Diabetes mellitus affects more than 347 million people worldwide, leading to the death of about 4.6 million people worldwide every year.[1] Ankle-brachial index screens have determined that the prevalence of peripheral vascular disease (PVD) among diabetics is approximately 20-30%.[2] Of those patients who develop critical limb ischemia, as many as 25% require amputation.[3] The current clinical standard of care for peripheral ischemia includes physical therapy, pharmacological interventions, endovascular stent placement and surgical bypass of stenosed arteries.[4] While these methods provide temporary relief of the patient’s symptoms, there are presently no durable, long-term treatment options for patients with severe PVD and accompanying peripheral ischemia. Regenerative strategies for inducing the growth of new blood vessels have the appeal of addressing the fundamental problems of diffuse disease and microvascular involvement that limit current therapies. Many attempts have been made to induce therapeutic angiogenesis; however, clinical trials with the delivery of growth factors[5], cytokines[6], viral delivery of growth factor genes[7], and implantation of bone marrow cells[8] have only achieved limited success in patients.
One potential reason for these therapeutic failures is that many of the co-morbidities that accompany peripheral ischemia, such as diabetes and hyperlipidemia, also create a state in which there is resistance to angiogenic stimulation. If ischemia occurs in a healthy person, compensatory mechanisms induce angiogenesis to restore perfusion. Thus, if a long-term ischemic condition persists it implies that endogenous pathways have been interrupted or are insufficient to restore blood flow. We have recently explored the concept of “growth factor resistance” in therapeutic angiogenesis and found that many co-receptors for growth factor signaling are altered in the diabetic disease state.[9] Among these co-receptors, syndecan-4 is a cell surface, transmembrane protein that is glycosylated with heparan sulfate glycosaminoglycans. Syndecan-4 acts a co-receptor for fibroblast growth factor-2 (FGF-2) and other growth factors, as well as regulating myriad cellular functions including migration, proliferation and homeostasis.[10] Here, we examined whether the delivery exogenous syndecan-4 could improve angiogenic therapy for ischemia in diabetes. We demonstrate that delivery of syndecan-4 proteoliposomes markedly improve revascularization in diabetic, hyperlipidemic animals and leads to immune modulation to increase macrophage polarization to the pro-angiogenic M2 phenotype.
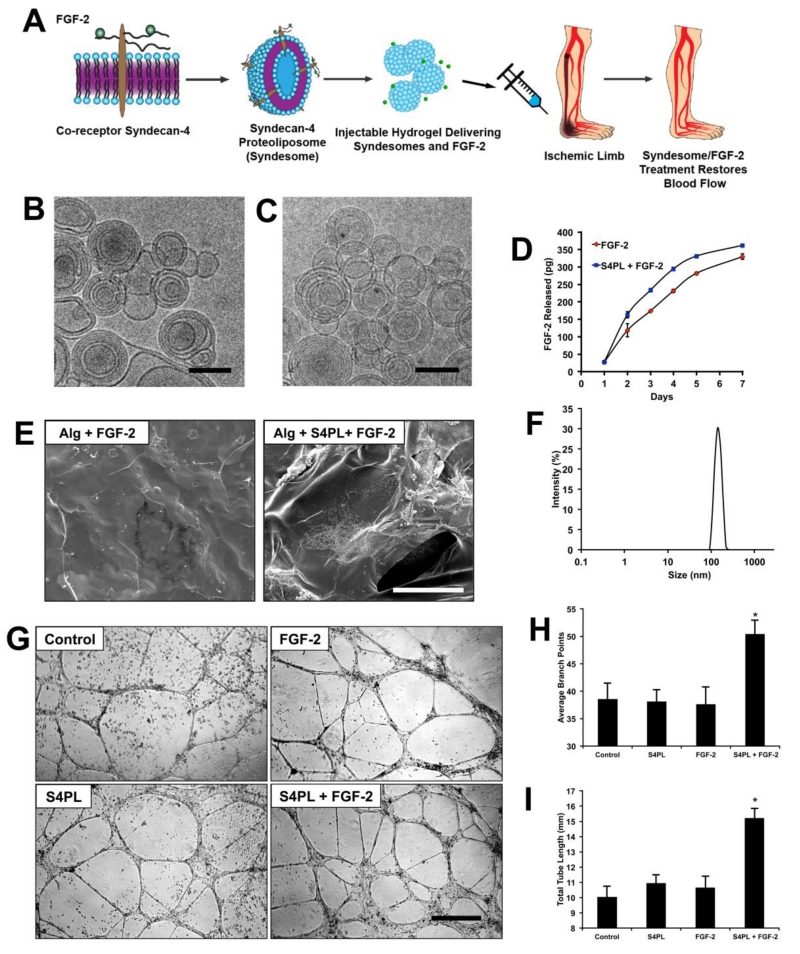
