Abstract
Primary glial and neuronal tumors of the ovary or peritoneum are rare neuroectodermal-type tumors similar to their counterparts in the central nervous system. We retrospectively reviewed 11 cases. These cases included 4 ependymomas, 6 astrocytic tumors and 1 neurocytoma. Patients’ age ranged from 9 to 50 years (mean, 26 years; median, 24 years). All ependymal tumors with detailed clinical history (n = 3) were not associated with any other ovarian neoplasm. In contrast, all astrocytic tumors were associated with immature teratoma (n = 4), mature cystic teratoma (n = 1) or mixed germ cell tumor (n = 1). The neurocytoma arose in association with mature teratomatous components in a patient with a history of treated mixed germ cell tumor. Immunohistochemical staining showed that 7 of 7 ependymal and astrocytic tumors (100%) were positive for glial fibrillary acidic protein, and 2 of 2 ependymomas (100%) were positive for both estrogen and progesterone receptors. The neurocytoma was positive for synaptophysin, and negative for S100 protein, glial fibrillary acidic protein and SALL4. No IDH1-R132H mutation was detected in 2 of 2 (0%) astrocytomas by immunohistochemistry. Next-generation sequencing was performed on additional 2 ependymomas and 2 astrocytomas, but detected no mutations in a panel of 50 genes that included IDH1, IDH2, TP53, PIK3CA, EGFR, BRAF and PTEN. Follow-up was available for 8 patients, with the follow-up period ranging from 4 to 59 months (mean, 15 months; median, 8.5 months), of which 3 had no evidence of disease and 5 were alive with disease. In conclusion, primary glial and neuronal tumors of the ovary can arise independently or in association with other ovarian germ cell tumor components. Pathologists should be aware of these rare tumors and differentiate them from other ovarian neoplasms. Even though an IDH1 or IDH2 mutation is found in the majority of WHO grade II and III astrocytomas, and in secondary glioblastomas arising from them, such mutations were not identified in our series, suggesting that these tumors are molecularly different from their CNS counterparts despite their morphologic and immunophenotypic similarities.
Keywords: neuroectodermal-type tumor, neurocytoma, ependymoma, astrocytoma, ovarian germ cell tumor
INTRODUCTION
Primary glial and neuronal tumors of the ovary and/or peritoneum are rare neuroectodermal-type tumors, morphologically similar to their counterparts in the central nervous system (CNS). (1) Neuroectodermal-type tumors of the ovary are divided into three groups: differentiated tumors, primitive tumors, and anaplastic tumors, with the latter two groups being associated with poorer prognosis. (2) Despite the rapid advance in our understanding of CNS glial and neuronal tumors, as reflected by the introduction of integrated, molecular signature-based definitions of many pediatric and adult entities in the forthcoming World Health Organization (WHO) 2016 Classification of Tumours of Central Nervous System (3), the corresponding tumors arising in extra-CNS locations have not been well studied.
The pathogenesis of glial and neuronal tumors of the gynecologic tract is largely poorly understood. They are considered to belong to the general category of teratomas (1); however, some tumors have been reported in association with epithelial ovarian tumors, adenosarcoma, or malignant mixed Müllerian tumors. (4–9) Clinical experience in the diagnosis and treatment of these neuroectodermal-type tumors of the ovary or peritoneum is limited.
In this study, we retrospectively reviewed 11 cases of primary glial or neuronal tumors of ovary/peritoneum, including 4 ependymomas, 6 astrocytic tumors and 1 neurocytoma, and correlated the clinical, pathologic, and molecular characteristics with their counterparts in the CNS.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board. We searched the database of the Department of Pathology at The University of Texas MD Anderson Cancer Center between 1988 and 2015, and identified 11 primary glial or neuronal tumors of the ovary/peritoneum. Metastatic tumors were excluded from the study. Clinical data, including demographic information, diagnosis and follow-up, were obtained from the medical records. Archived hematoxylin and eosin and immunohistochemistry (IHC) slides were retrieved and reviewed.
The following immunohistochemical stains were performed at outside institutions and were reviewed at our institution: CD57, CD99, desmin, epithelial membrane antigen (EMA), glial fibrillary acidic protein (GFAP), pancytokeratin, S100 protein and synaptophysin. Immunohistochemical stains performed at our institution included: calretinin (clone DC8, 1:120; Invitrogen, Carlsbad, CA, USA), CD99 (clone 12E7, 1:300; DAKO, Burlington, ON, Canada), EMA (clone GP1.4, 1:600; Novocastra, Leica Microsystems, Wetzlar, Germany), estrogen receptor (ER) (clone 6F11, diluted 1:35; Leica Microsystems, Wetzlar, Germany), GFAP (1:7000; BD Biosciences, Franklin Lakes, NJ, USA), inhibin (clone R1, 1:50; AbD Serotec, Oxford, UK), isocitrate dehydrogenase (IDH1-R132H) (clone H09, diluted 1:40; Dianova, Hamburg, Germany), keratin cocktail (AE1/AE3, 1:50, DAKO, Burlington, ON, Canada; Cam5.2, 1:50, BD Biosciences, Franklin Lakes, NJ, USA; MNF116, 1:50, DAKO, Burlington, ON, Canada; keratin 8 and 18, 1:25, Invitrogen, Carlsbad, CA, USA), Ki-67 (clone MIB-1, 1:100; DAKO, Burlington, ON, Canada), neurofilament (clone SEN28, 1:100; Invitrogen, Carlsbad, CA, USA), neuronal nuclear antigen (Neu-N) (clone A60, 1:50; Bio SB, Santa Barbara, CA, USA), phosphohistone H3 (pHH3) (1:400; Millipore, Billerica, MA, USA), progesterone receptor (PR) (clone PgR1294, 1:200; DAKO, Burlington, ON, Canada), S100 (clone 15E2E2, 1:900; BioGenex, Fremont CA, USA), Spalt-like transcription factor 4 (SALL4) (clone 6E3, 1:100; Biocare, Concord, CA, USA), synaptophysin (clone 27G12, 1:600; Novocastra, Leica Microsystems, Wetzlar, Germany). In addition, fluorescent in situ hybridization (FISH)-based 1p/19q codeletion was performed in case 9. Next-generation sequencing (NGS) was performed for 4 patients in a CLIA certified laboratory, using a targeted hot-spot panel of 50 genes (Ion AmpliSeq™ Cancer Hotspot Panel v2, ThermoFisher Scientific, Grand Island, NY, USA) on an Ion Torrent platform (Ion PGM™ System, ThermoFisher Scientific, Grand Island, NY, USA). The 50 genes were ABL1, AKT1, ALK, APC, ATM, BRAF, CDH1, CDKN2A, CSF1R, CTNNB1, EGFR, ERBB2, EZH2, FBXW7, FGFR1, FGFR2, FGFR3, FLT3, GNA11, GNAQ, GNAS, HNF1A, HRAS, IDH1, IDH2, JAK2, JAK3, KDR, KIT, KRAS, MET, MLH1, MPL, NOTCH1, NPM1, NRAS, PDGFRA, PIK3CA, PTEN, PTPN11, RB1, RET, SMAD4, SMARCB1, SMO, SRC, STK11, TP53 and VPL. The NGS reports were reviewed.
