Abstract
Rationale
Phendimetrazine appears to have limited abuse potential and reduces cocaine self-administration in preclinical studies. No human studies have evaluated the safety and tolerability of cocaine in combination with phendimetrazine, which is a necessary next step in evaluating the efficacy of phendimetrazine for treating cocaine use disorder.
Objectives
This study determined the safety and tolerability of acute cocaine doses during chronic phendimetrazine treatment.
Methods
Ten subjects completed this within-subjects, placebo-controlled, inpatient study. Subjects were maintained on ascending oral phendimetrazine doses (0, 70, 140 and 210 mg/day). After at least 7 maintenance days at each dose, subjects received ascending doses of intranasal cocaine (0, 10, 20, 40 and 80 mg), separated by 90 minutes, within one session.
Results
Cocaine produced prototypical cardiovascular and subject-rated effects (e.g., increased blood pressure and ratings of like drug). The cardiovascular effects of cocaine alone were not clinically significant for an acute drug response (e.g., average heart rate did not approach tachycardia, 100 beats/minute). Phendimetrazine enhanced peak heart rate following placebo and low cocaine doses, but these effects were also not clinically significant. Phendimetrazine was otherwise devoid of effects alone and did not alter the subject-rated effects of cocaine or hypothetical demand for cocaine on a purchase task.
Conclusions
Cocaine was safe and well tolerated during maintenance on a three-fold range of phendimetrazine doses. Given this safety profile, the reduced abuse potential of phendimetrazine and promising preclinical research, future human laboratory studies and possibly clinical trials should evaluate the efficacy of phendimetrazine for reducing cocaine use.
Keywords: Humans, Physiological Effects, Pharmacotherapy, Phendimetrazine, Cocaine
Introduction
The prevalence of cocaine use and cocaine use disorder has remained stable for the past five years (Center for Behavioral Health Statistics and Quality 2015), placing a substantial economic burden on the United States (Cartwright 2000). Effective behavioral treatments for cocaine use disorder have been developed (Dutra et al. 2008), but they have not been widely adopted within the treatment community. The Food and Drug Administration has also not approved any medications for treating cocaine use disorder despite being a research priority for nearly three decades (Schuster and Snyder 1989). Identifying an effective pharmacotherapy for cocaine use disorder would help reduce the public health burden posed by cocaine.
Translational studies show that monoamine agonists, in particular monoamine releasers, reduce cocaine use (Grabowski et al. 2004a; Herin et al. 2010; Mooney et al. 2009; Stoops and Rush 2013). d-Amphetamine treatment reduces cocaine self-administration in rats, monkeys and humans (Greenwald et al. 2010; Negus and Mello 2003a; 2003b; Rush et al. 2010; Thomsen et al. 2013) and also reduces the number of cocaine-positive urine samples provided by patients enrolled in clinical trials (Grabowski et al. 2001; 2004b). Many monoamine agonist drugs are considered to have high abuse potential (e.g., Stoops et al. 2004), which limits acceptance and adoption of this strategy by treatment providers. Identifying a monoamine releaser with reduced abuse potential that also reduces cocaine taking would significantly advance treatment of cocaine use disorder.
Phendimetrazine (Bontril®), a DEA schedule III medication in the United States, is used to treat obesity. Phendimetrazine is a weak monoamine releaser but is metabolized into phenmetrazine, a more potent norepinephrine and dopamine releaser (Chait et al. 1987; Corwin et al. 1987; Rothman et al. 2002). Phendimetrazine shares discriminative stimulus effects with d-amphetamine and cocaine (Banks et al. 2013a; de la Garza and Johanson 1987; Evans and Johanson 1987), but is a less potent locomotor stimulant than d-amphetamine and methamphetamine (Jones and Holtzman 1994). Phendimetrazine is a relatively weak reinforcer, maintaining drug self-administration only under select conditions (Corwin et al. 1987; Griffiths et al. 1979) and it has limited abuse potential in cocaine users (Bolin et al. In Press). Phendimetrazine also reduces cocaine self-administration in monkeys (Banks et al. 2013b, 2013c). In one study, 14 days of continuous treatment with a range of doses of intravenous phendimetrazine reduced cocaine self-administration in monkeys (Banks et al. 2013b). Decreases in cocaine self-administration were similar in magnitude to those produced by d-amphetamine. In the other study, a similar phendimetrazine treatment regimen also significantly reduced cocaine self-administration while increasing allocation of behavior to earning a concurrently available alternative food reinforcer (Banks et al. 2013c). These findings collectively suggest that phendimetrazine may have promise as a pharmacotherapy for cocaine use disorder.
