Abstract
Paracetamol is an analgesic commonly used by people of all ages, which is well documented to cause severe hepatotoxicity with acute over-exposures. The risk of hepatotoxicity from non-acute paracetamol exposures is less extensively studied, and this is the exposure most common in older adults. Evidence on the effectiveness of N-acetyl cysteine (NAC) for non-acute paracetamol exposures, in any age group, is lacking. This study aimed to examine the effect of long-term exposure to therapeutic doses of paracetamol and sub-acute paracetamol over-exposure, in young and old mice, and to investigate whether NAC was effective at preventing paracetamol hepatotoxicity induced by these exposures. Young and old male C57BL/6 mice were fed a paracetamol-containing (1.33g/kg food) or control diet for 6 weeks. Mice were then dosed orally 8 times over 3 days with additional paracetamol (250mg/kg) or saline, followed by either one or two doses of oral NAC (1200mg/kg) or saline. Chronic low-dose paracetamol exposure did not cause hepatotoxicity in young or old mice, measured by serum alanine aminotransferase (ALT) elevation, and confirmed by histology and a DNA fragmentation assay. Sub-acute paracetamol exposure caused significant hepatotoxicity in young and old mice, measured by biochemistry (ALT) and histology. Neither a single nor double dose of NAC protected against this toxicity from sub-acute paracetamol in young or old mice. This finding has important clinical implications for treating toxicity due to different paracetamol exposure types in patients of all ages, and implies a need to develop new treatments for sub-acute paracetamol toxicity.
Keywords: N-Acetyl Cysteine, Paracetamol, Hepatotoxicity, Chronic, Ageing, Supra-therapeutic
INTRODUCTION
Paracetamol is an analgesic commonly used by people of all ages, which is thought to be safe at low doses, but can cause severe hepatotoxicity in over-exposure situations (1). The majority of paracetamol toxicity studies have explored single, acute doses and there have been limited studies on the risk of paracetamol toxicity from chronic or repeated paracetamol exposure.
In humans, two studies found that compared to a single acute over-exposure, staggered over-exposures to paracetamol were associated with an increased risk of liver failure (2,3). Another found that 31-44% of patients taking the recommended therapeutic paracetamol dose (4g/day) for 14 days had increased serum alanine aminotransferase (ALT) concentrations, an indicator of liver damage (4). A case study (5) and a large-scale retrospective hospital study (6), highlighted the increased risk of chronic paracetamol over-exposure in patients taking multiple paracetamol-containing medications. There have been few animal studies of chronic paracetamol exposure. Two rat studies, and one mouse study, showed that low-dose paracetamol administered daily did not cause liver toxicity in the absence of other risk factors (7–9). Several rat and BALB/c mouse studies have found that pre-treatment with nontoxic doses of paracetamol for 4-8 days before exposure to a high dose of paracetamol can protect against hepatotoxicity, through mechanisms including reduction in cytochrome (CYP)2E1 activity, and increased glutathione (GSH) levels (10–12). However, another rat study found that pre-treatment 24 hours earlier with a moderate dose of paracetamol increased the degree of hepatotoxicity from a secondary paracetamol exposure, due to increased CYP2E1 activity (13). More research is needed to clarify the effect of species, timing, and dose on the effect of paracetamol pre-treatment on susceptibility to hepatotoxicity.
The pharmacokinetics of acute paracetamol exposure have been well established (1). At therapeutic doses, paracetamol is predominantly metabolized via the conjugation pathways (1) with approximately 5% oxidized by CYP2E1 to the toxic metabolite N-acetyl-p-benzoquinone imine (NAPQI) (14). With therapeutic paracetamol dosing, NAPQI is detoxified by conjugation with GSH (15). With paracetamol over-exposure, there is saturation of the conjugation metabolism pathways and GSH depletion resulting in build-up of the toxic NAPQI, which can result in hepatotoxicity (16).
Current treatment of paracetamol overdose to prevent hepatotoxicity is the glutathione precursor N-acetyl-cysteine (NAC) (17). NAC guidelines are designed for protection from a single high dose of paracetamol and there is little evidence to guide risk assessment and optimal treatment for staggered over-exposures, chronic exposure or repeated supra-therapeutic exposures (18). One animal study found that several weeks of NAC pre-treatment protected against toxicity induced by twice-weekly paracetamol dosing (19). However this study does not model the clinically relevant situation, in which a patient would have post rather than pre-treatment with NAC.
The risk of hepatotoxicity from paracetamol in old age is not well described. Animal studies have shown either increased risk of toxicity (20), or decreased susceptibility to acute paracetamol toxicity in old age (21,22), and there are no studies on risk of hepatotoxicity with chronic paracetamol exposure in old age. One clinical study showed that the older people are more likely to have chronic low-dose exposure or accidental over-exposure to paracetamol while younger people are more likely to have acute high-dose exposure (23). Furthermore, as older patients are more likely to be taking multiple medications including over the counter medications (24), there is the possibility that they are at increased risk of chronic high exposure due to multiple paracetamol-containing medications or other medications that can cause hepatotoxicity. As such understanding the effect of chronic or repeated paracetamol exposure on the risk of toxicity, and optimization of treatment for staggered or chronic paracetamol exposures, would be particularly of benefit to the older population.
This study models chronic and staggered sub-acute paracetamol exposures in mice, which are especially clinically relevant in the older population. The study aim was to examine the effect of chronic paracetamol and sub-acute paracetamol exposure in both young and old mice, and investigate whether NAC was effective at preventing paracetamol toxicity induced by chronic and sub-acute exposure.
MATERIALS AND METHODS
Animals
Male C57BL/6 mice were obtained from, and housed at the Kearns Facility (St Leonards, NSW). Mice were group housed in cages of 1-5 with ad libitum access to food (Rat and Mouse Premium Breeder Diet, Gordon's Specialty Stockfeeds, NSW, Australia) and water. Animal rooms were maintained on a 12 hour light/dark cycle at 20-22 degrees Celsius, and 30-70% humidity. Animals were randomly assigned to diet groups by cage, and then individually randomly assigned to treatment groups prior to the treatment day. Mice were euthanized if moribund, and where possible necropsied. Animal protocols were approved by the Animal Care Ethics Committee at Royal North Shore Hospital, and all animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” (National Institutes of Health).
Chronic and Sub-Acute Paracetamol Treatment of Mice
For the first cohort, mice were randomized at 16±1 weeks of age (young, n=59) or 107±2 weeks of age (old, n=56) to either a control diet (Standard Meat Free Mouse and Rat Feed, 20 % Protein, 60% Carbohydrate, 5% Fat, Specialty Feeds, Australia), or control diet supplemented with paracetamol at a concentration of 1.33g/kg feed, both diets fed ad libitum. Paracetamol tablets were ground, and incorporated into the food pellets by Specialty Feeds, Australia. All mice were fed control diet for two weeks prior to the experimental period to adjust to the new diet. Body weight for all mice, and food intake per cage were monitored weekly. Food intake per mouse (g/mouse/day) was approximated by dividing the cage intake by the number of mice per cage. Mice remained on their respective diets for a further six weeks.