We have previously shown that there is a reduction in protein expression of key growth factor signaling molecules including co-receptors like syndecan-4.[9] Syndecan-4 plays an important role in the FGF-2 signaling pathway.[10] Our strategy to circumvent the reduction in syndecan-4 levels was to deliver the missing syndecan-4 co-receptors embedded in a liposomal membrane to enhance the activity of FGF-2 therapy (Figure. 1A). We synthesized syndecan-4 proteoliposomes (S4PL) by incorporating the pure syndecan-4 protein in the 400nm liposomes by serial dilution. We used dynamic light scattering (DLS) to confirm the size of the syndecan-4 proteoliposomes that was found to be around 400 nm (Figure. 1F) and cryo-EM to verify the shape and size of the proteoliposomes (Figure. 1B, C). For delivery of the compounds in vivo, we decided to use an alginate hydrogel due to its biocompatibility, regulatory approval and ability to be made injectable. The release kinetics of FGF-2 from the alginate gels with or without syndecan-4 proteoliposomes (S4PL) was characterized and we found that S4PL with FGF-2 releases slightly more FGF-2 over 7 days (Figure. 1D). We probed the structural features of the alginate gels encapsulating the treatments before being used for the animal studies. Therefore we lyophilized the alginate gels and imaged them with SEM and found no significant structural differences (Figure. 1E). Finally, we performed an in vitro tubule formation assay using human, diabetic endothelial cells and found that syndecan-4 proteoliposomes improved the FGF-2 activity by 24 hours of treatment (Figure. 1G). Specifically, we found increased numbers of average branch points (Figure. 1H) and total tubule length (Figure. 1I).
Figure 1. Syndecan-4 proteoliposomes enhance tubule formation in diabetic endothelial cells.
(A) Graphical representation of the delivery mechanism where the recombinant syndecan-4 embedded in the membrane is co-delivered with the FGF-2. (B, C) Cryo EM image of the empty liposomes and syndecan-4 proteoliposomes, respectively. Bar = 100 nm. (D) Release kinetics of FGF-2 from alginate beads with FGF-2 only (red) or alginate with S4PL+FGF-2 (blue). (E) SEM (scanning electron microscopy) image of the lyophilized alginate gel with FGF-2 only or FGF-2 with syndecan-4 proteoliposomes. (F) Dynamic light scattering showing the size of the liposomes. (G) Tube formation assay on with control, FGF-2, syndecan-4 proteoliposomes (S4PL) and S4PL with FGF-2. (H, I) Average number of branch points and total tube length respectively in various treatment groups. *Statistically different from the all other treatment groups (p < 0.05; n = 10).