Prior to the evaluation of the pharmacotherapeutic efficacy of phendimetrazine in the human laboratory or clinical trials, a necessary next step is to evaluate whether phendimetrazine can safely be administered in combination with cocaine. Doing so is especially important because cocaine users treated with phendimetrazine will likely lapse during treatment. The purpose of the present experiment was to determine the safety and tolerability of acutely administered cocaine during phendimetrazine maintenance in current cocaine users. We hypothesized that cocaine would be safe and well tolerated during phendimetrazine maintenance.
Method
Subjects
In order to be eligible for the study, subjects had to be healthy and without contraindications to cocaine and phendimetrazine. Subjects also had to report recent use of cocaine, meet diagnostic criteria for a cocaine use disorder (i.e., abuse or dependence) according to a computerized Structured Clinical Interview for DSM-IV (SCID) that was reviewed by a psychiatrist or psychologist and provide a cocaine or benzoylecgonine positive urine sample during screening to verify cocaine use status. Screening procedures for all subjects included a medical history questionnaire, laboratory chemistries (e.g., blood chemistry screen, complete blood count and urinalysis), electrocardiogram and a brief psychiatric examination. Subjects were excluded from participation if a study physician deemed the screening results to be abnormal (e.g., if the electrocardiogram was determined to be outside normal limits). Subjects with histories of serious physical disease, current physical disease or current or past histories of serious psychiatric disorder (including current or past histories of substance abuse or dependence) that in the opinion of a study physician would interfere with study participation were also excluded. Female subjects had to be using an effective form of birth control (e.g., birth control pills, IUD, condoms or abstinence) in order to participate.
A total of thirteen subjects provided sober, written informed consent to participate in this within-subjects, placebo-controlled, inpatient study. All subjects completed the comprehensive medical and psychological screens described above. Before inpatient admission, one subject failed the medical screening and another subject elected not to participate. Eleven subjects were admitted to the inpatient unit for the study. Ten subjects (9 males, 1 female; 6 African American, 3 Caucasian, 1 Hispanic) meeting criteria for cocaine use disorder completed the study. The eleventh subject was discharged after his first experimental session that occurred during placebo maintenance due to persistent elevations in heart rate (i.e., above 99 beats per minute) following cocaine administration that prevented further dosing. Data from that subject were excluded from analysis. Subjects weighed 81 ± 18 kg on average (± SD) and were 43 ± 4 years of age upon screening. Subjects had 13 ± 1 years of education and Drug Abuse Screening Test (Skinner 1982) scores of 7 ± 2. All subjects were current crack cocaine users and reported using cocaine 11 ± 6 days in the month prior to screening. All subjects also had experience with intranasal cocaine, but preferred smoking cocaine. On a screening questionnaire that assessed route preference, subjects rated smoked cocaine at 2.8 ± 1.2 on a scale of 0 (Not at All) to 4 (Very Much So) and rated intranasal cocaine at 1.9 ± 1.0. Subjects reported experience with other drugs of abuse. All subjects were daily cigarette smokers (11 ± 8 cigarettes/day). Eight subjects reported weekly alcohol use (17 ± 13 standard drinks/week). In the month prior to screening, subjects reported cannabis use (n=8), opioid use (n=3), amphetamine use (n=2) and benzodiazepine use (n=1). One subject met DSM-IV criteria for non-physiological alcohol dependence and another met criteria for alcohol abuse. Study physicians cleared each of these subjects after it was determined that these diagnoses would not interfere with study participation. All subjects were paid for their participation.
General Procedures
Subjects were enrolled as inpatients at the University of Kentucky Chandler Medical Center Clinical Research Inpatient Unit (CRIU) for up to 38 days and participated in one drug-free practice and four experimental sessions. During inpatient admission, subjects received standard caffeine-free hospital meals. Urine samples were collected daily and expired breath samples were collected prior to each session to confirm drug and alcohol abstinence, respectively. Pregnancy tests were conducted daily on urine samples from the female subject. All tests were negative throughout her participation. When not in session, subjects could smoke cigarettes ad libitum as long as CRIU staff were available to escort them to the designated smoking area.
The Medical Institutional Review Board of the University of Kentucky approved this study, which was conducted in accordance with all relevant guidelines, including the 1964 Declaration of Helsinki.
Drug Maintenance Days
Drug maintenance began on the day immediately following the practice session and continued throughout the protocol. Placebo or phendimetrazine was administered orally at 0700 and 1900 h. All subjects were initially maintained on placebo, followed by ascending doses of phendimetrazine (35, 70 and 105 mg, administered twice per day, for a total daily dose of 0, 70, 140 and 210 mg). The selected maintenance period was seven days, but placebo maintenance lasted at least nine days to avoid conducting experimental sessions on weekends. After maintenance on placebo, subjects completed the first experimental session, described below, and then began maintenance on phendimetrazine the following day. After seven days of phendimetrazine maintenance (i.e., on the eighth day after beginning a phendimetrazine condition), subjects completed an experimental session and then proceeded to the next maintenance condition until study completion.