Following six weeks of their control or experimental diet, mice from each diet group were then further randomized to sub-acute paracetamol or saline treatment, given via oral gavage,. Mice were dosed an additional paracetamol dose (250mg/kg, Panadol Color-free Baby Drops, 100mg/ml, GlaxoSmithKline, Australia), or saline, three times per day for two days. The mice were gavaged at 8am, 1pm and 6pm. On the third day the mice were fasted from 6am, then gavaged with two more doses of paracetamol (250mg/kg), or saline, at 8am and 1pm. Mice were fasted before these final doses, to normalise the glutathione levels in the liver as a result of varied food consumption. For the paracetamol diet group only, immediately following the final sub-acute paracetamol dose, mice were also dosed, via oral gavage, NAC (1200mg/kg in saline, pH=7.0, Sigma-Aldrich, MO, USA) or saline. At 3pm these mice were given a second NAC dose (1200mg/kg), or saline, via oral gavage. This created eight treatment groups for each age group: control diet+saline (young n=8, old n=6), control diet+paracetamol (young n=8, old n=7), paracetamol diet+saline (young n=6, old n=6), paracetamol diet+paracetamol (young n=8, old n=7),, paracetamol diet+saline+NAC (young n=6, old n=6), paracetamol diet+paracetamol+NAC (young n=8, old n=7), paracetamol diet+saline+2xNAC (young n=7, old n=4), paracetamol diet+paracetamol+2xNAC (young n=7, old n=4). The NAC dose was chosen based on a previous study which saw protection against acute paracetamol toxicity with 1200mg/kg NAC given one hour post paracetamol (25).
For all mice, three hours after the final sub-acute paracetamol dose (4pm), each mouse was anaesthetized with an i.p. injection of ketamine (75 mg/kg) and xylazine (10 mg/kg). A midline laparotomy was performed and blood taken from the Inferior Vena Cava. The portal vein was then cannulated with an 23G intravenous catheter (BD, Sydney, Australia) through which the liver was perfused in-situ at 1–1.5 mL/min/g of liver with oxygenated Krebs-Henseleit bicarbonate buffer (95% O2–5% CO2, 37°C) to remove the blood. Sections of the liver were snap frozen in liquid nitrogen for biochemistry and enzymatic assays, or fixed in 10% neutral formalin for histopathological analysis. See Supplementary Table 1 for the experimental timeline.
Acute Paracetamol Treatment of Mice
A second cohort of young mice (n=13, age=14.7±2.4 weeks) fed standard diet (Rat and Mouse Premium Breeder Diet, Gordon's Specialty Stockfeeds, NSW, Australia) were treated with a single high dose of paracetamol, or saline, with or without a single dose of NAC. Prior to treatment mice were fasted overnight (16 hours), and then dosed with 700mg/kg paracetamol (Panadol Color-free Baby Drops, 100mg/ml, GlaxoSmithKline, Australia) or saline vehicle via oral gavage between 8am and 10am. Immediately following this mice were also dosed via oral gavage with NAC (1200mg/kg in saline, pH=7.0) or saline vehicle. Mice were allowed free access to food after dosing. Six hours after dosing mice were anaesthetized with an i.p injection of ketamine (75 mg/kg) and xylazine (10 mg/kg), a blood sample was taken, and the liver was perfused and samples collected as described above. See Supplementary Table 1 for the experimental timeline.
Serum Biochemistry
Blood was stored at 4°C for up to two hours and allowed to clot before separating the serum by centrifugation at 10,000g for 10 minutes. Serum was stored in aliquots at −80°C. Serum total protein, albumin, bilirubin, alkaline phosphatase (ALP), gamma glutamyltransferase (GGT), alanine aminotransferase (ALT), creatinine and serum paracetamol concentrations were measured by a National Association of Testing Authorities accredited hospital laboratory, PaLMS (Pacific Laboratory Medicine Services) at Royal North Shore Hospital (Sydney, Australia) using an Architect i1000SR immunoassay analyzer (Abbott Diagnostics, IL, USA). The lower limit of the paracetamol serum concentration test was 20μmol/L.
Histology
Liver samples for all mice, and spleens and macroscopically abnormal tissue from old mice with macroscopically suspected cancer, were assessed for histopathology. Fixed tissue was embedded in paraffin, 5μm sections were cut on a microtome and mounted on slides. Slides were stained with Haemotoxylin and Eosin in the National Association of Testing Authorities accredited hospital laboratory of the Pathology department of Royal Prince Alfred Hospital, Sydney, Australia. Histopathology was scored by an anatomical pathologist (CM), who was blinded to the treatment and age groups of the samples. Images were taken on an Olympus BX51 microscope connected to an Olympus DP26 camera (Olympus, Sydney, Australia).
Biochemical examination of frozen liver samples
Liver microsomes were isolated, and the CYP2E1 activity measured based on the absorption wavelength of p-aminophenol, which is converted from aniline via aniline hydroxylase activity, as described by Roberts et al. (1995) and Mach et al. (2014).
Liver concentrations of total glutathione were determined with a Glutathione Assay kit (#703002, Cayman Chemicals, MI, USA) according to the manufacturer's specifications.
DNA fragmentation was determined using an ELISA Cell Death detection kit (#11544675001, Roche, Switzerland) according to the manufacturer's specifications, and following sample preparation as described by Mach et al. (2015).
Statistics
Data are expressed as mean ± SEM unless otherwise indicated. Differences between mean values across treatment and age groups were calculated with one- two- or three-way ANOVA with Tukey's HSD post-hoc test where appropriate. A Kruskal-Wallis test was used to compare serum ALT for the acute paracetamol group as the data was not normally distributed. Chi-squared tests were used for comparisons of proportions across groups.
RESULTS
Animal Characteristics
Weights, food consumed and serum biochemistry results for each mouse group of cohort 1 are shown in Table 1. One-way ANOVA across the eight treatment groups for young and old mice, with Tukey's HSD post-hoc testing, showed no difference in animal weights, food consumed or any serum biochemistry results across any of the old groups. For the young mice, compared to control diet-saline dosed controls, there was a significant increase in liver weight (as a percentage of body weight), bilirubin and total protein, in some paracetamol treated groups. The paracetamol doses received in the diet ranged from 167 to 188 mg/kg/day (Table 1). Several old mice had suspected cancer, as assessed via histology (Table 1B). These mice were not excluded from analysis, in order to mimic a true model of an aged population with multimorbidity, and these mice were equally spread across the treatment groups.
Table 1.
