Abstract
Males and females respond differently to stroke. Moreover, females often experience worse long-term stroke outcomes. Fenofibrate, a peroxisome proliferator-activated receptor alpha (PPARα) agonist has been shown to improve stroke outcome and resolve neuroinflammation in male mice. The present study compares the effect of pretreatment with fenofibrate versus vehicle control in male and female mice during experimental stroke. Mice were treated with low-dose fenofibrate 30 minutes before and once a day for three additional days after stroke onset. We observed a reduction in infarct volume in male mice 96 hours post-stroke with low-dose fenofibrate pretreatment that was due to increase of an M2 macrophage phenotype in the brain and an increase in regulatory cells in the periphery. These outcomes were not replicated in females, likely due to the lower PPARα expression in cells and tissues in females vs males. We conclude that PPARα agonist treatment prior to stroke is neuroprotective in males but not females. These findings indicate PPARα as a probable mechanism of sex difference in stroke outcome and support the need for representation of females in stroke therapy research.
Keywords: Experimental stroke, Sex difference, Inflammation, PPARα, fenofibrate
Introduction
Stroke is a leading cause of death and disability in the United States (Mozaffarian et al. 2015) and the immune response plays an important role in stroke outcome. Following ischemic injury, peripheral leukocytes become activated and can infiltrate ischemic tissue where they perpetuate neurodegeneration and increase infarct volume (Offner et al. 2006a; Offner et al. 2006b; Seifert et al. 2012a; Seifert et al. 2012b). The immune response to stroke differs between males and females. For instance, male mice exhibit an initial increase in infarction characterized by an early and more robust recruitment of CD45highCD11b+ macrophages into post-ischemic brain relative to females (Banerjee et al. 2013; Dotson et al. 2015). If the spleen is removed prior to MCAO, sex differences in infarct and activated microglia/macrophages in the brain is lost due to, in part, a decrease of peripheral CD11b+ cells in males only (Dotson et al. 2015). Despite a poor initial stroke outcome in males, females often suffer from more long-term stroke effects leading to higher incidence of disability and a substandard quality of life (Di Carlo et al. 2003).
The difference in how males and females respond to stroke needs to be considered when defining successful stroke therapies. Hundreds of potential stroke therapies and neuroprotectants that have been successful in preclinical studies failed to be translated to successful stroke therapies in humans (Turner et al. 2013). A major contributing factor to the lack of clinical success in stroke therapy is the underrepresentation of females in therapy-based basic research.
A promising target for stroke therapy is peroxisome proliferator-activated receptor alpha (PPARα). PPARα is a nuclear hormone receptor and ligand-activated transcription factor that regulates lipid metabolism in the liver. PPARα also negatively regulates inflammation. PPARα ligands inhibit pro-inflammatory cytokines by interfering with the NF-κB signaling pathway (Delerive et al. 2001). In addition to inflammation, PPARα plays a role in oxidative stress and leukocyte-endothelial interactions (Marx et al. 1999; Poynter and Daynes 1998). PPARα is expressed in brain tissue such as neurons and astrocytes, cerebral vascular endothelial and in monocytes/macrophages (Bishop-Bailey 2000; Cullingford et al. 1998; Kainu et al. 1994). In the periphery, PPARα is expressed in the liver, the intestines, specifically the Peyer’s patch, and in the white pulp of the spleen (Braissant et al. 1996; Moreno et al. 2004). At a cellular level, PPARα is expressed in macrophages, T cells and B cells (Chinetti et al. 1998; Jones et al. 2002). Together, the regulation of inflammation, oxidative stress and leukocyte extravasation along with cerebral and peripheral expression has made PPARα an attractive target for therapy of ischemia.
PPARα agonist treatment has been effective at reducing infarction in males when given prior to stroke onset (Ouk et al. 2013). Specifically, PPARα agonist therapy before stroke reduces adhesion molecule expression in the brain thereby inhibiting early neutrophil infiltration and consequential microglia activation (Ouk et al. 2013). However, the effect of PPARα agonists on stroke has yet to be studied in females. Males and females differ in expression of PPARα receptors. In liver, PPARα mRNA and protein are expressed at higher level in the male and mediate several sex differences in metabolism (Jalouli et al. 2003). PPARα mRNA and protein are also expressed at higher basal levels in male vs. female lymphocytes (Dunn et al. 2007). Further, PPARα deficiency selectively alters male, but not female, T lymphocyte function and immune virulence (Dunn et al. 2007). Therefore, it is imperative that we examine how females respond to PPARα agonist stroke therapy.