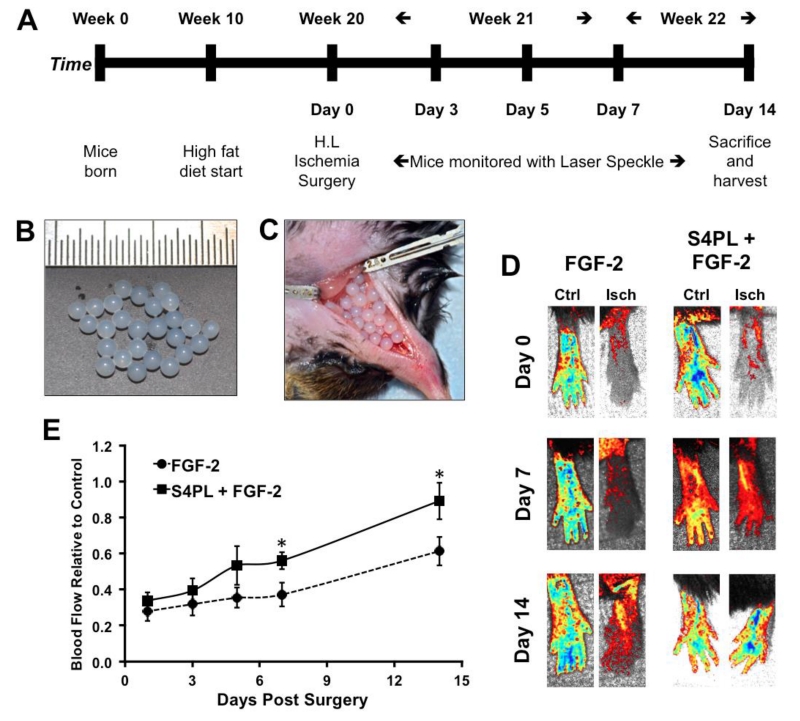
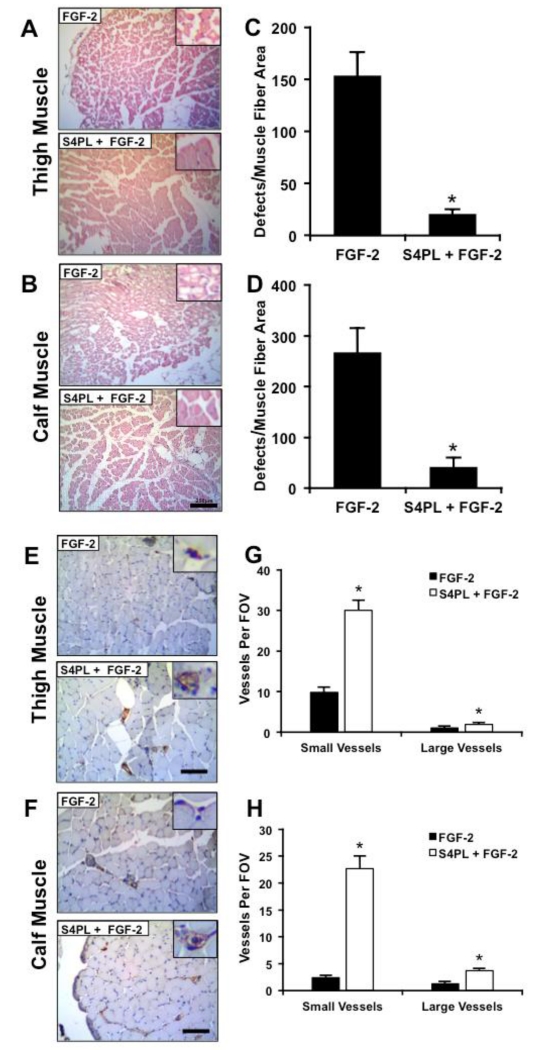
Ischemia is a major contributing factor to the development of non-healing wounds and diabetic ulcers.[11] To examine whether syndecan-4 proteoliposomes could significantly overcome growth factor resistance in an animal model, we placed ob/ob mice on high fat diet for 10 weeks to create a severe diseased state (Figure. 2A). We then induced hind limb ischemia in the mice through femoral artery ligation and subsequent removal of a portion of the artery. We implanted alginate beads containing FGF-2 or FGF-2 with syndecan-4 proteoliposomes in the ischemic region (Figure. 2B-D). We tracked the recovery in perfusion of the mice feet using laser speckle contrast imaging over 14 days (Figure. 2E). A quantitative analysis of the perfusion in the ischemic foot relative to the contralateral control foot revealed a marked increase in the perfusion in the mice treated with syndecan-4 proteoliposomes with FGF-2 (about 86% recovery for the syndecan-4 proteoliposomes/FGF-2 group versus approximately 50% recovery in the FGF-2 group; Figure. 2F). After 14 days, the mice were sacrificed and histological analysis of the thigh and calf muscles demonstrated a reduction in the ischemic changes in the muscle fibers for the syndesome treated mice (Figure. 3A-D). We performed immunostaining targeted for endothelial cells using a von Willebrand factor (vWF) antibody and found significantly increased vascularity for both large and small vessels in the thigh and calf muscles (Figure. 3E-H). The increase in vascularity in the thigh and calf muscle (Figure. 3G, H) was more pronounced compared to blood perfusion in the feet (Figure. 2E). This difference may be due to the presence of immature vessels or due to the fact that the speckle imaging method only measures perfusion in the skin whereas histological analysis can assess the entire tissue including the muscle.
