Abstract
Two repurposed medications have been proposed to treat cocaine abuse. Progesterone, a gonadal hormone, and atomoxetine, a medication commonly used to treat attention deficit/hyperactivity disorder, have both been separately shown to reduce cocaine self-administration and reinstatement (i.e. relapse). The goal of the present study was to examine sex differences in the individual effects of PRO and ATO as well as the combination PRO+ATO treatment on cocaine (COC), caffeine (CAF), and/or cue-primed reinstatement of cocaine-seeking. Adult male and female Wistar rats lever-pressed under a FR 1 schedule for cocaine infusions (0.4 mg/kg/inf). After 14 sessions of stable responding in daily 2-h sessions, rats underwent a 21-day extinction period when no drug or drug-related stimuli were present. Rats were then separated into four groups that received PRO (0.5 mg/kg) alone (PRO+SAL), ATO (1.5 mg/kg) alone (VEH+ATO), control (VEH+SAL) or combination (PRO+ATO) treatments prior to the reinstatement condition. Reinstatement of cocaine-seeking to cues and/or drug injections of cocaine or caffeine was tested after extinction. During maintenance, females self-administered more cocaine than males, but no sex differences were seen during extinction. Females showed greater cocaine-seeking than males after a CAF priming injection. Individual treatment with ATO did not decrease reinstatement under any priming condition; however, the combination treatment decreased cocaine-seeking under the COC+CUES priming condition in males, and both PRO alone and the combination treatment decreased cocaine-seeking in the CAF+CUES condition in females. Overall, PRO alone was only effective in reducing reinstatement in females, while the combination treatment was consistently effective in reducing reinstatement in both sexes.
Keywords: atomoxetine, caffeine, cocaine, combination treatment, progesterone, reinstatement
1. Introduction
One of the most difficult challenges in the treatment of drug addiction is relapse to drug use after abstinence (see Shaham and Miczek, 2003 for review). Cocaine users are particularly vulnerable to relapse (Ouimette et al., 2007; Volkow et al., 2006; Alleweireldt et al., 2001; Ciccocioppo et al., 2001), yet there are no effective pharmacological treatments for this phase of cocaine addiction (Bei and Mantsch, 2012; for review, see Zhen and Zhang, 2012; Mendelson and Mello, 1996). Novel approaches are being tested, such as repurposing other medications like atomoxetine (ATO) and progesterone (PRO), which are FDA-approved for treatment of other uses such as attention deficit/hyperactivity disorder (ADHD) treatment and contraception, respectively (Zlebnik and Carroll, 2014; Jones, 2012; Economidou et al., 2011; see Carroll and Anker, 2010; Anker and Carroll, 2011).
An emerging treatment for stimulant dependence, ATO, (Sofuoglu and Sewell, 2009; Somaini et al., 2011) is an FDA-approved medication used to treat ADHD (Bymaster et al., 2002), and it has shown promise in reducing stimulant dependence. In rodents, ATO reduced cocaine reinstatement elicited by cues (Zlebnik and Carroll, 2014; Economidou et al., 2011), nicotine withdrawal symptoms (Davis and Gould, 2007) and conditioned stimulus effects of nicotine (Reichel et al., 2007). In humans, ATO decreased both physiological (e.g., blood pressure increases) and subjective (e.g., pleasurable ratings) responses to d-amphetamine (Sofouglu et al., 2009). However, in some studies, no effects of ATO on stimulant use have been found (Levin et al., 2009; Walsh et al., 2013; Rush et al., 2011).
Another repurposed pharmacological treatment that has shown promise in decreasing stimulant addiction is PRO. Progesterone is used in oral contraceptives and in the maintenance of pregnancies (Jones et al., 2012). Initial evidence showed that, during stages of the estrous cycle when PRO is high and estradiol is low, cocaine self-administration was lower, and rats exhibited less cocaine-induced hyperactivity than during estrous stages when estradiol was high and progesterone was low (Lynch et al., 2000; Jackson et al., 2006; Sell et al., 2000). In humans, exogenous administration of PRO attenuated cocaine use and relapse to cocaine (Yonkers et al., 2014). In rodents, PRO treatment reduced reinstatement of cocaine-seeking (e.g., Anker et al., 2009; Feltenstein et al., 2009; for a review, see Carroll and Anker, 2010; Anker and Carroll, 2011), prevented the escalation of cocaine self-administration (Larson et al. 2007), decreased cocaine-induced stereotyped behaviors (Souza et al., 2014) and reduced impulsivity for cocaine self-administration (Smethells et al., under review).
Combination therapies, both behavioral and pharmacological, may be effective strategies for reducing cocaine dependence and relapse (Verrico et al., 2013; Smith et al., 2014), especially when employing medications that show efficacy when used alone (Stoops and Rush, 2014). Recent work in our laboratory indicated that combining a behavioral intervention (i.e. concurrent running in an attached exercise wheel) with PRO was more successful in preventing reinstatement to cocaine-seeking than either treatment alone (Zlebnik et al., 2014). In fact, the combination of wheel-running and ATO produced an additive effect of PRO and ATO on reinstatement of cocaine-seeking behavior (Zlebnik and Carroll, 2015). The purpose of this study was to extend these findings and examine a potential combined effect of ATO and PRO on reinstatement of cocaine-seeking. We hypothesized that ATO and PRO would produce a greater attenuation of reinstatement to cocaine (COC), caffeine (CAF) and/or cue primes than either pharmacological treatment alone.
Additionally, sex-specific effects of ATO, PRO, and their combination were examined. Sex differences were previously reported in the effects of PRO on cocaine dependence in humans, with PRO producing greater effects in females than males (Fox et al., 2013; Evans and Foltin, 2006; for review, see Quinones-Jenab and Jenab, 2010). Animal work with exogenous PRO similarly showed greater effects in females than males (Anker et al., 2009; Zlebnik et al., 2014). While there has been little research on sex differences in treatment effects of ATO on cocaine dependence, some studies involving the therapeutic potential of ATO on ADHD showed different treatment responsivity between sexes (Marchant et al., 2011; Robison et al., 2008). For example, women reported greater improvement on measures of ADHD symptoms than men, and in general, female animals were more responsive than males to treatments for drug-seeking behaviors (see reviews by Anker and Carroll, 2011; Carroll and Anker, 2010).
