Abstract
2- Deoxy–D-Glucose (2-DG) is being developed as a potential anticonvulsant and disease-modifying agent for epilepsy patients; however, during preclinical development, cardiac toxicity has been encountered in rats. This study was performed to determine whether cardiac troponin (cTnI and cTnT), Atrial Natriuretic Peptide (ANP), Brain Natriuretic Peptide (BNP), N-terminal pro-brain natriuretic peptide (NT-proBNP) and/or creatine kinase (CK) could be useful as indicators of 2-deoxy-D-glucose (2-DG) cardiac toxicity. In addition, this study also investigated the association of cardiac histopathological changes with these biomarkers. F344 rats (four/sex/group/sacrifice point) were gavaged with either vehicle or 2-DG (50, 125, or 375 mg/kg BID; total daily dose of 100, 250 or 750 mg/kg/day) for 7, 14, 21, or 45 days followed by a 15 day recovery. Dose dependent increases in NT pro-BNP and BNP plasma concentrations were observed. Following recovery period, the NT pro-BNP and BNP concentrations returned to baseline levels. There were no remarkable increases in CK, ANP, cTnI, or cTnT concentrations. There were no gross cardiac lesions observed at the necropsy. Microscopic findings of vacuolar degeneration and hypertrophy of the endothelial cells of the endocardium were present in the heart at doses of 250 and 750 mg/kg/day. Microscopic findings, in general, were associated with increases in NT pro-BNP levels. Cardiac toxicity appeared to be reversible. In conclusion, NT-proBNP and BNP are potential early biomarkers for 2-DG induced cardiac toxicity that can be useful to monitor 2-DG therapy in clinical trials.
Keywords: 2-Deoxy-D-glucose, NT pro-BNP, BNP, vacuolar degeneration
INTRODUCTION
The potential mechanisms associated with anticonvulsant and antiepileptic properties of 2-Deoxy–D-Glucose (2DG) were discovered while investigating mechanisms of ketogenic diet which has been used for decades as a treatment for intractable epilepsy in patients (1, 2). However, ingestion of even a small amount of carbohydrate can rapidly reduce effectiveness of the ketogenic diet and result in seizure recurrence (3). This clinical finding suggested that inhibition or reduction of glycolysis might have anticonvulsant effects; which was then confirmed in in-vitro and in-vivo experiments with 2-DG, a known inhibitor of glycolysis (2,4,5). These results suggest that 2-DG could be a promising, novel compound for further development and introduction into humans for anticonvulsant and antiepileptic therapy.
As an analogue of glucose, uptake of 2-DG into cells occurs through glucose transporters and is dependent on metabolic demand which is higher in neural circuitry undergoing synchronization during seizures. After uptake via the glucose transporter, 2-DG is phosphorylated at the 6 position to 2DG-6P. But, unlike glucose-6P, 2DG-6P cannot be further metabolized by glucose-6P isomerase (GPI) and is “trapped” in cells due to limited dephosphorylation by phosphatases, inhibiting subsequent steps of glycolysis (6).
Minor et al. (7) have performed a long-term dietary toxicity study of synthetic 2-DG at doses ranging from 20 to 300 mg/kg (0.04–0.6 % 2DG in the diet) in F344 and Brown Norway rats which identified cardiac toxicity associated with use of 2-DG as a potential calorie restriction mimetic and for other therapeutic applications. The study demonstrated that 2-DG increases mortality of male Fischer-344 rats, increases incidence of pheochromocytoma in the adrenal medulla, reduces weight gain secondary to reduced food intake, and increases vacuolation of cardiac myocytes with an increase in autophagic flux.
The findings of Minor et al. (2010) prompted a need to better understand 2-DG induced cardiac toxicity and to develop a cardiac safety biomarker for monitoring 2-DG therapy. Cardiac biomarkers e.g., cardiac troponin (cTnI and cTnT), Atrial Natriuretic Peptide (ANP), Brain Natriuretic Peptide (BNP), N-terminal pro-brain natriuretic peptide (NT-proBNP) and/or creatine kinase (CK) are proteins secreted into blood when myocardial injury occurs and play a crucial role in diagnosis, specific risk analysis, and personalized treatment of patients suffering from various cardiac anomalies (8). ANP is secreted by stretching of the atria or with increase in blood or extracellular fluid volume (9). BNP is another natriuretic hormone secreted by cardiac ventricles and functions locally to reduce ventricular fibrosis (10). BNP is synthesized and stored as prohormone, and after secretion is cleaved into equimolar amounts of active BNP and NT-proBNP. BNP and NT-proBNP are validated biomarkers for diagnosis and exclusion of congestive heart failure (11,12). Diagnosis of acute myocardial infarction can be achieved by use of troponins (13) and CK. The troponin complex has three components including cTnC, cTnI and cTnT that engage with components of tropomyosin and actin filaments to safeguard contraction of cardiac and striated muscles (14). All isoforms of cTnI are expressed solely in cardiomyocytes (15). CK is an 86-kilodalton dimeric enzyme found in heart muscle, brain, and skeletal muscle which catalyzes the reaction of creatine and adenosine triphosphate to form phosphocreatine and adenosine diphosphate to generate energy at a cellular level (16).
It has been clinically established that troponin serves as a biomarker for drugs causing cardiac toxicity (17). Troponin I and T secretion has been associated with neonatal rat cardiomyocyte damage (18). Empirically NT-pro BNP alone in human patients (19) or troponin T and NT pro-BNP both in rat models (20) have been observed to be early markers of anthracycline and amitriptyline induced cardiovascular toxicity respectively.
Previous studies have indicated that other cardiac biomarkers such as BNP and NT pro BNP secreted by heart ventricles due to muscular stretching or cardiac damage may be useful prognostic biomarkers for cardiac abnormalities (21,22). There are reports wherein NT pro-BNP has been found to be of prognostic value as an early marker in breast cancer patients on chemotherapy experiencing anthracycline induced cardiotoxicity (23).
The goal of this study was to reproduce 2-DG induced cardiac toxicity using a clinically relevant route/dosing schedule in a rat model and to identify potential cardiac biomarkers that can be useful to monitor cardiac toxicity during clinical evaluation of 2-DG.