Experimental Sessions
Subjects received the appropriate maintenance dose at 0700 on the morning of all experimental sessions. Subjects were allowed to smoke a cigarette prior to experimental sessions that started at 0830 h and were not allowed to smoke again until the session ended approximately 7 h later. Five intranasal cocaine doses were given during each session in ascending order 90 min apart (0, 10, 20, 40 and 80 mg). Subjects completed all experimental measures prior to drug administration and at 15 min intervals for 45 min to capture the peak effects of each cocaine dose (Cone 1995). If heart rate exceeded 130 beats per minute, systolic blood pressure exceeded 180 mmHg, diastolic blood pressure exceeded 120 mmHg or clinically significant ECG changes occurred following administration of cocaine at any point during the experiment, participation would have been terminated but no subject was excluded from further participation for exceeding these parameters. Medications were to be held if a subject's heart rate was ≥100 bpm, systolic pressure was ≥150 mmHg or diastolic pressure was ≥100 mmHg; once these measures fell below these cutoffs, medications could be administered. No doses were held or delayed for exceeding these criteria apart from those described above for the eleventh discharged subject.
Measures
Physiological measures (i.e., heart rate and blood pressure) were recorded using a digital monitor. Temperature was measured using a digital oral thermometer. Subject-rated measures included an Adjective Rating Scale (Oliveto et al. 1992) and a locally developed Drug-Effect Questionnaire (Rush et al. 2003). CRIU nursing staff completed the Udvalg for Kliniske UndersØgelser (UKU) Side Effects Scale (Lingjaerde et al. 1987) with the subjects and recorded weight daily.
Subjects also completed a cocaine purchase task prior to cocaine dose administration, but after the morning phendimetrazine maintenance dose, at the start of the experimental session for each phendimetrazine condition (Bruner and Johnson 2014). Subjects were asked to indicate the hypothetical number of cocaine “hits” (i.e., $5 units) they would purchase at monetary increments ranging from $0.01 to $1000/hit to examine economic demand for cocaine. All choices were hypothetical and were not purchased or administered.
Drug Administration
All drugs were administered in a double-blind fashion. Only study investigators and the Investigational Drug Service staff had access to dose orders in order to maintain the blind. These individuals did not interact with subjects during experimental sessions, nor did they collect experimental data. Immediate release phendimetrazine doses (35, 70 and 105 mg b.i.d.; KVK-Tech Inc., Newtown, PA) were prepared from commercially available drug in a gelatin capsule backfilled with cornstarch. Placebo capsules contained only cornstarch. The seven-day minimum maintenance period with twice daily dosing was selected to reach steady state phendimetrazine levels based upon its published half-life of approximately 9 h (Müller et al. 1975). Phenmetrazine has a variable half-life that is dependent upon urine pH, ranging from 16 up to 48 h (toxnet.nlm.gov 2015). By conducting cocaine experimental sessions after seven days of maintenance, phenmetrazine levels also should have approached steady state.
Cocaine doses (0, 10, 20, 40 and 80 mg) were prepared by combining the appropriate amount of cocaine HCl (Mallinckrodt, St. Louis, MO) with lactose to equal a total of 100 mg powder. During each administration, a nurse presented the subject with the powder, a mirror and a standard razor blade. The subject was instructed to divide the powder into two even “lines” and insufflate one line of powder through each nostril using a 65-mm plastic straw within 2 min.
Data Analysis
Physiological and subject-rated measures were analyzed as peak effect (i.e., the maximum score observed in the 45 min following each cocaine administration) using a two factor repeated-measures General Linear Model (GLM) with Phendimetrazine Dose (0, 70, 140 and 210 mg/day) and Cocaine Dose (0, 10, 20, 40 and 80 mg) as the factors (SPSS, IBM Corporation, Armonk, NY). F values from the GLM were used to determine statistical significance using Huynh-Feldt corrected degrees of freedom (for sphericity violations) and a threshold of p < 0.05.
Weight and data for items from the UKU were analyzed using a two factor repeated-measures GLM with Phendimetrazine Dose (0, 70, 140 and 210 mg/day) and Maintenance Day (1-8 [Day 8 represents data from the day of the experimental session]) as the factors. Because placebo maintenance was longer for all subjects, only the final 8 days of placebo maintenance were included in the analyses. Sex specific items on the UKU (e.g., menorrhagia), as well as items that had only “0” responses for all subjects (i.e., no subjects endorsed experiencing these effects throughout the study), were not included in the data analysis. F values from the GLM were used to determine statistical significance using Huynh-Feldt corrected degrees of freedom and a threshold of p < 0.05.
