Summary
We often experience troubled sleep in a novel environment [1]. This is called the first-night effect (FNE) in human sleep research and has been regarded as a typical sleep disturbance [2–4]. Here we show that the FNE is a manifestation of one hemisphere being more vigilant than the other as a night watch to monitor unfamiliar surroundings during sleep [5, 6]. Using advanced neuroimaging techniques [7, 8] as well as polysomnography, we found that the temporary sleep disturbance in the first sleep experimental session involves regional interhemispheric asymmetry of sleep depth [9]. The interhemispheric asymmetry of sleep depth associated with the FNE was found in the default-mode network (DMN) involved with spontaneous internal thoughts during wakeful rest [10, 11]. The degree of asymmetry was significantly correlated with the sleep-onset latency, which reflects the degree of difficulty of falling asleep and is a critical measure for the FNE. Furthermore, the hemisphere with reduced sleep depth showed enhanced evoked brain response to deviant external stimuli. Deviant external stimuli detected by the less-sleeping hemisphere caused more arousals and faster behavioral responses than those detected by the other hemisphere. None of these asymmetries was evident during subsequent sleep sessions. These lines of evidence are in accord with the hypothesis that troubled sleep in an unfamiliar environment is an act for survival over an unfamiliar and potentially dangerous environment by keeping one hemisphere partially more vigilant than the other hemisphere as a night watch which wakes the sleeper up when unfamiliar external signals are detected.
Results and Discussion
Does sleep disturbance caused by an unfamiliar environment, that is, the FNE have only negative effects? It has been suggested that a function of partial sleep such as unilateral hemispheric sleep in some birds and marine mammals is a protective mechanism to compensate for risks during sleep [5, 6]. This led us to ask whether the FNE is involved in some type of interhemispheric sleep to be vigilant in one brain hemisphere in humans as a protective mechanism.
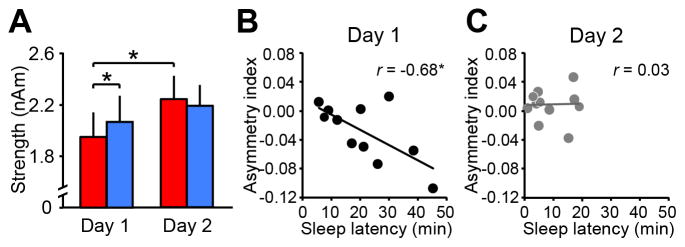
In Experiment 1 (see Supplemental Experimental Procedures), we tested whether a regional interhemispheric asymmetry occurs with the FNE using an advanced neuroimaging technique that combines magnetoencephalography (MEG), structural magnetic resonance imaging (MRI), and polysomnography (PSG) in the sleeping brain. We investigated slow-wave activity (SWA), which is a spontaneous brain oscillation (1–4 Hz) in non-rapid eye movement (NREM) sleep. The reason that we focused on SWA is that it is the only sleep characteristic, which reflects the depth of sleep [9, 12] and is supported by cross-species studies of local sleep including those for mammals and birds. Since SWA in humans originates in cortical regions including the brain network such as DMN [10, 11, 13], we hypothesize that the regional interhemispheric SWA occurs in brain networks while the FNE occurs. To test this hypothesis, we measured SWA from 4 brain networks (including the DMN, Figure S1) during the first sleep session in which the FNE occurs and the second sleep session in which the FNE does not occur (Table S1). We conducted a 4-way repeated measures ANOVA on SWA with the factors being network, hemisphere (left vs. right), sleep stage (slow-wave sleep vs. stage 2 sleep), and sleep session (day 1 vs. day 2). A factor of sleep stage was included because the strength of SWA, or sleep depth, should be different between sleep stages. If regional interhemispheric asymmetry of SWA occurs with the FNE in a certain network, this should manifest as an interaction among the factors. We indeed found the 4-way interaction significant (F3,30=4.45, p=0.011, Figure S1, Table S2). The hemisphere x sleep session interaction was significant only in the DMN among the 4 networks during slow-wave sleep (F1,10=10.03, p=0.010, Table S2). Further analyses (Table S2) indicated that SWA in the left DMN on day 1 was significantly smaller than SWA in the right DMN on day 1 (Figure 1A; t10=2.59, p=0.027, d=0.8) and was also significantly smaller than SWA in the left DMN on day 2 (t10=2.69, p=0.023, d=0.8). There was no significant difference between days in the right DMN (t10=0.97, p=0.355, n.s.). SWA associated with K-complexes did not show any hemispheric asymmetry (Figure S2).