EXPERIMENTAL DESIGN
Twenty rats/sex were assigned to each dose group including control, low, mid and high. Animals were gavaged either vehicle or 2DG at target doses of 50, 125, or 350 mg/kg twice daily (BID), for total daily doses of 100, 250, or 750 mg/kg 2DG, respectively (Table 1). Animals received a dose volume of 10 mL/kg per administration approximately 6 hours apart. On the day of scheduled necropsy, animals received only the morning dose of vehicle or test article, and core groups were terminated approximately 4 hours post-dose.
Table 1.
Experimental Design
| Group | Target Dosea (mg/kg/day) |
Total Number Of Animals (M/F)b |
Number of Core/Recovery
Rats For Scheduled Necropsy (M/F) |
|||||
|---|---|---|---|---|---|---|---|---|
| Day 7 |
Day 14 |
Day 21 |
Day 22 |
Day 45 |
Day 60 |
|||
| 1-Vehicle | 0 | 20/20 | 4/4 | 4/4 | 4/4 | 0/0 | 4/4 | 4/4 |
| 2-Low | 100 | 20/20 | 4/4 | 4/4 | 4/4 | 0/0 | 4/4 | 4/4 |
| 3-Mid | 250 | 20/20 | 4/4 | 4/4 | 4/4 | 0/0 | 4/4 | 4/4 |
| 4-High | 750 | 20/20 | 4/4 | 4/4 | 4/4 | 0/3 | 0/0 | 4/4 |
Total dose per day. Groups 1, 2, 3, and 4 were administered target doses of 0, 50, 125, and 375 mg/kg/dose twice daily, respectively. Target dose volumes for all animals in Groups 1 through 4 was 10 mL/kg.
Total number of animals includes those died prior to scheduled termination.
Endpoints reported in this study included cardiac biomarkers, and anatomical pathology of heart tissue. The four rats/sex/dose group/day, were scheduled for terminal necropsy on Study Days 7, 14, 21, and 45, with four/sex recovery animals per dose group scheduled for terminal necropsy on Study Day 60. Due to unscheduled deaths in the high dose group (750 mg/kg/day), surviving animals were either maintained without dosing until the end of the recovery period on Study Day 60 (four per sex), or were scheduled for early termination and necropsy on Study Day 22 (three females).
MATERIALS AND METHODS
Formulation Preparation
The test article formulations were prepared weekly at concentrations of 0, 5, 12.5, and 37.5 mg/mL of test article (purity ≥ 98%) in deionized water. Test article was purchased from Sigma-Aldrich, Saint Louis, MO. Dose formulation concentration analyses were performed for the first preparation using HPLC/UV detection. In addition, 9 days formulation stability of 5, 25 and 125 mg/mL 2DG concentration was determined when stored at room temperature and protected from light. Target doses were 0, 100, 250 and 750 mg/kg/day and target formulation concentrations were 0, 5, 12.5, and 37.5 mg/mL.
Test System
A total of 80 Fischer-344 rats/sex of approximately 9 weeks of age with 127–232 g (males) and 104–156 g (females) weights were purchased from Charles River Laboratories, Inc., (Stone Ridge, NY). Prior to use on the study, all animals were quarantined for 8 days to evaluate health status.
Housing and Environmental Conditions
Polycarbonate cages with hardwood bedding were used to house individual animals according to current Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International standards and current requirements stated in the Guide for the Care and Use of Laboratory Animals [National Research Council (NRC)]. Twelve hours of light/dark cycle and a minimum of ten room air changes per hour with the room temperature and relative humidity of 64 to 79°F and 30 to 70 percent respectively were used. Cages and feeders were changed and sanitized at least weekly.
Food and Water
A pelleted certified feed (Harlan Teklad 2018C) was fed to all rats ad libitum. Water was provided ad libitum from the West Jefferson, Ohio, municipal water supply facility. The water conformed to the current United States Environmental Protection Agency (EPA) drinking water standards. No known or reported contaminants in either the water or feed affected the study results or interpretations.
Group Assignment and Animal Identification
Animals were identified by cage card with a pre-test identification number and permanent tail tattoo. Cage cards were color coded by group. Body weights were obtained from individual animals and group assignments were made using the Xybion Next Generation PATH/TOX System which ensured similar group mean body weights by sex.
Clinical Observations
During the study, twice daily observations for morbidity / moribundity and mortality were performed on all animals with additional cage-side observations at least once each week.
Cardiac Biomarkers Analysis
Whole blood was collected under CO2/O2 anesthesia into tubes containing EDTA as an anticoagulant on study days 2, 7, 14, 21, 22, 45, and 60. Specimens were collected and preserved as single aliquots and stored in a freezer set to maintain −70°C. Serum was collected in tubes containing serum separator gel. The cardiac safety biomarkers analyzed were cTnI, cTnT, BNP, NT-proBNP, ANP and CK. The BNP, NT-proBNP, and cTnI, and cTnT were analyzed using biomarker kits from Mesoscale Diagnostics (catalog numbers of K153KFD, K153JKD, and K15161C respectively) while ANP was analyzed using biomarker kit from Shanghai BlueGene Biotech (catalog number of E02A0493). The BNP, NT-proBNP, cTnI, and cTnT assay plates were read by the MesoScale Diagnostic’s Sector Imager 2400 model 1250 and the ANP assay plates were read by the μQuant plate reader using KC4 software. The data were then transferred to Softmax Pro 5 for generation of the reference curves and sample analysis. All assay reference curves were qualified to determine if the assay kits were performing as per the manufacturer instructions. Each reference curve was analyzed a minimum of twelve times in duplicate to demonstrate reproducibility.
Necropsy
Core study animals were necropsied at approximately 4 hours post-dose on Study Days 7, 14, 21, 22, 45 and remaining four recovery animals/sex/group were necropsied on Study Day 60. All animals were individually weighed, anesthetized with CO2/O2 for blood collection prior to necropsy.
Every necropsy comprised an examination of the external body surface; all orifices including the cranial, thoracic, abdominal, and pelvic cavities and their contents. Heart tissues were collected and preserved in 10 percent neutral buffered formalin (NBF).