RESULTS
We identified 11 patients with glial and neuronal tumors of the ovary, including 4 ependymomas, 6 astrocytic tumors and 1 neurocytoma (Table 1). Patients’ age ranged from 9 to 50 years (mean, 26 years; median, 24 years). The majority of patients presented with a pelvic mass with or without suspicious omental implant or peritoneal metastasis. Since several cases were consult cases submitted by outside institutions, the data regarding tumor stage was not always available. Follow-up was available for 8 patients (range: 4–59 months; mean, 15 months; median, 8.5 months), of which 3 had no evidence of disease and 5 were alive with disease at last follow-up.
Table 1.
Clinical Features of Primary Glial and Neuronal Tumors of the Ovary or Peritoneum.
| PT | Age, yrs | Neuroectodermal tumor type, location | Other ovarian neoplasm | Surgery | Adjuvant therapy | Follow-up |
|---|---|---|---|---|---|---|
| Ependymal tumors | ||||||
| 1 | 35 | Ependymoma, surface of ovary and peritoneum | Unknown | RSO | Unknown | NA |
| 2 | 50 | Ependymoma, ovary and peritoneum | None | RSO, previous LSO and hysterectomy for fibroid | Yes, radiation | AWD, residual peritoneal and omental disease (CT scan), 59 months |
| 3 | 36 | Anaplastic ependymoma, broad ligament, ovary and peritoneum | None | TAH-BSO, OM | Yes, carboplatin and etoposide | AWD, persistent ependymoma, 9 months |
| 4 | 29 | Anaplastic ependymoma, ovary and peritoneum | None | BSO, OM | Yes, BEP | AWD, residual disease in abdomen and pelvis (CT scan), 4.5 months |
|
| ||||||
| Astrocytic tumors | ||||||
| 5 | 38 | Low-grade astrocytoma, omentum, peritoneum and etc. | Mixed germ cell tumor, immature teratoma and yolk sac tumor | TAH-BSO, OM | Yes, BEP, paclitaxel and carboplatin, bevacizumab | NA |
| 6 | 24 | Anaplastic astrocytoma/high-grade glioma, right ovary | Mature cystic teratoma | RSO | No | NED, 5 months |
| 7 | 17 | High-grade glioma/astrocytoma, right ovary | Immature teratoma, low-grade | RSO | No | NED, 8 months |
| 8 | 19 | High-grade glioma/astrocytoma, left ovary | Immature teratoma, high-grade | LSO | Yes, BEP | NED, 18 months |
| 9 | 9 | High-grade glioma/astrocytoma, right ovary | Immature teratoma, low-grade | Unknown | Unknown | NA |
| 10 | 10 | High-grade glioma/astrocytoma arising from gliomatosis peritonei | Immature teratoma, high-grade; gliomatosis peritonei | Cytoreductive surgery; previous history of LSO, OM | Yes, HIPEC and radiation (etoposide and cisplatin before the diagnosis of glioma) | AWD, residual tumor in the pelvis (CT scan), 4 months (10 months after the diagnosis of ovarian immature teratoma) |
|
| ||||||
| Neuronal tumors | ||||||
| 11 | 22 | Neurocytoma, low grade, arising in the background of mature teratoma component in the diaphragm | Mixed germ cell tumor (immature teratoma and yolk sac tumor) | RSO, OM | Yes, BEP chemotherapy | AWD, mature teratomas only, 12 months (20 months after the diagnosis of ovarian mixed germ cell tumor) |
Abbreviations: PT, patient; yrs, years; RSO, right salpingo-oophorectomy; NA, not available; LSO, left salpingo-oophorectomy; AWD, alive with disease; CT, computed tomography; TAH, total abdominal hysterectomy; BSO, bilateral salpingo-oophorectomy; OM, omentectomy; BEP, bleomycin, etoposide, cisplatin; NED, no evidence of disease; HIPEC, hyperthermic intraperitoneal chemotherapy.
Ependymal Tumors
Four ependymal tumors were identified, including 2 low-grade ependymomas and 2 anaplastic ependymomas. Patients’ age ranged from 29 to 50 years (mean, 37.5 years; median, 35.5 years). Three patients presented with pelvic masses and one patient presented with an alleged broad ligament abscess (case 3). Grossly, case 2 was a 13.0–centimeter pink-tan mass; case 3 was a 7.0-centimeter pink-tan mass; case 4 was an 18.0-centimeter ovoid, ragged, pink mass (stage pT3c). Microscopically, the ependymomas exhibited round to oval nuclei with a fine “salt and pepper” chromatin pattern, perivascular pseudorosette formation, and rare true ependymal rosette formation. Two cases (cases 3 and 4) also showed areas of elevated mitotic activity, consistent with anaplastic ependymoma. In addition, vascular proliferation and necrosis were present in case 3, but not case 4. Multiple architectural patterns, including classical, papillary, and clear cell areas were present (Figure 1). Importantly, all ependymomas involved both the ovary and the peritoneum, and none of them was associated with another ovarian neoplasm. The tumor cells were typically positive for GFAP, ER, PR and EMA (perinuclear dot-like pattern) (Table 2). The mutational status of 50 genes, including IDH1, IDH2, PIK3CA, and PTEN, was assessed by next-generation sequencing in 1 low-grade ependymoma and 1 anaplastic ependymoma. No mutations in the covered coding regions of these genes were identified in either of the 2 cases.