Animal Characteristics – Young and Old Mice.
| Control Diet | Paracetamol Diet | |||||||
|---|---|---|---|---|---|---|---|---|
| Saline | APAP | Saline | APAP | Saline + NAC | APAP + NAC | Saline + 2 x NAC | APAP + 2 x NAC | |
| Young Mice | n=8 | n=8 | n=6 | n=8 | n=6 | n=8 | n=7 | n=7 |
| Age (weeks) | 22.9 (0.1) | 22.3 (0.3) | 23.2 (0.2) | 22.1 (0.5) | 23.0 (0.3) | 22.2 (0.4) | 21.1 (0.3) | 21.1 (0.0) |
| Paracetamol dose, week 6 of diet (mg/kg mouse/day) | - | - | 174.6 (5.7) | 187.8 (10.8) | 173.4 (5.1) | 167.3 (4.4) | 187.1 (3.1) | 187.6 (3.9) |
| Weight pre-subacute dosing (g) | 29.6 (0.6) | 28.8 (0.6) | 30.7 (0.7) | 28.4 (0.8) | 28.1 (0.6) | 30.6 (0.6) | 28.2 (0.7) | 27.5 (0.7) |
| Weight post-subacute dosing (g) | 27.6 (0.6) | 25.0 (0.9) | 28.2 (0.4) | 24.9 (0.7) | 25.8 (1.2) | 27.3 (0.6) | 26.3 (0.7) | 24.7 (0.6) |
| Food eaten, day 2 of subacute dosing (g/mouse/day) | 2.2 (0.2) | 2.1 (0.3) | 2.6 (0.4) | 2.6 (0.2) | 2.5 (0.3) | 1.9 (0.1) | 2.3 (0.5) | 2.3 (0.7) |
| Liver weight (% body weight) | 3.8 (0.1) | 4.3 (0.3) | 3.7 (0.2) | 4.6 (0.2) | 4.3 (0.2) | 4.8 (0.1)* | 3.9 (0.2) | 4.8 (0.3)*# |
| Total protein (g/L) | 44.7 (0.9) | 49.3 (1.3) | 45.5 (2.2) | 49.5 (1.5) | 44.0 (1.9) | 50.7 (1.0)*# | 49.1 (1.2) | 53.7 (1.4)* |
| Albumin (g/L) | 24.3 (0.8) | 26.4 (0.7) | 25.0 (1.0) | 26.3 (0.8) | 25.2 (0.2) | 26.3 (0.3) | 26.0 (0.7) | 28.0 (0.6)* |
| Bilirubin (μmol/L) | 1.4 (0.3) | 3.2 (0.3)*# | 3.0 (0.4) | 5.0 (1.1)* | - | 3.6 (0.2)* | 2.7 (0.3) | 3.5 (0.2)* |
| ALP (U/L) | 99.7 (9.4) | 96.7 (6.9) | 112.5 (4.2) | 93.8 (12.3) | 104.6 (6.9) | 110.6 (5.2) | 110.4 (2.2) | 110.8 (6.7) |
| GGT (U/L) | 3.0 (0.0) | 3.0 (0.0) | 3.0 (0.0) | 3.0 (0.0) | 3.0 (0.0) | 3.0 (0.0) | 3.0 (0.0) | 3.0 (0.0) |
| Creatinine (μmol/L) | 32.0 (1.7) | 32.5 (2.9) | 29.5 (1.7) | 30.7 (0.7) | 27.0 (0.0) | 30.2 (0.5) | 28.4 (1.1) | 29.0 (0.6) |
| Old Mice | n=6 | n=7 | n=6 | n=7 | n=6 | n=7 | n=4 | n=4 |
| Age at sac (weeks) | 114.2 (0.1) | 114.3 (0.1) | 114.1 (0.1) | 114.0 (0.1) | 114.1 (0.1) | 114.5 (0.1) | 107.6 (0.5) | 108.6 (0.9) |
| Paracetamol dose, week 6 of diet (mg/kg mouse/day) | - | - | 140.6 (9.4) | 126.9 (5.8) | 133.1 (3.7) | 139.6 (6.9) | 151.8 (13.9) | 159.5 (9.8) |
| Weight pre-subacute dosing (g) | 29.6 (2.2) | 28.3 (2.2) | 32.0 (1.5) | 34.2 (0.7) | 32.0 (0.4) | 32.1 (0.6) | 31.6 (1.4) | 28.4 (2.2) |
| Weight post-subacute dosing (g) | 28.6 (2.0) | 26.5 (1.6) | 32.0 (1.6) | 30.6 (0.9) | 29.9 (0.3) | 28.6 (0.5) | 30.6 (1.3) | 26.1 (1.6) |
| Food eaten, day 2 of subacute dosing (g/mouse/day) | 2.8 (0.3) | 2.2 (0.3) | 2.8 (0.6) | 1.5 (0.4) | 1.7 (0.3) | 1.8 (0.3) | 2.5 (0.5) | 2.7 (0.6) |
| Liver weight (% body weight) | 4.1 (0.3) | 4.5 (0.2) | 4.4 (0.2) | 4.6 (0.2) | 6.7 (1.4) | 4.8 (0.4) | 4.2 (0.2) | 5.1 (0.2) |
| Total protein (g/L) | 48.6 (1.7) | 50.0 (1.7) | 49.4 (1.1) | 47.8 (2.1) | 48.2 (1.1) | 48.8 (1.1) | 51.3 (0.8) | 52.3 (2.2) |
| Albumin (g/L) | 23.8 (0.7) | 23.7 (1.4) | 23.2 (0.5) | 23.1 (1.1) | 23.0 (0.4) | 23.3 (0.5) | 25.5 (0.3) | 25.0 (0.7) |
| Bilirubin (μmol/L) | 2.2 (0.5) | 2.7 (0.6) | 2.0 (0.4) | 2.6 (0.5) | 3.2 (0.5) | 3.6 (0.8) | 2.5 (0.3) | 3.0 (0.4) |
| ALP (U/L) | 99.6 (12.7) | 100.8 (12.2) | 90.6 (4.7) | 115.6 (17.4) | 173.7 (75.8) | 115.9 (21.1) | 146.3 (21.6) | 144.3 (7.6) |
| GGT (U/L) | 3.0 (0.0) | 3.0 (0.0) | 3.0 (0.0) | 3.0 (0.0) | 3.7 (0.7) | 3.0 (0.0) | 3.0 (0.0) | 3.0 (0.0) |
| Creatinine (μmol/L) | 46.3 (15.3) | 24.7 (2.2) | 35.7 (4.2) | 37.7 (6.2) | 31.7 (1.2) | 30.5 (1.2) | 40.8 (7.8) | 40.5 (13.2) |
| Suspected cancer (n, %) | 1 (16.7) | 0 (0) | 0 (0) | 0 (0) | 1 (16.7) | 1 (14.3) | 0 (0) | 0 (0) |
All values are mean (SEM) or n (% of group).
p<0.05 compared to control diet, saline group
p<0.05 compared to corresponding saline group.
NAC, N-acetyl cysteine; APAP, paracetamol; ALP, alkaline phosphatase; GGT, gamma glutamyltransferase
Several mice died or were moribund, and thus euthanized, during the experiment. One young mouse on paracetamol diet, died after two days of sub-acute paracetamol dosing (suspected severe paracetamol-induced liver toxicity, serum ALT concentration=32545 U/L, 80% liver necrosis). Three old mice on control diet, and three old mice on paracetamol diet died before the subacute dosing was started (unable to be necropsied). Three old mice fed paracetamol diet, died after starting the sub-acute dosing regimen, though they were receiving saline, and are not suspected to have had paracetamol-induced liver toxicity (one unable to be necropsied; one suspected kidney failure, serum creatinine=348 μmol/L, ALT=63 U/L; one suspected liver cancer, ALT=78 U/L).
