given on treatment content, frequency, and/or duration of the treatment episode. The duration of both the intake session and each intervention session of physical therapy was 30 min. The duration of the treatment episode was determined by the physical therapist and varied according to the individual needs and recovery of the patients. The contrast between the Coach2Move strategy and usual care physical therapy is shown in Table 1.
Study parameters
Measurements were performed at baseline (t0), and 3 (t1) and 6 months after baseline (t2). The main study outcome is PA at 6 months as measured using the LASA Physical Activity Questionnaire (LAPAQ).31 Two primary outcomes were computed from the LAPAQ data: total PA and moderate intensity PA in minutes per day. The latter outcome includes all domains of the LAPAQ except light household chores (i.e. cooking and washing dishes). This outcome was used because most PA standards prescribe moderate intensity PA that does not include light household work.1, 15 Secondary outcomes included mobility (Get Up & Go Test in seconds, 6 min walking test on a 10 m circuit, the 10 m walking test—walking speed in metres per second)32, 33, 34), quality of life (Short Form‐36, score 0–100),25 frailty (Evaluative Frailty Index for PA, score 0.00–1.00),35 fatigue (Numeric Rating Scale—fatigue, score 0–10),36 and patient‐specific complaints (score 0–10).37 The patient‐specific complaints questionnaire asks the patient to select the three most debilitating activity problems (e.g. stair climbing, walking outside the house, standing up from a chair, etc.) and to rate their experienced disability on a scale of 0 (no disability) to 10 (not possible to perform the activity mentioned). Multi‐morbidity was registered using the Cumulative Illness Rating Scale‐Geriatrics.38 To monitor falls, a question focusing on the number of falls in the preceding 3 months was asked.
For the economic evaluation, healthcare utilization was registered by means of a questionnaire. The questionnaire considered the type and number of health care used including primary care, analgesics, hospital stay, nursing home stay, use of residential facilities, home care (household and nursing), and the purchase of assistive devices in the 3 months prior to administering the questionnaire (details: Appendices A and B). The number of physical therapy sessions was registered by the physical therapy practices, including the prolonged intake in the Coach2Move group. Besides analgesics, other medications were not taken into account because we did not expect there to be any benefit for medication consumption as a consequence of physical therapy over a 6 months period.
Statistical analysis
The study was designed to have 80% power at a significance level of 0.05 and was based on data from our pilot study and literature. The necessary sample size was 130 patients taking into account a dropout rate of 20%. The sample size calculation has been described in detail elsewhere.23 We expected the Coach2Move strategy to increase PA 1.68 times more than usual care physical therapy. This expectation was based on the findings of our pilot study (3.35‐fold increase in PA),22 and improvements in PA noted in the literature based on a standard exercise intervention (1.2‐fold increase).39 Because we hypothesized a possible overestimation of the effects found in our pilot study as compared with other studies, and taking into account possible contamination, the expected difference in effectiveness was determined.23
Statistical analyses were performed based on the intention to treat principle. Descriptive statistics were used to describe the study groups. Linear mixed models with therapy practice as a random factor and repeated measurements structure at subject level (baseline, 3 and 6 months) were used to take into account correlation of measurements over time. Each of the outcomes was added as a dependent variable, and group was taken as a fixed variable. Physical therapy practice was taken as random effect in this model to account for clustering of patients. Mean differences between groups at each measurement point are presented with 95% confidence intervals. Effect sizes (Cohen's d) were calculated by dividing the mean difference in effect between the Coach2Move and the usual care physical therapy group by the pooled standard deviation. In a post hoc analysis, we compared the proportion of patients who did not fulfil the PA standard at baseline but who did so at 6 months (responders), and we determined the proportion of participants that reported one or more falls. The number of responders and fallers was compared between groups using a χ2 test.
Cost‐utility analysis was performed from a societal perspective. Cost prices were determined using standard unit cost prices according to the Dutch guidelines for costing research,40 or real cost prices were determined using activity‐based costing (Appendix A). Costs were computed by multiplying the cost prices with the usage frequency as reported by the patients in the healthcare utilization questionnaire with the exception of the frequency of physical therapy treatment that was based on registration by the involved physical therapy practices. The cost price for physical therapy in the Coach2Move group was higher than the cost price for usual care physical therapy conforming to the higher rates for specialized physical therapists as advised by the Dutch Society for Physical Therapy (Appendix A). The extra time taken for the prolonged intake in the Coach2Move group was included in the analyses. Only costs that could be related to physical therapy were taken into account. For example, orthopaedic surgery was included, whilst chemotherapy was excluded. In respect of drug use, only analgesics were taken into account. Quality of health status was analysed by transforming the SF‐36 scores to SF‐6D scores that were subsequently transformed into quality‐adjusted life years (QALYs) using the trapezium method.41 This means that the total change in SF‐6D score was approximated as a trapezoid: the area under the curve was calculated by dividing the curve into aggregated trapeziums or segments. Because we needed to correct for coincidental unbalanced allocation in an observed confounder (baseline cost), as well as the potential cluster effects (physical therapy practice), a regression‐based approach to cost‐effectiveness analysis was applied.42 Therefore, the net monetary benefit statistic (NMB) was used, defined as follows: NMB = willingness to pay (WTP * Effect) − Costs. QALY was used as the indicator of effect, and six threshold values of WTP were defined (Table 5). The decision rule states that the option with the highest NMB is the most cost effective given the specific WTP.42 NMBs were calculated at patient level, and subsequently, NMB differences between groups for different WTP thresholds were statistically tested using mixed model analyses in which physical therapy practice was included as a random factor; a correction for difference in baseline values (costs and SF‐6D) was applied and bootstrapped 200 times.
Table 5.