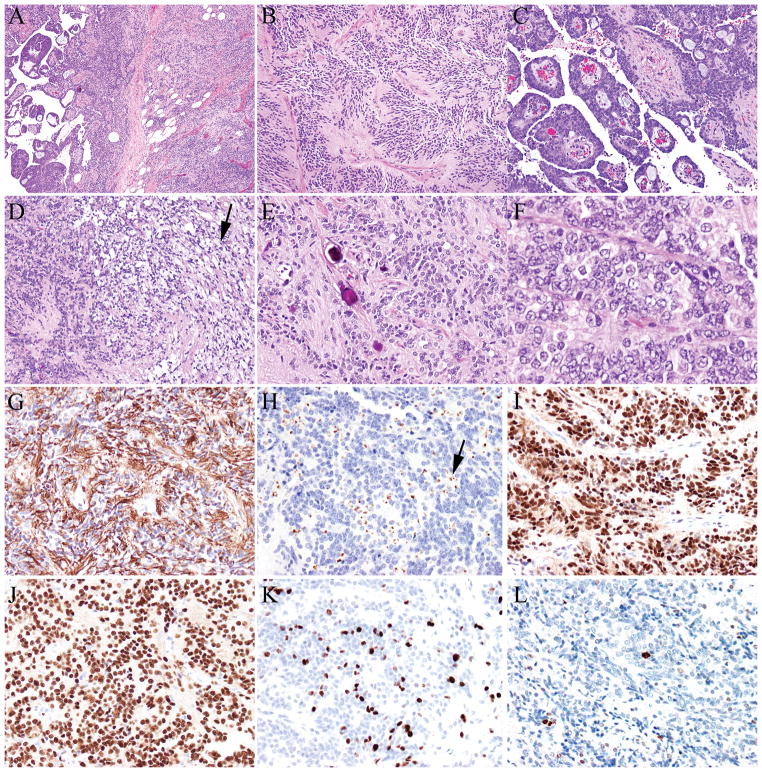
FIGURE 1.
A–F, Anaplastic ependymoma (A) showing multiple architectural patterns, including classical areas with perivascular pseudorosettes (B), papillary (C), and clear cell areas (D, black arrow). Psammoma bodies (E) were present. Some areas showed increased cellularity, nuclear atypia, and elevated mitotic activity (F). Tumor cells were positive for glial fibrillary acidic protein (G), epithelial membrane antigen (perinuclear dot-like pattern, black arrow) (H), estrogen receptor (I) and progesterone receptor (J), and demonstrated a high proliferative index (K- Ki-67/MIB-1). Phosphohistone H3 (pHH3) highlighted mitotic figures (L).
Table 2.
Immunohistochemical and Molecular Results of Primary Glial and Neuronal Tumor of the Ovary or Peritoneum.*
| PT | GFAP | IDH1 mutation | IDH2 mutation | Ki-67 | Others |
|---|---|---|---|---|---|
| 1 | NA | NA | NA | NA | NA |
| 2† | Positive | Negative (NGS) | Negative (NGS) | NA | Positive for ER and PR; Negative for EMA, CD99, desmin, synaptophysin, pancytokeratin, CD57 and S100 protein. |
| 3 | Positive | NA | NA | 14.4% | Focal positive for EMA (perinuclear dot-like pattern) and keratin cocktail; negative for inhibin and calretinin. |
| 4† | Positive | Negative (NGS) | Negative (NGS) | 21.7% | Positive for ER, PR, EMA (perinuclear dot-like pattern); focal positive for CD99 and S100 protein; negative for SALL4, synaptophysin and keratin cocktail; pHH3 highlighted 9 mitotic figures per 10 high-power fields. |
| 5 | Positive | Negative (IHC) | NA | 2.5% | Positive for S100 protein; negative for desmin; pHH3 highlighted very few mitotic figures. |
| 6 | Positive | NA | NA | NA | Negative for synaptophysin, Neu-N and neurofilament. |
| 7† | Positive | Negative (NGS) | Negative (NGS) | 20% | Positive for S100 protein; negative for synaptophysin and Neu-N. |
| 8 | NA | NA | NA | NA | NA |
| 9# | Positive | Negative (IHC) | NA | NA | NA |
| 10† | NA | Negative (NGS) | Negative (NGS) | 22.2% | pHH3 highlighted 5–7 mitotic figures per 10 high-power fields. |
| 11 | Negative | Negative (IHC) | NA | 0.9% | Positive for synaptophysin, negative for S100 protein and SALL4. |
Abbreviations: PT, patient; GFAP, glial fibrillary acidic protein; IDH, isocitrate dehydrogenase; NA, not available; NGS, next-generation sequencing; IHC, immunohistochemistry; ER, estrogen receptor; PR, progesterone receptor; EMA, epithelial membrane antigen; pHH3, phosphohistone H3.
Some Immunohistochemical studies were performed by the outside institutions (submitted for review).
Next-generation sequencing (targeted hot-spot 50-gene panel) was performed in Cases 2, 4, 7 and 10, but detected no mutations in a panel of 50 genes that included IDH1, IDH2, TP53, PIK3CA, EGFR, BRAF, PTEN and etc.
1p/19q co-deletion was not identified in case 9.
The patients underwent either unilateral or bilateral salpingo-oophorectomy with or without hysterectomy. Three patients with adequate follow-up information were alive with disease at 4.5, 9 and 59 months, respectively (Table 1).