In the present study, we compare PPARα agonist therapy during stroke to vehicle control in male and female mice. Mice were treated via intraperitoneal (i.p.) injection with low-dose fenofibrate 30 minutes prior to transient MCAO, and then each day after until brain and spleens were harvested at 96 hours post-stroke. We found that males, but not females, showed improved infarct size 96 hours after stroke onset with low-dose fenofibrate. Males had an increase in M2 macrophage gene expression in ischemic tissue when treated with fenofibrate while females exhibited an increase in pro-inflammatory cytokine gene expression. Finally, while fenofibrate decreased inflammatory cytokine production in peripheral immune cells after stroke of both males and females, the frequency of regulatory cells in the spleen was increased in males but decreased in female mice. These data indicate that males and females respond to PPARα activation differently and perpetuate the crucial need for representation of females in stroke therapy research.
Materials and Methods
Ethics Statement
The study was conducted in accordance with National Institutes of Health guidelines for the use of experimental animals, and the protocols were approved by the Portland Veteran Affairs Medical Center Institutional Animal Care and Use Committee, protocol # 2840-12, local database ID # 2840 and the Oregon Health and Science University Animal Care and Use Committee, protocol # IS00003885.
Experimental Animals
Male and female C57BL/6J wild-type (WT) mice (The Jackson Laboratory, Sacramento, CA, USA) weighing 20-25 g were housed in a climate-controlled room on a 12-hour light/dark cycle. Food and water were provided ad libitum.
Middle Cerebral Artery Occlusion Model
Transient focal cerebral ischemia was induced in male and female mice for 1 hour by reversible right middle cerebral artery occlusion (MCAO) under isoflurane anesthesia followed by 96 hours of reperfusion as described previously (Zhang et al. 2008). Head and body temperature were controlled at 37.0 ± 1.0°C throughout MCAO surgery with a warm water blanket and a heating lamp ~18 inches above the mouse. Occlusion and reperfusion were verified in each animal by Laser Doppler Flowmetry (LDF) (Model DRT4, Moor Instruments Ltd., Wilmington, DE). The common carotid artery was exposed and the external carotid artery was ligated and cauterized. Unilateral MCAO was accomplished by inserting a 6-0 nylon monofilament surgical suture (ETHICON, Inc., Somerville, NJ, USA) with a heat-rounded and silicone-coated (Xantopren comfort light, Heraeus, Germany) tip into the internal carotid artery via the external carotid artery stump. Adequacy of MCAO was confirmed by monitoring cortical blood flow at the onset of the occlusion with a LDF probe affixed to the skull. Animals were excluded if mean intra-ischemic LDF was greater than 30% pre-ischemic baseline LDF. At 1 hour of occlusion, the occluding filament was withdrawn to allow for reperfusion. Mice were then allowed to recover from anesthesia and survived for 96 hours of reperfusion.
Fenofibrate treatment
Mice were randomized to receive 0.2mL (300μg) fenofibrate or 0.2mL vehicle (10% DMSO+ 2% tween 80 in sterile saline) by i.p. injection 30 minutes after stroke surgery and once a day for three additional days. Mice were euthanized at 96 hours after stroke onset.
Infarct Volume Analysis
Brains were harvested after 96 hours of reperfusion and sliced into five 2-mm-thick coronal sections for staining with 1.2% 2,3,5-triphenyltetrazolium chloride (TTC; Sigma, St. Louis, MO, USA) in saline as described previously (Hurn et al. 2007). The 2-mm brain sections were incubated in 1.2% TTC for 15 min at 37°C, and then fixed in 10% formalin for 24 hours. Infarct volume was measured using digital imaging and image analysis software (Systat, Inc., Point Richmond, CA, USA). To control for edema, infarct volume (cortex, striatum, and hemisphere) was determined by subtraction of the ipsilateral noninfarcted regional volume from the contralateral regional volume. This value was then divided by the contralateral regional volume and multiplied by 100 to yield regional infarct volume as a percent of the contralateral region.