Net monetary benefit and incremental net monetary benefit
| WTP (€) | Coach2Move | Usual care PT | ||
|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Incremental NMB | P value | |
| 2000 | −1931 (−2702 to −1161) | −2800 (−3601 to −1999) | 869 (120 to 1618) | 0.023 |
| 5000 | −816 (−1599 to −33) | −1698 (−2513 to −885) | 882 (153 to 1612) | 0.018 |
| 10 000 | 1042 (236 to 1848) | 137 (−700 to 975) | 905 (117 to 1993) | 0.024 |
| 20 000 | 4759 (3903 to 5614) | 3810 (2921 to 4698) | 949 (72 to 1827) | 0.034 |
| 30 000 | 8475 (7565 to 9386) | 7482 (6536 to 8427) | 994 (113 to 1875) | 0.027 |
| 50 000 | 15 909 (14 878 to 16 940) | 14 825 (13 754 to 15 897) | 1083 (−111 to 2277) | 0.075 |
P values are based on linear mixed model analysis adjusted for baseline values that was bootstrapped 200 times.
An NMB > 0 represents a cost‐effective intervention.
The incremental NMB gives the difference between the NMB of the Coach2Move group compared with the usual care PT group. A positive value is in favour of the Coach2Move group.
CI, confidence interval; NMB, net monetary benefit; PT, physical therapist; WTP, willingness to pay.
In a post hoc analysis, the proportion of patients that had to deal with one or more major incident (visit emergency department, hospital admission, nursing home admission, and temporary stay in residential care facility) or died was compared between groups using a χ 2 test.
Results
Between September 2012 and November 2013, 361 potentially eligible patients were informed about the study (Figure 1). Participation was declined by 122 patients, and 109 patients did not fulfil the inclusion criteria. One hundred and thirty eligible patients were randomly assigned to the intervention group (n = 64) and the control group (n = 66). One of the patients assigned to the usual care physical therapy group was excluded after randomization based on additional information about the patients' health status that did not meet the inclusion criteria. In both intervention groups, one patient declined the allocated intervention because the health insurance did not cover treatment. Some patients in both groups were not able or willing to perform the walking tests at follow‐up measurement. There were no missing data on the questionnaires.
Figure 1.
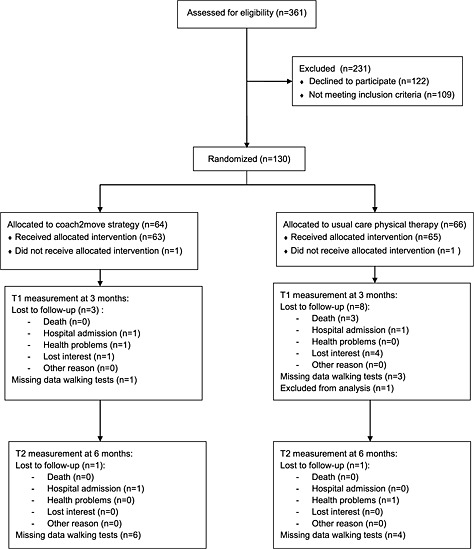
Trial flowchart.
Demographic characteristics of participants are shown in Table 2. There were no statistically significant differences between groups at baseline.
Table 2.
Demographic characteristics and baseline scores
| Coach2Move (n = 64) | Usual care PT (n = 65) | |
|---|---|---|
| Demographic characteristics | n (%) or mean (SD) | n (%) or mean (SD) |
| Age (years) | 78.4 (5.5) | 78.6 (5.5) |
| Men | 16/64 (25%) | 20/65 (31%) |
| CIRS co‐morbidity score, higher score, and more co‐morbidity | 7.27 (3.2) | 6.25 (2.6) |
| Educational level | ||
| High | 5/49 (10%) | 5/60 (8%) |
| Middle | 7/49 (14%) | 10/60 (15%) |
| Low | 37/49 (76%) | 45/60 (77%) |
| Marital status: having a partner | 31/64 (48%) | 35/65 (54%) |
| Living in a residential care facility | 3/64 (5%) | 2/65 (3%) |
| Post‐operative status | 15/64 (23%) | 13/65 (20%) |
| Baseline values at the outcome measures | ||
| Total PA (minutes per day) | 87.0 (56.6) | 87.9 (61.0) |
| Moderate intensity PA (minutes per day) | 28.8 (29.7) | 35.4 (35.4) |
| Frailty (0.00–1.00, higher score, more frailty) | 0.34 (0.1) | 0.30 (0.1) |
| Quality of life and physical subscale (0–100, higher score, and better quality of life) | 30.8 (9.3) | 34.0 (10.8) |
| Quality of life and mental subscale (0–100, higher score, and better quality of life) | 56.7 (8.8) | 56.2 (10.8) |
| Walking speed (m/s) | 0.83 (0.3) | 0.83 (0.3) |
| Mobility (s, Get Up & Go Test) | 34.7 (15.0) | 35.1 (18.1) |
| Walking distance (m) | 225.1 (117.3) | 240.4 (102.9) |
| Patient‐specific complaints (0–10, higher score, more complaints) | 7.6 (3.1) | 7.2 (2.1) |
| Fatigue (0–10, higher score, more fatigue) | 5.2 (2.2) | 5.2 (2.2) |
| Primary care costs (€) | 88.54 (161.6) | 71.98 (128.3) |
| Physical therapy costs (€)a | 30.80 (135.5) | 23.82 (87.6) |
| Medication costs (€) | 19.52 (125.3) | 1.45 (4.2) |
| Hospital care costs (€) | 1875.06 (4254.5) | 1639.69 (3642.2) |
| Outpatient care costs (€) | 2392.09 (8852.3) | 2553.20 (6268.4) |
| Home care costs (€) | 1213.41 (2027.4) | 1026.43 (1477.4) |
| Assistive devices costs (€) | 113.09 (415.3) | 38.49 (150.2) |
| Total costs (€) | 6466.60 (11154.1) | 5398.06 (8321.3) |
No significant between‐group differences were observed at baseline. Costs apply to the 3 months preceding baseline.
CIRS, cummulative illness rating scale; PA, physical activity; PT, physical therapist; SD, standard deviation.