Astrocytic Tumors
Six primary ovarian/peritoneal astrocytomas were identified (Table 1), including one low-grade and five high-grade gliomas/astrocytomas. Patients’ age ranged from 9 to 38 years (mean, 19.5 years; median, 18 years). All 6 patients also had coexistent ovarian germ cell tumors, including immature teratoma (n = 4), mature cystic teratoma (n = 1), and mixed germ cell tumor (n = 1). In 4 patients, astrocytomas arose from ovary, and in the other 2 patients they arose from peritoneal implants (n = 1) or from gliomatosis peritonei (mature glial tissue in the peritoneal cavity) (n = 1). Of note, case 10 was also included in our recently published case series about gliomatosis peritonei. (10) Grossly, case 5 was a 7.5-centimeter multi-nodular mass, and multiple peritoneal nodules were also present; case 7 was a 16-centimeter solid and cystic mass with a smooth surface; case 8 was a 12-centimeter fleshly light-tan mass; case 9 was a 8.0-centimeter fungating pink and yellow-tan mass protruding from the luminal surface of the cystic wall of a 19.0-centimeter immature cystic teratoma. Of note, since astrocytoma usually arises from an ovarian germ cell tumor, it may not be possible to accurately measure the size of the astrocytoma, and the above measurement may also include the ovarian germ cell tumor component.
Microscopically, low-grade astrocytoma displayed moderately increased cellularity, low to moderate nuclear atypia, and very rare mitotic figures (Figure 2). In contrast, high-grade astrocytomas showed marked nuclear atypia and prominently elevated mitotic activity (Figure 3). Vascular proliferation and/or necrosis were identified in 4 tumors (cases 7–10), similar to CNS glioblastoma. Immunophenotyping results are shown in Table 2. No IDH1-R132H mutations were identified in 4 of 4 astrocytomas (0%) by either immunohistochemistry (n = 2) or NGS (n = 2). In the latter 2 cases, NGS failed to identify any mutations in the covered coding regions of the 50-gene panel, including IDH1, IDH2, PIK3CA, and PTEN.
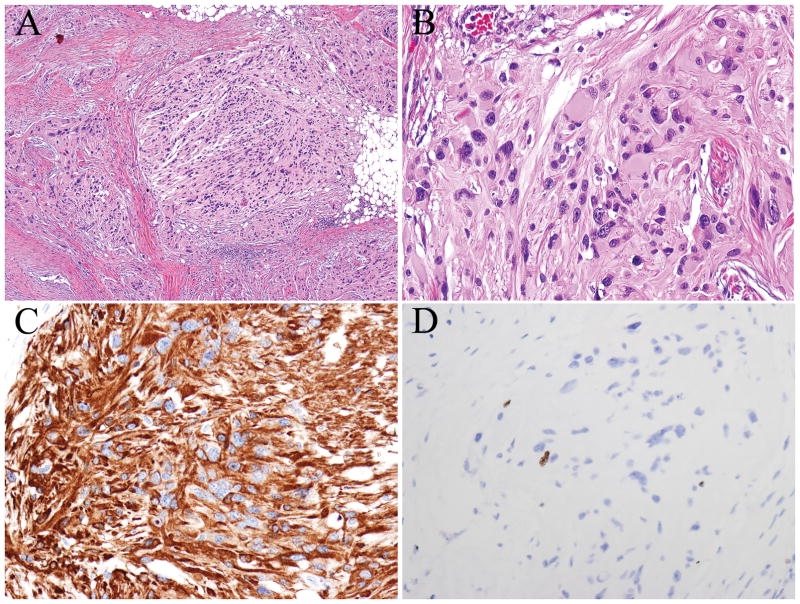
FIGURE 2.
A, Low-grade astrocytoma showing moderately increased cellularity, gemistocytic morphology, nuclear atypia, and very rare mitotic figures (A–B). Tumor cells expressed glial fibrillary acidic protein (C) and demonstrated a low proliferative index (D- Ki-67/MIB-1).
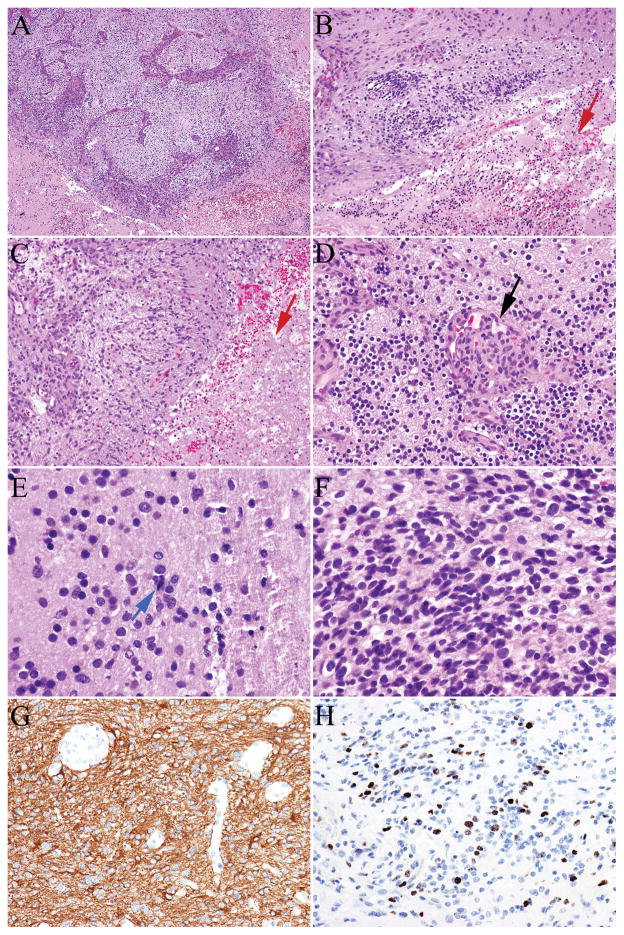
FIGURE 3.
A-D, High-grade astrocytoma (A–F) had increased cellularity, nuclear atypia, necrosis (B–C, red arrow), florid microvascular proliferation (D, black arrow), and brisk mitotic activity (E, blue arrow). Tumor cells expressed glial fibrillary acidic protein (G) and demonstrated a high proliferative index (H–Ki-67/MIB-1).
Among 5 patients with operative notes available, all patients underwent unilateral salpingo-oophorectomy, or bilateral salpingo-oophorectomy, hysterectomy and omentectomy, and one patient had a previous history of left salpingo-oophorectomy for primary ovarian germ cell tumor and underwent cytoreductive surgery for the glioma (Table 1). Among 4 patients with follow-up data, 3 patients had no evidence of disease at 5, 8 and 18 months after the primary ovarian/peritoneal astrocytoma diagnosis. One patient was alive with disease 4 months after the astrocytoma diagnosis.