Assessment of Toxicity with Chronic Paracetamol Exposure
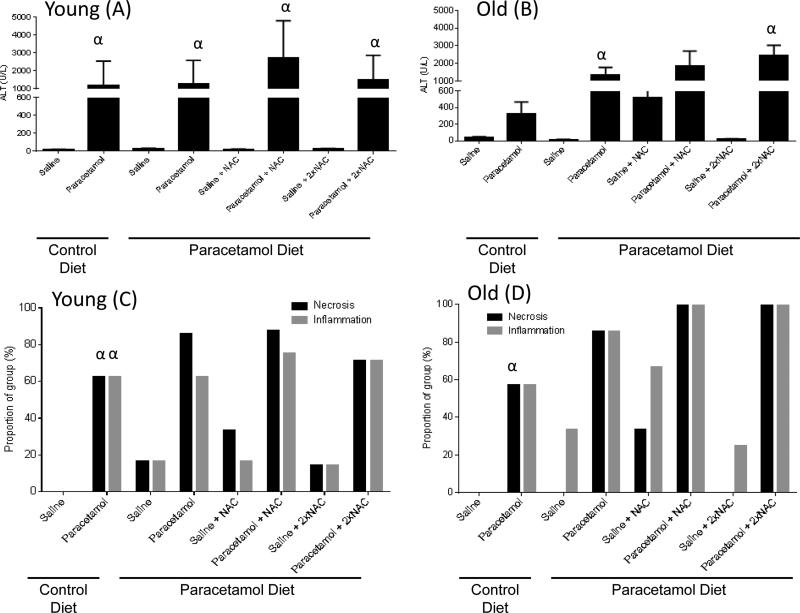
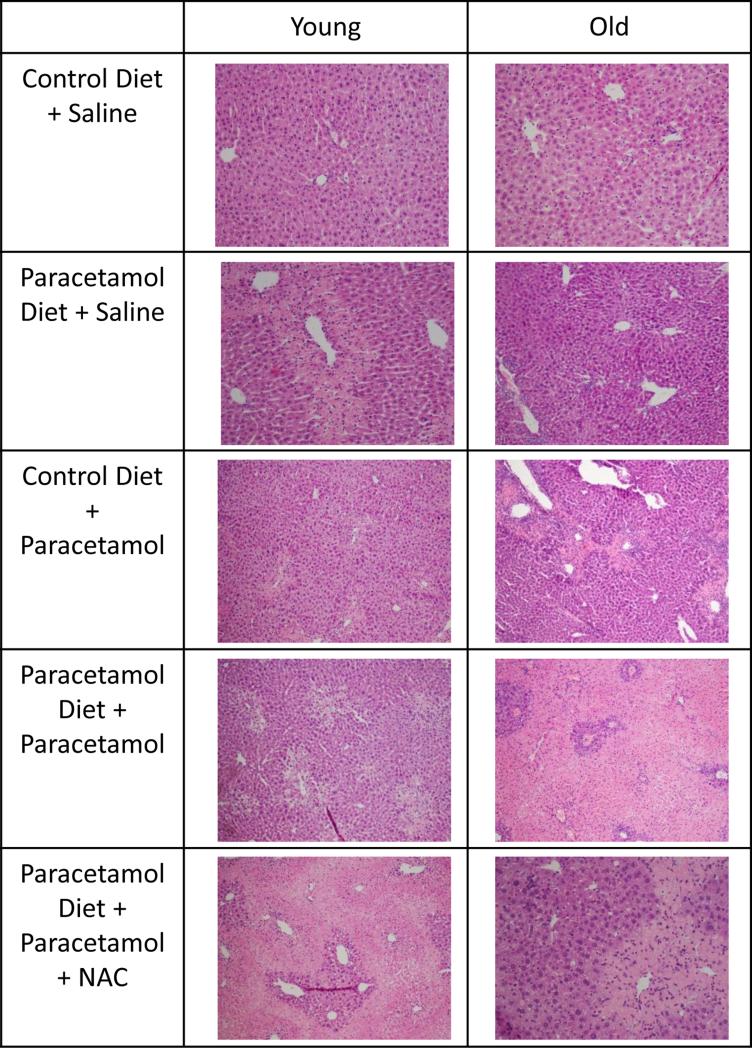
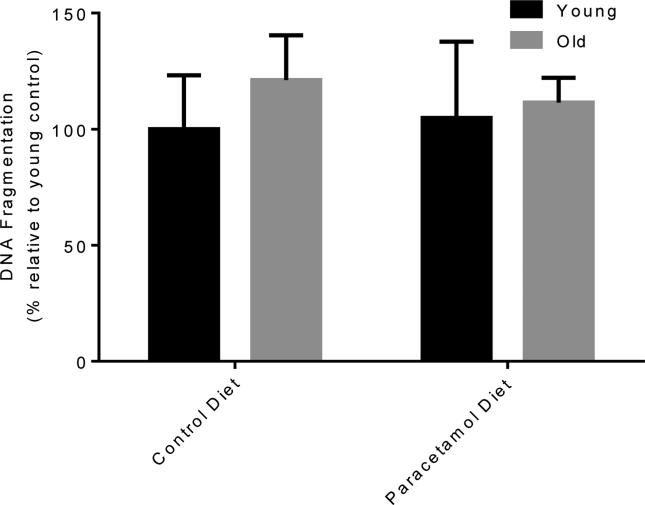
Chronic dietary paracetamol exposure did not result in an increase in serum ALT in young or old mice (Figure 1A and B). To determine whether there was low-grade biomolecular liver damage that serum ALT did not reveal, we investigated the level of DNA fragmentation in the livers of young and old, control or paracetamol diet, saline treated mice groups (Figure 2). There was no increase in DNA fragmentation with dietary paracetamol exposure in young or old mice (young p=0.91, old p=0.72). For those mice receiving sub-acute saline, with the paracetamol diet compared to the control diet, there was no significant difference in the proportion of young or old mice with necrosis (Figure 1C and D), and a trend towards an increase in the proportion of young and old mice with inflammation (young p=0.14, old p=0.12) (Figure 1C and D). Representative histology images are shown in Figure 3.
Figure 1.
Serum alanine aminotransferase (ALT) concentration and histology grading of necrosis and inflammation for young (A, C, respectively) and old (B, D, respectively) mice fed either a control diet or paracetamol containing (1.33g/kg feed) diet for 6 weeks, then treated with 3 days of saline or paracetamol (250mg/kg × 3/day) plus a single or double dose of N-Acetyl Cysteine (NAC) (1200mg/kg) for the paracetamol diet group. Serum ALT activity data expressed as mean ± SEM. Histology data are presented as prevalence % of group. αp<0.05 compared to corresponding saline-treated group
Figure 2.
Representative Haemotoxylin and Eosin stained liver histology images for young and old mice fed either a control diet or paracetamol containing (1.33g/kg feed) diet for 6 weeks, then treated with 3 days of saline or paracetamol (250mg/kg × 3/day) plus a single dose of N-Acetyl Cysteine (NAC) (1200mg/kg) for the paracetamol diet group. Images taken at 100-200X.
Figure 3.
DNA Fragmentation for young and old mice fed either a control diet or paracetamol containing (1.33g/kg feed) diet for 6 weeks, then treated with 3 days of saline. Data expressed as % relative to young control diet ± SEM.