Figure 2. Syndecan-4 proteoliposomes increase blood flow in the ischemic hind limb of ob/ob mice on a high fat diet.
(A) Study design of the in vivo experiment. (B) Treatments were encapsulated in 4% alginate beads. (C) The beads were placed in the incision site after the femoral artery ligation. (D) Laser speckle contrast imaging of the perfusion of the feet of mice following induction of ischemia. (E) Quantitative analysis of perfusion of the ischemic foot normalized to the contralateral control foot. *Statistically different from the FGF-2 only treatment group (p < 0.05; n = 8).
Figure 3. Syndecan-4 proteoliposomes with FGF-2 reduce muscle damage and enhance angiogenesis in the ischemic tissue.
(A, B) Histological sections from the thigh and calf muscle of the ischemic limbs (Bar = 250 μm). Inset is magnified threefold. (C, D) Quantification of the number of muscle fiber with defects in the thigh and calf muscle of the mice respectively. (E, F) Immunostaining for von Willebrand factor (vWF), which is a marker for endothelial, cells in calf muscle sections from the ischemic hind limbs (Bar = 125 μm). Inset is magnified threefold. (G, H) Quantification of the number of vessels per field of view (20× magnification image) in thigh and calf muscle sections. *Statistically different from the FGF-2 only treatment group (p < 0.05; n = 8).
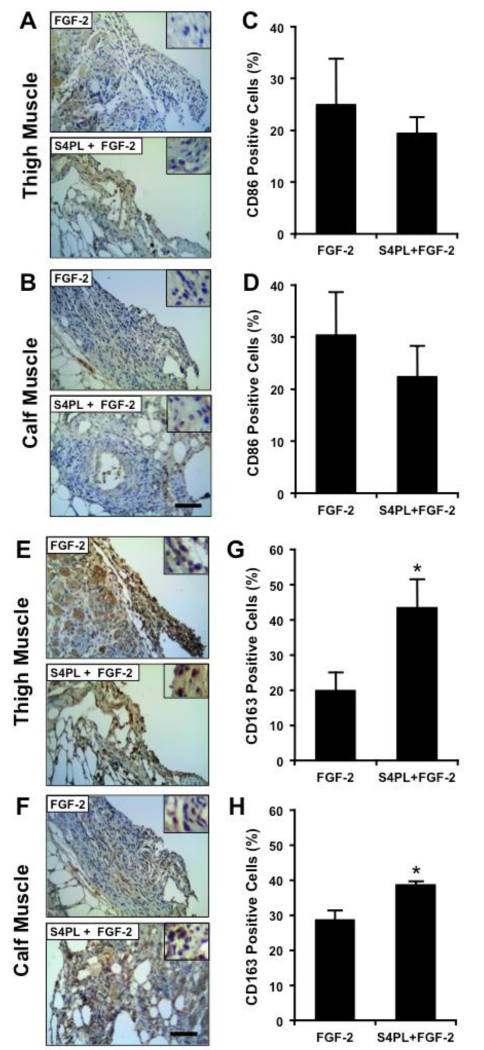
Macrophages are a key cell type in orchestrating the angiogenic and healing responses in ischemic tissues.[12] We used immunostaining to examine the expression of the M1 macrophage marker CD86 and the M2 macrophage marker CD163 in the tissue infiltrates surrounding the ischemic injury in the ob/ob mice after 14 days. There was a non-significant trend of decreased CD86 in both the thigh and calf muscles of the syndesome and FGF-2 treated mice in comparison to those treated with FGF-2 alone in both thigh (Figure. 4A, C) and calf muscle (Figure. 4B, D). In addition, we found an increase in the M2 macrophage marker CD163 in the thigh (Figure. 4E, G) and calf muscle (Figure. 4F, H) of the mice treated with syndecan-4 proteoliposomes and FGF-2. Notably, there was a significant number of CD163 positive M2 macrophages even at day 14 following ligation, suggesting that active healing was still taking place. These cells would likely aid healing and may be present late into the revascularization process due to the obese ob/ob mouse model used in the studies.