Figure 1. SWA asymmetry in the DMN during slow-wave sleep in association with the FNE.
(A) SWA in the DMN during slow-wave sleep. Red bars show the left hemisphere, and the blue bars show the right hemisphere. The values are mean ± SEM. Asterisks indicate a significant difference in the post-hoc tests after the 4-way repeated measures ANOVA (*p<0.05). (B) Scatter plots for the asymmetry index of DMN SWA against the sleep-onset latency for day 1 (r=−0.68, p=0.022), and (C) day 2 (r=0.03, p=0.935, n.s.). *p<0.05. The correlation coefficient on day 1 was significantly different from day 2 (zpf10=−1.99, p=0.046). See Figure S1 for SWA in other networks, details of ANOVA results and details of the asymmetry index. See also Figure S2 for additional data on SWA.
We further examined the relationship between the degree of interhemispheric asymmetry of SWA in the DMN and the degree of the FNE. We obtained an asymmetry index for SWA strength ([left SWA - right SWA]/[left SWA + right SWA]) for the DMN during slow-wave sleep (Figure S1). If SWA asymmetry for the DMN is associated with the reduced sleep quality in the first sleep session, the asymmetry index should be significantly correlated with the sleep-onset latency, which is a sensitive parameter for the presence of the FNE [3, 14]. A strong and significant negative correlation between these measures was found on day 1, but not on day 2 (Figures 1B&C). The correlations were significantly different between days.
To our best knowledge, regional asymmetric SWA associated with the FNE has never been reported in humans. Why was this not found in previous studies? First, visual inspection of PSG did not detect any hemispheric asymmetry in the apparent amplitude of SWA in the current data. Second, frequency analyses on sensor-space MEG failed to reveal regional hemispheric asymmetry of SWA (Figure S2). It may be difficult to detect the regional asymmetric SWA in a specific network such as the DMN in association with the FNE, unless sleeping brain activities are examined across different sleep sessions, hemispheres and brain networks with high spatial resolution.
Is the regionally lighter sleep in the left hemisphere shown in the results of Experiment 1 on day 1 related to higher vigilance to external signals? In Experiment 2, we addressed this question using an oddball paradigm, where the amplitude of the brain response evoked by rare stimuli correlates with vigilance [15, 16] with a new group of subjects (see Supplemental Experimental Procedures). If one hemisphere is more vigilant than the other on day 1, the amplitude of the brain response should be larger in the hemisphere than in the other hemisphere on day 1. Both infrequent deviant and frequent standard beeps were presented every 1s monaurally while subjects were asleep.
We conducted a 3-way repeated measures ANOVA on the mean amplitude of evoked brain responses with the factors of sound type, hemisphere, and sleep session during slow-wave sleep (Figure S3) in which regional asymmetric SWA was found in Experiment 1. The results indicated that the mean amplitude of brain responses to deviant sounds was significantly augmented in the left hemisphere compared to the right on day 1 (Figure 2A; t12=2.92, p=0.013, d=0.9), but not on day 2 (t12=1.37, p=0.195, n.s.). The amplitude of the brain response to the deviant sounds in the left hemisphere was significantly reduced on day 2 (t12=3.18, p=0.008, d=1.0), while there was no significant difference between days in the right hemisphere (t12=0.66, p=0.523, n.s.). No significant difference was found in the brain responses to the standard sound between the hemispheres or between sleep sessions (Figure 2B). Thus, hemispheric asymmetry in the brain responses was specific to the deviant sounds on day 1. These results indicate that the left hemisphere was more vigilant than the right when the FNE occurred. Hemispheric asymmetry in the brain responses to the deviant sounds was not found during wakefulness or stage 2 sleep (Figure S3).