Assessment of Toxicity with Sub-acute Paracetamol Exposure
All young mouse groups treated with sub-acute paracetamol had increased serum ALT concentrations compared to their corresponding saline-treated groups (p<0.05, Figure 1A). For old mice, sub-acute paracetamol treatment significantly increased ALT compared to the corresponding saline treated group only for the paracetamol diet+paracetamol group and paracetamol diet+paracetamol+2xNAC group (p<0.05). There was a trend towards increased serum ALT with sub-acute paracetamol treatment in the other old groups compared to saline controls (control diet+paracetamol group p=0.09, paracetamol diet+paracetamol+NAC group p=0.19) (Figure 1B). Histological grading also showed an increase in the proportion of mice with necrosis and inflammation, for all sub-acute paracetamol treated groups compared to the saline-treated groups (young necrosis p=0.002, inflammation p=0.001; old necrosis p=0.001, inflammation p=0.06). Representative histology images are shown in Figure 3. A 3-way ANOVA of age, diet and treatment group of all mice, showed that treatment was the only significant factor associated with serum ALT concentration, with all age and diet interaction terms non-significant, implying that neither the age nor diet group of the mice affected the degree of sub-acute paracetamol-induced toxicity.
Assessment of Toxicity with Paracetamol Exposure Plus N-Acetyl Cysteine
In our study, neither treatment with a single nor double dose of NAC was able to reduce the degree of toxicity induced by sub-acute paracetamol dosing. A two-way ANOVA for each age group, when only considering those mice on the paracetamol diet, of sub-acute paracetamol/saline treatment and NAC treatment, showed that the interaction term between sub-acute treatment and NAC, for ALT serum concentration, was not significant (Figure 1A and B). There was also no reduction in the proportion of necrosis or inflammation seen in the groups treated with either a single or double dose of NAC plus paracetamol, compared to paracetamol alone (young necrosis p=0.72, inflammation p=0.69; old necrosis p=0.23, inflammation p=0.23). Representative histology images are shown in Figure 3.
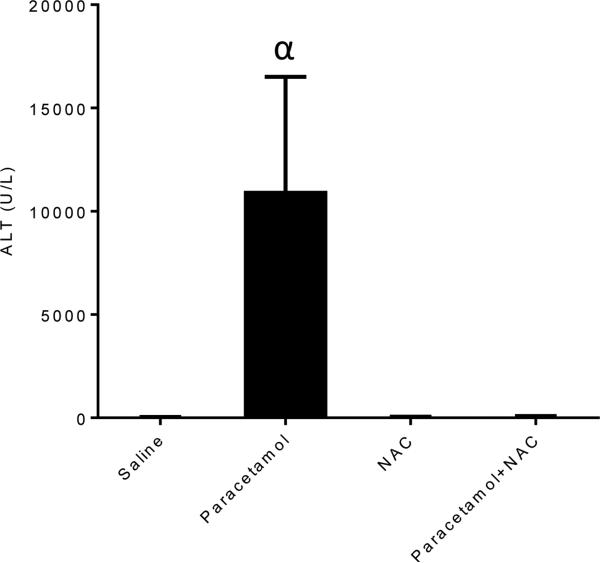
In order to determine that NAC was protective with an acute paracetamol exposure in our mouse model, we treated a second cohort of young mice with acute paracetamol only, with or without concurrent NAC (Figure 4). Treatment with acute paracetamol resulted in increased ALT serum concentration, compared to saline treated controls, whilst concurrent NAC treatment maintained ALT serum concentration at control levels (p=0.024) (Figure 4). Acute paracetamol treatment did not result in detectable necrosis six hours after treatment in any mice, with one mouse showing mild detectable inflammation after paracetamol treatment.
Figure 4.
Serum alanine aminotransferase (ALT) concentration for young mice treated with an acute dose of paracetamol (700mg/kg) or saline, then a single dose of N-Acetyl Cysteine (1200mg/kg) or saline. Data expressed as mean ± SEM. αp<0.05 compared to corresponding saline-treated group.
Serum Paracetamol Levels, Liver Glutathione Levels and CYP2E1 Activity
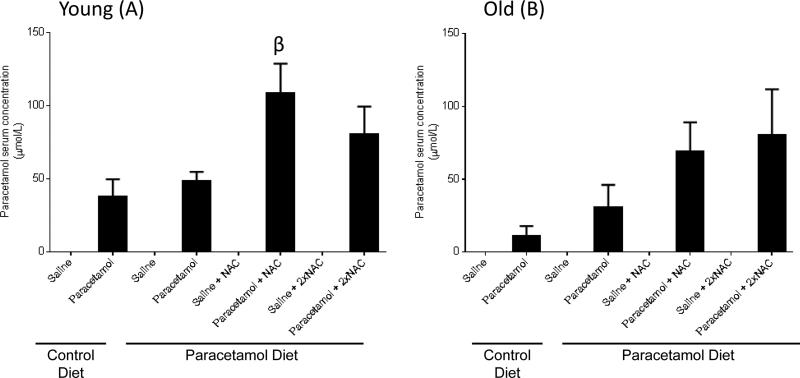
We also assessed the serum paracetamol levels for all young (Figure 5A) and old (Figure 5B) mouse groups treated with sub-acute paracetamol or saline. In all saline treated mice, even those receiving chronic paracetamol in the diet, paracetamol serum levels were below the detectable limit of the test. A one-way ANOVA across treatment groups for each mouse group, identified a significant increase in paracetamol serum level for young mice treated with sub-acute paracetamol plus NAC, compared to those treated with sub-acute paracetamol only (Figure 5A) (p=0.036).
Figure 5.
Paracetamol serum concentrations for young (A) and old (B) mice fed either a control diet or paracetamol containing (1.33g/kg feed) diet for 6 weeks, then treated with 3 days of saline or paracetamol (250mg/kg × 3/day) plus a single or double dose of N-Acetyl Cysteine (NAC) (1200mg/kg) for the paracetamol diet group. Data are expressed as mean ± SEM. βp<0.05 compared to control diet+saline group
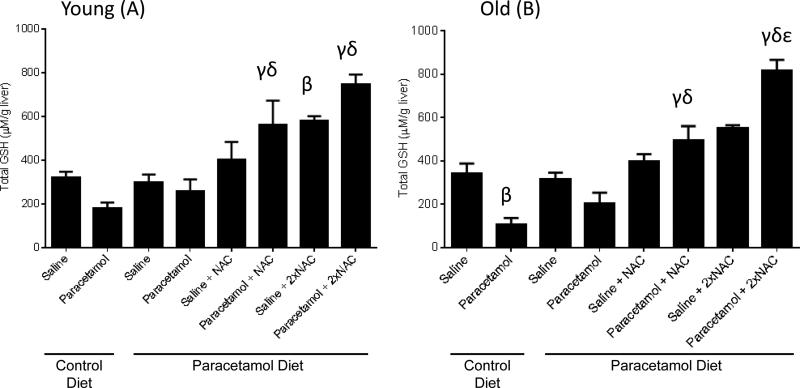
Total liver glutathione levels were reduced with sub-acute paracetamol treatment in control fed old mice (p=0.01), but not young mice (p=0.55) (Figure 6). In both young and old mice, there was a significant increase in GSH levels with both a single and double dose of NAC, in combination with sub-acute paracetamol treatment, compared to sub-acute paracetamol treatment alone, for mice on either a control or paracetamol diet (Figure 6).
Figure 6.