Figure 4. Syndesome and FGF-2 treated limbs have increased staining for M2 macrophage markers.
(A, B) Thigh and calf muscle sections immunostained for CD86 (M1 macrophage marker). (C, D) Quantification of percentage of CD86 positive cells in the thigh and calf muscle. (E, F) Immunostaining for CD163 (M2 macrophage marker) in the thigh and calf muscle. (G, H) Quantification of percentage of CD163 positive cells in the thigh and calf muscle sections. *Statistically different from FGF-2 only group (p <0.05; n = 8). Scale bars = 125 μm and insets are magnified by threefold.
In this study, we have shown that co-therapy with syndecan-4 is effective in enhancing revascularization in the ischemic hind limb of diabetic, obese ob/ob mice. We have previously demonstrated that this mouse model is resistant to growth factor therapy and has reduced expression of syndecan-4.[9] Here, we examined whether delivery of exogenous syndecan-4 could improve therapeutic angiogenesis in the diabetic disease state. We demonstrate that the syndecan-4 proteoliposomes increase the recovery of perfusion and vessel density following femoral ligation in diabetic, ob/ob mice. This increased angiogenic response was accompanied by an increase in markers for M2-polarized macrophages that have been associated with neovascularization. Together, these results demonstrate that co-delivery of syndecan-4 significantly improves the therapeutic potential of FGF-2 in a diabetic model of peripheral ischemia. As syndecan-4 is a major co-receptor in many growth factor signaling pathways, the therapy may have many applications in treating the complications of diabetes including enhancing non-healing wounds and blood vessel growth in myocardial ischemia.
EXPERIMENTAL SECTION
Preparation of syndecan-4 proteoliposomes
To prepare liposomes, stock solutions (10 mg/ml) of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), cholesterol and sphingomyelin were prepared in chloroform. The lipids were mixed in the volumetric ratio 2:1:1:1 in a round bottom flask and the chloroform was removed on rotatory evaporator attached to a vacuum pump. Liposomes were resuspended in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer by vortexing, sonication and freeze thawing. Finally an extruding device (Avanti Polar Lipids) with polycarbonate membranes (400 nm) was used to prepare liposomes. A mild detergent, n-octyl-β-D-glucopyranoside (0.1% w/v) was added to both the liposomes and the recombinant syndecan-4. The protein and liposomes were then combined and the detergent was removed by timed serial dilution, dialysis and treatment with Biobeads (Biorad).
Characterization of proteoliposomes
The size of the liposomes was measured using dynamic light scattering (DLS) using a Zetasizer Nano ZS (Malvern). The instrument was calibrated for size using polystyrene beads (54 nm) before measurement. For imaging with cryo-electron microscopy, the liposomes were plunge-frozen in liquid ethane on carbon holey film grids as previously described (R2×2 Quantifoil®; Micro Tools GmbH, Jena, Germany).[13] The grids were transferred to a cryo-specimen holder (Gatan 626) under liquid nitrogen and put in a microscope (JEOL 2100 LaB6, 200 keV). Grids were maintained at close to liquid nitrogen temperatures during EM session (−172°C to −180°C). Liposomes were imaged at 20,000× EM magnification with a CCD camera (UltraScan 895, GATAN, Inc.) using low-dose imaging procedure. Images were acquired with less than 20 electrons/Å2 electron dose.
Preparation and characterization of alginate gels
A sodium alginate (Sigma) solution (4% w/v) was prepared in sterile water. The syndecan-4 proteoliposomes and FGF-2 were mixed in the alginate solution as needed. The treatments were loaded in syringes (5 ml) with a 20G needle and extruded drop wise as beads into calcium chloride solution (1.1%) and crosslinked for 1 hour at 4°C. These gels were either used for the release studies or the in vivo hind limb ischemia study.
Tube Formation Assay
Human microvascular endothelial cells isolated from adult skin of type 2 diabetes patients were purchased from Lonza. They were grown in MCDB 131 media with endothelial supplements (Lonza). The cells (passage 2) were grown on growth factor reduced Matrigel (Corning) with various treatments and the plates were imaged over a period of 24 hours using an inverted phase contrast microscope (Nikon). The length of the tubule, number of branch points and the total number of tubules were quantified on Metamorph (Molecular Devices).