It is included in ‘primary care’.
The primary and secondary outcomes at the 3 and 6 months follow‐up are presented in Table 3. The estimated between‐group difference at 6 months on the primary outcome was 17.9 min per day (95% CI 4.0 to 34.9, P = 0.012) for moderate intensity PA and 14.1 min per day (95% CI −6.6 to 34.9, P = 0.182) for total PA in favour of the Coach2move intervention. For frailty, the estimated difference was −0.03 (95%CI: −0.06 to −0.00; P = 0.027). The effect size (Cohen's d) for total PA was 0.23 (95%CI: −0.14, 0.60), for moderate intensity PA 0.38 (95% CI: 0.01, 0.74), and for frailty, −0.23 (95% CI: −0.59, 0.13). Significant within‐group differences were found on all outcomes except for the psychological subscale of the SF‐36 and fatigue in the Coach2Move group. The usual care physical therapy group improved significantly on total PA, all mobility‐related outcomes, the physical subscale of the SF‐36, and patient‐specific complaints (Table 3).
Table 3.
Primary and secondary outcomes of the intention‐to‐treat analysis at 3 and 6 months after baseline
| n | Baseline | n | 3 months | n | 6 months | Difference between groups at 3 months | Difference between groups at 6 months | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (95% CI) | Mean (95% CI) | Mean difference (95% CI) | P value | Mean difference (95% CI) | P value | ||||
| Total physical activity (min/day) | ||||||||||
| Coach2Move | 64 | 87.0 (56.6) | 61 | 117.9 (97.3 to 138.5)b | 60 | 118.4 (97.8 to 139.0)b | 8.8 (−11.9 to 29.5) | 0.40 | 14.1 (−6.6 to 34.9) | 0.182 |
| Usual care PT | 65 | 87.9 (61.0) | 57 | 109.1 (94.4 to 123.8) a | 56 | 104.3 (89.6 to 119.0)a | ||||
| Moderate intensity physical activity (min/day) | ||||||||||
| Coach2Move | 64 | 28.8 (29.7) | 61 | 55.0 (41.3 to 68.7)b | 60 | 62.7 (49.0 to 76.4)b | 9.9 (−3.9 to 23.7) | 0.160 | 17.9 (4.0 to 31.7) | 0.012 |
| Usual care PT | 65 | 35.4 (35.4) | 57 | 45.1 (35.3 to 54.9) | 56 | 44.8 (35.0 to 54.6) | ||||
| Frailty (0.00–1.00; higher score, more frailty) | ||||||||||
| Coach2Move | 64 | 0.34 (0.1) | 57 | 0.24 (0.22 to 0.27)b | 60 | 0.25 (0.23 to 0.27)b | −0.02 (−0.00 to 0.00) | 0.090 | −0.03 (−0.06 to −0.00) | 0.027 |
| Usual care PT | 65 | 0.30 (0.1) | 65 | 0.27 (0.25 to 0.29)a | 56 | 0.28 (0.26 to 0.30) | ||||
| Waling speed (m/s) | ||||||||||
| Coach2Move | 64 | 0.83 (0.3) | 60 | 0.98 (0.91 to 1.05)b | 54 | 0.99 (0.92 to 1.06)b | 0.05 (−0.02 to 0.22) | 0.178 | 0.04 (−0.03 to 0.11) | 0.28 |
| Usual care PT | 65 | 0.83 (0.3) | 56 | 0.93 (0.88 to 0.98)b | 52 | 0.95 (0.90 to 1.0)b | ||||
| Mobility (s, Get Up & Go Test) | ||||||||||
| Coach2Move | 64 | 34.7 (15.0) | 60 | 30.7 (26.4 to 35.0)b | 54 | 30.8 (26.3 to 35.4)b | −0.4 (−4.8 to 4.0) | 0.85 | −0.7 (−5.0 to 3.9) | 0.80 |
| Usual care PT | 65 | 35.1 (18.1) | 56 | 31.1 (28.0 to 34.2)b | 52 | 31.4 (28.3 to 34.5)b | ||||
| Walking distance (m) | ||||||||||
| Coach2Move | 64 | 225.1 (117.3) | 60 | 278.4 (252.3 to 304.5)b | 54 | 288.6 (261.9 to 316.3)b | 5.2 (−21.1 to 31.5) | 0.70 | 0.8 (−26.1 to 27.6) | 0.96 |
| Usual care PT | 65 | 240.4 (102.9) | 56 | 273.2 (254.2 to 292.2)b | 52 | 287.8 (268.6 to 307.0)b | ||||
| Quality of life—physical subscale (0–100, higher score, better quality of life) | ||||||||||
| Coach2Move | 64 | 30.8 (9.3) | 61 | 40.2 (36.9 to 43.5)b | 60 | 42.2 (38.9 to 45.5)b | −0.3 (−3.6 to 3.0) | 0.86 | 3.1 (−0.2 to 6.4) | 0.07 |
| Usual care PT | 65 | 34.0 (10.8) | 57 | 40.5 (38.1 to 42.9)b | 56 | 39.1 (36.7 to 41.5)b | ||||
| Quality of life—psychological subscale (0–100, higher score, better quality of life) | ||||||||||
| Coach2Move | 64 | 56.7 (8.8) | 61 | 54.8 (51.5 to 58.1) | 60 | 54.4 (51.0 to 57.7) | 2.2 (−1.1 to 5.6) | 0.184 | −0.9 (−4.2 to 2.5) | 0.62 |
| Usual care PT | 65 | 56.2 (10.8) | 57 | 52.5 (50.1 to 54.9)b | 56 | 55.2 (52.8 to 57.6) | ||||
| Patient‐specific complaints (0–10, higher score, more complaints) | ||||||||||
| Coach2Move | 64 | 7.6 (3.1) | 64 | 5.0 (3.9 to 6.0)b | 60 | 4.6 (3.6 to 5.7)b | −0.3 (−1.4 to 0.7) | 0.54 | −0.7 (−1.7 to 0.4) | 0.20 |
| Usual care PT | 65 | 7.2 (2.1) | 57 | 5.3 (4.6 to 6.0)b | 56 | 5.3 (4.6 to 6.0)b | ||||
| Fatigue (0–10, higher score, more fatigue) | ||||||||||
| Coach2Move | 64 | 5.2 (2.2) | 64 | 4.5 (3.7 to 5.4) | 60 | 4.4 (3.5 to 5.2) | −0.5 (−1.4 to 0.4) | 0.27 | −0.2 (−1.1 to 0.6) | 0.62 |
| Usual care PT | 65 | 5.2 (2.2) | 60 | 5.0 (4.0 to 5.2) | 56 | 4.6 (4.0 to 5.2) | ||||
Means and mean differences between and within groups were estimated based on linear mixed models for repeated measurement.