The goal of the present study was to examine sex-specific effects of ATO, PRO and combination PRO+ATO treatments on reinstatement of cocaine-seeking behavior generated by cues-, cocaine- and caffeine-priming conditions, and a drug+cues condition. Caffeine and caffeine+cues were used as priming conditions, as caffeine produced robust reinstatement of cocaine-seeking (Regier et al., 2014; Weerts and Griffith, 2003; Green and Schenk, 2002; Schenk et al., 1996; Worley et al., 1994). However, it is not known whether there are sex differences in response to this priming condition. It was hypothesized that females would be more sensitive to the priming effects of caffeine and caffeine with cues, as female rats generally show enhanced sensitivity to drug and/or cue-induced reinstatement compared to males (Anker et al., 2009; Kerstetter et al., 2008; Lynch and Carroll, 2000; Anker and Carroll, 2010). These results will provide useful information for designing sex-specific treatments to prevent relapse to drug-seeking. It was also predicted that females would be more responsive to single PRO and ATO treatments as well as their combinations, as in previous studies female rats showed greater responsivity to drug abuse treatments than males (see reviews by Anker and Carroll, 2011; Carroll and Anker, 2010).
2. Materials and Methods
2.1 Animals
Forty-five female and forty-three male adult Wistar rats (weighing 200-224 and 250-274 g on arrival and with age ranges of 63-77 days) from Harlan Sprague-Dawley Inc. (Madison, WI, USA) were used in the present study. Initially, rats were pair-housed in plastic cages and allowed ad libitum access to food (Teklad 2018, Harlan Laboratories, Madison, WI, USA) and water. They were habituated to the facility for at least 3 days before being beginning the experiment. All experiments took place during the light phase of the cycle (lights on from 0600-1800h). Rooms were maintained at 24° C with 40-50% humidity.
Once the experiments began, rats were transferred to the operant conditioning chambers, where they were single-housed for the duration of the study. Rats were allowed free access to water, but they were restricted to 16 g (female) or 20 g (male) of food per day based on previous work (Lynch et al., 2002). Experimental sessions were started at 0900 h, and food was given post session at 1515 h. Body weights were recorded weekly, and rat health was checked daily. All experiments were approved by the Institutional Animal Care and Use Committee (Protocol #1307-30762A) in compliance with the Guide for the Care and Use of Animals (National Research Council, 2011).
2.2 Apparatus
Rats were housed in custom-built octagonal operant conditioning chambers previously described by Anker et al. (2007) and each chamber was contained in a sound-attenuating wooden box with a ventilation fan. There were two levers on opposite sides of the chamber with LED stimulus lights above each lever along with a house light (4.76 W) in an upper corner. A syringe pump (PHM-100, Med Associates. St. Albans, VT) delivered cocaine infusions via a swivel-tether system (375/22PS, Instech, Plymouth Meeting, PA, USA; C313CS-MN, Plastics One, Roanoke, VA, USA). The tether was attached to the rat by a harness (CIH95AB, Instech). MED-PC IV software running on PC computers controlled all experiments and collected data.
2.3 Drugs
Cocaine HCl (National Institute of Drug Abuse, Research Triangle Institute, Research Triangle Park, NC) was dissolved in sterile saline to a concentration of 1.6 mg cocaine HCl/1 ml saline. Heparin (5 USP/ml) was added to enhance catheter patency. Cocaine was infused at 0.025 ml/s, and the duration of the infusion was set based on the weight of the rat (1s/100 g), resulting in a delivery of a standard 0.4 mg/kg dose. Progesterone (Sigma Aldrich, St. Louis, MO) was dissolved in peanut oil (Sigma Aldrich, VEH, 0.625 mg/ml) and administered subcutaneously (s.c.) at a final dose of 0.5 mg/kg that produced physiologically-relevant levels of PRO in previous studies (Jackson et al., 2006; White and Uphouse, 2004) and previously decreased drug-seeking behaviors in our laboratory (Zlebnik et al., 2014; Anker et al., 2012). Additionally, in a dose-response study, 0.5 mg/kg PRO significantly decreased cocaine-induced hyperlocomotion (Niyomchai et al., 2005). Atomoxetine HCl (ATO, Tocris Biosciences, Bristol, UK) was dissolved in sterile saline to reach a concentration of 3 mg/ml and administered intraperitoneally (i.p.) at a final dose of 1.5 mg/kg. This dose was chosen based on our previous work in both female and male rats showing that ATO at 1.5 mg/kg reduced cocaine-induced reinstatement in both high and low-impulsive rats (Zlebnik and Carroll, 2015), and similar doses affected the reinforcement value of cocaine but not food (Economidou et al, 2011). Both treatment drugs (PRO and ATO) and their corresponding vehicles (peanut oil and saline [SAL], given s.c. and i.p. respectively) were acutely administered 30 min prior to session (08:30) on priming sessions, and treatment was not given before saline priming sessions. Reinstatement doses of cocaine (COC, 10 mg/kg) and caffeine (CAF, 5 mg/kg) were administered i.p. at the start of the session (09:00).
2.4 Surgical Procedures
Rats were surgically implanted with a chronic indwelling jugular catheter in a procedure developed by Weeks (1972) and modified by Carroll et al. (1981), Lynch and Carroll (1999) and Zlebnik et al. (2010). Rats were first anesthetized using a 9:1 ketamine/xylazine mixture for males (90 mg/kg ketamine, 10 mg/kg xylazine) and a 6:1 mixture for females (60 mg/kg ketamine, 10 mg/kg xylazine). Atropine (0.15 ml of 0.4 mg/ml) was given as a respiration aide. A chronic indwelling polyurethane catheter (15 cm long, Plastics One, Roanoke, VA) was then implanted from the right jugular vein to the right atrium. The distal end of the catheter led subcutaneously to a medial incision (1 cm rostral to the scapulae) attached to the harness. Post-surgery, rats were given buprenorphine (0.05 mg/ml) twice daily for 3 days, during which no testing was performed. During recovery days, enrofloxacin (10 mg/kg) was flushed through the catheters and ibuprofen (50 mg/kg) was given in the drinking water. Heparin (10 IU/kg) was flushed daily through the catheters to prevent clotting. Patency was checked weekly with a ketamine (10 mg/kg)/midazolam (0.5 mg/kg)/saline mixture, with weights recorded at the same time. Patency was assumed if the loss of a righting reflex was observed within 10 sec after administration of the ketamine mixture. If there was no loss of a righting reflex after 2 checks, a second catheter was implanted in the left jugular vein identical to the procedures described above.
2.5 Procedures
2.5.1 Acquisition and maintenance
After catheter implantation and post-surgery recovery, rats were trained to lever-press for cocaine in their operant conditioning chambers. Sessions were conducted once every day and lasted 6 h. At session start, the house light was illuminated, and rats were allowed access to two levers, one active and one inactive. An active lever press led to an infusion of cocaine on a fixed ratio 1 (FR 1) schedule and illumination of the stimulus lights above the active lever for the duration of the infusion. A response on the inactive lever was recorded, and it resulted in illumination of the corresponding stimulus lights but did not produce an infusion. If rats self-administered the maximum number of infusions during acquisition (i.e. 40 infusions), the session was considered complete, and all lights and levers were turned off.