Figure 2. Brain responses during slow-wave sleep.
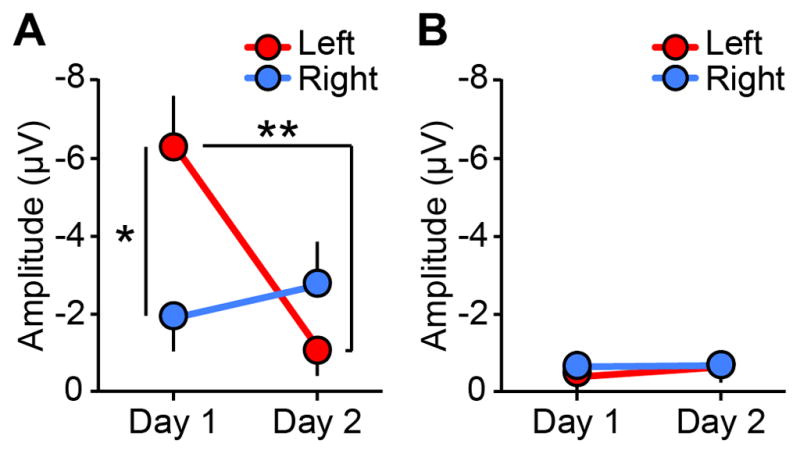
(A) The amplitudes of the brain responses to deviant sounds in the left (red) and right (blue) hemispheres (μV). Asterisks indicate a significant difference in the post-hoc tests after the 3-way repeated measures ANOVA (**p<0.01, *p<0.05). (B) The amplitudes of the brain responses to standard sounds in the left (red) and right (blue) hemispheres (μV). The values are mean ± SEM. See Figure S3 for additional information regarding brain responses including detailed results of the ANOVA, timecourse, and the brain responses in other sleep stages.
Next, we found that enhanced vigilance in the left hemisphere resulted in more arousals. An arousal is defined as an abrupt and short shift of EEG frequency [17]. We counted how often arousals occurred per minute following a deviant sound during slow-wave sleep. Arousals occurred more frequently on day 1 than day 2 (Figure 3A). Given that an arousal was induced by a monaural deviant sound, we examined whether the arousal occurrence depended on the contralateral hemisphere to the ear to which the deviant sound was presented. Here, a trial on which a deviant sound was presented to the right (left) ear is called a left- (right-) hemisphere trial [18]. The arousal occurrence% following the left-hemisphere trials occupied more than 80% of the total arousals on day 1 and was significantly larger than chance (Figure 3B; Wilcoxon signed-rank test, z12=3.22, p=0.001). The arousal occurrence% following the left-hemisphere trials was significantly larger on day 1 than on day 2 (Figure 3B; z12=2.49, p=0.013). However, this left-hemisphere dominance in the arousal occurrence vanished on day 2 (z12=0.32, p=0.751, n.s.). These results indicate that the left hemisphere showed more arousals with deviant external stimuli than the right hemisphere during sleep on day 1 when the FNE occurred.
Figure 3. Arousals followed by deviant sounds presented during slow-wave sleep.