Neuronal Tumor
Only one neurocytoma was identified in our archival search. A 22-year-old woman had a previous history of ovarian mixed germ cell tumor with immature teratoma and yolk sac tumor components, and received 6 cycles of bleomycin, etoposide, cisplatin (BEP) chemotherapy. Eight months after the diagnosis of the original ovarian mixed germ cell tumor, follow-up computed tomography (CT) scan of abdomen showed multiple peritoneal nodules, and biopsy demonstrated a mature teratoma component. Moreover, a low-grade neurocytoma arising from the mature teratomatous elements was diagnosed on a diaphragmatic implant. Microscopically, the tumor measured 2.0-millimeter and formed a distinct nodule. There was no other intermixing teratoma component. The histological features of the tumor included monotonously rounded nuclei with perinuclear cytoplasmic halos, and low mitotic activity (Figure 4). The tumor was adjacent to a cyst lined by ependyma-like cells, similar to central neurocytoma of the brain, which commonly arises adjacent to the lateral or third ventricle. The tumor cells strongly immunoexpressed synaptophysin, but not GFAP, S100 protein, IDH1-R132H, or SALL4. The quantitative Ki-67 proliferative index was 0.9% (Table 2). The patient underwent unilateral salpingo-oophorectomy and omentectomy. Later, she was also diagnosed with growing teratoma syndrome. She was alive with disease 12 months after the diagnosis of neurocytoma (20 months after the diagnosis of ovarian mixed germ cell tumor).
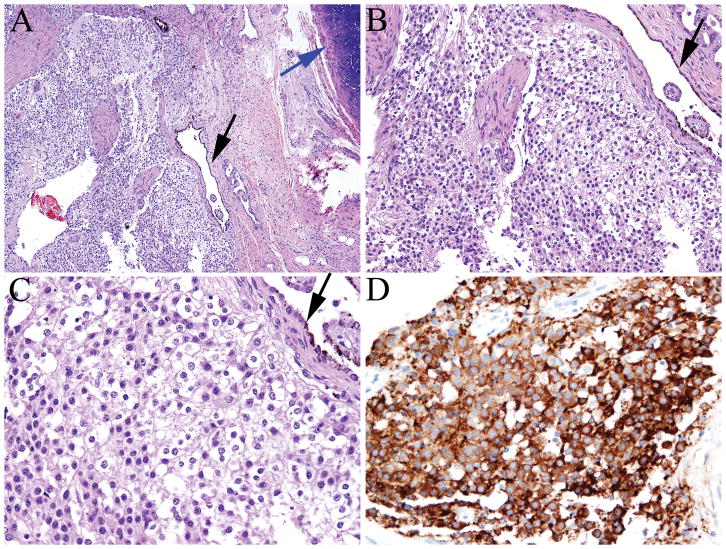
FIGURE 4.
A–B, Neurocytoma exhibits round, uniform nuclei, perinuclear cytoplasmic halos, and a low mitotic count (A–C). The tumor was adjacent to a cyst lined by ependyma-like cells (black arrow). Tumor cells showed strong synaptophysin expression (D).
DISCUSSION
Primary neuroectodermal tumors of the ovary can be parsed into three groups: 1) differentiated tumors, resembling ependymoma or astrocytoma of the CNS; 2) primitive tumors, resembling embryonal tumor with multilayered rosettes (comprising the historical entities of medulloepithelioma, ependymoblastoma, and embryonal tumor with abundant neuropil and true rosettes), neuroblastoma, or medulloblastoma; 3) anaplastic tumors, resembling glioblastoma. (2) Our study represents one of the most comprehensive studies of this rare class of tumors, which has provided collective data on pathology, grading, molecular signature and therapeutic interventions. Our analysis has demonstrated several important differences between the primary CNS tumor cohort and the primary ovarian/peritoneum tumor cohort in term of diagnostic terminology, biology and clinical behavior. First, although the two groups share very similar morphologic features, the four-tier WHO grading system (grades I-IV) for CNS gliomas has not been validated for extra-CNS gliomas. (11, 12) We thus choose to use a simple two-tier system (low-grade, high-grade) for extra-CNS tumor grade stratification. Secondly, despite morphologic similarity, primary ovarian/peritoneum astrocytomas do not exhibit the canonical IDH mutations that define the majority of WHO grade II and III CNS diffuse astrocytomas. Finally, with respect to treatment and prognosis, diffuse gliomas of the CNS cannot be cured by surgical resection secondary to their widely infiltrative growth pattern (i.e., infiltration along axons and dendrites), and ependymomas also can be difficult to totally extirpate without significant morbidity because of location in proximity to eloquent CNS centers; however, in sharp contrast, glial and neuronal tumors of the ovary can be extensively debulked and often totally resected through surgery, although it may not be possible to completely resect tumors with peritoneal dissemination. This feature is likely a major contributor to the difference in recurrence-free and overall survival between CNS patients and ovarian patients.