During self-administration training, rats were given 3 free infusions of cocaine and ground food was placed on the active lever every 2 h until rats met the criterion of 40 infusions/6-h session. Once the criteria were met, acquisition was complete, session length was decreased to 2 h, and the maximum number of infusions was increased to 500 per session to prevent any possibility of overdose, while allowing for high levels of self-administration. Rats were allowed to self-administer cocaine in the 2-h daily sessions until the maintenance phase was complete. The maintenance phase criteria consisted of 10-14 sessions of 20 or more infusions per day and an active/inactive lever press ratio of at least 3:1. Only the first 10 sessions of maintenance were used to examine group differences, as not all rats completed the full 14 sessions due to catheter patency issues. Any rats that did not complete this phase were excluded from the data analysis.
2.5.2 Extinction
After the maintenance phase, rats were placed into extinction; whereby, lever presses were recorded, but the house light was turned off and lever presses on both the active and inactive levers resulted in no infusions and no illumination of the stimulus lights. Each session lasted 2 h. The extinction phase lasted 21 days and once completed, rats were divided into 4 groups based on treatment to be received during reinstatement: VEH+SAL, VEH+ATO, PRO+SAL, PRO+ATO.
2.5.3 Reinstatement
Reinstatement was conducted within subjects in a counterbalanced fashion. Cocaine-seeking behavior was induced by i.p. priming injections (e.g. COC and CAF), cues previously paired with COC (e.g. stimulus lights and infusion pump) or a combination of both. Overall, 5 different priming conditions were used: COC (10 mg/kg), CUES, CAF (5 mg/kg), COC+CUES, CAF+CUES. Each reinstatement session was preceded by a control session when rats received either saline priming injections of equivalent volumes and/or no cues. Acute treatment with ATO and PRO (and their corresponding control groups) was given 30 min prior to the beginning of each reinstatement session.
2.6 Statistics
All data are expressed as mean ± SEM. Two-way ANOVA were used to examine maintenance and extinction, with sex and sessions as independent variables. Number of total infusions was the dependent measure during maintenance, and number of total responses on the active lever was the dependent measure during extinction and reinstatement. Individual reinstatement conditions were analyzed using a mixed-model three-way ANOVA with sex and treatment as between-group variables and saline (pre) and drug/cue prime (post) conditions as the within-group variable. A main effect of cue/drug priming condition was used to determine whether there was significant reinstatement during each of the priming conditions in comparison to the preceding saline day. If reinstatement was observed, subsequent comparisons were made with pre-planned post hoc Tukey comparisons to examine differences between the treatment groups. An outlier analysis was performed within each reinstatement condition due to substantial within group variance, and any outliers above or below 2 standard deviations from the mean were excluded (data from 2 males and 4 females were excluded across several reinstatement conditions). An alpha value of ≤0.05 was used to indicate significance. Statistical analyses were run using GraphPad Prism version 5.
3. Results
3.1 Maintenance
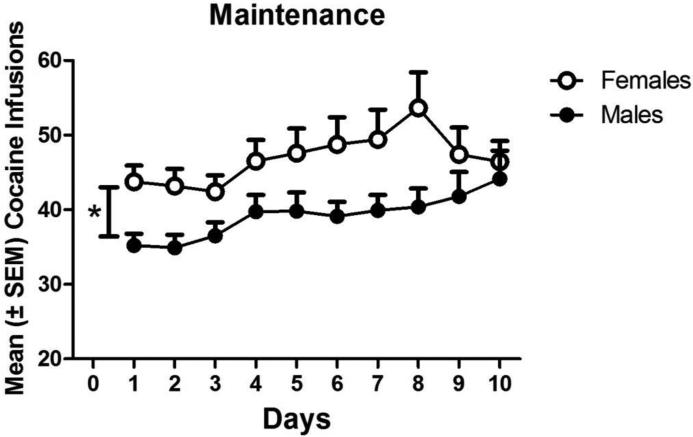
Figure 1 indicates that there were sex differences in mean infusions earned (± SEM) between males and females across the 10 maintenance sessions. Data from the first 5 sessions of maintenance for some animals were previously presented in Swalve et al. (2015). There was a significant main effect of sex on total infusions [F(1, 87)=6.772, p < 0.01] and a main effect of session [F(9, 783)=3.301, p < 0.001], but there was no interaction. Thus, over the 10 sessions, females had significantly more infusions than males, and total infusions in both males and females increased over sessions.
Figure 1.
Mean (±SEM) cocaine (0.4 mg/kg, IV) infusions over 10 sessions of the maintenance period (2 h daily sessions). The vertical bar with an asterisk indicates a difference between males (n=43) and females (n=45) on total infusions during the 10 sessions of maintenance. Data from some animals on the first 5 sessions of maintenance has been previously published (Swalve et al, 2015) * = p < 0.05.
3.2 Extinction
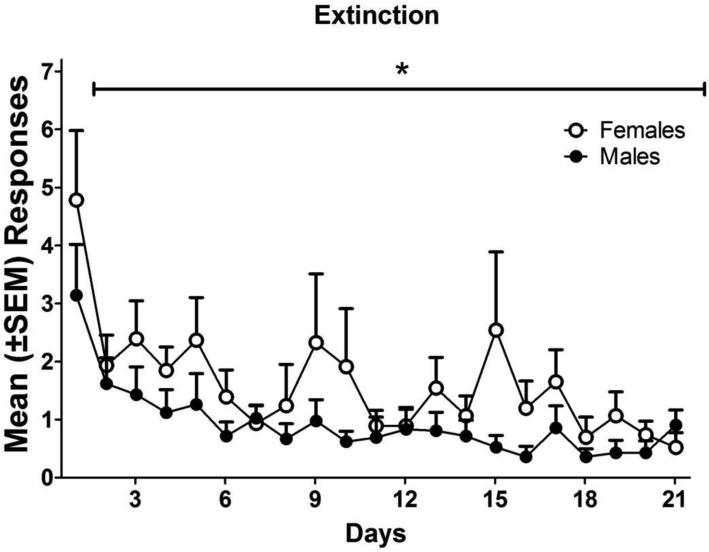
Figure 2 shows the mean number of responses (± SEM) across the 21 extinction sessions. There was no main effect of sex on extinction responses and no interaction, but there was a main effect of sessions [F(20, 1720)=4.424, p < 0.001]. Both males and females decreased responding during extinction over sessions.
Figure 2.
Mean (±SEM) lever presses over the 21 sessions of extinction. The horizontal bar with an asterisk indicates a significant decrease in responding over 21 sessions of extinction in both males (n=43) and females (n=45). * = p < 0.05.