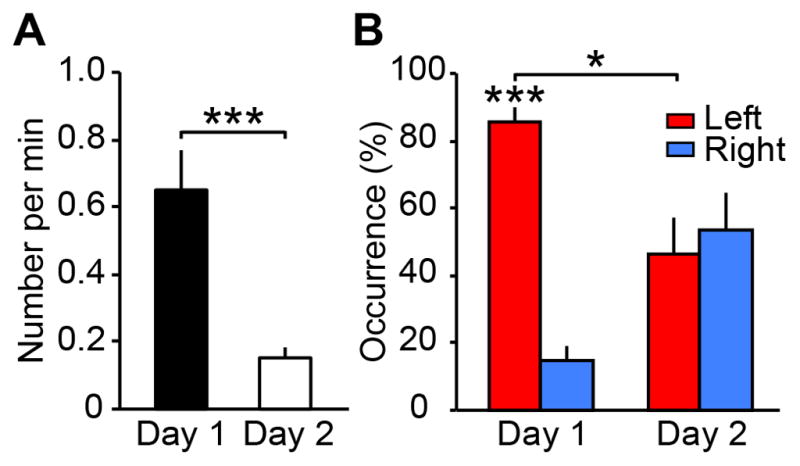
(A) The total number of arousals per min during slow-wave sleep. There was a significant difference in the number of arousals per min between days (Wilcoxon signed-rank test, z12=3.18, p=0.002). (B) The percentage of arousal occurrences that followed left-hemisphere trials (red) and right-hemisphere trials (blue). The values are mean ± SEM. ***p<0.005, *p<0.05, the false discovery rate (FDR) was controlled to be at 0.05.
Does the vigilant hemisphere on day 1 produce faster behavioral responses to deviant external stimuli than on day 2? If the FNE plays a role as a protective mechanism such as a night watch rather than showing a merely disrupted sleep, a faster behavioral response should be generated from sleep upon the detection of deviant external stimuli in the first sleep session. In Experiment 3, we asked a new group of subjects to lightly tap fingers when they heard sounds while they were sleeping, using an oddball paradigm similar to Experiment 2. First, a larger number of subjects were woken by left-hemisphere trials than right-hemisphere trials on day 1 compared to day 2 (Figure 4A). Second, the reaction time from a deviant sound to tap was significantly faster on day 1 than day 2 (Figure 4B). Third, this faster response on day 1 was mainly driven by the shorter time from a deviant sound to awakening while the brain was still asleep and not by the time from awakening to tapping, by the left hemisphere (Figure S4), indicating that subjects woke up faster after the deviant sound. Importantly, the faster awakening was correlated with the asymmetry index of regional SWA (Supplemental Experimental Procedures) on day 1, but not on day 2 (Figure S4). These results demonstrate that the FNE is linked to faster awakening upon detection of deviant stimuli during sleep by a more vigilant hemisphere, and suggest that the FNE is involved in a protective mechanism.
Figure 4. Awakening and behavioral responses followed by deviant sounds.
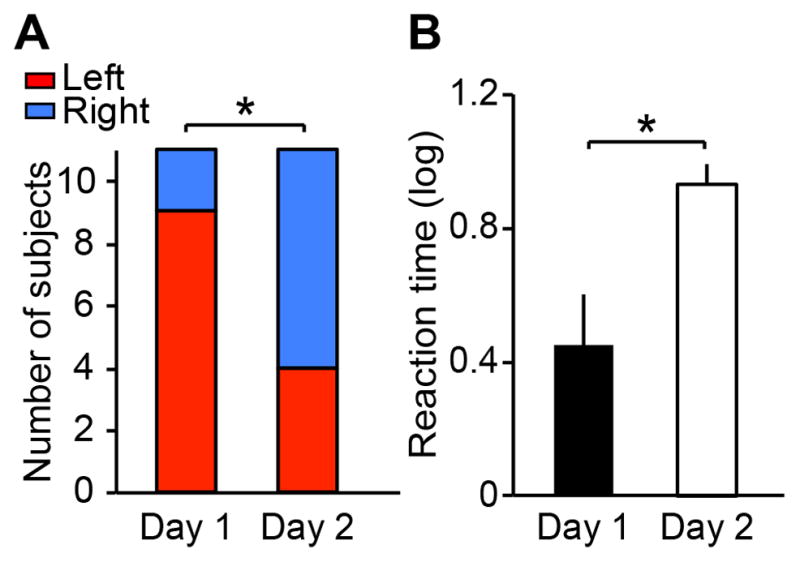
(A) The number of subjects who were woken in the left- (red) and right- (blue) hemisphere trials. The numbers of subjects who were woken on the left- and right hemisphere trials were significantly different between days (McNemar’s test, p=0.033). The number of subjects who were woken on the left-hemisphere trials was significantly larger than chance on day 1 (Wilcoxon signed-rank test, z10=2.11, p=0.035). (B) The reaction time between the deviant sound and the finger tapping. The reaction time was significantly faster on day 1 than day 2 (Wilcoxon signed-rank test, z10=2.31, p=0.021). The values are mean ± SEM. *p<0.05. See Figure S4 for additional information on the reaction time.