Hind limb ischemia model
All animal experiments were performed with the approval of the Institutional Animal Care and Use Committee (IACUC) of University of Texas at Austin and in accordance with NIH guidelines “Guide for Care and Use of Laboratory Animals” for animal care. All the animal experiments were performed on a diabetic, obese and hyperlipidemic ob/ob mouse model (B6.Cg-Lepob/J; Jackson Laboratories). The mice were fed a high fat diet (D12331; Research Diets) for 10 weeks before performing the hind limb ischemia studies. To induce ischemia in the hind limb of the mice, an incision was made along the midline of the thigh and the femoral artery was separated from the vein and nerve. The artery was ligated with two 6-0 silk sutures as described in our previous study.[14] Alginate beads were then applied directly to the region surrounding the femoral artery and the incision site was closed using surgical staples. The feet of the mice were imaged at 1, 3, 5, 7 and 14 days using laser speckle contrast imaging as described below. At day 14, mice were euthanized and the gastrocnemius (calf) and quadriceps (thigh) muscles of both ischemic and contralateral control limbs were harvested. Tissues were snap frozen in liquid N2-chilled isopentane and stored at −80°C until further analysis.
Laser speckle contrast imaging
A custom-made laser speckle contrast imager (LSCI) was used to image the tissue blood flow non-invasively over the course of the study as previously described.[15] The sample was illuminated with a laser diode (Thor Labs, 785nm, 50mW) and the images captured using a Zoom-7000 lens (Navitar) linked to a Bassler CCD camera (Graftek). The blood perfusion in the ischemic limb (hind-limb ischemia surgery) was quantified relative of the contralateral control limb. Both limbs were imaged simultaneously using a diffusely focused laser.
Histological analysis and immunostaining
The frozen tissues were fixed in paraformaldehyde (4% w/v) and transferred into ethanol (70% w/v). The samples were then paraffin embedded and sectioned using a microtome. Muscle sections were then stained with Hematoxylin and Eosin (H&E) stain for anatomical features.[9] The samples were also immunostained using antibodies staining for von Willebrand factor (1:1000; DAKO), CD86 (1:250; Bioss) and CD163 (1:100; Bioss). The staining was detected using DAKO Envision kit using a 3,3′-di-amino benzidine (DAB) substrate. The vessel density was quantified by manually measuring the positively stained cells on Metamorph (Molecular Devices). Cells positive for CD86 or CD163 positive cells were quantified using the online tool “ImmunoRatio,” an automated image analysis software for DAB immunostained images.[16]
ACKNOWLEDGEMENTS
The authors gratefully acknowledge support through the American Heart Association (10SDG2630139), the Welch Foundation (F-1836) and the NIH Director’s New Innovator Grant (1DP2 OD008716-01) to A.B.B. This work was also supported through the NIH (EB-011556, NS-078791, NS-082518), NSF (CBET-0644638), American Heart Association (14EIA18970041), and the Coulter Foundation grants to A.K.D. A.B.B and S.D. are guarantors of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
CONFLICTS OF INTEREST.
The authors have filed a patent application on the compounds and methods described in this work.