Cocaine purchase task data were analyzed using the exponential demand equation (Hursh and Silberberg 2008):
where Q = consumption at each unit price; Q0 = derived intensity of demand (consumption at unconstrained price); k = a constant that denotes the range of consumption values in log units (set to k = 4 for the current analysis); C = the unit price of the commodity (price/$5 unit); and α = derived essential value (a measure of elasticity of demand). Additional parameters were computed from purchase task values including: Demand Breakpoint (the first price at which consumption was suppressed to zero), Omax (maximum expenditure) and Pmax (price at maximum expenditure). Multilevel models with purchase task outcomes (Level 1) nested within subjects (Level 2) were estimated in the NLME package in R statistical software (Pinheiro et al. 2015). The rationale for this approach was two-fold: 1) to account for missing data from two subjects due to technical errors and 2) to account for the nesting of demand outcomes across dose within subjects. To examine the effects of phendimetrazine dose on economic demand outcomes (Q0, Omax, Pmax, Demand Breakpoint, and Alpha), Phendimetrazine Dose (0, 70, 140, and 210 mg) was entered as a categorical, fixed main effect. Random intercept models were fit and significant dose effects examined by comparing model coefficients with Placebo as a reference condition. Effects were considered statistically significant for p < 0.05.
Results
Physiological Measures
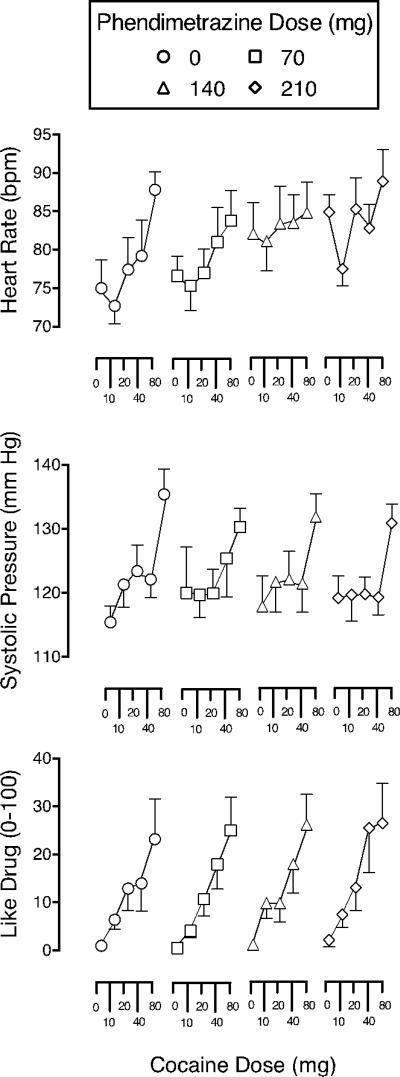
Significant main effects of Phendimetrazine Dose (F = 3.26) and Cocaine Dose (F = 8.24) were observed for peak heart rate. As shown in Figure 1, cocaine increased heart rate in a dose-related manner. Phendimetrazine increased peak heart rate for placebo and lower cocaine doses, but average peak heart rate did not exceed 90 beats per minute (bpm). Significant main effects of Cocaine Dose were also observed on systolic (F = 10.49) and diastolic (F = 15.59) pressure, but there was no effect of phendimetrazine on these measures. Cocaine increased systolic and diastolic pressure in a dose-related manner as shown in Figure 1 for systolic pressure. There were no significant effects observed for temperature or weight, nor were there any significant interactions for any of the measures.
Fig. 1.
Peak effect dose-response functions for cocaine following maintenance on placebo (circles), 70 mg phendimetrazine (squares), 140 mg phendimetrazine (triangles) and 210 mg phendimetrazine (diamonds) for Heart Rate (top graph), Systolic Blood Pressure (middle graph) and subject ratings of Like Drug (bottom graph). X-axis: Intranasal cocaine dose. Brackets indicate 1 S.E.M. A significant effect of phendimetrazine dose was observed on Heart Rate. No effect of phendimetrazine dose was detected on Systolic Blood Pressure or subject ratings of Like Drug.
Subject-Rated Measures
Significant main effects of Cocaine Dose were observed for the Stimulant Subscale of the Adjective Rating Scale and eleven items from the Drug-Effect Questionnaire (Active, Alert, Energetic; Any Effect; Good Effects; High; Irregular or Racing Heartbeat; Like Drug; Rush; Stimulated; Talkative, Friendly; Willing to Pay For and Willing to Take Again; F values > 3.65). There was no effect of Phendimetrazine Dose on these outcomes. No other statistically significant effects were observed. Figure 1 shows representative data for Like Drug, indicating that cocaine produced dose-related increases in subject-rated measures that were unchanged by phendimetrazine.