3.3 Reinstatement
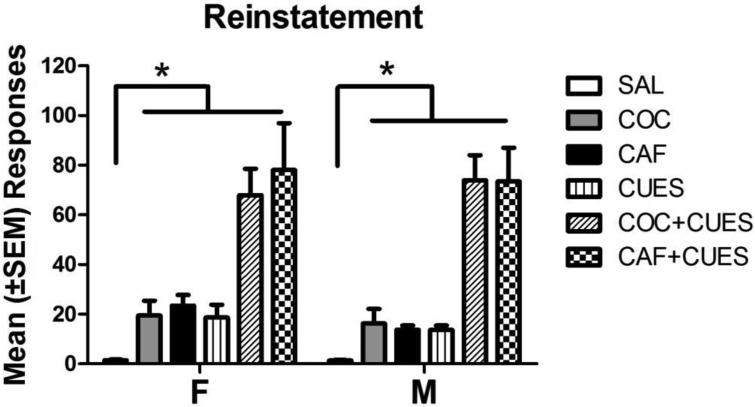
Figure 3 illustrates active lever-pressing during all reinstatement conditions. Individual three-way analyses of each reinstatement condition showed a main effect of reinstatement under COC [F(1,80)=16.71, p<0.001], CUES, [F(1,81)=26.98, p<0.001], CAF [F(1,81)=54.54, p<0.001], COC+CUES [F(1,79)=97.05, p<0.001], and CAF+CUES [F(1,78)=155.76, p<0.001] priming conditions. This effect was seen in both males and females.
Figure 3.
Mean (±SEM) active lever presses during reinstatement conditions in males and females * = p < 0.05. The saline days were averaged for display, as they were not significantly different. The bar with an asterisk represents differences in responding after all reinstatement conditions in males and females compared to the saline conditions.
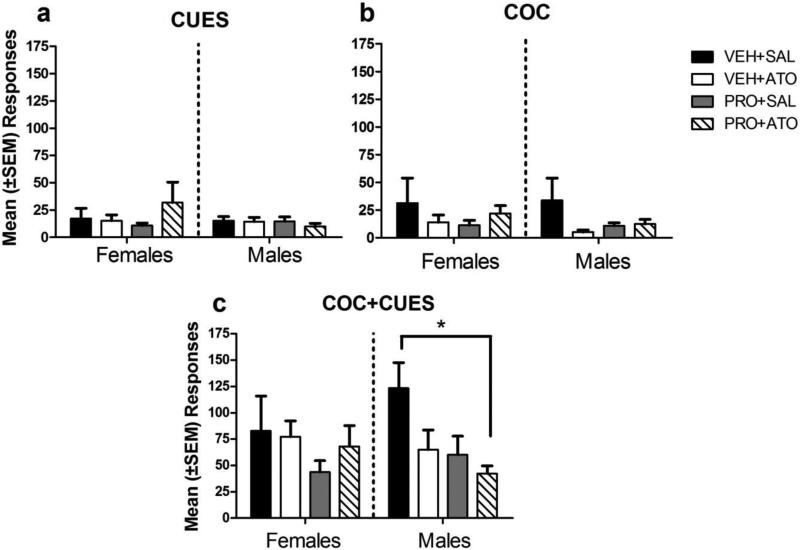
Figure 4 illustrates the results of preplanned post-hoc Tukey comparisons of reinstatement responding after the COC+CUES, CUES and COC priming conditions. There was an interaction of reinstatement X treatment during the COC+CUES session [F(3,79)=3.00, p < 0.05], but there was no interaction with sex and no three-way interaction. The combination treatment PRO+ATO significantly reduced responding compared to the control VEH+SAL condition during the COC+CUES reinstatement session (p<0.05). There were no significant differences between treatment groups (ps>0.05). This finding occurred only in the COC+CUES condition and only in males.
Figure 4.
Mean (±SEM) lever presses during a) CUES reinstatement session compared to b) COC and c) COC+CUES sessions. The bar with an asterisk represents differences in responding during the COC+CUES reinstatement session. Sample sizes for the VEH+SAL, VEH+ATO, PRO+SAL and PRO+ATO groups were 11, 11, 12, and 11 for females and 12, 10, 11, and 10 for males, respectively. * = p < 0.05.
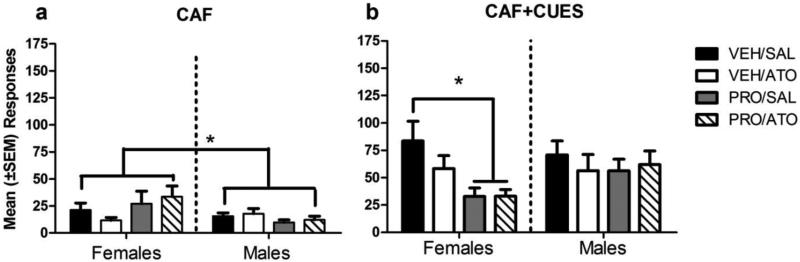
Figure 5a illustrates that females had significantly more reinstatement responses than males after a CAF priming injection under all treatment conditions. There was an interaction of reinstatement X sex for the CAF session [F(1,81)=4.00, p < 0.05]. There were no other significant interactions during the CAF session (ps>0.05). However, there were no interactions with sex on the saline, COC, CUES, or the combination COC+CUES and CAF+CUES reinstatement days. Females responded more to CAF than males.
Figure 5.
Mean (±SEM) lever presses during the a) CAF and b) CAF+CUES reinstatement session. The bars with an asterisk show a significant difference in responding during the CAF reinstatement session between males and females and a difference between treatment groups during the CAF+CUES reinstatement session. Sample sizes for the VEH+SAL, VEH+ATO, PRO+SAL and PRO+ATO groups were 11, 11, 12, and 11 for females and 12, 10, 11, and 10 for males, respectively. * = p < 0.05.
Figure 5b shows that reinstatement responding during the CAF+CUES condition was significantly decreased by both PRO alone and PRO+ATO in females. There were no significant differences between treatment groups (ps>0.05) and no interactions of drug, sex, or treatment X reinstatement during the CAF+CUES session; however, post hoc analyses indicated a significant effect of treatment in females. Both PRO+SAL and PRO+ATO decreased responding under the CAF+CUES condition. There were no treatment differences in males.
4. Discussion
This study showed significant sex differences in maintenance infusions of cocaine, reinstatement of cocaine-seeking after a caffeine priming condition, and response to treatments. Females had higher levels of cocaine infusions than males, and they were more sensitive than males to caffeine-primed reinstatement of cocaine-seeking behavior. These results were consistent with previous reports of sex differences in reinstatement of cocaine-seeking under similar experimental conditions (Anker et al., 2009). Individual treatment of ATO did not significantly decrease reinstatement after any priming condition. The PRO alone treatment and combination treatments (PRO+ATO) significantly reduced reinstatement in females during the CAF+CUES condition, while only the combination treatment attenuated responding under the COC+CUES reinstatement condition in males. Taken together, these results suggest that females are more likely to use greater amounts of cocaine and reinstate drug use after caffeine exposure than males. This study is the first to show that caffeine produces sex-specific effects on reinstatement of cocaine-seeking after extinction. Additionally, combination treatments may be more effective than individual pharmacological treatments for some forms of relapse, and the efficacy of treatments for some aspects of relapse depends on sex.