Difference at 3 months: all outcomes, except the Short Form‐36—physical subscale—favour the Coach2Move group.
Difference at 6 months: all outcomes, except the Short Form‐36—psychological subscale—favour the Coach2Move group.
CI, confidence interval; PT, physical therapist; SD, standard deviation.
Within group difference P < 0.05.
Within group difference P < 0.01.
Post hoc analyses showed no difference in the proportion of participants that reported one or more fall incidents. In the Coach2Move group, 18 participants fell once during the 6 months follow‐up, and nine participants had more than one fall incident. For the usual care physical therapy group, 13 participants reported one fall incident, and nine fell more than once. The number of patients who were not physically active at moderate intensity for 30 min per day at baseline, but were at 6 months, was significantly higher in the Coach2Move group (35%) compared with the usual care physical therapy group (18%) [χ 2 test (P = 0.037)].
Tables 4 and 5 show the results in terms of cost effectiveness. Treatment according to the Coach2Move strategy resulted in both statistically significant savings of €848.8 (95% CI: −1607 to −90, P = 0.028) and higher QALYs (mean difference: 0.02, 95% CI: 0.00 to 0.03, P = 0.011). Table 5 shows that the NMB of the Coach2Move approach was higher than the NMB of the usual care physical therapy group at every WTP threshold of the pre‐specified range. The incremental NMB was in favour of the Coach2Move strategy with a probability of the Coach2Move strategy being cost effective compared with usual care physical therapy of over 95% (Figure 2).
Table 4.
Aggregated costs and quality‐adjusted life years at 6 months
| Coach2Move: aggregated outcome at 6 months (n = 60) | Usual care PT: aggregated outcome at 6 months (n = 55) | Mean difference between groups (95% CI) | P value | |||
|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | |||
| Primary care (€) | 574.6 | 453.2 to 696.1 | 671.2 | 545.8 to 796.7 | −96.6 (−231.0 to 37.7) | 0.157 |
| PT (€)a | 467.7 | 345.2 to 590.3 | 569.9 | 443.5 to 696.3 | −102.2 (−229.7 to 25.3) | 0.115 |
| Analgetics | 5.7 | −28.5 to 39.9 | 37.2 | 1.6 to 72.8 | −31.5 (−77.9 to 15.0) | 0.182 |
| Hospital care (€) | 308.6 | 13.8 to 603.4 | 372.0 | 64.1 to 679.9 | −63.4 (−489.9 to 363.1) | 0.77 |
| Outpatient care (€) | 9.5 | −240.5 to 259.5 | 269.6 | 8.4 to 530.8 | −260.1 (−622.4 to 102.2) | 0.158 |
| Home care (€) | 1785.5 | 1405.9 to 2165.2 | 2076.4 | 1679.8 to 2473.0 | −290.8 (−840.7 to 259.0) | 0.28 |
| Assistive devices (€) | 30.4 | −17.6 to 78.4 | 51.8 | 1.8 to 101.8 | −21.4 (−89.0 to 46.2) | 0.53 |
| Total (€) | 2675.6 | 1911.5 to 3439.7 | 3524.4 | 2730.2 to 4318.6 | −848.8 (−1607 to −90) | 0.028 |
| QALYs (0.00–0.50) | 0.37 | 0.36 to 0.39 | 0.35 | 0.34 to 0.37 | 0.02 (0.00 to 0.03) | 0.011 |
Costs are estimated based on a linear mixed model with adjustments for baseline. Total costs and QALYs are based on a linear mixed model with adjustments for baseline values that was bootstrapped 200 times. A negative mean difference represents lower costs for the Coach2Move group; a positive mean difference represents higher costs for the Coach2Move group. A positive difference in QALY indicates an improvement of the Coach2Move group compared with the usual care group.
Mean difference on all outcomes favour the Coach2Move group. Costs in €.
CI, confidence interval; PT, physical therapist; QALYs, quality‐adjusted life years.
It is included in ‘primary care’.
Figure 2.

Incremental net monetary benefit and acceptability curve.
Frequencies of healthcare use are laid out in Appendix B. The mean number of physical therapy sessions was significantly lower (P = 0.003) in the Coach2Move group (mean: 11 including prolonged intake, SD: 4.5; median: 11) than in the usual care physical therapy group (mean: 17, SD: 15.0; median: 12). At the first follow‐up visit after 3 months (t1), 55% (n = 35) of the Coach2Move participants had finished their physical therapy treatment. In the usual care group, 36% (n = 24) had finished after 3 months. At the end of this study (t2‐6 months), all but one Coach2Move participant no longer received physical therapy treatment. Four patients in the usual care physical therapy continued to visit their physical therapist. On average, the usual care physical therapy group had a higher dosage of treatment than the Coach2Move group. The proportion of patients who had to deal with one or more major incidents (visit to emergency department, hospital admission, nursing home admission, and temporary stay in residential care facility) or who had died was significantly higher in the usual care physical therapy group than in the Coach2Move group: 22% and 8%, respectively (P = 0.028).