Heart tissues from all groups necropsied were processed to slides and stained with hematoxylin and eosin for histopathologic examination.
Statistical Analysis
Statistical differences for cTnI, cTnT, BNP, NT-proBNP, and ANP data were assessed using Student T test assuming equal variance (MicroSoft Excel Program). CK data were analyzed based on homogeneity / non homogeneity by Bartlett’s test. All data are expressed as mean ± standard deviation and the statistical significance level was set at p ≤ 0.05.
RESULTS
Dose Formulation Analysis
Formulations of 2-DG prepared in deionized water were within 10 percent of targeted concentrations of 0, 5, 12.5, and 37.5 mg/mL with a 9 day stability confirmed for 5, 25, and 125 mg/mL 2-DG concentration when stored at room temperature and protected from light.
Survival and clinical observations
In the high dose group (750 mg/kg/day), one male (Study day 19), two males (Study day 21), one male and one female (Study day 22) were found dead. Due to severe toxicity, dose administration for the high dose treatment was stopped on Study Day 21 and remaining three females were sacrificed on Study day 22. Clinical observations of lethargy, rough coat, thin appearance, and reduced/absent feces were observed in animals in the high dose (750 mg/kg/day) group during Study day 18 through day 22.
Cardiac Biomarkers
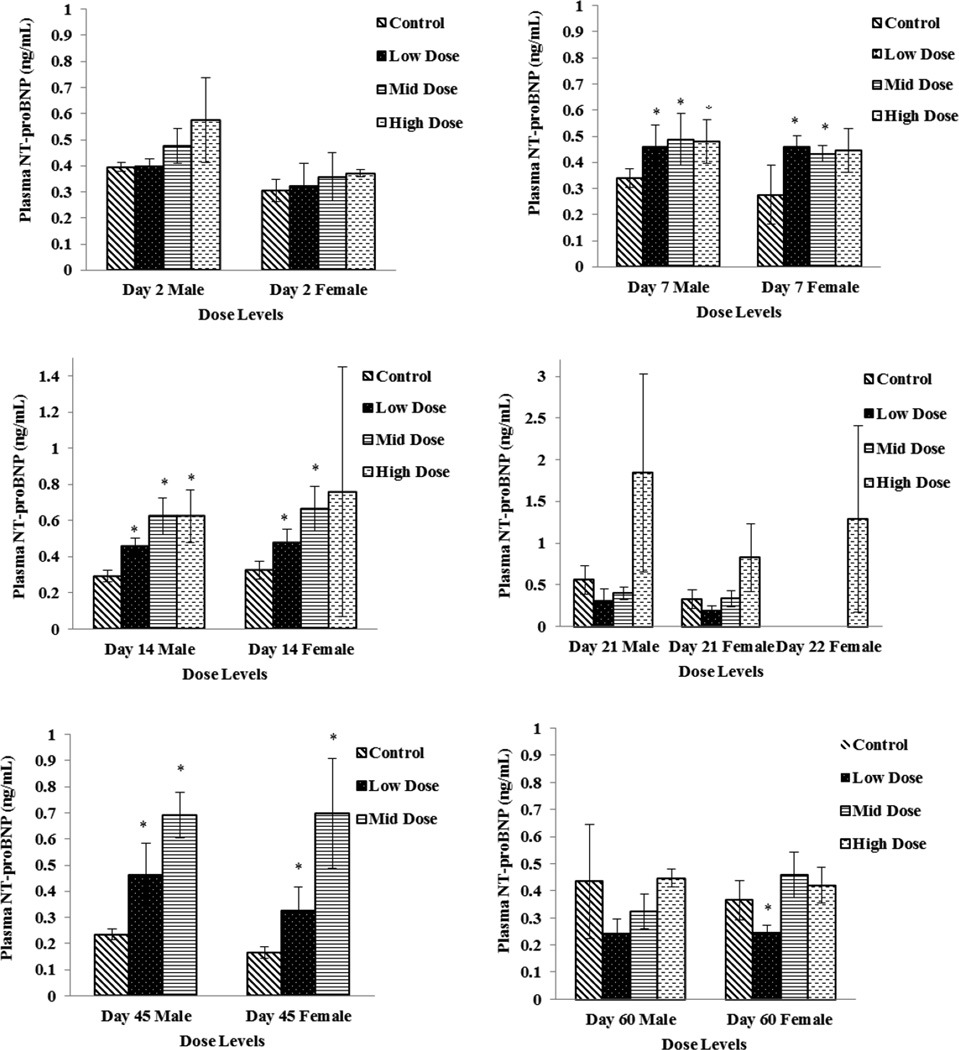
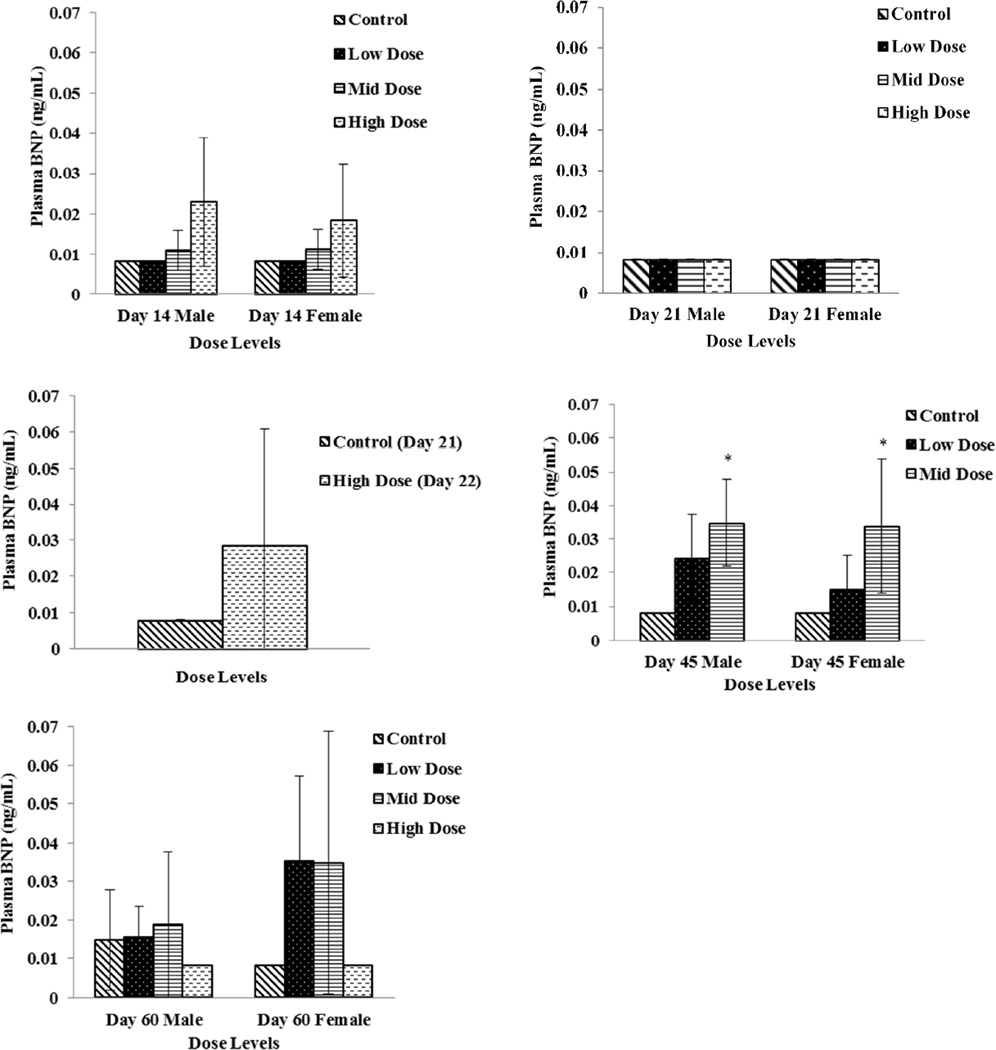
Plasma specimens were evaluated for CK, ANP, BNP, NT-proBNP, cTnI, and cTnT concentrations as potential cardiac toxicity biomarkers. There were no remarkable changes in ANP, cTnI, or cTnT concentrations. Plasma concentrations of NT-proBNP were significantly increased (p ≤ 0.05) on Study Day 7, 14 and on Study Day 45 in all dose groups except for high dose females (Fig 1). Although