The origin of extra-CNS glial and neuronal tumors is not clear. One study observation is that although astrocytomas and neurocytoma were often associated with ovarian germ cell tumors, there was no such association with ependymomas, although rare cases of ependymoma associated with ovarian teratoma have been previously reported by others. (13, 14) Earlier studies have reported primary ovarian ependymoma (2, 13, 15–24), primary peritoneal ependymoma, (25, 26) , and primary ependymoma of the broad ligament. (27–29) Extra-axial ependymomas may show different morphological and immunophenotypic features from CNS ependymomas. Idowu and colleagues (30) demonstrated that extra-axial ependymomas more frequently express cytokeratin markers, such as 34betaE12, CK18, CAM 5.2 and CK7, compared to CNS ependymomas. In addition, ovarian and peritoneal ependymomas are frequently positive for ER and PR; thus these tumors can potentially be treated with hormonal therapy.(15–17, 31) On the other hand, astrocytic and neuronal tumors are often associated with ovarian germ cell tumors, but may also arise from metastatic tumor implants (i.e., peritoneal or omental implant) or from gliomatosis peritonei, which is characterized by mature glial tissue in the peritoneal cavity, usually present in patients with ovarian immature teratoma. (10, 32, 33)
From a diagnostic point of view, the differential diagnosis of glial and neuronal tumors of the ovary/peritoneum includes other primary ovarian neoplasms as well as metastatic neoplasms. Low-grade ependymoma may be misdiagnosed as low-grade endometrioid carcinoma or serous borderline tumor, and high-grade ependymoma may be misdiagnosed as high-grade serous carcinoma. In this setting, the presence of pseudorosettes and/or ependymal rosettes supports the diagnosis of ependymoma. In addition, immunohistochemical staining may be helpful in making a correct diagnosis. The combination of GFAP and other markers, including WT-1 and PAX8, may be helpful in problematic situations. High-grade serous carcinoma is typically positive for WT-1 and PAX8, whereas gliomas are usually strongly and diffusely positive for GFAP. Sex cord-stromal tumors, such as Sertoli-Leydig cell tumor or sclerosing stromal tumors, are also in the differential diagnosis; immunoexpression of inhibin and calretinin can be helpful as sex cord-stromal tumors usually show immunoreactivity for these two markers.
Previously, Hirschowitz and colleagues described a case of neurocytoma arising from a mature cystic teratoma in a 23-year-old woman.(35) Although neurocytoma can be confused with oligodendroglioma (i.e., both tumors demonstrate round nuclei with perinuclear halo), neurocytoma is typically negative for GFAP and positive for synaptophysin, whereas oligodendroglioma demonstrates the opposite immunophenotype. Interestingly, like their CNS counterparts, the neurocytoma we report and that reported by Hirschowitz and colleagues both arose adjacent to an ependyma-lined cyst, mimicking the presence of a ventricle. A final diagnostic caveat is that there was one previous case report documenting a CNS central neurocytoma with peritoneal cavity dissemination (34); thus, the rare event of metastasis from a CNS primary should be kept in mind and excluded.
In the forthcoming WHO 2016 Classification of Tumours of Central Nervous System, critical molecular signature will be incorporated into the definition of diffuse gliomas and embryonal tumors. For example, IDH mutational status will be incorporated into the definition and name of diffuse astrocytomas (e.g., diffuse astrocytoma, IDH-Mutant), and demonstration of IDH mutation and 1p/19q codeletion will define the highly clinically homogeneous entity of “Oligodendroglioma, IDH-mutant and 1p/19q codeleted”. (3, 36, 37) Moreover, molecular diversity has been demonstrated in diffuse gliomas that are otherwise indistinguishable by morphology. For example, pediatric diffuse gliomas of the CNS have a very different biology compared to adult diffuse gliomas, with Histone H3.3 mutations, not IDH mutations, serving as the driver mutations in a large subset of pediatric diffuse gliomas. (38–40) None of our interrogated ovarian astrocytic tumors exhibited IDH mutation (Table 2). Because of the limited sample number, it is uncertain if the lack of an IDH mutation signature is a characteristic of primary ovarian gliomas or, if so, what the molecular drivers are. Similarly, ependymomas of the CNS exhibit different molecular characteristics based on anatomic origin. Supratentorial ependymomas frequently carry C11orf95-RELA fusions that cause constitutive activation of the nuclear factor-κB signaling pathway. In contrast, posterior fossa ependymomas are divided into two distinct molecular and prognostic subgroups: group A – characterized by epigenetic changes, increased activation of several cancer signaling pathways, and a more aggressive prognosis, and group B – characterized by lack of epigenetic alterations, enriched in chromosomal number variations, and a good prognosis. (41–46) In stark contrast, little is known about the genetic and epigenetic changes in extra-CNS ependymomas. Next-generation sequencing, performed on 2 ependymomas in our study, failed to detect mutations in the coding regions of a panel of 50 genes, including IDH1, IDH2, PIK3CA, and PTEN. While these data strongly suggests that ovarian ependymomas may not carry mutations similar to those observed in CNS counterparts, such a conclusion awaits validation from a larger cohort.
In conclusion, our findings provide updated information on rare glial and neuronal tumors of the gynecologic tract (summarized in Table 3). Further molecular studies are needed to elucidate the molecular drivers of gynecologic glial/neuronal tumors and attendant prognostic and therapeutic associations. Owing to the rarity of glial and neuronal tumors of the ovary/peritoneum, a multi-institutional study would be helpful to further understanding of this rare tumor class.
Table 3.
Key Features of Primary Glial Neoplasms of the Ovary or Peritoneum.
| CNS glial neoplasms | Ovarian/peritoneum glial neoplasms | |
|---|---|---|
| Location | May involve areas that control critical functions that cannot be resected with free margins. | Extensive surgery is usually possible for ovarian tumors, but may not be possible for tumors with peritoneal dissemination. |
| Histologic features | See the description in the main text. | Resembles their counterparts in the CNS. Astrocytic tumors are usually associated with ovarian germ cell tumors, but ependymomas are usually not associated with other ovarian neoplasms. |
| Immunophenotype | Immunostains for ER and PR are positive only in a small percentage of CNS ependymomas. (29) | Immunostains for ER and PR are frequently positive in ovarian/peritoneal ependymomas. |
| Molecular findings | Characteristic recurrent molecular alternations are identified (i.e., IDH mutations, etc). | No IDH1 or IDH2 mutations were detected in the present study. |
| Treatment | Surgical resection, chemoradiation. | Surgical resection (i.e., USO, TAH-BSO, or cytoreduction), followed by HIPEC, chemotherapy or radiation. * |
Abbreviations: CNS, central nervous system; ER, estrogen receptor; PR, progesterone receptor; IDH, isocitrate dehydrogenase; USO, unilateral salpingo-oophorectomy; TAH-BSO, total abdominal hysterectomy and bilateral salpingo-oophorectomy; HIPEC, hyperthermic intraperitoneal chemotherapy.
Patients may receive chemotherapy for the treatment of ovarian germ cell tumors that are associated with gliomas.