UKU Side Effect Items
No statistically significant effects were observed for UKU side effect items.
Cocaine Purchase Task
Data from one subject were deemed non-systematic across all doses by standard criteria and removed from further purchase task analysis (Stein et al. 2015). The exponential demand equation provided an excellent fit to the remaining individual data (median R2 = .87, interquartile range = .81 to .92; see Figure 2) that did not differ as a function of phendimetrazine dose. Demand values did not differ by Phendimetrazine Dose for any demand parameter.
Fig. 2.
Economic demand curves for cocaine ($5 units) following maintenance on placebo (circles), 70 mg phendimetrazine (squares), 140 mg phendimetrazine (triangles), and 210 mg phendimetrazine (diamonds). Representative curves were fit to mean data using the exponential demand question (Hursh and Silberberg 2008). Individual values indicate mean and error bars indicate 1 S.E.M. Phendimetrazine did not impact Cocaine Purchase Task outcomes.
Discussion
The results of the present experiment indicate that acute administration of an eight-fold range of cocaine doses is safe and tolerable during oral maintenance on a three-fold range of phendimetrazine doses. Intranasal cocaine produced prototypical stimulant-like effects (e.g., increased blood pressure and subject ratings of Like Drug) and phendimetrazine generally did not alter these effects. Phendimetrazine enhanced the peak heart rate observed following placebo and lower cocaine doses, but phendimetrazine did not increase the peak heart rate observed following the highest dose of cocaine (i.e., peak heart rate was approximately 90 bpm across all phendimetrazine dose conditions following administration of 80 mg intranasal cocaine). Moreover, average peak heart rate did not approach tachycardia (i.e., above 100 bpm) for any cocaine-phendimetrazine dose combination. No serious or unexpected adverse events occurred, there were no statistically significant effects observed on UKU side effect scale items and there was no change in subjects’ weight across phendimetrazine maintenance conditions.
The safe profile observed following administration of the combination of cocaine with phendimetrazine is particularly notable because of the selected maintenance doses and regimen. First, the three-fold range of maintenance doses included the highest clinically recommended daily dose (i.e., 210 mg). Second, using a seven-day maintenance period allowed for steady state phendimetrazine/phenmetrazine blood levels to be reached, matching putative clinical use of phendimetrazine for cocaine use disorder. Third, phendimetrazine doses were administered immediately prior to experimental sessions, ensuring that the effects of cocaine were tested while phendimetrazine/phenmetrazine plasma concentrations were elevated. Together, these design features and results indicate that phendimetrazine is safe and would be well tolerated in future studies evaluating its efficacy as a pharmacotherapy for cocaine use disorder.
Phendimetrazine maintenance did not change the subject-rated effects of cocaine. These results are not necessarily unexpected because the abuse related effects of cocaine are difficult to alter (e.g., Foltin et al. 2015). Attenuating the subject-rated effects of cocaine may be a desired effect of a potential pharmacotherapy, but an effective medication may reduce cocaine use through a number of other mechanisms, including attenuating the reinforcing effects of cocaine, reducing withdrawal or remediating cognitive impairment (Comer et al. 2008; Haney 2009; Haney and Spealman 2008; Rush and Stoops 2012; Sofuoglu et al 2013). Phendimetrazine attenuates the reinforcing effects of cocaine in monkeys (Banks et al. 2013b; 2013c) and may also remediate cognitive deficits as has been frequently observed with other drugs that act in monoamine systems (Sofuoglu et al. 2013), indicating that further testing may be warranted for cocaine use disorder. Although phendimetrazine did not attenuate the subject-rated effects of cocaine, it also did not enhance the subject-rated effects of cocaine. Enhancement of subject-rated drug effects is an undesirable outcome for cocaine pharmacotherapies (Foltin and Fischman 1994).
Phendimetrazine did not alter hypothetical economic demand outcomes for standardized $5 hits on the cocaine purchase task. The predictive validity of hypothetical economic demand tasks for screening medications remains largely undetermined, as is the case with all human laboratory screens for stimulant use disorder pharmacotherapies in the absence of an approved, effective medication. However, the results observed here indicate that such measures can be implemented in human laboratory medications development studies with consistency across measurements (i.e., demand curves and demand fit were similar across phendimetrazine conditions for this nearly five week study). These measures provide complimentary information on the subject-rated value of cocaine under various medication maintenance conditions, although neither subject-rated or hypothetical purchase task outcomes were affected by phendimetrazine dosing in the present study. It is important to note the limitation that this cocaine purchase task only evaluated demand for hypothetical cocaine, rather than the doses of cocaine administered in this study. Whether phendimetrazine would change economic demand for sampled doses has yet to be evaluated. Overall, the lack of effect on phendimetrazine on subject-rated measures and the cocaine purchase task should not overly dampen enthusiasm for future research because this experiment was designed to determine whether phendimetrazine could be safely combined with cocaine rather than the efficacy of phendimetrazine for managing cocaine use disorder.