Discussion
Our study shows that the patient‐centred Coach2Move strategy is effective in enhancing moderate intensity PA and in reducing frailty in older adults with mobility problems. In addition, Coach2Move has lower costs, higher benefits, and fewer incidents compared with usual physical therapy management.
Both studied interventions used physical therapy strategies (e.g. strength training, fitness training, and balance training) and were effective in increasing mobility that is a clear and objective indicator of muscle functioning (sarcopenia) and total physical functioning. Older adults in the Coach2move group, however, were able to use these capacities to increase activities in daily life over a period of 6 months and to decrease frailty, whereas in 55% of cases, the intervention had already been completed within 3 months. The Coach2Move intervention was tailored to personal impairments, disabilities, and participation problems that were systematically diagnosed in an extensive intake. The patient was involved in clinical decision‐making, and, most importantly, the physical and social environment was involved in the treatment process, a factor that probably supported the retention and, sometimes even the improvement, in functioning, even after physical therapy had ended.
Exercise and PA are thought to be the most important interventions to ameliorate frailty because of the positive influence on multiple frailty‐related factors such as muscle strength, activity level, and walking speed.5, 7 The direct effect on frailty is an important and promising result of our study. Most studies only consider physical aspects of frailty, whilst research has shown that psychological and social factors also influence frailty status.34 A recent study showed that a PA intervention reduced physical frailty in older adults and emphasized the potential importance of PA in reducing frailty and improving health status in older adults.8 Our study supports these findings and is the first study to show a reduction in frailty as measured on multiple dimensions. Because frailty is a powerful predictor of adverse health outcome, reducing frailty could possibly have large positive effects on health status and for a longer period of time. However, we do not know if the magnitude of the difference in frailty score is large enough to be clinically relevant. However, it should be mentioned that both groups showed significant improvements in mobility compared with baseline, which were systematically larger in the Coach2Move group (although not significant). This needs to be further assessed in future longitudinal studies.
In a meta‐analysis of our research group, we could not confirm an increase in PA as a result of physical exercise therapy in community‐dwelling older adults with mobility problems and/or multi morbidity.2 A recent study that compared a group exercise programme with a home‐based exercise programme coached by a mentor with a usual care control group in older adults over 65 years old found an increase of 15 min of PA per day in the group exercise programme and a 9% increase in the proportion of participants that fulfilled PA standards.43 In our study, we found significant improvements on all outcomes in both groups. However, we found a much larger increase in the Coach2Move group: the Coach2Move group increased moderate intensity PA with over 30 min per day. Given the internationally accepted PA standards of 30 min a day,1 these findings are likely to be clinically relevant. A proportion of 35% of the participants in the Coach2Move group did not fulfil the PA standard at baseline but did so at 6 months. In addition, the participants in the present study were of higher age and had already existing mobility problems. This means that we judge the increased PA as clinically relevant when taking into account the relatively high age and high number of co‐morbidities.
In contrast to other studies, we also found healthcare cost savings. At baseline, both groups had made huge healthcare costs in the preceding 3 months. At 6 months follow‐up, the costs had reduced enormously in both groups, but significantly more in the Coach2Move group. These large differences between baseline and follow‐up costs may be explained by the fact that a number of patients started physical therapy after a fall incident, a hospital admission, or surgery that increased costs. In addition, we also believe that treatment by a physical therapist reduces the number of other health professionals needed and improves mobility and thereby reducing, for example, the risk of fall incidents. Moreover, the Coach2Move strategy focuses on self‐management in which patients are empowered to take responsibility for their own health, possibly explaining the additional cost advantage for the Coach2Move group.
Inconsistent results have been found in respect of the effectiveness of patient‐centred care.44, 45 It has been shown that standard protocols are not sufficient to treat older adults with multi‐morbidity.46 Integrating the principles of patient‐centred care into clinical reasoning can offer a solution for physical therapy by combining the knowledge from evidence‐based physical therapy protocols, the clinical experience of physical therapists, and patient preferences and needs. Our study shows that a patient‐centred physical therapy approach is feasible and effective for older adults with mobility problems, thus emphasizing the importance of a shift towards patient‐centred care in order to improve healthcare efficiency.
Contamination between the two participating physical therapists in one practice might have been an issue. We believe that the risk of contamination was minimized because specific skills and knowledge are needed to apply the Coach2Move strategy. Even though the Coach2Move strategy uses the same exercise modalities and therefore, intuitively, seems very similar to usual care physical therapy, research has shown that physical therapists do not generally set patient‐centred goals when improving PA and physical therapists are not trained to specifically stimulate shared decision‐making and to apply coaching techniques that increase self‐management.47
Even though we applied broad inclusion and exclusion criteria as present in typical daily clinical practice, we had some difficulty with the inclusion of patients. Many potential participants declined to participate mostly because they preferred a specific therapist and did not want to be randomized, which possibly leads to selection bias and may limit the generalizability of our study findings. Also, informal caregivers were not taken into account in the cost‐effectiveness analysis because we did not expect informal caregivers to make a difference to the incremental cost‐effectiveness analysis. Consequently, the shown cost‐effectiveness of the Coach2Move strategy is mostly based on costs from a healthcare perspective and, to a lesser extent, on costs from a societal perspective. Retrospectively, it would have been better to include informal caregivers in our cost‐effectiveness evaluation. Another limitation of the present study is the lack of a control group that did not receive an intervention to account for natural recovery. In addition, the follow‐up period of 6 months is relatively short.
Our study shows that older adults with mobility problems are able to safely increase PA in their own environment and decrease their level of frailty when they are adequately coached by a physical therapist that has the knowledge and skills to take health status, personal needs, preferences, facilitators, and barriers into account. Moreover, this study emphasizes the potential cost‐effectiveness of patient‐centred care in frail–older adults. The results of this study justify a future study with long‐term follow‐up to replicate the present findings. In addition, the Coach2Move strategy could be used as an example to incorporate patient‐centred strategies in daily physical therapy care. The positive effect found on frailty status emphasizes the importance of PA promotion in older adults, and therefore PA should be encouraged by health authorities. We have shown that not only healthy older adults benefit from PA but also frail–older adults with mobility limitations.