The elevated self-administration of cocaine in females compared to males was consistent with previous research. For example, after initiation of use, maintenance levels of cocaine self-administration were typically higher in females than males (Zhou et al., 2014; Peterson et al. 2014; Anker et al. 2011; Cummings et al. 2011; Lynch, 2008; Lynch and Carroll 1999). However, in a few studies, no sex differences were found (Westenbroek et al. 2013; Kosten and Zhang 2008; Jackson et al. 2006; Cosgrove et al. 2002; Roberts et al. 1989). Broadly, these results are consistent with the majority of the literature showing a sex difference where females self-administer more cocaine than males (see Anker and Carroll, 2011).
Reinstatement of cocaine-seeking after a CAF priming injection has been well-established (Regier et al., 2014; Weerts and Griffith, 2003; Green and Schenk, 2002; Schenk et al., 1996; Worley et al., 1994), yet sex-specific effects of CAF as a priming agent have not been studied. The present study was the first to show that females had enhanced responding, compared to males, on the cocaine-associated lever after a priming injection of CAF (5 mg/kg). This finding is consistent with both clinical and preclinical reports. In clinical research, females were more vulnerable than males to relapse of cocaine use after a stressful life event or an increase in depressive symptoms (Fox and Sinha, 2009; Elman et al., 2001; McKay et al., 1996). Similarly, female rats frequently displayed enhanced reinstatement under several priming conditions such as cocaine (Anker et al., 2009; Kerstetter et al., 2008; Lynch and Carroll, 2000) and a pharmacological stressor, such as yohimbine (Anker and Carroll, 2010). However, male and female rats showed similar levels of reinstatement under the cocaine-associated cue condition (Zhou et al., 2014), suggesting that sex differences are dependent on the priming condition. The significance of these findings for humans is that a legal, commonly available stimulant, caffeine, may be capable of triggering relapse to cocaine-seeking behavior, particularly in females.
There were no significant sex differences in reinstatement after a cocaine priming condition; whereas, Kippin et al. (2005) and Lynch and Carroll (2000) both showed higher cocaine-primed reinstatement in female rats compared to males. This inconsistency is most likely due to a relatively low level of reinstatement responding under the COC and CUES priming conditions, potentially due to session length. Session length was 2 h in the present study compared to 6+ h in previous work (Lynch and Carroll, 2000; Zlebnik et al., 2014), and that may have limited both levels of reinstatement and the expression of sex differences. In extended access sessions, active lever-pressing following COC (Zlebnik et al. 2010) and CUES priming conditions (Zlebnik and Carroll, 2015) was greater than the level of reinstatement found in this study. Sex differences are often found in long-access conditions compared to shorter sessions (Carroll et al. 2002; Campbell et al. 2002; Lynch et al. 2000; Lynch & Carroll 1999; Fuchs et al. 2005; Roth & Carroll 2004; Caine et al. 2004; Roberts et al. 1989). Thus, the difference in session length between our study and previous work could have contributed to the lower levels of reinstatement in this study.
The mechanism behind the enhanced reinstatement to caffeine in females is currently unknown, although there are two possibilities derived from the literature. One hypothesis is that the interoceptive effects of caffeine and cocaine are similar, thus caffeine may elicit cocaine-seeking via generalization between the two substances. There are mechanistic similarities between caffeine and cocaine, and these pharmacological similarities contribute to the effects of caffeine on cocaine-seeking and cocaine self-administration (Weerts and Griffith, 2003; Green and Schenk, 2002; Knapp et al., 2001; Schenk and Partridge, 1999). Additionally, in a discrimination procedure in both humans and rats, caffeine substitutes for cocaine (Oliveto et al., 1998; Harland et al., 1989). Generalization between caffeine and cocaine would explain the elevated CAF-priming effect in female rats, as they typically show greater COC-induced reinstatement than males (Anker et al., 2009; Kerstetter et al., 2008; Lynch and Carroll, 2000). However, responding to the COC priming condition did not show sex differences in the present study, possibly due to a floor effect, as responding in both groups during this condition was relatively low. Another hypothesis is that caffeine is functioning as a form of pharmacological stressor, as stress-induced reinstatement of cocaine-seeking is more common in females than males (Anker and Carroll, 2010) and high doses of caffeine in humans induce symptoms of anxiety (Lara, 2010). However this is unlikely, as this dose of caffeine is relatively low and a significantly higher dose produced no effect on anxiety (Bhattacharya et al., 1997).
Another finding from the present study indicated that dual therapies were consistently more effective than monotherapies. The lack of therapeutic effects solely by ATO in the present study is not uncommon, as ATO has shown mixed effects on decreasing stimulant use (Levin et al., 2009; Walsh et al., 2013; Rush et al., 2011). A previous study indicated that ATO alone decreased reinstatement (Zlebnik and Carroll, 2015), but Broos and colleagues (2015) showed no effect on reinstatement if rats had received extinction training. Similarly, exogenous PRO reduced some forms of reinstatement (i.e. yohimbine, cocaine, cocaine+cue, yohimbine+cue) but not others (e.g. cue, Zlebnik et al., 2014). We found similar effects here, as PRO alone decreased reinstatement in the CAF+CUES condition but not the COC+CUES condition. In many studies with males there have been no effects of PRO (Feltenstein et al., 2009), or only minor therapeutic effects (Anker et al., 2009), and that is consistent with the present findings.
In contrast, the combination of PRO and ATO was effective at decreasing reinstatement responding in the COC+CUES condition in males. Similarly, previous work indicates that combinations of wheel-running and ATO (Zlebnik et al., 2015; Zlebnik et al., 2014) or wheel-running and PRO (Zlebnik et al., 2014) were more effective than single therapies. These findings are consistent with a recent clinical review suggesting that combination treatments for stimulant disorders may hold promise (Stoops and Rush, 2014). For example, cocaine self-administration was not decreased by either amphetamine or butorphanol, but it was significantly attenuated by the combination treatment (Smith et al., 2014). Combination treatments should be further explored as some treatments could be more efficacious when combined.