Total liver glutathione (GSH) concentrations for young (A) and old (B) mice fed either a control diet or paracetamol containing (1.33g/kg feed) diet for 6 weeks, then treated with 3 days of saline or paracetamol (250mg/kg × 3/day) plus a single or double dose of N-Acetyl Cysteine (NAC) (1200mg/kg) for the paracetamol diet group. Data are expressed as mean ± SEM. βp<0.05 compared to control diet+saline group; γp<0.05 compared to control diet+paracetamol group; δp<0.05 compared to paracetamol diet+paracetamol group; εp<0.05 compared to paracetamol diet+paracetamol+NAC group
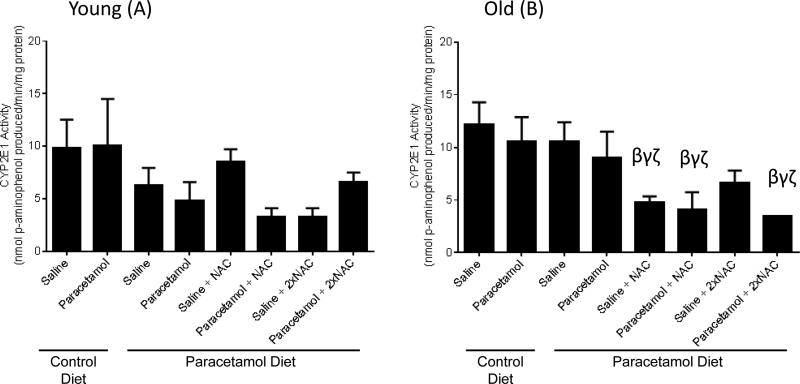
Old mice treated with saline+NAC, paracetamol+NAC or paracetamol+2×NAC had significantly lower CYP2E1 activity levels than old mice treated with saline only (on either control or paracetamol diet). There was no significant change in CYP2E1 activity for young mice with paracetamol or NAC dosing (Figure 7), although a similar trend towards reduced CYP2E1 levels with NAC treatment was seen for some groups.
Figure 7.
Liver cytochrome (CYP)2E1 activity for young (A) and old (B) mice fed either a control diet or paracetamol containing (1.33g/kg feed) diet for 6 weeks, then treated with 3 days of saline or paracetamol (250mg/kg × 3/day) plus a single or double dose of N-Acetyl Cysteine (NAC) (1200mg/kg) for the paracetamol diet group. Data are expressed as mean ± SEM. βp<0.05 compared to control diet+saline group; γp<0.05 compared to control diet+paracetamol group; ζp<0.05 compared to paracetamol diet+saline group.
A three-way ANOVA of age, diet and treatment group showed that there was no significant diet or age effect on paracetamol serum level, total hepatic GSH levels or CYP2E1 activity.
DISCUSSION
Although clinically there are a variety of exposures to paracetamol (staggered, chronic, accidental, supra-therapeutic as well as acute high-dose), there has been very minimal research on the risk of toxicity across these different paracetamol exposures, and how this changes with age. Furthermore, the evidence for the effectiveness of the first-line clinically used paracetamol toxicity treatment, NAC, against non-acute paracetamol exposures is lacking. In this study we successfully modelled three clinically relevant paracetamol exposure situations, in young and old mice: chronic exposure to therapeutic levels of paracetamol, sub-acute exposure to supra-therapeutic doses of paracetamol over 3 days (most often an accidental overdose due to confusion with multiple paracetamol containing medications or dose escalation for unrelieved pain), and a combination of these two exposures. We also tested whether NAC administered after sub-acute dosing to mimic the human clinical situation, would protect against hepatotoxicity. We found that chronic low-dose paracetamol exposure did not cause hepatotoxicity, as measured by serum ALT. Three days of sub-acute exposure caused significant hepatotoxicity, and neither a single nor double dose of NAC protected against this toxicity in young or old mice.
Chronic paracetamol exposure in the diet for six weeks did not cause clinically detectable hepatotoxicity in young or old mice. There was no elevation in serum ALT, or DNA fragmentation, in the paracetamol diet group compared to those fed a control diet. Interestingly, a small number of animals in the paracetamol diet fed groups without sub-acute paracetamol treatment had histological evidence of necrosis and/or inflammation. Although this did not achieve statistical significance., it does suggest that the paracetamol diet may have caused some low-level damage in some mice which is not measurable in the circulation, at least not after 6 weeks of treatment. Previous animal studies that have dosed once daily, via intraperitoneal injection or oral gavage, for 30-99 days with 75-300mg/kg showed no evidence of toxicity as assessed by liver function tests (7–9). Our study, which modeled a more clinically relevant exposure to paracetamol at regular intervals over a full day, as it was consumed in the diet, rather than once daily, appears to confirm these findings. Paracetamol serum levels were below the detectable level for paracetamol diet groups in the current study, despite the mice consuming daily paracetamol doses of up to 188mg/kg. Perhaps future studies modeling clinically therapeutic levels of paracetamol ingestion, may need to use higher daily doses of paracetamol, or a longer treatment period, in order to detect circulating levels of liver toxicity markers.
Sub-acute paracetamol exposure over three days caused significant hepatotoxicity in young and old mice, as measured by biochemistry and histology. However, there was no clear combinatorial toxicity effect of paracetamol exposure in the diet plus sub-acute paracetamol exposure, although dietary pre-exposure to paracetamol certainly did not protect against subsequent sub-acute exposure in this study, as has been seen with higher dose pre-treatment in other studies (10–12). The low daily dose received in the current study may not have been enough to induce the protective pharmacokinetic changes that were seen in the previous studies, as we saw no change in total glutathione or CYP2E1 activity with chronic paracetamol treatment.
The pre-treatment protective effect seen in the previous studies (10–12), was also not seen with the sub-acute paracetamol treatment in the current study. Although the mice were receiving doses of 250mg/kg three times per day for three days, we did not see the protective pharmacokinetic changes seen with pre-treatment by the other studies. In our study, total liver glutathione concentration was unchanged with sub-acute paracetamol treatment in most groups, which is consistent with previous acute paracetamol studies for this time frame (15,28). CYP2E1 activity was not changed with sub-acute paracetamol treatment in any group. Previous acute studies have found CYP2E1 activity to be reduced with 400mg/kg after 4 hours in mice (29). In the studies of multiple paracetamol dose treatment, Shayiq et al. (1999) found that eight days of increasingly high daily doses of paracetamol treatment in BALB/c mice resulted in increased total liver glutathione levels, and reduced CYP2E1 activity, which in term contributed to protection against toxicity from a larger acute dose on the ninth day. However it is important to note that genetic background can affect the outcomes, as Shayig and colleagues saw 100% mortality 24 hours after a dose of 500mg/kg paracetamol in BALB/c mice, whilst the C57BL/6 mice in the current study received 750mg/kg per day for three days with zero mortality. The other multiple paracetamol dose studies were conducted in rats, which also have altered paracetamol pharmacokinetics and toxicology compared to C57BL/6 mice (28), and found protection from toxicity with four days of low dose pre-treatment (10,11), but increased toxicity with one moderate pre-treatment dose 18 hours before a second dose (13). These results demonstrate that time-frame, dose, species and genetic background can significantly affect paracetamol pharmacokinetics and toxicity, and more research is needed to clarify the effect of multi-day paracetamol dosing on susceptibility to hepatotoxicity.