Contributors
N. d. V., J. B. S., and R. N. S. conceived and designed the study and obtained funding. N. d. V. was responsible for trial management, recruitment, and data collection under supervision from J. B. S., P. W., and R. N. R. N. S. trained participating (geriatric) physical therapists, and N. d. V. performed the coaching of the geriatric physical therapists during the trial. Data collection was supported by research assistants. R. A. and E. A. were involved in statistical analyses to determine effectiveness and cost‐effectiveness, respectively. N. d. V., J. B. S., P. W., M. O. R., and R. N. S. interpreted the data. N. d. V. drafted the manuscript with contributions from all other authors. The authors read and approved the final manuscript.
Conflicts of interest
All authors declare that there are no financial or non‐financial competing interests to declare in relation to this manuscript.
Acknowledgements
We thank the Dutch Association for Physical Therapy in Geriatrics (NVFG) for supporting this study. We thank Hanna van Eijsden and Sandra Bolder for their help with data collection, and Elly van Selst for her support in coaching the PTs. Finally, we thank all participating physical therapy practices, physical therapists, and patients for participating in this study.
This trial is registered in The Netherlands National Trial Register (registration number: NTR3527). This study is part of the designing optimal interventions for physical therapy (DO‐IT) research programme. DO‐IT is funded by the Royal Dutch Society for Physical Therapy (KNGF). This randomized controlled trial is co‐financed by The Netherlands Organization for Health Research and Development (ZonMw, project number: 171201010). All authors declare that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle (von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7–8).
Healthcare consumption and costs
| Resource use | Coach2Move (n = 60) | Usual care PT (n = 55) | Unit cost (€) | ||
|---|---|---|---|---|---|
| Patients that used resource number (%) | Units used mean (SD) | Patients that used resource number (%) | Units used mean (SD) | ||
| Primary care | |||||
| Physical therapy* (including group | 11 (5) | 17 (12) | Geriatric PT: 46 per session1 | ||
| physical therapy) | Usual care PT: 36 per session2 | ||||
| <5 sessions | 5 (8.3) | 2 (3.6) | |||
| 5–12 sessions | 40 (66.7) | 28 (50.9) | Group physical therapy: 15 per person per session 3 | ||
| 13–18 sessions | 10 (16.7) | 10 (18.2) | |||
| >18 sessions | 5 (8.3) | 15 (27.3) | |||
| General practitioner (including home visits) | 2 (2) | 2 (2) | 28 per visit2 | ||
| Home visit: 43 per visit2 | |||||
| 0 visits | 10 (16.7) | 8 (14.5) | |||
| 1 visit | 12 (20.0) | 18 (32.7) | |||
| 2 visits | 15 (25.0) | 16 (29.1) | |||
| >2 visits | 23 (38.3) | 13 (23.6) | |||
| Other allied healthcare practitioners (specialized nurse, occupational therapist, chiropractor, acupuncture, and podiatrist) | 22 (36.7) | 1.5 (3.4) | 8 (14.5) | 0.9 (3.6) | Allied health practitioners: 36 per visit2 |
| Specialized nurse: 9 per visit4 | |||||
| Pain medication | 19 (31.7) | 162.91 (292.54) | 19 (34.5) | 109.43 (212.80) | Dependent on type: 0.21–315 |
| Hospital care | |||||
| Consultation specialized physician | 24 (40.0) | 0.9 (1.5) | 26 (47.3) | 0.84 (1.1) | 64 per visit2 |
| Diagnostics (x‐ray, MRI) | 8 (13.3) | 0.17 (0.46) | 9 (16.4) | 0.25 (0.87) | X‐ray: 474 |
| MRI: 2004 | |||||
| Emergency department | 3 (5.0) | 0.05 (0.22) | 4 (7.3) | 0.11 (0.46) | 151 per visit2 |
| Hospital admission | 2 (3.3) | 0.25 (1.6) | 5 (9.1) | 0.65 (2.44) | 435 per day2 |
| Other hospital care (pain treatment and surgery) | 2 (3.3) | 0.02 (0.13) | 1 (1.8) | 0.04 (0.27) | Pain treatment 120.54 |
| Surgery: dependent on type of surgery: 1375–10 9254 | |||||
| Outpatient care | |||||
| Nursing home admission | 0 (0) | 0 (0) | 1 (1.8) | 0.76 (5.67) | 238 per day2 |
| Residential home admission | 1 (1.7) | 0.2 (1.5) | 2 (3.6) | 0.87 (4.79) | 90 per day2 |
| Home care | |||||
| Home care (nurse) | 13 (21.7) | 23.56 (69.59) | 17 (30.9) | 19.51 (40.12) | 44 per hour2 |
| Home care (housework) | 35 (58.3) | 37.39 (45.94) | 40 (72.7) | 36.2 (43.67) | 24 per hour2 |
| Meal service (home or residential home/community centre) | 9 (15.0) | 18.87 (51.18) | 9 (16.4) | 14.81 (46.00) | At home: 5 per meal6 |
| At community center: 7 per meal6 | |||||
| Assistive devices | |||||
| Cane | 6 (10.0) | 0.13 (0.43) | 1 (1.8) | 0.02 (0.13) | 136 |
| Crutches | 2 (3.3) | 0.07 (0.43) | 1 (1.8) | 0.04 (0.27) | 236 |
| Walker | 5 (8.3) | 0.08 (0.28) | 4 (7.3) | 0.07 (0.26) | 906 |
| Other | 2 (3.3) | 0.03 (0.18) | 4 (7.3) | 0.04 (0.19) | Wheelchair: 1996 |
| Orthopaedic shoes: 12006 | |||||
| Other: 40–12006 | |||||
| Death | 0 (0.0) | — | 3 (5.5) | — | — |
| Number of incidents (emergency department visits, hospital admissions, nursing home admissions, residential home admissions, and death) | 6 (10.0) | — | 15 (27.3) | — | — |
PT, physical therapist; SD, standard deviation; MRI, magnetic resonance imaging.