a dose dependent increasing trend was also observed with BNP concentrations, significant difference compared to control was noticed only on Day 45 (Fig 2). Elevations in NT-proBNP in individual subjects were, in general, accompanied by vacuolar degenerative (atrium) histological changes. On Study Day 14, this association was noticed in 3 of 4 males and 1 of 4 females in high dose (750 mg/kg/day) group, and 1 of 4 females in mid dose (250 mg/kg/day) group; on Study Day 21, 2 of 4 males and 4 of 4 females in high dose (750 mg/kg/day) group; on Study Day 45, 3 of 4 males and 1 of 4 females in mid dose (250 mg/kg/day). By Study Day 60 following 15 days of recovery period, significant increases in NT pro-BNP and BNP concentration levels were not observed (Fig 1 and 2).
Figure 1.
Plasma NT pro-BNP levels in Low Dose (100 mg/kg/day), Mid Dose (250 mg/kg/day), and High Dose (750mg/kg/day) rats. When N > 2, an asterisk (*) indicates a statistically (p ≤ 0.05) difference from the control group
Figure 2.
Plasma BNP levels in Low Dose (100 mg/kg/day), Mid Dose (250 mg/kg/day), and High Dose (750mg/kg/day) rats. When N > 2, an asterisk (*) indicates a statistically (p ≤ 0.05) difference from the control group
Creatine Kinase
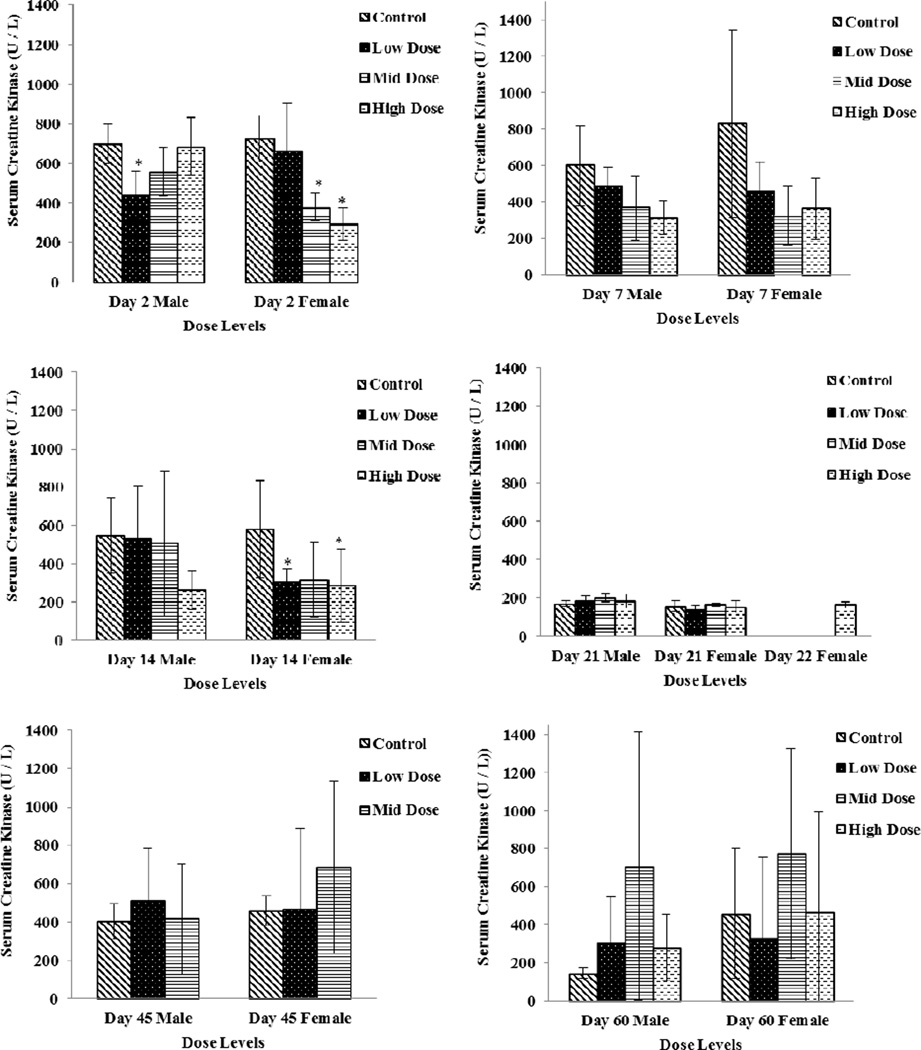
CK activity decreased sporadically in several dose groups (males and females) with return to baseline levels compared to the vehicle group on Study Days 45 and 60 (Fig 3). In males, the change was −37 percent in the low dose (100 mg/kg/day) group on Study Day 2. In females, changes were noted on Study Day 2 in the mid and high dose groups (− 47 and −59 percent for the 250 and 750 mg/kg/day groups, respectively) and on Study Day 14 in the low dose and high dose groups (− 47 and −51 percent, for the 100 and 750 mg/kg/day groups, respectively).