Acknowledgments
Source of Funding:
This work was supported in part by grant from the Cancer Prevention and Research Institute of Texas, the MD Anderson Cancer Center SPORE in Ovarian Cancer (National Institute of Health Grant), a Sister Institution Grant from MD Anderson Cancer Center Global Academic Programs. Drs. Li Liang and Adriana Olar are supported by the training grant T32CA163185 from NIH/NCI.
We thank Joseph Munch from the Department of Scientific Publications at The University of Texas MD Anderson Cancer Center for editing the manuscript.
Footnotes
Conflicts of Interest The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
References
- 1.Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO classification of tumours of female reproductive organs. Lyon: International Agency for Research on Cancer; 2014. International Agency for Research on Cancer., World Health Organization. [Google Scholar]
- 2.Kleinman GM, Young RH, Scully RE. Primary neuroectodermal tumors of the ovary. A report of 25 cases. Am J Surg Pathol. 1993;17:764–778. doi: 10.1097/00000478-199308000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Louis DN, Perry A, Burger P, et al. International Society Of Neuropathology--Haarlem consensus guidelines for nervous system tumor classification and grading. Brain Pathol. 2014;24:429–435. doi: 10.1111/bpa.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsuura Y, Kitajima M, Hachisuga T, et al. Malignant mixed mullerian tumor with malignant neuroectodermal components (teratoid carcinosarcoma) of the ovary: Report of a case with clinicopathologic findings. J Obstet Gynaecol Res. 2010;36:907–911. doi: 10.1111/j.1447-0756.2010.01238.x. [DOI] [PubMed] [Google Scholar]
- 5.Mott RT, Murphy BA, Geisinger KR. Ovarian malignant mixed mesodermal tumor with neuroectodermal differentiation: a multifaceted evaluation. Int J Gynecol Pathol. 2010;29:234–238. doi: 10.1097/PGP.0b013e3181bd413c. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Galvis OF, Cabrera-Ozoria C, Fernandez JA, et al. Malignant Mullerian mixed tumor of the ovary associated with yolk sac tumor, neuroepithelial and trophoblastic differentiation (teratoid carcinosarcoma) Int J Gynecol Pathol. 2008;27:515–520. doi: 10.1097/PGP.0b013e31817b06c7. [DOI] [PubMed] [Google Scholar]
- 7.Tanimoto A, Arima N, Hayashi R, et al. Teratoid carcinosarcoma of the ovary with prominent neuroectodermal differentiation. Pathol Int. 2001;51:829–832. doi: 10.1046/j.1440-1827.2001.01275.x. [DOI] [PubMed] [Google Scholar]
- 8.Ehrmann RL, Weidner N, Welch WR, et al. Malignant mixed mullerian tumor of the ovary with prominent neuroectodermal differentiation (teratoid carcinosarcoma) Int J Gynecol Pathol. 1990;9:272–282. doi: 10.1097/00004347-199007000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Shintaku M, Mise Y. Mullerian adenosarcoma with a neuroectodermal component associated with an endometriotic cyst of the ovary: a case report. Pathol Int. 2012;62:271–275. doi: 10.1111/j.1440-1827.2011.02782.x. [DOI] [PubMed] [Google Scholar]
- 10.Liang L, Zhang Y, Malpica A, et al. Gliomatosis peritonei: a clinicopathologic and immunohistochemical study of 21 cases. Mod Pathol. 2015;28:1613–20. doi: 10.1038/modpathol.2015.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuller GN, Scheithauer BW. The 2007 Revised World Health Organization (WHO) Classification of Tumours of the Central Nervous System: newly codified entities. Brain Pathol. 2007;17:304–307. doi: 10.1111/j.1750-3639.2007.00084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spaulding R, Alatassi H, Stewart Metzinger D, et al. Ependymoma and carcinoid tumor associated with ovarian mature cystic teratoma in a patient with multiple endocrine neoplasia I. Case Rep Obstet Gynecol. 2014;2014:712657. doi: 10.1155/2014/712657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stolnicu S, Furtado A, Sanches A, et al. Ovarian ependymomas of extra-axial type or central immunophenotypes. Hum Pathol. 2011;42:403–408. doi: 10.1016/j.humpath.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Auerbach R, Mittal K, Schwartz PE. Estrogen and progestin receptors in an ovarian ependymoma. Obstet Gynecol. 1988;71:1043–1045. [PubMed] [Google Scholar]
- 16.Carr KA, Roberts JA, Frank TS. Progesterone receptors in bilateral ovarian ependymoma presenting in pregnancy. Hum Pathol. 1992;23:962–965. doi: 10.1016/0046-8177(92)90414-x. [DOI] [PubMed] [Google Scholar]
- 17.Deval B, Rousset P, Bigenwald C, et al. Treatment of ovarian anaplastic ependymoma by an aromatase inhibitor. Obstet Gynecol. 2014;123:488–491. doi: 10.1097/AOG.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 18.Erdogan G, Ozel E, Pestereli HE, et al. Ovarian ependymoma. APMIS. 2005;113:301–303. doi: 10.1111/j.1600-0463.2005.apm_10.x. [DOI] [PubMed] [Google Scholar]
- 19.Fan F, Hernandez-Rios P, Damjanov I, et al. Metastasis of ovarian ependymoma to the liver diagnosed by fine needle aspiration cytology. Acta Cytol. 2006;50:709–710. [PubMed] [Google Scholar]
- 20.Garcia-Barriola V, De Gomez MN, Suarez JA, et al. Ovarian ependymoma. A case report. Pathol Res Pract. 2000;196:595–599. doi: 10.1016/S0344-0338(00)80035-7. [DOI] [PubMed] [Google Scholar]
- 21.Harieaswer S, Sinha R, Elabassy M. Ovarian ependymoma: appearance on magnetic resonance imaging. Int J Gynecol Pathol. 2006;26:710–713. doi: 10.1080/01443610600940372. [DOI] [PubMed] [Google Scholar]
- 22.Hirahara F, Yamanaka M, Miyagia E, et al. Pure ovarian ependymoma: report of a case treated with surgery, chemotherapy, irradiation and hyperthermotherapy. Eur J Obstet Gynecol Reprod Biol. 1997;75:221–223. doi: 10.1016/s0301-2115(97)00134-6. [DOI] [PubMed] [Google Scholar]