The regional interhemispheric asymmetry in SWA, brain responses, and behavioral outcomes were observed specifically during slow-wave sleep associated with the FNE. We suggest that this sleep stage specificity is related to increased vigilance and responsiveness for a night watch as a counter measure to the vulnerability of this sleep stage [19, 20]. Unihemispheric sleep has been linked to a protective mechanism in some birds and marine mammals to monitor the environments and detect predators [5, 6, 21]. Although the interhemispheric asymmetry of SWA in our study is more regionally restricted compared to animals [5, 6, 21], we speculate that the regional interhemispheric asymmetry of SWA in humans is also linked with a protective mechanism, which is sensitive to potential danger in an unfamiliar sleeping environment and the increased need for vigilance during sleep.
Some forms of regional interhemispheric asymmetry of SWA have already been reported in humans. However, they were observed in entirely different conditions and may have fundamentally different mechanisms from the form of asymmetry found in this study. First, regional interhemispheric asymmetry of SWA in prolonged wakefulness [22, 23] and stimulation to a unilateral cortical region [24, 25] have been reported and are regarded as the rationale for the sleep homeostasis hypothesis [9, 26]. According to this hypothesis, regional SWA is modulated by a homeostatic need for SWA accumulated in a brain region during prior wakefulness. However, the sleep homeostasis hypothesis cannot account for the present results. The sleep homeostasis hypothesis predicts that decreased SWA in the left DMN should result from the decreased use of the region during prior wakefulness. However, we did not systematically manipulate to use, or stimulate, cortical regions including the left DMN during prior wakefulness. Second, increased stress and/or anxiety in novel environments during wakefulness increased SWA during subsequent sleep in animals [27]. However, the present finding that the SWA decreased in the left DMN on day 1 does not match the increased-stress/anxiety account. Importantly, the FNE does not necessarily accompany increased anxiety or discomfort level (see Table S3 [4]). Third, patients with sleep apnea and insomnia show regional interhemispheric asymmetry in SWA [28–31]. However, the asymmetry is not specific to the DMN or the FNE in these studies. Finally, the present study recruited young healthy subjects, not clinical populations. Thus, it is unlikely that the forms of asymmetry in the above three cases have the same underlying mechanism as the asymmetry found in our study.
Our study suggests that the DMN works as a night watch in an unfamiliar environment to protect the sleeper. The DMN has been associated with mind wandering, or simulation and evaluation of upcoming events apart from execution of a current task [10, 32]. While general connectivity in the brain is mostly broken during sleep [33, 34], the DMN is not completely shut off and works as a network showing reduced connectivity during sleep [35–37]. These unique characteristics of the DMN may fit well with the role of a night watch. However, since the number of brain networks examined in the current study was limited, the possibility of involvements of other intrinsic networks [34, 38, 39] cannot be rejected. Moreover, the DMN may not work solely as a night watch, but may cooperate with the subcortical circuits that play important roles in the regulation of sleep and wakefulness [40]. More studies are needed to fully understand neural mechanisms underlying the night watch system associated with the FNE.