Figure 3.
Serum Creatine Kinase levels in Low Dose (100 mg/kg/day), Mid Dose (250 mg/kg/day), and High Dose (750mg/kg/day) rats. When N > 2, an asterisk (*) indicates a statistically (p ≤ 0.05) difference from the control group. Photomicrographs of sections of the cardiac atrium with H&E stain, 10X; control (left) and high dose (right).
Necropsy Gross Observations and Histopathology 7, 14, and 21 Study Day Necropsies
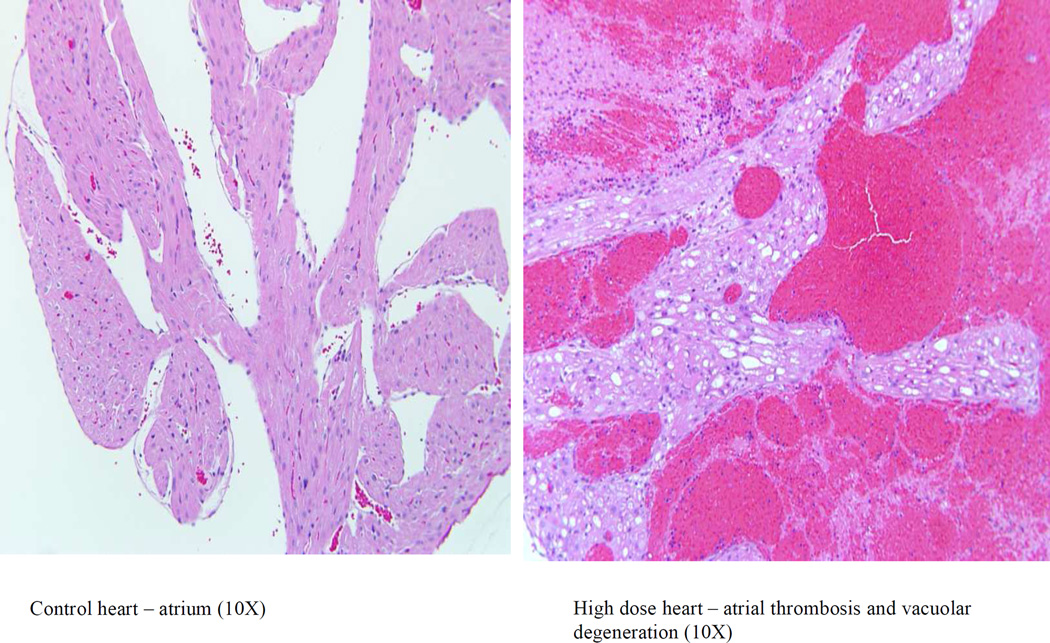
No 2-DG related gross observations were noted at necropsy. Hearts from animals administered 2-DG for 7, 14, and 21 days were examined. Cardiac lesions are summarized in Table 2. Beginning on Study Day 14 in males at 750 mg/kg/day and in females at 250 and 750 mg/kg/day, test article associated microscopic findings of vacuolar degeneration (Figure 4) and hypertrophy of the endothelial cells of the endocardium were present in the heart of animals administered 2-DG. Vacuolar degeneration in the unilateral or bilateral atrial myocardium, and occasionally within the ventricular myocardium, was characterized by vacuoles within the myofibers often replacing the entire fiber with displacement of the fiber nuclei. Sporadically, vacuoles within atrial tissue were associated with either atrophy or complete loss/replacement of the myofiber as was noticed in 2-DG administered animals in the mid and high dose (250 and 750 mg/kg/day) groups on Study Day 14 (3 of 4 males and 2 of 4 females in high dose group and 1 of 4 females in mid dose group), and Study Day 21 (4 of 4 males and females in high dose group with the highest incidence). Vacuolar degeneration was coded as minimal (Grade 1) for involvement of up to 50 percent of one atrium (usually the left); mild (Grade 2) for either involvement of 100 percent of one atrium or up to 50 percent of both atria; moderate (Grade 3) for 100 percent involvement of one atrium and up to 50 percent of the other; and marked (Grade 4) when the lesion approached maximal, involving 100 percent of both atria. Atrial vacuolar degeneration was often associated with endothelial cell hypertrophy of the atrial endocardium and less frequently within the ventricular endocardium.
Table 2.