While our findings of stronger effects of combination treatments are similar to those of previous studies, we only found a significant effect of PRO alone in females in CAF+CUES reinstatement condition. This is contrary to previous reports from our laboratory showing a significant attenuation of cocaine- and combination cocaine and cue-primed reinstatement after exogenously administered progesterone (Anker et al., 2007; Anker et al., 2009; Zlebnik et al., 2014). These discrepant results for the cocaine priming conditions are most likely due to type of treatment. Anker et al. (2007, 2009) used chronic treatment, compared to the acute treatment prior to reinstatement used in the present study, and they found significant effects of individual treatments. Significant reinstatement was found in all conditions; thus, the individual therapies should have been able to reduce responding if they were effective treatments for COC-primed reinstatement. Thus, the combination treatment may be more effective at acutely decreasing relapse to cocaine-seeking than individual therapies. However, this study used only one dose of PRO and ATO; thus, it may be possible that beneficial effects of both individual and combination treatments may be found at higher or lower doses of both drugs and future research is warranted.
5. Conclusion
Overall, these results suggest that combination pharmacotherapies were consistently more efficacious in preventing reinstatement (e.g., relapse) of cocaine-seeking behavior than individual pharmacological treatments. Individual treatment with PRO was effective in reducing only one type of reinstatement (i.e. CAF+CUES) and only in females. Sex differences occurred, but they varied with the type of stimuli used. Females responded more during a CAF priming condition than males. Additionally, females were more responsive to treatments during a CAF+CUES priming condition while males were more responsive to treatment during COC+CUES. Further research examining the interaction of monotherapies, including an examination of different doses, is warranted, and may advance treatment. As PRO is already commonly prescribed in some oral contraceptives (Jones et al., 2012), using this as an individual treatment in females, and as a combination therapy, may be particularly easy to implement. Thus, clinical research on the combination of ATO and PRO on cocaine abuse and relapse is strongly suggested.
Highlights.
Female rats self-administered more cocaine than males.
Female rats were more sensitive to caffeine reinstatement than males.
Individual treatment with progesterone decreased reinstatement in females.
The combination of progesterone and atomoxetine decreased reinstatement.
Male and female rats differentially responded to combination treatments.
Acknowledgements
The authors are grateful to Jared Mitchell and Heather Veglahn for their assistance with data collection and Dr. Lynn Eberly for the randomization table. This study was supported by NIH/NIDA P50 DA033942 (MEC), F31 DA036248 (NEZ), University of Minnesota Academic Health Center, and NIDA training grant T32 DA007097 (JRS; Dr. Thomas Molitor, PI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alleweireldt AT, Weber SM, Neisewander JL. Passive exposure to a contextual discriminative stimulus reinstates cocaine-seeking behavior in rats. Pharmacol Biochem Behav. 2001;69:555–560. doi: 10.1016/s0091-3057(01)00573-1. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Holtz NA, Carroll ME. Effects of progesterone on escalation of iv cocaine self-administration in rats selectively bred for high (HiS) and low (LoS) saccharin intake. Behav Pharmacol. 2012;23(2):205. doi: 10.1097/FBP.0b013e32834f9e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Biological Basis of Sex Differences in Psychopharmacology. Springer Berlin Heidelberg; 2011. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. pp. 73–96. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Zlebnik NE, Navin SF, Carroll ME. Responding during signaled availability and nonavailability of iv cocaine and food in rats: age and sex differences. Psychopharmacology. 2011;215(4):785–799. doi: 10.1007/s00213-011-2181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. The role of progestins in the behavioral effects of cocaine and other drugs of abuse: human and animal research. Neurosci Biobehav Rev. 2010;35(2):315–333. doi: 10.1016/j.neubiorev.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Holtz NA, Zlebnik N, Carroll ME. Effects of allopregnanolone on the reinstatement of cocaine-seeking behavior in male and female rats. Psychopharmacology. 2009;203(1):63–72. doi: 10.1007/s00213-008-1371-9. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Larson EB, Gliddon LA, Carroll ME. Effects of progesterone on the reinstatement of cocaine-seeking behavior in female rats. Exp Clin Psychopharmacol. 2007;15(5):472. doi: 10.1037/1064-1297.15.5.472. [DOI] [PubMed] [Google Scholar]
- Bhattacharya SK, Satyan KS, Chakrabarti A. Anxiogenic action of caffeine: an experimental study in rats. J Psychopharmacol. 1997;11:219–224. doi: 10.1177/026988119701100304. [DOI] [PubMed] [Google Scholar]
- Broos N, Loonstra R, Mourik Y, Schetters D, Schoffelmeer AN, Pattij T, De Vries TJ. Subchronic administration of atomoxetine causes an enduring reduction in context-induced relapse to cocaine seeking without affecting impulsive decision making. Addict Biol. 2014 doi: 10.1111/adb.12168. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Perry KW. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacol. 2002;27(5):699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Caine SB, Bowen CA, Yu G, Zuzga D, Negus SS, Mello NK. Effect of gonadectomy and gonadal hormone replacement on cocaine self-administration in female and male rats. Neuropsychopharmacol. 2004;29(5):929–42. doi: 10.1038/sj.npp.1300387. [DOI] [PubMed] [Google Scholar]
- Campbell UC, Morgan AD, Carroll ME. Sex differences in the effects of baclofen on the acquisition of intravenous cocaine self-administration in rats. Drug Alcohol Dep. 2002;66(1):61–69. doi: 10.1016/s0376-8716(01)00185-5. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav. 2010;58(1):44–56. doi: 10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology. 2002;161(3):304–313. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- Carroll ME, France CP, Meisch RA. Intravenous self-administration of etonitazene, cocaine and phencyclidine in rats during food deprivation and satiation. J Pharmacol Exp Ther. 1981;217(2):241–247. [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci USA. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Hunter RG, Carroll ME. Wheel-running attenuates intravenous cocaine self-administration in rats: sex differences. Pharmacol Biochem Behav. 2002;73(3):663–671. doi: 10.1016/s0091-3057(02)00853-5. [DOI] [PubMed] [Google Scholar]