- 23.Komuro Y, Mikami M, Sakaiya N, et al. Tumor imprint cytology of ovarian ependymoma. A case report. Cancer. 2001;92:3165–3169. doi: 10.1002/1097-0142(20011215)92:12<3165::aid-cncr10111>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 24.Morovic A, Damjanov I. Neuroectodermal ovarian tumors: a brief overview. Histol Histopathol. 2008;23:765–771. doi: 10.14670/HH-23.765. [DOI] [PubMed] [Google Scholar]
- 25.Mogler C, Kohlhof P, Penzel R, et al. A primary malignant ependymoma of the abdominal cavity: a case report and review of the literature. Virchows Arch. 2009;454:475–478. doi: 10.1007/s00428-009-0744-8. [DOI] [PubMed] [Google Scholar]
- 26.Verdun TP, Owen DA. Primary peritoneal ependymoma. Pathol Res Pract. 2015;211:268–270. doi: 10.1016/j.prp.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Bell DA, Woodruff JM, Scully RE. Ependymoma of the broad ligament. A report of two cases. Am J Surg Pathol. 1984;8:203–209. doi: 10.1097/00000478-198403000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Matsuyama A, Hisaoka M, Yamamoto I, et al. Extraspinal ependymoma of the broad ligament. Pathol Int. 2010;60:241–244. doi: 10.1111/j.1440-1827.2009.02509.x. [DOI] [PubMed] [Google Scholar]
- 29.Whittemore DE, Grondahl RE, Wong K. Primary extraneural myxopapillary ependymoma of the broad ligament. Arch Pathol Lab Med. 2005;129:1338–1342. doi: 10.5858/2005-129-1338-PEMEOT. [DOI] [PubMed] [Google Scholar]
- 30.Idowu MO, Rosenblum MK, Wei XJ, et al. Ependymomas of the central nervous system and adult extra-axial ependymomas are morphologically and immunohistochemically distinct--a comparative study with assessment of ovarian carcinomas for expression of glial fibrillary acidic protein. Am J Surg Pathol. 2008;32:710–718. doi: 10.1097/PAS.0b013e318159a2b4. [DOI] [PubMed] [Google Scholar]
- 31.Yoffe R, Khakoo Y, Dunkel IJ, et al. Recurrent ependymoma treated with high-dose tamoxifen in a peripubertal female: Impact on tumor and the pituitary-ovarian axis. Pediatr Blood Cancer. 2007;49:758–760. doi: 10.1002/pbc.20647. [DOI] [PubMed] [Google Scholar]
- 32.Dadmanesh F, Miller DM, Swenerton KD, et al. Gliomatosis peritonei with malignant transformation. Mod Pathol. 1997;10:597–601. [PubMed] [Google Scholar]
- 33.Shefren G, Collin J, Soriero O. Gliomatosis peritonei with malignant transformation: a case report and review of the literature. Am J Obstet Gynecol. 1991;164:1617–1620. doi: 10.1016/0002-9378(91)91445-3. discussion 1620–1611. [DOI] [PubMed] [Google Scholar]
- 34.Coelho Neto M, Ramina R, de Meneses MS, et al. Peritoneal dissemination from central neurocytoma: case report. Arq Neuropsiquiatr. 2003;61:1030–1034. doi: 10.1590/s0004-282x2003000600028. [DOI] [PubMed] [Google Scholar]
- 35.Hirschowitz L, Ansari A, Cahill DJ, et al. Central neurocytoma arising within a mature cystic teratoma of the ovary. Int J Gynecol Pathol. 1997;16:176–179. doi: 10.1097/00004347-199704000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Olar A, Wani KM, Alfaro-Munoz KD, et al. IDH mutation status and role of WHO grade and mitotic index in overall survival in grade II–III diffuse gliomas. Acta Neuropathol. 2015;129:585–596. doi: 10.1007/s00401-015-1398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olar A, Sulman EP. Molecular Markers in Low-Grade Glioma-Toward Tumor Reclassification. Semin Radiat Oncol. 2015;25:155–163. doi: 10.1016/j.semradonc.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartzentruber J, Korshunov A, Liu XY, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482:226–231. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- 39.Diaz AK, Baker SJ. The genetic signatures of pediatric high-grade glioma: no longer a one-act play. Semin Radiat Oncol. 2014;24:240–247. doi: 10.1016/j.semradonc.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fontebasso AM, Liu XY, Sturm D, et al. Chromatin remodeling defects in pediatric and young adult glioblastoma: a tale of a variant histone 3 tail. Brain Pathol. 2013;23:210–216. doi: 10.1111/bpa.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Versteeg R. Cancer: Tumours outside the mutation box. Nature. 2014;506:438–439. doi: 10.1038/nature13061. [DOI] [PubMed] [Google Scholar]
- 42.Parker M, Mohankumar KM, Punchihewa C, et al. C11orf95-RELA fusions drive oncogenic NF-kappaB signalling in ependymoma. Nature. 2014;506:451–455. doi: 10.1038/nature13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pietsch T, Wohlers I, Goschzik T, et al. Supratentorial ependymomas of childhood carry C11orf95-RELA fusions leading to pathological activation of the NF-kappaB signaling pathway. Acta Neuropathol. 2014;127:609–611. doi: 10.1007/s00401-014-1264-4. [DOI] [PubMed] [Google Scholar]
- 44.Witt H, Mack SC, Ryzhova M, et al. Delineation of two clinically and molecularly distinct subgroups of posterior fossa ependymoma. Cancer cell. 2011;20:143–157. doi: 10.1016/j.ccr.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wani K, Armstrong TS, Vera-Bolanos E, et al. A prognostic gene expression signature in infratentorial ependymoma. Acta Neuropathol. 2012;123:727–738. doi: 10.1007/s00401-012-0941-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoffman LM, Donson AM, Nakachi I, et al. Molecular sub-group-specific immunophenotypic changes are associated with outcome in recurrent posterior fossa ependymoma. Acta Neuropathol. 2014;127:731–745. doi: 10.1007/s00401-013-1212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]