Microscopic Cardiac Lesions in Males and Females Administered 2DG for 14, and 21/22, and 45 Days
| Study Day | Observation | Group 1 (Control) n = 4 |
Group 2 (100 mg/kg) n = 4 |
Group 3 (250 mg/kg) n = 4 |
Group 4 (750 mg/kg) n = 4 |
|
|---|---|---|---|---|---|---|
| Male | 14 | Vacuolar Degeneration, Atrium | 0 | 0 | 0 | 3 (1.0)a |
| 21 | Hypertrophy, Endocardium | 0 | 0 | 0 | 3 (1.3) | |
| Mineralization | 0 | 0 | 1 (1.0) | 1 (1.0) | ||
| Vacuolar Degeneration, Atrium | 0 | 0 | 0 | 4 (2.5) | ||
| 22b/45 | Hypertrophy, Endocardium | 0 | 0 | 0 | 4 (1.5) | |
| Mineralization | 0 | 0 | 1 (2.0) | 1 (1.0) | ||
| Necrosis | 0 | 0 | 0 | 4 (1.0) | ||
| Thrombosis, Atrium | 0 | 0 | 0 | 4 (1.5) | ||
| Vacuolar Degeneration, Atrium | 0 | 0 | 3 (1.0) | 4 (2.0) | ||
| Female | 14 | Vacuolar Degeneration, Atrium | 0 | 0 | 1 (1.0) | 2 (1.5) |
| Hypertrophy, Endocardium | 0 | 0 | 0 | 3 (1.0) | ||
| 21 | Vacuolar Degeneration, Atrium | 0 | 0 | 0 | 4 (2.3) | |
| Hypertrophy, Endocardium | 0 | 0 | 0 | 3 (1.0) | ||
| 22b /45 | Hypertrophy, Endocardium | 0 | 0 | 1 (1.0) | 2 (1.5) | |
| Mineralization | 0 | 0 | 0 | 1 (1.0) | ||
| Necrosis | 0 | 0 | 0 | 1 (1.0) | ||
| Thrombosis, Atrium | 0 | 0 | 0 | 1 (2.0) | ||
| Vacuolar Degeneration, Atrium | 0 | 0 | 1 (1.0) | 4 (2.5) |
Number present (average severity).
Group 4 animals were necropsied on Study Day 22 or found dead prior to Study Day 22.
Figure 4.
Photomicrographs of sections of the cardiac atrium with H&E stain, 10 ; control (left) and high dose(right).
Cardiac myofiber inflammation was seen in some animals from all groups, including controls, and was composed of mixed inflammatory infiltrates associated with scattered, fragmented, or sometimes missing, myofibers and was not considered to be test article related. This lesion was indistinguishable from spontaneous cardiomyopathy which is considered a normal background finding in rats of this age.
Cardiac mineralization occurred in one mid dose (250 mg/kg/day) and one high dose (750 mg/kg/day) male rat. The mineralization consisted of replacement of either myofibers (when associated with degeneration) or mineralization of the elastic fibers at the base of the aorta. The mineralization was considered a secondary effect and not directly related to administration of 2DG.There were no test article related changes noted in the low dose, 100 mg/kg/day animals.
45 Day Interval Necropsy (High Dose Necropsied on Study Day 22 and Early Deaths)
Heart tissue lesions are summarized in Table 2. Heart lesions in animals administered 2-DG for 45 days were similar but more marked than rats administered 2-DG for up to 21 days (surviving high dose terminated at 22 days and early death high dose animals).
Heart lesions such as vacuolar degeneration of the atrium, endocardial (endothelial cell) hypertrophy, inflammation, and mineralization observed in high and mid dose animals were similar to that described in the 7, 14, and 21 day animals. Vacuolar degeneration and endocardial (endothelial cell) hypertrophy were considered test article related. In one mid dose animal (250 mg/kg/day) vacuolar degeneration of the atrium of the heart and other cardiac changes were also noted. In high dose animals, thrombosis with associated necrosis, occurred in the left atrium in those animals found dead. In animals with minimal (Grade 1) atrial thrombosis, up to 50 percent of the left atrial lumen was occluded; mild (Grade 2) affected the entire left atrium; Grade 3 was coded to include the entire left atrium and up to 50 percent of the right atrium.
Study Day 60 Recovery Necropsy (45 Day Dosing with 15 Day Recovery; High Dose Group Concluded Dosing on Study Day 21 and Necropsied on Study Day 60)
There were no vacuolar degenerative changes in myocardium noted in this group except for a single male rat in the high dose group, suggesting that test article associated cardiac alterations were reversible (Table 3).
Table 3.
Microscopic Cardiac Lesions in Recovery Males and Femalesa
| Male | Observation |
Group
1 (Control) n = 4 |
Group 2 (100 mg/kg) n = 4 |
Group 3 (250 mg/kg) n = 4 |
Group 4 (750 mg/kg) n = 4 |
| Vacuolar Degeneration, Atrium | 0 | 0 | 0 | 1 (1.0)b | |
| Female | Vacuolar Degeneration, Atrium | 0 | 0 | 0 | 0 |
| Mineralization | 1 (1.0) | 0 | 0 | 0 |
Dose administration in Group 4 animals was terminated on Study Day 21. Groups 1, 2, 3 were terminated on Day 60. NE = Not examined.
Number present (average severity).
DISCUSSION/CONCLUSION
The study was conducted to see whether 2-DG induced cardiac toxicity could be reproduced using clinically relevant route and dosing schedule in a rat model to identify potential cardiac biomarkers that can be useful to monitor cardiac toxicity during its clinical evaluation. 2-DG is a known glycolysis pathway inhibitor and this glycolytic property of 2-DG has been used to develop anticonvulsant and antiepileptic therapy. 2-DG also has been used or evaluated previously for several indications such as an agent for measurement and imaging of regional glucose utilization by positron emission tomography (PET Scan) and an adjunct agent in chemotherapy of human cancers.
Minor et al., have performed a long-term dietary toxicity study of 2-DG in rats that showed 2-DG increases in mortality, increases incidence of pheochromocytoma in the adrenal medulla, reduces weight gain secondary to reduced food intake and increases in vacuolation of cardiac myocytes with an increase in autophagic flux. Decreases in the mean arterial blood pressure followed by intravenous administration of 2-DG was observed in anaesthetized rats (24,25).
Current sub-chronic study further characterizes 2-DG induced cardiac toxicity and identifies potential cardiac biomarkers that might be helpful to monitor cardiac toxicity during clinical trials. Dosing formulations were analyzed for 2-DG concentrations and were found to be within ±10% of targeted concentrations confirming the accuracy of dosing formulations. Clinical signs were noticed only in high dose group animals that included lethargy, rough coat, thin appearance, hunched posture, and reduced/absent feces. By day 22 of the treatment, four males and one female rat died from the high dose group indicating males are more sensitive to 2-DG toxicity. Increased sensitivity of male rats to 2-DG toxicity was also observed by Minor et al. (7). This sex difference in toxicity could be accounted for higher metabolic demand in male rats compared to females as 2-DG is a known inhibitor of glycolysis.