- Cummings JA, Gowl BA, Westenbroek C, Clinton SM, Akil H, Becker JB. Effects of a selectively bred novelty-seeking phenotype on the motivation to take cocaine in male and female rats. Biol Sex Differ. 2011;2(3) doi: 10.1186/2042-6410-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. Atomoxetine reverses nicotine withdrawal-associated deficits in contextual fear conditioning. Neuropsychopharmacol. 2007;32(9):2011–2019. doi: 10.1038/sj.npp.1301315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Dalley JW, Everitt BJ. Selective norepinephrine reuptake inhibition by atomoxetine prevents cue-induced heroin and cocaine seeking. Biol Psychiatry. 2011;69(3):266–274. doi: 10.1016/j.biopsych.2010.09.040. [DOI] [PubMed] [Google Scholar]
- Elman I, Karlsgodt KH, Gastfriend DR. Gender differences in cocaine craving among non-treatment-seeking individuals with cocaine dependence. Am J Drug Alcohol Abuse. 2001;27(2):193–202. doi: 10.1081/ada-100103705. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacol. 2006;31(3):659–674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Byrd EA, Henderson AR, See RE. Attenuation of cocaine-seeking by progesterone treatment in female rats. Psychoneuroendocrinology. 2009;34(3):343–352. doi: 10.1016/j.psyneuen.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Sofuoglu M, Morgan PT, Tuit KL, Sinha R. The effects of exogenous progesterone on drug craving and stress arousal in cocaine dependence: impact of gender and cue type. Psychoneuroendocrinology. 2013;38(9):1532–1544. doi: 10.1016/j.psyneuen.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Sinha R. Sex differences in drug-related stress-system changes: implications for treatment in substance-abusing women. Harv Rev Psychiatry. 2009;17(2):103–119. doi: 10.1080/10673220902899680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Mehta RH, Case JM, See RE. Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology. 2005;179(3):662–672. doi: 10.1007/s00213-004-2080-7. [DOI] [PubMed] [Google Scholar]
- Green TA, Schenk S. Dopaminergic mechanism for caffeine-produced cocaine seeking in rats. Neuropsychopharmacol. 2002;26(4):422–30. doi: 10.1016/S0893-133X(01)00343-8. [DOI] [PubMed] [Google Scholar]
- Harland RD, Gauvin DV, Michaelis RC, Carney JM, Seale TW, Holloway FA. Behavioral interaction between cocaine and caffeine: a drug discrimination analysis in rats. Pharmacol Biochem Behav. 1989;32(4):1017–1023. doi: 10.1016/0091-3057(89)90075-0. [DOI] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacol. 2006;31(1):129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Jones J, Mosher W, Daniels K. Current contraceptive use in the United States, 2006–2010, and changes in patterns of use since 1995. Natl Health Stat Report. 2012;60:1–25. [PubMed] [Google Scholar]
- Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE. Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology. 2008;198(1):63–75. doi: 10.1007/s00213-008-1089-8. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, Mehta RH, Case JM, Parker MP, Bimonte-Nelson HA, See RE. Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus. Psychopharmacology. 2005;182(2):245–252. doi: 10.1007/s00213-005-0071-y. [DOI] [PubMed] [Google Scholar]
- Knapp CM, Foye MM, Cottam N, Ciraulo DA, Kornetsky C. Adenosine agonists CGS 21680 and NECA inhibit the initiation of cocaine self-administration. Pharmacol Biochem Behav. 2001;68(4):797–803. doi: 10.1016/s0091-3057(01)00486-5. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Zhang XY. Sex differences in non-reinforced responding for cocaine. Am J Drug Alcohol Abuse. 2008;34(4):473–488. doi: 10.1080/00952990802082206. [DOI] [PubMed] [Google Scholar]
- Lara DR. Caffeine, mental health, and psychiatric disorders. J Alzheimers Dis. 2010;20(Suppl 1):S239–48. doi: 10.3233/JAD-2010-1378. [DOI] [PubMed] [Google Scholar]
- Levin FR, Mariani JJ, Secora A, Brooks D, Cheng WY, Bisaga A, Hennessy G. Atomoxetine treatment for cocaine abuse and adult attention deficit/hyperactivity disorder (ADHD): A preliminary open trial. J Dual Diagn. 2009;5(1):41–56. doi: 10.1080/15504260802628767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology. 2008;197(2):237–246. doi: 10.1007/s00213-007-1028-0. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Arizzi MN, Carroll ME. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology. 2000;152(2):132–139. doi: 10.1007/s002130000488. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology. 2000;148(2):196–200. doi: 10.1007/s002130050042. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Regulation of intravenously self-administered nicotine in rats. Exp Clin Psychopharmacol. 1999;7(3):198. doi: 10.1037//1064-1297.7.3.198. [DOI] [PubMed] [Google Scholar]
- Marchant BK, Reimherr FW, Halls C, Williams ED, Strong RE, Kondo D, Robison RJ. Long-term open-label response to atomoxetine in adult ADHD: influence of sex, emotional dysregulation, and double-blind response to atomoxetine. Atten Defic Hyperact Disord. 2011;3(3):237–244. doi: 10.1007/s12402-011-0054-2. [DOI] [PubMed] [Google Scholar]
- McKay JR, Rutherford MJ, Cacciola JS, Kabasakalian-McKay R, Alterman AI. Gender differences in the relapse experiences of cocaine patients. J Nerv Ment Dis. 1996;184(10):616–622. doi: 10.1097/00005053-199610000-00006. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guidelines for the Care and Use of Laboratory Animals. 8th edn. The National Academic Press; Washington, DC: 2011. [Google Scholar]
- Niyomchai T, Russo SJ, Festa ED, Akhavan A, Jenab S, Quiñones-Jenab V. Progesterone inhibits behavioral responses and estrogen increases corticosterone levels after acute cocaine administration. Pharmacol Biochem Behav. 2005;80(4):603–610. doi: 10.1016/j.pbb.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Oliveto AH, McCance-Katz E, Singha A, Hameedi F, Kosten TR. Effects of d-amphetamine and caffeine in humans under a cocaine discrimination procedure. Behav Pharmacol. 1998;9(3):207–17. [PubMed] [Google Scholar]
- Ouimette P, Coolhart D, Funderburk JS, Wade M, Brown PJ. Precipitants of first substance use in recently abstinent substance use disorder patients with PTSD. Addict Behav. 2007;32:1719–1727. doi: 10.1016/j.addbeh.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Peterson AB, Hivick DP, Lynch WJ. Dose-dependent effectiveness of wheel running to attenuate cocaine-seeking: impact of sex and estrous cycle in rats. Psychopharmacology. 2014;231(13):2661–2670. doi: 10.1007/s00213-014-3437-1. [DOI] [PubMed] [Google Scholar]