*Only number of PT consults significantly differed between the Coach2Move group and the usual care PT group.
Sources for cost prices:
1Standard unit cost price: Dutch guideline for costing research corrected for higher rate for specialized PTs (Royal Dutch Association for Physical Therapy).
2Standard unit cost price: Dutch guideline for costing research.
3Cost price (Royal Dutch Association for Physical Therapy) for groups of five to 10 people.
4Dutch Healthcare Authority
5Cost price (www.medicijnkosten.nl)
6Real cost price
de Vries, N. M. , Staal, J. B. , van der Wees, P. J. , Adang, E. M. M. , Akkermans, R. , Olde Rikkert, M. G. M. , and Nijhuis‐van der Sanden, M. W. G. (2016) Patient‐centred physical therapy is (cost‐) effective in increasing physical activity and reducing frailty in older adults with mobility problems: a randomized controlled trial with 6 months follow‐up. Journal of Cachexia, Sarcopenia and Muscle, 7: 422–435. doi: 10.1002/jcsm.12091.
References
- 1. Chodzko‐Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, et al American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc 2009;41:1510–1530. [DOI] [PubMed] [Google Scholar]
- 2. de Vries NM, van Ravensberg CD, Hobbelen JSM, Olde Rikkert MG, Staal JB, Nijhuis‐van der Sanden MW. Effects of physical exercise therapy on mobility, physical functioning, physical activity and quality of life in community‐dwelling older adults with impaired mobility, physical disability and/or multi‐morbidity: a meta‐analysis. Ageing Res Rev 2012;11:136–149. [DOI] [PubMed] [Google Scholar]
- 3. Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS et al Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA 2014;311:2387–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cesari M, Landi F, Vellas B, Bernabei R, Marzetti E. Sarcopenia and physical frailty: two sides of the same coin. Front Aging Neurosc 2014;6:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Landi F, Marzetti E, Martone AM, Bernabei R, Onder G. Exercise as a remedy for sarcopenia. Curr Opin Clin Nutr Metabol Care 2014;17:25–31. [DOI] [PubMed] [Google Scholar]
- 6. Coventry PA, Bower P, Keyworth C, Kenning C, Knopp J, Garrett C et al The effect of complex interventions on depression and anxiety in chronic obstructive pulmonary disease: systematic review and meta‐analysis. PLoS One 2013;8e60532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Theou O, Stathokostas L, Roland KP, Jakobi JM, Patterson C, Vandervoort AA et al The effectiveness of exercise interventions for the management of frailty: a systematic review. J Aging Res 2011;2011:569194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cesari M, Vellas B, Hsu FC, Newman AB, Doss H, King AC et al A physical activity intervention to treat the frailty syndrome in older persons‐results from the LIFE‐P study. J Gerontol A Biol Sci Med Sci 2015;70:216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Landi F, Abbatecola AM, Provinciali M, Corsonello A, Bustacchini S, Manigrasso L et al Moving against frailty: does physical activity matter? Biogerontology 2010;11:537–545. [DOI] [PubMed] [Google Scholar]
- 10. Clegg A, Young J, Iliffe S, Olde Rikkert MG, Rockwood K. Frailty in elderly people. Lancet 2013;381:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J et al Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 12. Gobbens RJ, Luijkx KG, Wijnen‐Sponselee MT, Schols JM. Toward a conceptual definition of frail community dwelling older people. Nurs Outlook 2010;58:76–86. [DOI] [PubMed] [Google Scholar]
- 13. Gobbens RJ, Luijkx KG, Wijnen‐Sponselee MT, Schols JM. Towards an integral conceptual model of frailty. J Nutr Health Aging 2010;14:175–181. [DOI] [PubMed] [Google Scholar]
- 14. Jefferis BJ, Sartini C, Lee IM, Choi M, Amuzu A, Gutierrez C et al Adherence to physical activity guidelines in older adults, using objectively measured physical activity in a population‐based study. BMC Pub Health 2014;14:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hildebrandt VH, Bernaards CM, Stubbe JH. Trendrapport bewegen en gezondheid 2010–2011. Leiden: TNO; 2013. [Google Scholar]
- 16. Baert V, Gorus E, Mets T, Geerts C, Bautmans I. Motivators and barriers for physical activity in the oldest old: a systematic review. Ageing Res Rev 2011;10:464–474. [DOI] [PubMed] [Google Scholar]
- 17. Heath GW, Parra DC, Sarmiento OL, Andersen LB, Owen N, Goenka S et al Evidence‐based intervention in physical activity: lessons from around the world. Lancet 2012;380:272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hill KD, Hunter SW, Batchelor FA, Cavalheri V, Burton E. Individualized home‐based exercise programs for older people to reduce falls and improve physical performance: a systematic review and meta‐analysis. Maturitas 2015;82:72–84. [DOI] [PubMed] [Google Scholar]
- 19. Scholl I, Zill JM, Harter M, Dirmaier J. An integrative model of patient‐centeredness—a systematic review and concept analysis. PLoS One 2014;9e107828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Campbell NC, Murray E, Darbyshire J, Emery J, Farmer A, Griffiths F et al Designing and evaluating complex interventions to improve health care. BMJ 2007;334:455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Vries NM, van Ravensberg CD, Hobbelen JS, van der Wees PJ, Olde Rikkert MG, Staal JB et al The Coach2Move approach: development and acceptability of an individually tailored physical therapy strategy to increase activity levels in older adults with mobility problems. J Geriatr Phys Ther 2015;38:169–182. [DOI] [PubMed] [Google Scholar]