Plasma specimens were evaluated for CK, ANP, BNP, NT-proBNP, cTnI, and cTnT, concentrations as potential cardiac toxicity biomarkers. Plasma concentrations of NT pro-BNP were significantly increased in a dose-dependent manner on Study Days 7, 14 and 45.On Study Day 21 although dose-dependent increases were observed, the changes were not statistically different compared to the concurrent control group possibly due to higher control levels and/or variability. BNP concentrations were also statistically increased on Study Day 45. ‥ The increases in NT pro-BNP levels were often associated with microscopic myocardial vacuolar degeneration. It appears that NT pro-BNP is more sensitive to early 2-DG induced cardiac injury compared to BNP, as more robust changes with NT pro-BNP were noticed which may be accounted for a longer biological half-life of NT-proBNP than that of BNP (approx. 70 min vs. 20 min) (26). Elevated levels of NT pro BNP and BNP were decreased by the end of recovery period (day 60) which corresponded with the microscopic evidence of recovery in heart tissue. There were no remarkable changes observed in CK, cTnI, or cTnT concentrations indicating 2-DG may be causing no or minimal necrotic changes such as myocardial infarction as these markers are more likely be indicative of cell death. There were no significant changes observed in ANP levels. More robust changes observed in NT pro-BNP and BNP levels indicating 2-DG induced cardiac toxicity could be related to cardiac hypertrophy/dilatation of the heart leading to stretching of cardiac tissues and releasing these markers into the blood. This is the first evidence of altered serum NT- proBNP and BNP levels in rats in response to 2-DG toxicity indicating their usefulness in clinical trials of 2-DG as potential cardiac safety biomarkers. If 2-DG induced cardiac toxicity in rat is relevant to human, these biomarkers may need to be qualified/validated as per the FDA Guidance. It is quite possible that 2-DG induced cardiac toxicity could be limited to rodents due to higher metabolic demand, hence, further evaluation of 2-DG toxicity in higher order animal species such as dog or monkey would be helpful.
There were no 2-DG related gross lesions noted at necropsy. Test article related microscopic findings in the heart were noted beginning on exposure Study Day 14 in the 250 and 750 mg/kg/day groups. Vacuolar degeneration of the atrial myocardium associated with endocardial (endothelial cell) hypertrophy was the most significant early lesion. By Study Day 22, four male rats and one female rat administered high dose (750 mg/kg/day) of 2-DG had died likely due to cardiac failure associated with atrial thrombosis. In the mid dose (250 mg/kg/day) group at Study Day 45, 2-DG related findings consisted of vacuolar degeneration and endocardial (endothelial cell) hypertrophy of the heart. Cardiotoxicity noted in the present study corroborated with findings of Minor et al. (7) and Lane et al. (27).
Treatment related cardiac lesions were not evident in the low-dose (100 mg/kg/day) group. The onset of treatment-related lesions in heart tissue occurred in the mid-dose (250 mg/kg/day) group females following 14 consecutive days of exposure to 2DG, and in mid-dose group males after 21 consecutive days of exposure. Onset of treatment-related cardiac lesions in both males and females of the high-dose (750 mg/kg/day) group occurred after 14 days of exposure. Notable progression of treatment-related lesions, in both incidence and severity, was observed in the heart tissue of mid- and high-dose group animals from Study Day 14 to the end of exposure on Study Days 22 (high-dose group) and 45 (mid-dose group). Following a recovery period of 15 days, treatment-related cardiac lesions were completely remodeled in the mid-dose group animals. After 39 days of recovery, a majority of treatment-related cardiac lesions were remodeled in the high-dose group animals, with one incidence of vacuolar degeneration (atrium) persisting among high dose males.
2-DG-induced cardiac toxicity was reproduced in a rat model demonstrating association of elevated levels of NT pro-BNP and BNP (NT pro-BNP being more sensitive) with microscopic findings in heart (myocardial vacuolar degeneration). Following a recovery period, treatment-related cardiac lesions showed complete recovery (mid-dose group) or trend towards recovery (high-dose group) indicating 2-DG-induced cardiac toxicity is reversible. Furthermore, NT pro –BNP, and BNP are potential early biomarkers for 2-DG induced cardiac toxicity that can be useful to monitor 2-DG therapy during its clinical evaluation.
Acknowledgments
Authors thank Alan Koester and other Battelle staff for conducting study.
FUNDING
This work was supported by NCI-Leidos Contract No. HHSN261200800001E, and NINDS under BrIDGS/NCATS Program.
Footnotes
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: TPS holds a use patent for 2-DG.