- Quinones-Jenab V, Jenab S. Progesterone attenuates cocaine-induced responses. Horm Behav. 2010;58(1):22–32. doi: 10.1016/j.yhbeh.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Regier PS, Claxton AB, Zlebnik NE, Carroll ME. Cocaine-, caffeine-, and stress-evoked cocaine reinstatement in high vs. low impulsive rats: treatment with allopregnanolone. Drug Alcohol Depend. 2014;143:58–64. doi: 10.1016/j.drugalcdep.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Linkugel JD, Bevins RA. Nicotine as a conditioned stimulus: Impact of attention-deficit/hyperactivity disorder medications. Exp Clin Psychopharmacol. 2007;15(5):501. doi: 10.1037/1064-1297.15.5.501. [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Bennett SAL, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology. 1989;98(3):408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Robison RJ, Reimherr FW, Marchant BK, Faraone SV, Adler LA, West SA. Gender Differences in 2 Clinical Trials of Adults With Attention-Deficit/Hyperactivity Disorder: A Retrospective Data Analysis.(CME). J Clin Psychiatry. 2008;69(2):213–221. doi: 10.4088/jcp.v69n0207. [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology. 2004;172(4):443–449. doi: 10.1007/s00213-003-1670-0. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Lile JA, Glaser PE, Hays LR. Physiological and subjective effects of acute intranasal methamphetamine during atomoxetine maintenance. Pharmacol Biochem Behav. 2011;100(1):40–47. doi: 10.1016/j.pbb.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Partridge B. Cocaine-seeking produced by experimenter-administered drug injections: dose-effect relationships in rats. Psychopharmacology. 1999;147(3):285–290. doi: 10.1007/s002130051169. [DOI] [PubMed] [Google Scholar]
- Schenk S, Worley CM, McNamara C, Valadez A. Acute and repeated exposure to caffeine: effects on reinstatement of extinguished cocaine-taking behavior in rats. Psychopharmacology. 1996;126(1):17–23. doi: 10.1007/BF02246406. [DOI] [PubMed] [Google Scholar]
- Sell SL, Scalzitti JM, Thomas ML, Cunningham KA. Influence of ovarian hormones and estrous cycle on the behavioral response to cocaine in female rats. J Pharmacol Exp Ther. 2000;293(3):879–886. [PubMed] [Google Scholar]
- Shaham Y, Miczek KA. Reinstatement—toward a model of relapse. Psychopharmacology. 2003;168(1-2):1–2. [Google Scholar]
- Smith MA, Pennock MM, Pitts EG, Walker KL, Lang KC. The effects of amphetamine, butorphanol, and their combination on cocaine self-administration. Behav Brain Res. 2014;274:158–163. doi: 10.1016/j.bbr.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Poling J, Hill K, Kosten T. Atomoxetine attenuates dextroamphetamine effects in humans. Am J Drug Alcohol Abuse. 2009;35(6):412–416. doi: 10.3109/00952990903383961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Sewell RA. REVIEW: Norepinephrine and stimulant addiction. Addict Biol. 2009;14(2):119–129. doi: 10.1111/j.1369-1600.2008.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Poling J, Gonzalez G, Gonsai K, Oliveto A, Kosten TR. Progesterone effects on cocaine use in male cocaine users maintained on methadone: A randomized, double-blind, pilot study. Exp Clin Psychopharmacol. 2007;15(5):453. doi: 10.1037/1064-1297.15.5.453. [DOI] [PubMed] [Google Scholar]
- Somaini L, Donnini C, A Raggi M, Amore M, Ciccocioppo R, A Saracino M, Gerra G. Promising medications for cocaine dependence treatment. Recent Pat CNS Drug Discov. 2011;6(2):146–160. doi: 10.2174/157488911795933893. [DOI] [PubMed] [Google Scholar]
- Souza MFD, Couto-Pereira NS, Freese L, Costa PA, Caletti G, Bisognin KM, Barros HMT. Behavioral effects of endogenous or exogenous estradiol and progesterone on cocaine sensitization in female rats. Braz J Med Biol Res. 2014;47(6):505–514. doi: 10.1590/1414-431X20143627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Rush CR. Combination pharmacotherapies for stimulant use disorder: a review of clinical findings and recommendations for future research. Expert Rev Clin Pharmacol. 2014;7(3):363–374. doi: 10.1586/17512433.2014.909283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swalve N, Smethells JR, Carroll ME. Sex differences in the acquisition and maintenance of cocaine and nicotine self-administration in rats. Psychopharmacology. 2015:1–9. doi: 10.1007/s00213-015-4183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrico CD, Haile CN, Newton TF, Kosten TR, De La Garza R. Pharmacotherapeutics for substance-use disorders: a focus on dopaminergic medications. Expert Opin Investig Drugs. 2013;22(12):1549–1568. doi: 10.1517/13543784.2013.836488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Middleton LS, Wong CJ, Nuzzo PA, Campbell CL, Rush CR, Lofwall MR. Atomoxetine does not alter cocaine use in cocaine dependent individuals: A double blind randomized trial. Drug Alcohol Depend. 2013;130(1):150–157. doi: 10.1016/j.drugalcdep.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JB, Mantsch JR. L-tetrahydropalamatine: a potential new medication for the treatment of cocaine addiction. Future Med Chem. 2012;4(2):177–186. doi: 10.4155/fmc.11.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks JR. Long-term intravenous infusion. Methods Psychobiol. 1972;2:155–168. [Google Scholar]
- Weerts EM, Griffiths RR. The adenosine receptor antagonist CGS15943 reinstates cocaine-seeking behavior and maintains self-administration in baboons. Psychopharmacology. 2003;168(1-2):155–163. doi: 10.1007/s00213-003-1410-5. [DOI] [PubMed] [Google Scholar]
- Westenbroek C, Perry AN, Becker JB. Pair housing differentially affects motivation to self-administer cocaine in male and female rats. Behav Brain Res. 2013;252:68–71. doi: 10.1016/j.bbr.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley CM, Valadez A, Schenk S. Reinstatement of extinguished cocaine-taking behavior by cocaine and caffeine. Pharmacol Biochem Behav. 1994;48(1):217–221. doi: 10.1016/0091-3057(94)90519-3. [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Forray A, Nich C, Carroll KM, Hine C, Merry BC, Sofuoglu M. Progesterone for the reduction of cocaine use in post-partum women with a cocaine use disorder: a randomised, double-blind, placebo-controlled, pilot study. The Lancet Psychiatry. 2014;1(5):360–367. doi: 10.1016/S2215-0366(14)70333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Zhan CG. Are pharmacokinetic approaches feasible for treatment of cocaine addiction and overdose? Future Med Chem. 2012;4(2):125–128. doi: 10.4155/fmc.11.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Pruitt C, Shin CB, Garcia AD, Zavala AR, See RE. Fos expression induced by cocaine-conditioned cues in male and female rats. Brain Struct Funct. 2014;219(5):1831–1840. doi: 10.1007/s00429-013-0605-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Carroll ME. Effects of the combination of wheel running and atomoxetine on cue-and cocaine-primed reinstatement in rats selected for high or low impulsivity. Psychopharmacology. 2015;232(6):1049–1059. doi: 10.1007/s00213-014-3744-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Saykao AT, Carroll ME. Effects of combined exercise and progesterone treatments on cocaine seeking in male and female rats. Psychopharmacology. 2014;231(18):3787–3798. doi: 10.1007/s00213-014-3513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Anker JJ, Gliddon LA, Carroll ME. Reduction of extinction and reinstatement of cocaine seeking by wheel running in female rats. Psychopharmacology. 2010;209(1):113–125. doi: 10.1007/s00213-010-1776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]