NAC did not protect against hepatotoxicity induced by sub-acute paracetamol exposure in young or old mice. As seen in a second cohort of mice (Figure 4), and in previous studies (18,25), concurrent dosing of acute paracetamol and similar doses of NAC does protect against hepatotoxicity. NAC treatment did result in increased total liver glutathione concentrations in all mice, and decreased CYP2E1 activity, in old mice. These potentially protective pharmacokinetic changes, did not translate into protection in this study. It is likely that with three days of dosing, the paracetamol-induced liver damage has progressed too far for these mechanisms to be protective. The high prevalence of inflammation in the sub-acute paracetamol treated groups would imply that the damage has progressed beyond the early stages of covalent protein NAPQI binding damage, and into the induction of inflammatory damage (14). A recent mouse study found that with delayed treatment, NAC also did not protect against acute paracetamol induced hepatotoxicity (30). NAC treatment also resulted in increased serum paracetamol levels in the current study, which may be explained in part by the decrease in CYP2E1 activity or potentially reduced hepatic uptake of paracetamol. Perhaps, with concurrent paracetamol and NAC gavaging, there was also reduced NAC absorption that may have contributed to the lack of protection seen in this study. This reduced absorption may also contribute to the decrease in CYP2E1 activity observed with NAC dosing. It has previously been shown that CYP2E1 activity is initially down-regulated in acute paracetamol toxicity, and subsequently returns to normal levels (29). In the current study, we may have captured this time-point. This finding of a lack of protection from NAC against sub-acute paracetamol dosing, confirms the clinical observations of, and provides pre-clinical evidence that, for those who have taken staggered doses of paracetamol over several days, NAC may not be effective at preventing paracetamol hepatotoxicity. The high rate of side-effects associated with NAC (31) emphasises the importance of optimising NAC treatment and not using it in patients for whom it will be ineffective or unnecessary (32). This finding has important clinical implications in treating different types of paracetamol exposures, and is particularly important for older patients, as they are more likely to have the type of exposures, as explored here, against which NAC does not protect.
Recent studies have identified new, more specific, markers of drug-induced liver damage that are detectable at any earlier timepoint (3,33). Although we are confident that the large serum ALT increases in the current study represent the extent of liver damage, and this was confirmed by histology, it would be interesting in future studies to measure some of these newly identified markers such as serum microRNA(miR)-122, high mobility group box-1 and keratin-18 (34). Unlike recent observations with isoniazid we did not observe a difference in the type of histological damage with old age, for paracetamol (35). Further investigation of the changes to the histopathology of paracetamol toxicity with both age and exposure type would be interesting in future studies. Furthermore, this study is limited to a single time-point assessment of toxicity, and it would be interesting to assess toxicity over a time-course with sub-acute paracetamol dosing to determine whether liver injury reaches a peak after a certain number of doses, or is cumulative over many doses. In addition, the optimal time-frame for NAC treatment to prevent hepatotoxicity could also be identified with a time-course assessment.
In conclusion, this study found that, in young and old mice, sub-acute paracetamol exposure causes severe hepatotoxicity, which NAC does not prevent. Furthermore, chronic low-level paracetamol exposure does not cause hepatotoxicity in young or old mice. Although it is re-assuring that chronic therapeutic paracetamol exposure does not cause toxicity, the clinical implications of NAC not protecting against sub-acute paracetamol induced toxicity are alarming, and highlight the need to develop new treatments for the growing cohort of older patients with sub-acute paracetamol induced hepatotoxicity.
Supplementary Material
ACKNOWLEDGMENTS
SJM and RdC are supported by the Intramural Research Program of the National Institute on Ageing, National Institutes of Health.
AEK is supported by a National Health and Medical Research Council (NHMRC) biomedical postgraduate scholarship.
This project was partly funded by an Australian Association of Gerontology RM Gibson Scientific Research Grant and by the Penney Ageing Research Unit, Royal North Shore Hospital.
REFERENCES
- 1.Burke A, Smyth E, FitzGerald G. Goodman and Gilman's The Therapeutic Basis of Therapeutics. 11th ed. McGraw-Hill; 2009. [Google Scholar]
- 2.Craig DGN, Bates CM, Davidson JS, Martin KG, Hayes PC, Simpson KJ. Overdose pattern and outcome in paracetamol-induced acute severe hepatotoxicity. Br J Clin Pharmacol. 2011;71(2):273–82. doi: 10.1111/j.1365-2125.2010.03819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferner R, Dear J, Bateman D. Management of paracetamol poisoning. BMJ. 2011;342:d2218. doi: 10.1136/bmj.d2218. [DOI] [PubMed] [Google Scholar]
- 4.Watkins PB, Kaplowitz N, Slattery JT, Colonese CR, Colucci SV, Stewart PW, et al. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: a randomized controlled trial. JAMA. 2006;296(1):87–93. doi: 10.1001/jama.296.1.87. [DOI] [PubMed] [Google Scholar]
- 5.Lane JE, Belson MG, Brown DK, Scheetz A. Chronic acetaminophen toxicity: A case report and review of the literature. J Emerg Med. 2002;23(3):253–6. doi: 10.1016/s0736-4679(02)00526-7. [DOI] [PubMed] [Google Scholar]
- 6.Civan JM, Navarro V, Herrine SK, Riggio JM, Adams P, Rossi S. Patterns of acetaminophen use exceeding 4 grams daily in a hospitalized population at a tertiary care center. Gastroenterol Hepatol. 2014;10(1):27–34. [PMC free article] [PubMed] [Google Scholar]
- 7.Yisarakun W, Supornsilpchai W, Chantong C, Srikiatkhachorn A, Maneesri-le Grand S. Chronic paracetamol treatment increases alterations in cerebral vessels in cortical spreading depression model. Microvasc Res. 2014;94:36–46. doi: 10.1016/j.mvr.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Kondo K, Yamada N, Suzuki Y, Toyoda K, Hashimoto T, Takahashi A, et al. Enhancement of acetaminophen-induced chronic hepatotoxicity in restricted fed rats: a nonclinical approach to acetaminophen-induced chronic hepatotoxicity in susceptible patients. J Toxicol Sci. 2012;37(5):911–29. doi: 10.2131/jts.37.911. [DOI] [PubMed] [Google Scholar]