References
- 1.Vamecq J, Vallée L, Lesage F, Gressens P, Stables JP. Antiepileptic popular ketogenic diet: emerging twists in an ancient story. Prog Neurobiol. 2005;75:1–28. doi: 10.1016/j.pneurobio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Stafstrom CE, Ockuly JC, Murphree L, Valley MT, Roopra A, Sutula TP. Anticonvulsant and antiepileptic actions of 2-deoxy-D-glucose in epilepsy models. Ann. Neurol. 2009;65:435–447. doi: 10.1002/ana.21603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huttenlocher PR. Ketonemia and seizures: metabolic and anticonvulsant effects of two ketogenic diets in childhood epilepsy. Pediatr Res. 1976;10:536–540. doi: 10.1203/00006450-197605000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Hu XL, Cheng X, Fei J, Xiong ZQ. Neuron-restrictive silencer factor is not required for the antiepileptic effect of the ketogenic diet. Epilepsia. 2011;52:1609–1616. doi: 10.1111/j.1528-1167.2011.03171.x. [DOI] [PubMed] [Google Scholar]
- 5.Garriga-Canut M, Schoenike B, Qazi R, Bergendahl K, Daley TJ, Pfender RM, Morrison JF, Ockuly J, Stafstrom C, Sutula T, Roopra A. 2-Deoxy-D-glucose reduces epilepsy progression by NRSF-CtBP-dependent metabolic regulation of chromatin structure. Nat Neurosci. 2006;9:1382–1387. doi: 10.1038/nn1791. [DOI] [PubMed] [Google Scholar]
- 6.Horton RW, Meldrum BS, Bachelard HS. Enzymic and cerebralmetabolic effects of 2-deoxy-D-glucose. J. Neurochem. 1973;21:507–520. doi: 10.1111/j.1471-4159.1973.tb05996.x. [DOI] [PubMed] [Google Scholar]
- 7.Minor RK, Smith DL, Jr, Sossong AM, Kaushik S, Poosala S, Spangler EL, Roth GS, Lane M, Allison DB, de Cabo R, Ingram DK, Mattison JA. Chronic ingestion of 2-deoxy-D-glucose induces cardiac vacuolization and increases mortality in rats. Toxicol Appl Pharmacol. 2010;243:332–339. doi: 10.1016/j.taap.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh V, Martinezclark P, Pascual M, Shaw ES, O'Neill WW. Cardiac biomarkers - the old and the new: a review. Coron Artery Dis. 2010;21:244–256. doi: 10.1097/MCA.0b013e328338cd1f. [DOI] [PubMed] [Google Scholar]
- 9.Dietz JR. Mechanisms of atrial natriuretic peptide secretion from the atrium. Cardiovasc Res. 2005;68:8–17. doi: 10.1016/j.cardiores.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Potter LR, Yoder AR, Flora DR, Antos LK, Dickey DM. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb Exp Pharmacol. 2009;191:341–366. doi: 10.1007/978-3-540-68964-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Omland T, Aakvaag A, Bonarjee VV, Caidahl K, Lie RT, Nilsen DW, Sundsfjord JA, Dickstein K. Plasma brain natriuretic peptide as an indicator of left ventricular systolic function and long-term survival after acute myocardial infarction. Comparison with plasma atrial natriuretic peptide and N-terminal proatrial natriuretic peptide. Circulation. 1996;93:1963–1969. doi: 10.1161/01.cir.93.11.1963. [DOI] [PubMed] [Google Scholar]
- 12.Kang YJ. Toxic responses of the heart and vascular system. In: Klaassen CD, editor. Casarett and Doull’s Toxicology. The Basic Science of Poisons. Blacklick, OH: The McGraw-Hill Companies, Inc.; 2008. pp. 699–740. [Google Scholar]
- 13.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50:2173–2195. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Takeda S, Yamashita A, Maeda K, Mae´da Y. Structure of the core domain of human cardiac troponin in the Ca(2R)- saturated form. Nature. 2003;424:35–41. doi: 10.1038/nature01780. [DOI] [PubMed] [Google Scholar]
- 15.Perry SV. Troponin I: inhibitor or facilitator. Mol Cell Biochem. 1999;190:9–32. [PubMed] [Google Scholar]
- 16.Nanji AA. Serum creatine kinase isoenzymes: a review. Muscle Nerve. 1983;6:83–90. doi: 10.1002/mus.880060203. [DOI] [PubMed] [Google Scholar]
- 17.Adamcova M, Sterba M, Simunek T, Potacova A, Popelova O, Mazurova Y, Gersl V. Troponin as a marker of myocardiac damage in drug-induced cardiotoxicity. Expert Opin Drug Saf. 2005;4:457–472. doi: 10.1517/14740338.4.3.457. [DOI] [PubMed] [Google Scholar]
- 18.Hessel MH, Michielsen EC, Atsma DE, Schalij MJ, van der Valk EJ, Bax WH, Hermens WT, van Dieijen-Visser MP. van der Laarse. Release kinetics of intact and degraded troponin I and T after irreversible cell damage. Exp Mol Pathol. 2008;85:90–95. doi: 10.1016/j.yexmp.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Horacek JM, Pudil R, Jebavy L, Tichy M, Zak P, Maly J. Assessment of anthracycline-induced cardiotoxicity with biochemical markers. Exp Oncol. 2007;29:309–313. [PubMed] [Google Scholar]
- 20.Sorodoc V, Sorodoc L, Ungureanu D, Sava A, Jaba IM. Cardiac troponin T and NT-proBNP as biomarkers of early myocardial damage in amitriptyline-induced cardiovascular toxicity in rats. Int J Toxicol. 2013;32:351–357. doi: 10.1177/1091581813503888. [DOI] [PubMed] [Google Scholar]
- 21.De Lemos JA, McGuire DK, Drazner MH. B-type natiuretic peptide in cardiovascular disease. Lancet. 2003;362:316. doi: 10.1016/S0140-6736(03)13976-1. [DOI] [PubMed] [Google Scholar]
- 22.Weber M, Hamm C. Role of B-type natriuretic peptide (BNP) and NTproBNP in clinical routine. Heart. 2006;92:843–849. doi: 10.1136/hrt.2005.071233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romano S, Fratini S, Ricevuto E, Procaccini V, Stifano G, Mancini M, Di Mauro M, Ficorella C, Penco M. Serial measurements of NT-proBNP are predictive of not-high-dose anthracycline cardiotoxicity in breast cancer patients. Br J Cancer. 2011;105:1663–1668. doi: 10.1038/bjc.2011.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vijayaraghavan R, Kumar D, Dube SN, Singh R, Pandey KS, Bag BC, Kaushik MP, Sekhar K, Dwarakanath BS, Ravindranath T. Acute toxicity and cardio-respiratory effects of 2-deoxy-D-glucose: a promising radio sensitiser. Biomed Environ Sci. 2006;19:96–103. [PubMed] [Google Scholar]
- 25.Pandey R, Choudhry PK, Deshpande SB. 2-Deoxy-D-glucose alters the cardio-respiratory parameters in anaesthetized rats. Indian J Physiol Pharmacol. 2008;52:243–248. [PubMed] [Google Scholar]
- 26.Gaggin HK, Januzzi JL., Jr Biomarkers and diagnostics in heart failure. Biochim Biophys Acta. 2013;1832(12):2442–2450. doi: 10.1016/j.bbadis.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Lane MA, Ingram DK, Roth GS. 2-Deoxy-D-glucose feeding in rats mimics physiological effects of calorie restriction. J Anti-Aging Med. 1998;1:327–337. [Google Scholar]