Why was the left hemisphere more vigilant than the right when the FNE occurred? At least a few possibilities arise. First, the overall functional connectivity between the DMN and other regions is stronger in the left hemisphere than the right [39]. The stronger connectivity to other regions may be useful for a night watch and faster responses to risk factors. Direct stimulation by transcranial magnetic stimulation [33] might be useful for further investigations. Second, we measured brain activity only during the first sleep cycle. This leaves the possibility that during the first sleep cycle the left hemisphere is vigilant but vigilant hemispheres alternate with different cycles. Future studies are needed to examine these possibilities.
In summary, the present study has demonstrated that when we are in a novel environment, interhemispheric asymmetry occurs in regional SWA, vigilance, and responsiveness, as a night watch to protect ourselves.
Experimental procedures
A total of 35 young and healthy subjects participated in the study. All subjects gave written informed consent for their participation in experiments. The research protocols were approved by the institutional review boards. All experiments had 2 sleep sessions, which were conducted approximately a week apart. See Supplemental Experimental Procedures for more details.
Supplementary Material
Acknowledgments
The authors thank Mary A. Carskadon, Aaron V. Berard, Jonathan Dobres, and Kazuhisa Shibata for their helpful comments on an earlier draft.
This work was supported by grants to T.W. and Y.S. (NIH R01MH091801, R01EY015980, R01EY019466, R01EY018334, and NSF BCS 1539717). This work also involved the use of instrumentation supported by the NCRR Shared Instrumentation Grant Program and High-End Instrumentation Grant Program; specifically, grant numbers S10RR014978, S10RR021110 and S10RR023401. This research was carried out in part at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies, NCRR P41RR14075. Part of this research was also conducted using computational resources and services at the Center for Computation and Visualization, Brown University.
Footnotes
Author Contributions
M.T. and Y.S. designed the research. M.T. and J.B. performed the experiments and analyzed the data. M.T., T.W., and Y.S. wrote the manuscript.
Conflict of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Masako Tamaki, Email: tamaki@brown.edu.
Ji Won Bang, Email: ji_won_bang@brown.edu.
Takeo Watanabe, Email: takeo_watanabe@brown.edu.
Yuka Sasaki, Email: yuka_sasaki@brown.edu.
References
- 1.Roth T, Stubbs C, Walsh JK. Ramelteon (TAK-375), a selective MT1/MT2-receptor agonist, reduces latency to persistent sleep in a model of transient insomnia related to a novel sleep environment. Sleep. 2005;28:303–307. [PubMed] [Google Scholar]
- 2.Agnew HW, Jr, Webb WB, Williams RL. The first night effect: an EEG study of sleep. Psychophysiology. 1966;2:263–266. doi: 10.1111/j.1469-8986.1966.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 3.Tamaki M, Nittono H, Hayashi M, Hori T. Examination of the first-night effect during the sleep-onset period. Sleep. 2005;28:195–202. doi: 10.1093/sleep/28.2.195. [DOI] [PubMed] [Google Scholar]
- 4.Tamaki M, Nittono H, Hori T. The first-night effect occurs at the sleep-onset period regardless of the temporal anxiety level in healthy students. Sleep Biol Rhythms. 2005;3:92–94. [Google Scholar]
- 5.Rattenborg NC, Lima SL, Amlaner CJ. Half-awake to the risk of predation. Nature. 1999;397:397–398. doi: 10.1038/17037. [DOI] [PubMed] [Google Scholar]