- 9.de Meijer VE, Kalish BT, Meisel J a., Le HD, Puder M. Dietary Fish Oil Aggravates Paracetamol-Induced Liver Injury in Mice. J Parenter Enter Nutr. 2012;37(2):268–73. doi: 10.1177/0148607112450735. [DOI] [PubMed] [Google Scholar]
- 10.Ghanem CI, Ruiz ML, Villanueva SSM, Luquita M, Llesuy S, Catania V a., et al. Effect of repeated administration with subtoxic doses of acetaminophen to rats on enterohepatic recirculation of a subsequent toxic dose. Biochem Pharmacol. 2009;77(10):1621–8. doi: 10.1016/j.bcp.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 11.O'Brien PJJ, Slaughter MRR, Swain A, Birmingham JMM, Greenhill RWW, Elcock F, et al. Repeated acetaminophen dosing in rats: adaptation of hepatic antioxidant system. Hum Exp Toxicol. 2000;19:277–83. doi: 10.1191/096032700678815918. [DOI] [PubMed] [Google Scholar]
- 12.Shayiq RM, Roberts DW, Rothstein K, Snawder JE, Benson W, Ma X, et al. Repeat exposure to incremental doses of acetaminophen provides protection against acetaminophen-induced lethality in mice: An explanation for high acetaminophen dosage in humans without hepatic injury. Hepatology. 1999;29(2):451–63. doi: 10.1002/hep.510290241. [DOI] [PubMed] [Google Scholar]
- 13.Kim SJ, Lee MY, Kwon DY, Kim SY, Kim YC. Alteration in metabolism and toxicity of acetaminophen upon repeated administration in rats. J Pharmacol Sci. 2009;111(2):175–81. doi: 10.1254/jphs.09151fp. [DOI] [PubMed] [Google Scholar]
- 14.Holt MP, Ju C. Mechanisms of drug-induced liver injury. AAPS J. 2006;8(1):E48–54. doi: 10.1208/aapsj080106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell J, Jollow D, Potter W, Gillette J, Brodie B. Acetaminophen-Induced Hepatic Necrosis IV. Protective Role of Glutathione. J Pharmacol Exp Ther. 1973;187(1):211–7. [PubMed] [Google Scholar]
- 16.Larson AM. Acetaminophen Hepatotoxicity. Clin Liver Dis. 2007;11(3):525–48. doi: 10.1016/j.cld.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Corcoran GB, Racz WJ, Smith CV, R MJ. Effects of N-Acetylcysteine on Acetaminophen Covalent Binding and Hepatic Necrosis in Mice. J Pharmacol Exp Ther. 1985;232(3):864–72. [PubMed] [Google Scholar]
- 18.Daly FFS, Fountain JS, Murray L, Graudins A, Buckley N a. Guidelines for the management of paracetamol poisoning in Australia and New Zealand - Explanation and elaboration. Med J Aust. 2008;188(5):296–301. doi: 10.5694/j.1326-5377.2008.tb01625.x. [DOI] [PubMed] [Google Scholar]
- 19.Chen P, Pang V, Jeng Y, Chen T, Hu F, Chi W, et al. Establishment of a Standardized Animal Model of Chronic Hepatotoxicity Using Acetaminophen-lnduced Hepatotoxicity in the Evaluation of Hepatoprotective Effects of Health Food. J Food Drug Anal. 2012;20(1):41. [Google Scholar]
- 20.Tarloff JB, Khairallah E a, Cohen SD, Goldstein RS. Sex- and age-dependent acetaminophen hepato- and nephrotoxicity in Sprague-Dawley rats: role of tissue accumulation, nonprotein sulfhydryl depletion, and covalent binding. Fundam Appl Toxicol. 1996;30(1):13–22. doi: 10.1006/faat.1996.0038. [DOI] [PubMed] [Google Scholar]
- 21.Rikans LE, Moore DR. Acetaminophen hepatotoxicity in aging rats. Drug Chem Toxicol. 1988;11(3):237–47. doi: 10.3109/01480548809017880. [DOI] [PubMed] [Google Scholar]
- 22.Mach J, Huizer-Pajkos A, Cogger VC, McKenzie C, Le Couteur DG, Jones BE, et al. The effect of aging on acetaminophen pharmacokinetics, toxicity and Nrf2 in fischer 344 rats. Journals Gerontol Ser A Biol Sci Med Sci. 2014;69(4):387–97. doi: 10.1093/gerona/glt095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kane A, Mitchell SJ, Carroll PR, Matthews S, Hilmer SN. Characteristics of older and younger patients with suspected paracetamol toxicity. Australas J Ageing. 2012;31(3):190–3. doi: 10.1111/j.1741-6612.2012.00598.x. [DOI] [PubMed] [Google Scholar]
- 24.Qato DM, Alexander GC, Conti RM, Johnson M, Schumm P, Lindau ST. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA. 2008;300(24):2867–78. doi: 10.1001/jama.2008.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.James LP, McCullough SS, Lamps LW, Hinson J a. Effect of N-acetylcysteine on acetaminophen toxicity in mice: Relationship to reactive nitrogen and cytokine formation. Toxicol Sci. 2003;75(2):458–67. doi: 10.1093/toxsci/kfg181. [DOI] [PubMed] [Google Scholar]
- 26.Roberts BJ, Shoaf SE, Song BJ. Rapid changes in cytochrome P4502E1 (CYP2E1) activity and other P450 isozymes following ethanol withdrawal in rats. Biochem Pharmacol. 1995;49(11):1665–73. doi: 10.1016/0006-2952(95)00098-k. [DOI] [PubMed] [Google Scholar]
- 27.Mach J, Huizer-Pajkos A, Kane A, Jones B, McKenzie C, Mitchell SJS, et al. The effect of aging on mitochondrial and cytosolic hepatic intrinsic death pathway and apoptosis associated proteins in Fischer 344 rats. Exp Gerontol. 2015;67:54–61. doi: 10.1016/j.exger.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGill MR, Williams CD, Xie Y, Ramachandran a, Jaeschke H. Acetaminophen-induced liver injury in rats and mice: comparison of protein adducts, mitochondrial dysfunction, and oxidative stress in the mechanism of toxicity. Toxicol Appl Pharmacol. 2012;264:387–94. doi: 10.1016/j.taap.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snawder JE, Roe AL, Benson RW, Roberts DW. Loss of CYP2E1 and CYP1A2 activity as a function of acetaminophen dose: relation to toxicity. Biochem Biophys Res Commun. 1994;203(1):532–9. doi: 10.1006/bbrc.1994.2215. [DOI] [PubMed] [Google Scholar]
- 30.Soeda J, Mouralidarane A, Ray S, Novelli M, Thomas S, Roskams T, et al. The beta-adrenoceptor agonist isoproterenol rescues acetaminophen-injured livers through increasing progenitor numbers by Wnt in mice. Hepatology. 2014;60:1023–34. doi: 10.1002/hep.27266. [DOI] [PubMed] [Google Scholar]
- 31.Zyoud SH, Awang R, Sulaiman SAS, Al-Jabi SW. N-acetylcysteine-induced headache in hospitalized patients with acute acetaminophen overdose. Fundam Clin Pharmacol. 2011;25(3):405–10. doi: 10.1111/j.1472-8206.2010.00831.x. [DOI] [PubMed] [Google Scholar]
- 32.Bateman DN. Paracetamol poisoning: beyond the nomogram. Br J Clin Pharmacol. 2015;80(1):45–50. doi: 10.1111/bcp.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rumack BH, Bateman DN. Acetaminophen and acetylcysteine dose and duration: past, present and future. Clin Toxicol. 2012;50(2):91–8. doi: 10.3109/15563650.2012.659252. [DOI] [PubMed] [Google Scholar]
- 34.Antoine DJ, Dear JW, Lewis PS, Platt V, Coyle J, Masson M, et al. Mechanistic biomarkers provide early and sensitive detection of acetaminophen-induced acute liver injury at first presentation to hospital. Hepatology. 2013;58(2):777–87. doi: 10.1002/hep.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mach J, Mitchell SJ, Phillips L, Kane A, Jones B, Cabo R De, et al. The effect of ageing on isoniazid pharmacokinetics and hepatotoxicity in Fischer 344 rats. Fundam. Clin. Pharmacol. 2015;30:23–34. doi: 10.1111/fcp.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.