There are three other limitations that should be noted. First, the use of ascending doses of both phendimetrazine and cocaine might have introduced possible order effects, including the development of tolerance or carryover effects (e.g., vasoconstriction), which might have limited the ability to detect an attenuation of the subject-rated effects of cocaine by higher phendimetrazine doses. The ascending dose order was necessitated by the goals of the study (i.e., to evaluate the safety and tolerability of these combinations), but future research should build on this study by testing phendimetrazine and cocaine combinations in random order, now that the safety and tolerability of the combinations have been established. Although carryover effects are possible, the likelihood is minimal because (a) at least 90 minutes separated each dose administration and (b) the pharmacodynamic effects observed here are comparable in magnitude to those observed in other studies that administered similar, single doses (Rush et al. 2010; Ongoing; Stoops et al. 2012a; 2012b). A second limitation is that cocaine produced relatively low-level peak subject-rated effects. Higher cocaine doses that better approximate “street” cocaine use or cocaine administered by other routes frequently do not result in subject ratings that are much greater than those observed here (Collins et al. 2007; Comer et al. 2013; Haney et al. 2011; Walsh et al. 2001). Subject ratings of drug effects may suffer from a ceiling effect. Third, plasma phendimetrazine/phenmetrazine levels were not determined, but dosing was guided based on published pharmacokinetic profiles to reach steady state.
This study demonstrated that cocaine is safe and well tolerated when combined with phendimetrazine, but the utility of phendimetrazine for cocaine use disorder remains to be determined. The promising preclinical findings (Banks et al. 2013b, 2013c), limited abuse potential (Bolin et al., In Press; Corwin et al. 1987; Griffiths et al. 1979) and safe profile reported here indicate that further research is worthwhile to evaluate phendimetrazine as a pharmacotherapy for cocaine use disorder. Future studies should first further evaluate the influence of phendimetrazine on the effects of cocaine, including the reinforcing effects (Comer et al. 2008; Haney 2009; Haney and Spealman 2008), under controlled human laboratory conditions using random dosing order. This work should be followed by the conduct of Phase II and III clinical trials in treatment-seeking subjects should the human laboratory results prove positive.
Acknowledgments
The authors gratefully acknowledge the staff of the University of Kentucky Laboratory of Human Behavioral Pharmacology for technical assistance, the staff of the University of Kentucky Center for Clinical and Translational Science Clinical Research Inpatient Unit for medical assistance and the University of Kentucky Investigative Drug Service for preparation of study medications. This study complied with all laws of the United States of America.
The authors gratefully acknowledge research support from the National Institute on Drug Abuse (R01DA036553) and from the National Center for Advancing Translational Sciences (UL1TR000117) of the National Institutes of Health. These funding agencies had no role in study design, data collection or analysis, or preparation and submission of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest Statement: The authors declare no relevant conflicts of interest.
References
- Banks ML, Blough BE, Fennell TR, Snyder RW, Negus SS. Role of phenmetrazine as an active metabolite of phendimetrazine: evidence from studies of drug discrimination and pharmacokinetics in rhesus monkeys. Drug Alcohol Depend. 2013a;130:158–166. doi: 10.1016/j.drugalcdep.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Negus SS. Effects of 14-day treatment with the schedule iii anorectic phendimetrazine on choice between cocaine and food in rhesus monkeys. Drug Alcohol Depend. 2013b;131:204–213. doi: 10.1016/j.drugalcdep.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Fennell TR, Snyder RW, Negus SS. Effects of phendimetrazine treatment on cocaine vs food choice and extended-access cocaine consumption in rhesus monkeys. Neuropsychopharmacology. 2013c;38:2698–2707. doi: 10.1038/npp.2013.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolin BL, Stoops WW, Sites JP, Rush CR. Abuse potential of oral phendimetrazine in cocaine-dependent individuals: implications for agonist-like replacement therapy. J Addict Med. doi: 10.1097/ADM.0000000000000206. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner NR, Johnson MW. Demand curves for hypothetical cocaine in cocaine-dependent individuals. Psychopharmacology (Berl) 2014;231:889–97. doi: 10.1007/s00213-013-3312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright WS. Cocaine medications, cocaine consumption and societal costs. Pharmacoeconomics. 2000;18:405–13. doi: 10.2165/00019053-200018040-00008. [DOI] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality [19 December 2015];Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health (HHS Publication No. SMA 15-4927, NSDUH Series H-50) 2015 from http://www.samhsa.gov/data/
- Chait LD, Uhlenhuth EH, Johanson CE. Reinforcing and subjective effects of several anorectics in normal human volunteers. J Pharmacol Exp Ther. 1987;242:777–783. [PubMed] [Google Scholar]