REFERENCES
- [1].American Diabetes Association Diabetes Care. 2013;36:1033. [Google Scholar]
- [2].Marso SP, Hiatt WR. J Am Coll Cardiol. 2006;47:921. doi: 10.1016/j.jacc.2005.09.065. [DOI] [PubMed] [Google Scholar]
- [3].O’Hare AM, Glidden DV, Fox CS, Hsu CY. Circulation. 2004;109:320. doi: 10.1161/01.CIR.0000114519.75433.DD. [DOI] [PubMed] [Google Scholar]
- [4].Weinberg MD, Lau JF, Rosenfield K, Olin JW. Nat Rev Cardiol. 2011;8:429. doi: 10.1038/nrcardio.2011.81. [DOI] [PubMed] [Google Scholar]
- [5].Lederman RJ, Mendelsohn FO, Anderson RD, Saucedo JF, Tenaglia AN, Hermiller JB, Hillegass WB, Rocha-Singh K, Moon TE, Whitehouse MJ, Annex BH, Investigators T. Lancet. 2002;359:2053. doi: 10.1016/s0140-6736(02)08937-7. [DOI] [PubMed] [Google Scholar]; Lazarous DF, Unger EF, Epstein SE, Stine A, Arevalo JL, Chew EY, Quyyumi AA. J Am Coll Cardiol. 2000;36:1239. doi: 10.1016/s0735-1097(00)00882-2. [DOI] [PubMed] [Google Scholar]
- [6].van Royen N, Schirmer SH, Atasever B, Behrens CY, Ubbink D, Buschmann EE, Voskuil M, Bot P, Hoefer I, Schlingemann RO, Biemond BJ, Tijssen JG, Bode C, Schaper W, Oskam J, Legemate DA, Piek JJ, Buschmann I. Circulation. 2005;112:1040. doi: 10.1161/CIRCULATIONAHA.104.529552. [DOI] [PubMed] [Google Scholar]
- [7].Nikol S, Baumgartner I, Van Belle E, Diehm C, Visona A, Capogrossi MC, Ferreira-Maldent N, Gallino A, Wyatt MG, Wijesinghe LD, Fusari M, Stephan D, Emmerich J, Pompilio G, Vermassen F, Pham E, Grek V, Coleman M, Meyer F, investigators T. Mol Ther. 2008;16:972. doi: 10.1038/mt.2008.33. [DOI] [PubMed] [Google Scholar]; Morishita R, Makino H, Aoki M, Hashiya N, Yamasaki K, Azuma J, Taniyama Y, Sawa Y, Kaneda Y, Ogihara T. Arterioscler Thromb Vasc Biol. 2011;31:713. doi: 10.1161/ATVBAHA.110.219550. [DOI] [PubMed] [Google Scholar]; Kusumanto YH, van Weel V, Mulder NH, Smit AJ, van den Dungen JJ, Hooymans JM, Sluiter WJ, Tio RA, Quax PH, Gans RO, Dullaart RP, Hospers GA. Hum Gene Ther. 2006;17:683. doi: 10.1089/hum.2006.17.683. [DOI] [PubMed] [Google Scholar]; Shigematsu H, Yasuda K, Iwai T, Sasajima T, Ishimaru S, Ohashi Y, Yamaguchi T, Ogihara T, Morishita R. Gene Ther. 2010;17:1152. doi: 10.1038/gt.2010.51. [DOI] [PubMed] [Google Scholar]
- [8].Matoba S, Tatsumi T, Murohara T, Imaizumi T, Katsuda Y, Ito M, Saito Y, Uemura S, Suzuki H, Fukumoto S, Yamamoto Y, Onodera R, Teramukai S, Fukushima M, Matsubara H. Am Heart J. 2008;156:1010. doi: 10.1016/j.ahj.2008.06.025. [DOI] [PubMed] [Google Scholar]
- [9].Das S, Singh G, Baker AB. Biomaterials. 2014;35:196. doi: 10.1016/j.biomaterials.2013.09.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Elfenbein A, Simons M. J Cell Sci. 2013;126:3799. doi: 10.1242/jcs.124636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bruhn-Olszewska B, Korzon-Burakowska A, Gabig-Ciminska M, Olszewski P, Wegrzyn A, Jakobkiewicz-Banecka J. Acta Biochim Pol. 2012;59:507. [PubMed] [Google Scholar]
- [12].Koh TJ, DiPietro LA. Expert Rev Mol Med. 2011;13:e23. doi: 10.1017/S1462399411001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sherman MB, Guenther RH, Tama F, Sit TL, Brooks CL, Mikhailov AM, Orlova EV, Baker TS, Lommel SA. J Virol. 2006;80:10395. doi: 10.1128/JVI.01137-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jang E, Albadawi H, Watkins MT, Edelman ER, Baker AB. Proc Natl Acad Sci U S A. 2012;109:1679. doi: 10.1073/pnas.1117885109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Parthasarathy AB, Tom WJ, Gopal A, Zhang X, Dunn AK. Opt Express. 2008;16:1975. doi: 10.1364/oe.16.001975. [DOI] [PubMed] [Google Scholar]
- [16].Tuominen VJ, Ruotoistenmaki S, Viitanen A, Jumppanen M, Isola J. Breast Cancer Res. 2010;12:R56. doi: 10.1186/bcr2615. [DOI] [PMC free article] [PubMed] [Google Scholar]