- 6.Lilly J. Animals in aquatic environments: adaptation of mammals to the ocean. In: Dill DB, Adolph EF, Wilber CG, editors. Handbook of Physiology, Section 4, Adaptation to the environment. Washington, DC: American Physiological Society; 1964. pp. 741–757. [Google Scholar]
- 7.Lin FH, Witzel T, Hamalainen MS, Dale AM, Belliveau JW, Stufflebeam SM. Spectral spatiotemporal imaging of cortical oscillations and interactions in the human brain. Neuroimage. 2004;23:582–595. doi: 10.1016/j.neuroimage.2004.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahveninen J, Lin FH, Kivisaari R, Autti T, Hamalainen M, Stufflebeam S, Belliveau JW, Kahkonen S. MRI-constrained spectral imaging of benzodiazepine modulation of spontaneous neuromagnetic activity in human cortex. Neuroimage. 2007;35:577–582. doi: 10.1016/j.neuroimage.2006.12.033. [DOI] [PubMed] [Google Scholar]
- 9.Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 10.Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nobili L, Ferrara M, Moroni F, De Gennaro L, Russo GL, Campus C, Cardinale F, De Carli F. Dissociated wake-like and sleep-like electro-cortical activity during sleep. Neuroimage. 2011;58:612–619. doi: 10.1016/j.neuroimage.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 13.Murphy M, Riedner BA, Huber R, Massimini M, Ferrarelli F, Tononi G. Source modeling sleep slow waves. Proc Natl Acad Sci USA. 2009;106:1608–1613. doi: 10.1073/pnas.0807933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamaki M, Nittono H, Hayashi M, Hori T. Spectral analysis of the first-night effect on the sleep-onset period. Sleep Biol Rhythms. 2005;3:122–129. doi: 10.1093/sleep/28.2.195. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen-Bohlman L, Knight RT, Woods DL, Woodward K. Differential auditory processing continues during sleep. Electroencephalogr Clin Neurophysiol. 1991;79:281–290. doi: 10.1016/0013-4694(91)90124-m. [DOI] [PubMed] [Google Scholar]
- 16.Michida N, Hayashi M, Hori T. Effects of hypnagogic imagery on the event-related potential to external tone stimuli. Sleep. 2005;28:813–818. doi: 10.1093/sleep/28.7.813. [DOI] [PubMed] [Google Scholar]
- 17.Iber C, Ancoli-Israel SS, Chesson A, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. Westchester: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 18.Kimura D. Functional asymmetry of the brain in dichotic listening. Cortex. 1967;3:163–178. [Google Scholar]
- 19.Rechtschaffen A, Hauri P, Zeitlin M. Auditory awakening thresholds in REM and NREM sleep stages. Percept Mot Skills. 1966;22:927–942. doi: 10.2466/pms.1966.22.3.927. [DOI] [PubMed] [Google Scholar]
- 20.Williams HL, Hammack JT, Daly RL, Dement WC, Lubin A. Responses to auditory stimulation, sleep loss and the EEG stages of sleep. Electroencephalogr Clin Neurophysiol. 1964;16:269–279. doi: 10.1016/0013-4694(64)90109-9. [DOI] [PubMed] [Google Scholar]
- 21.Lyamin OI, Manger PR, Ridgway SH, Mukhametov LM, Siegel JM. Cetacean sleep: an unusual form of mammalian sleep. Neurosci Biobehav Rev. 2008;32:1451–1484. doi: 10.1016/j.neubiorev.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrara M, De Gennaro L, Curcio G, Cristiani R, Bertini M. Interhemispheric asymmetry of human sleep EEG in response to selective slow-wave sleep deprivation. Behav Neurosci. 2002;116:976–981. doi: 10.1037//0735-7044.116.6.976. [DOI] [PubMed] [Google Scholar]
- 23.Achermann P, Finelli LA, Borbely AA. Unihemispheric enhancement of delta power in human frontal sleep EEG by prolonged wakefulness. Brain Res. 2001;913:220–223. doi: 10.1016/s0006-8993(01)02796-2. [DOI] [PubMed] [Google Scholar]