- Collins SL, Evans SM, Foltin RW, Haney M. Intranasal cocaine in humans: effects of sex and menstrual cycle. Pharmacol Biochem Behav. 2007;86:117–24. doi: 10.1016/j.pbb.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Ashworth JB, Foltin RW, Johanson CE, Zacny JP, Walsh SL. The role of human drug self-administration procedures in the development of medications. Drug Alcohol Depend. 2008;96:1–15. doi: 10.1016/j.drugalcdep.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Mogali S, Saccone PA, Askalsky P, Martinez D, Walker EA, Jones JD, Vosburg SK, Cooper ZD, Roux P, Sullivan MA, Manubay JM, Rubin E, Pines A, Berkower EL, Haney M, Foltin RW. Effects of acute oral naltrexone on the subjective and physiological effects of oral D-amphetamine and smoke cocaine in cocaine abusers. Neuropsychopharmacology. 2013;38:2427–38. doi: 10.1038/npp.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone EJ. Pharmacokinetics and pharmacodynamics of cocaine. J Anal Toxicol. 1995;19:459–78. doi: 10.1093/jat/19.6.459. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Woolverton WL, Schuster CR, Johanson CE. Anorectics: effects on food intake and self-administration in rhesus monkeys. Alcohol Drug Res. 1987;7:351–361. [PubMed] [Google Scholar]
- de la Garza R, Johanson CE. Discriminative stimulus properties of intragastrically administered d-amphetamine and pentobarbital in rhesus monkeys. J Pharmacol Exp Ther. 1987;243:955–962. [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165:179–87. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- Evans SM, Johanson CE. Amphetamine-like effects of anorectics and related compounds in pigeons. J Pharmacol Exp Ther. 1987;241:817–825. [PubMed] [Google Scholar]
- Foltin RW, Fishman MW. Effects of buprenorphine on the self-administration of cocaine by humans. Behav Pharmacol. 1994;5:79–89. doi: 10.1097/00008877-199402000-00009. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Hany M, Rubin E, Reed SC, Vadhan N, Balter R, Evans SM. Development of translational preclinical models in substance abuse: Effects of cocaine administration on cocaine choice in humans and non-human primates. Pharmacol, Biochem, Behav. 2015;134:12–21. doi: 10.1016/j.pbb.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, Moeller FG. Dextroamphetamine for cocaine-dependence treatment: a double-blind randomized clinical trial. J Clin Psychopharmacol. 2001;21:522–526. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav. 2004a;29:1439–64. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Stotts A, Cowan K, Kopecky C, Dougherty A, Moeller FG, Hassan S, Schmitz J. Agonist-like or antagonist-like treatment for cocaine dependence with methadone for heroin dependence: two double-blind randomized clinical trails. Neuropsychopharmacology. 2004b;29:969–981. doi: 10.1038/sj.npp.1300392. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Lundahl LH, Steinmiller CL. Sustained release d-amphetamine reduces cocaine but not ‘speedball’-seeking in buprenorphine-maintained volunteers: a test of dual-agonist pharmacotherapy for cocaine/heroin polydrug abusers. Neuropsychopharmacology. 2010;35:2624–37. doi: 10.1038/npp.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Bradford LD, Brady JV. Predicting the abuse liability of drugs with animal drug self-administration procedures: psychomotor stimulants and hallucinogens. In: Thompson T, Dews PB, editors. Advances in behavioral pharmacology. Vol. 2. Academic Press; New York: 1979. pp. 164–208. [Google Scholar]
- Haney M. Self-administration of cocaine, cannabis and heroin in the human laboratory: benefits and pitfalls. Addcit Biol. 2009;14:9–21. doi: 10.1111/j.1369-1600.2008.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Rubin E, Foltin RW. Aripiprazole maintenance increases smoked cocaine self-administration in humans. Psychopharmacology (Berl) 2011;216:379–87. doi: 10.1007/s00213-011-2231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Spealman R. Controversies in translation research: drug self-administration. Psychopharmacology (Berl) 2008;199:403–19. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herin DV, Rush CR, Grabowski J. Agonist-like pharmacotherapy for stimulant dependence: preclinical, human laboratory, and clinical studies. Ann N Y Acad Sci. 2010;1187:76–100. doi: 10.1111/j.1749-6632.2009.05145.x. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115:186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Jones DN, Holtzman SG. Influence of naloxone upon motor activity induced by psychomotor stimulant drugs. Psychopharmacology (Berl) 1994;114:215–224. doi: 10.1007/BF02244839. [DOI] [PubMed] [Google Scholar]
- Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. 1987;334:1–100. doi: 10.1111/j.1600-0447.1987.tb10566.x. [DOI] [PubMed] [Google Scholar]
- Mooney ME, Herin DV, Schmitz JM, Moukaddam N, Green CE, Grabowski J. Effects of oral methamphetamine on cocaine use: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2009;101:34–41. doi: 10.1016/j.drugalcdep.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller OF, Hundt HK, Gosling JA. Availability of phendimetrazine from sustained and non-sustained action formulations. S Afr Med J. 1975;49:135–9. [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine-and food-maintained responding under a second-order scheduled in rhesus monkeys. Drug Alcohol Depend. 2003a;70:39–52. doi: 10.1016/s0376-8716(02)00339-3. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine-and food-maintained responding under a progressive-ratio schedule in rhesus monkeys. Psychopharmacology (Berl) 2003b;167:324–32. doi: 10.1007/s00213-003-1409-y. [DOI] [PubMed] [Google Scholar]
- Oliveto AH, Bickel WK, Hughes JR, Shea PJ, Higgins ST, Fenwick JW. Caffeine drug discrimination in humans: acquisition, specificity and correlation with self-reports. J Pharmacol Exp Ther. 1992;261:885–894. [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team nlme: Linear and nonlinear mixed effects models. R package version 3.1-122. 2015 http://CRAN.R-project.org/package=nlme.