- 23. de Vries NM, Staal JB, Teerenstra S, Adang EM, Rikkert MG, Nijhuis‐van der Sanden MW. Physiotherapy to improve physical activity in community‐dwelling older adults with mobility problems (Coach2Move): study protocol for a randomized controlled trial. Trials 2013;14:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khokhar SR, Stern Y, Bell K, Anderson K, Noe E, Mayeux R et al Persistent mobility deficit in the absence of deficits in activities of daily living: a risk factor for mortality. J American Geriatr Soc 2001;49:1539–1543. [DOI] [PubMed] [Google Scholar]
- 25. Montero‐Odasso M, Bergman H, Beland F, Sourial N, Fletcher JD, Dallaire L. Identifying mobility heterogeneity in very frail older adults. Are frail people all the same? Arch Gerontol Geriatr 2009;49:272–277. [DOI] [PubMed] [Google Scholar]
- 26. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007;62:722–727. [DOI] [PubMed] [Google Scholar]
- 27. Schenkman M, Deutsch JE, Gill‐Body KM. An integrated framework for decision making in neurologic physical therapist practice. Phys Ther 2006;86:1681–1702. [DOI] [PubMed] [Google Scholar]
- 28. Rothstein JM, Echternach JL, Riddle DL. The hypothesis‐oriented algorithm for clinicians II (HOAC II): a guide for patient management. Phys Ther 2003;83:455–470. [PubMed] [Google Scholar]
- 29. de Vries NM, Staal JB, van Ravensberg CD, Hobbelen JS, Olde Rikkert MG, Nijhuis‐van der Sanden MW. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev 2011;10:104–114. [DOI] [PubMed] [Google Scholar]
- 30. Leeden MvdS JB, Beekman E, Hendriks E, Mesters I, de Rooij M, de Vries N, Werkman M, de Graaf‐Peters V, de Bie R, Nijhuis‐van der Sanden R, Dekker J. Development of a framework to describe goals and content of exercise interventions in physical therapy: a mixed method approach including a systematic review. Phys Ther Rev 2014;19:1–14. [Google Scholar]
- 31. Stel VS, Smit JH, Pluijm SM, Visser M, Deeg DJ, Lips P. Comparison of the LASA Physical Activity Questionnaire with a 7‐day diary and pedometer. J Clin Epidemiol 2004;57:252–258. [DOI] [PubMed] [Google Scholar]
- 32. Podsiadlo D, Richardson S. The timed ‘Up & Go’: a test of basic functional mobility for frail elderly persons. J American Geriatr Soc 1991;39:142–148. [DOI] [PubMed] [Google Scholar]
- 33. Butland RJ, Pang J, Gross ER, Woodcock AA, Geddes DM. Two‐, six‐, and 12‐minute walking tests in respiratory disease. BMJ 1982;284:1607–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rossier P, Wade DT. Validity and reliability comparison of 4 mobility measures in patients presenting with neurologic impairment. Arch Phys Med Rehabil 2001;82:9–13. [DOI] [PubMed] [Google Scholar]
- 35. de Vries NM, Staal JB, Olde Rikkert MG, Nijhuis‐van der Sanden MW. Evaluative frailty index for physical activity (EFIP): a reliable and valid instrument to measure changes in level of frailty. Phys Ther 2013;93:551–561. [DOI] [PubMed] [Google Scholar]
- 36. Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Med Care 1992;30:473–483. [PubMed] [Google Scholar]
- 37. Beurskens AJ, de Vet HC, Koke AJ, Lindeman E, van der Heijden GJ, Regtop W et al A patient‐specific approach for measuring functional status in low back pain. J Manipulative Physiol Ther 1999;22:144–148. [DOI] [PubMed] [Google Scholar]
- 38. Beloosesky Y, Weiss A, Mansur N. Validity of the medication‐based disease burden index compared with the Charlson comorbidity index and the cumulative illness rating scale for geriatrics: a cohort study. Drugs Aging 2011;28:1007–1014. [DOI] [PubMed] [Google Scholar]
- 39. Rubenstein LZ, Josephson KR, Trueblood PR, Loy S, Harker JO, Pietruszka FM et al Effects of a group exercise program on strength, mobility, and falls among fall‐prone elderly men. J Gerontol A Biol Sci Med Sci 2000;55:M317–M321. [DOI] [PubMed] [Google Scholar]
- 40. LHakkaart‐van Roijen L, Tan SS, Bouwmans CAM. Dutch guideline for costing research: College voor zorgverzekeringen. 2010.
- 41. Atkinson K. An Introduction to Numerical Analysis, 2nd ed. New York: John Wiley & Sons; 1989. [Google Scholar]
- 42. Drummond MF, Sculpher MJ, Torrance GW, O'Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes, Third ed. New York: Oxford University Press; 2005. [Google Scholar]
- 43. Iliffe S, Kendrick D, Morris R, Masud T, Gage H, Skelton D et al Multicentre cluster randomised trial comparing a community group exercise programme and home‐based exercise with usual care for people aged 65 years and over in primary care. Health Technol Assessment 2014;18:1–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rathert C, Wyrwich MD, Boren SA. Patient‐centered care and outcomes: a systematic review of the literature. Medical care research and review: MCRR 2013;70:351–379. [DOI] [PubMed] [Google Scholar]
- 45. Dwamena F, Holmes‐Rovner M, Gaulden CM, Jorgenson S, Sadigh G, Sikorskii A et al Interventions for providers to promote a patient‐centred approach in clinical consultations. The Cochrane Database Syst Rev 2012;12CD003267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lugtenberg M, Burgers JS, Clancy C, Westert GP, Schneider EC. Current guidelines have limited applicability to patients with comorbid conditions: a systematic analysis of evidence‐based guidelines. PLoS One 2011;6e25987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nessen T, Opava CH, Martin C, Demmelmaier I. From clinical expert to guide: experiences from coaching people with rheumatoid arthritis to increased physical activity. Phys Ther 2014;94:644–653. [DOI] [PubMed] [Google Scholar]