- 24.Kattler H, Dijk DJ, Borbely AA. Effect of unilateral somatosensory stimulation prior to sleep on the sleep EEG in humans. J Sleep Res. 1994;3:159–164. doi: 10.1111/j.1365-2869.1994.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 25.Cottone LA, Adamo D, Squires NK. The effect of unilateral somatosensory stimulation on hemispheric asymmetries during slow wave sleep. Sleep. 2004;27:63–68. doi: 10.1093/sleep/27.1.63. [DOI] [PubMed] [Google Scholar]
- 26.Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Tang X, Xiao J, Parris BS, Fang J, Sanford LD. Differential effects of two types of environmental novelty on activity and sleep in BALB/cJ and C57BL/6J mice. Physiol Behav. 2005;85:419–429. doi: 10.1016/j.physbeh.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Abeyratne UR, Swarnkar V, Hukins C, Duce B. Interhemispheric asynchrony correlates with severity of respiratory disturbance index in patients with sleep apnea. IEEE Trans Biomed Eng. 2010;57:2947–2955. doi: 10.1109/TBME.2010.2060197. [DOI] [PubMed] [Google Scholar]
- 29.Rial R, Gonzalez J, Gene L, Akaarir M, Esteban S, Gamundi A, Barcelo P, Nicolau C. Asymmetric sleep in apneic human patients. Am J Physiol Regul Integr Comp Physiol. 2013;304:R232–237. doi: 10.1152/ajpregu.00302.2011. [DOI] [PubMed] [Google Scholar]
- 30.St-Jean G, Turcotte I, Perusse AD, Bastien CH. REM and NREM power spectral analysis on two consecutive nights in psychophysiological and paradoxical insomnia sufferers. Int J Psychophysiol. 2013;89:181–194. doi: 10.1016/j.ijpsycho.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Kovrov GV, Posokhov SI, Strygin KN. Interhemispheric EEG asymmetry in patients with insomnia during nocturnal sleep. Bull Exp Biol Med. 2006;141:197–199. doi: 10.1007/s10517-006-0126-z. [DOI] [PubMed] [Google Scholar]
- 32.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 33.Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–2232. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- 34.Spoormaker VI, Schroter MS, Gleiser PM, Andrade KC, Dresler M, Wehrle R, Samann PG, Czisch M. Development of a large-scale functional brain network during human non-rapid eye movement sleep. J Neurosci. 2010;30:11379–11387. doi: 10.1523/JNEUROSCI.2015-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larson-Prior LJ, Zempel JM, Nolan TS, Prior FW, Snyder AZ, Raichle ME. Cortical network functional connectivity in the descent to sleep. Proc Natl Acad Sci USA. 2009;106:4489–4494. doi: 10.1073/pnas.0900924106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horovitz SG, Braun AR, Carr WS, Picchioni D, Balkin TJ, Fukunaga M, Duyn JH. Decoupling of the brain’s default mode network during deep sleep. Proc Natl Acad Sci USA. 2009;106:11376–11381. doi: 10.1073/pnas.0901435106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samann PG, Tully C, Spoormaker VI, Wetter TC, Holsboer F, Wehrle R, Czisch M. Increased sleep pressure reduces resting state functional connectivity. MAGMA. 2010;23:375–389. doi: 10.1007/s10334-010-0213-z. [DOI] [PubMed] [Google Scholar]
- 38.Larson-Prior LJ, Zempel JM, Nolan TS, Prior FW, Snyder AZ, Raichle ME. Cortical network functional connectivity in the descent to sleep. Proc Natl Acad Sci USA. 2009;106:4489–4494. doi: 10.1073/pnas.0900924106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu H, Stufflebeam SM, Sepulcre J, Hedden T, Buckner RL. Evidence from intrinsic activity that asymmetry of the human brain is controlled by multiple factors. Proc Natl Acad Sci USA. 2009;106:20499–20503. doi: 10.1073/pnas.0908073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.