- Rush CR, Bolin BL, Stoops WW, Lile JA, Rayapati AO, Hays LR. Influence of phentermine-topiramate combinations on cocaine self administration. doi: 10.1016/j.drugalcdep.2020.108413. Ongoing. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW. Agonist replacement therapy for cocaine dependence: A Translational review. Future Med Chem. 2012;4:245–65. doi: 10.4155/fmc.11.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Hays LR, Glaser PEA, Hays LS. Risperidone attenuates the discriminative-stimulus effects of d-amphetamine in humans. J Pharmacol Exp Ther. 2003;306:195–204. doi: 10.1124/jpet.102.048439. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Sevak RJ, Hays LR. Cocaine choice in humans during D-amphetamine maintenance. J Clin Psychopharmacol. 2010;30:152–9. doi: 10.1097/JCP.0b013e3181d21967. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Katsnelson M, Vu N, Partilla JS, Dersch CM, Blough BE, Baumann MH. Interaction of the anorectic medication, phendimetrazine, and its metabolites with monoamine transporters in rat brain. Eur J Pharmacol. 2002;447:51–57. doi: 10.1016/s0014-2999(02)01830-7. [DOI] [PubMed] [Google Scholar]
- Schuster CR, Snyder M. NIDA's medication development program. NIDA Res Monogr. 1989;95:64–73. [PubMed] [Google Scholar]
- Skinner HA. The drug abuse screening test. Addict Behav. 1982;7:363–71. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, DeVito EE, Waters AJ, Carroll KM. Cognitive enhancement as a treatment for drug addictions. Neuropharmacology. 2013;64:452–63. doi: 10.1016/j.neuropharm.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JS, Koffarnus MN, Snider SE, Quisenberry AJ, Bickel WK. Identification and management of nonsystematic purchase task data: toward best practices. Exp Clin Psychopharmcol. 2015;23:377–86. doi: 10.1037/pha0000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Glaser PE, Fillmore MT, Rush CR. Reinforcing, subject-rated, performance and physiological effects of methylphenidate and d-amphetamine in stimulant abusing humans. J Psychopharmacol. 2004;18(40):534–43. doi: 10.1177/0269881104047281. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Glaser PE, Hays LR, Rush CR. Alternative reinforcer response cost impacts cocaine choice in humans. Prog Neuropsychopharm Biol Psychiatry. 2012a;36:189–93. doi: 10.1016/j.pnpbp.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Glaser PE, Hays LR, Rush CR. Influence of acute bupropion pre-treatment on the effects of intranasal cocaine. Addiction. 2012b;107:1140–7. doi: 10.1111/j.1360-0443.2011.03766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Rush CR. Agonist replacement for sitmulant dependence: a review of clinical research. Curr Pharm Des. 2013;19(40):7026–35. doi: 10.2174/138161281940131209142843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Barrett AC, Negus SS, Caine SB. Cocaine versus food choice procedure in rats: environmental manipulations and effects of amphetamine. J Exp Anal Behav. 2013;99(2):211–33. doi: 10.1002/jeab.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- toxnet.nlm.gov [19 December 2015];Phenmetrazine. 2015 from http://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+3156.
- Walsh SL, Geter-Douglas B, Strain EC, Bigelow GE. Enadoline and butorphanol: evaluation of kappa-agonists on cocaine pharmacodynamics and cocaine self-administration in humans. J Pharmacol Exp Ther. 2001;299:147–58. [PubMed] [Google Scholar]