Leukocyte isolation from brains and spleen
Spleens from individual mice were removed and a single-cell suspension was prepared by passing the tissue through a 100 μm nylon mesh (BD Falcon, Bedford, MA). The cells were washed using RPMI 1640 and the red blood cells lysed using 1× red blood cell lysis buffer (eBioscience, Inc., San Diego, CA) and incubated for 1 min at room temperature. The cells were then washed with RPMI 1640, counted on a Cellometer Auto T4 cell counter (Nexcelom, Lawrence, MA), and resuspended in staining medium (PBS containing 0.1% NaN3 and 1% bovine serum albumin (Sigma, Illinois)) for flow cytometry. The brain was divided into the ischemic (right) and nonischemic (left) hemispheres for analysis. The brain tissue was digested for 60 min with 1 mg/ml Type IV collagenase (Sigma Aldrich, St. Louis, MO) and DNase I (50 mg/ml, Roche Diagnostics, Indianapolis, IN) at 37°C with intermittent shaking. Samples were mixed with a 1 ml pipette every 15 min. The cell suspension was washed 1× in RPMI, resuspended in 80% Percoll, overlayed with 40% Percoll and centrifuged for 30 min at 1600 RPM. The cells were then washed twice with RPMI 1640 and resuspended in staining medium for flow cytometry.
Analysis of cell populations by flow cytometry
Antibodies were purchased (BD Biosciences, San Jose, CA or eBioscience, Inc., San Diego, CA). Four-color (FITC, PE, APC, PECy5 and/or PerCP) fluorescence flow cytometry analyses were performed, determining cell phenotypes from spleen and brain. Roughly 2×105 brain cells and 1×106 splenocytes were washed with staining medium, blocked with anti-mouse CD16/CD32 Mouse BD Fc Block™ (BD Biosciences, San Jose) and then incubated with varying combinations of the following monoclonal antibodies: CD11b (MAC-1), CD45 (Ly-5), CD3 (145-2C11), CD11c (HL-3), CD19 (1D3), CD4 (GK1.5), CD8 (53-6.7), CD122 (TM-β1), CD44 (IM7), CD69 (H1.2F3), Ly6G (RB6-8C5) and CD25 (7D4) for 20 min at 4°C. 7-AAD was used to identify dead cells. CD4+ regulatory T cells were identified using anti-Foxp3 (FJK-16s) and accompanying Fixation/Permeabilization reagents as per manufacturer’s instructions (eBioscience, Inc., San Diego, CA). Isotype matched mAb served as a negative control. Data were collected with BD AccuriTM C6 software on a BD AccuriTM C6 (BD Biosciences, San Jose, CA).
Intracellular staining
Spleen cells from individual mice were cultured at 1×106 cells/well in a 24-well culture plate in stimulation medium (RPMI, 1% sodium pyruvate, 1% L-glutamine, 0.4% 2-β-mercaptoethanol, 2% FBS) with PMA (50 ng/mL), ionomycin (500 ng/mL) and brefeldin A (1 μl/mL) (all reagents from Sigma-Aldrich, St. Louis, MO) for 4 hours at 37°C. Cells were blocked and surface stained (as described above), then fixed and permeabilized using the Cytofix/Cytoperm kit (BD Biosciences), according to manufacturer’s instructions. Fixed cells were washed with 1x permeabilization buffer (BD Biosciences) and incubated with the following antibodies: TNF-a, IL-10 or CD206. Isotype matched antibodies served as negative controls to establish background staining levels. Data were collected with BD AccuriTM C6 software on a BD AccuriTM C6 (BD Biosciences, San Jose, CA).
Real-time PCR
Ischemic hemispheres and were harvested from mice 96 hours after MCAO, frozen in liquid nitrogen and stored at −80°C until processed. Spleens were processed as described above and 1×106 cells were frozen in liquid nitrogen and stored at −80°C until processed. Total RNA was isolated using the RNeasy Mini Kit according to the manufacturer's instructions. (Qiagen, Valencia, CA). cDNA was synthesized using the SuperScript II Reverse Transcriptase cDNA synthesis kit (Life Technologies, Grand Island, NY). Quantitative realtime PCR was performed using the StepOnePlus Real-Time PCR System with TaqMan primers for mouse immune response (Life Technologies, Grand Island, NY). Primers used are as follows: IL-6 (Mm00446190_m1), TNFα (Mm00443258_m1), IL-1β (Mm00434228_m1), iNOS (Mm00440502_m1), MMP-9 (Mm00442991_m1), CD206 (Mm01329362_m1), ICAM-1 (Mm00516023_m1), VCAM-1 (Mm01320970_m1), p-Selectin (Mm01295931_m1). GAPDH housekeeping gene was used as an endogenous control. Results were analyzed using ExpressionSuite Software (Life Technologies, Grand Island, NY).
Statistical Analysis
Data are presented as mean ±SEM. Statistical comparisons were made between vehicle and fenofibrate for infarct, gene expression and cellular subtype. Statistical differences were performed with the Student’s t-test using Prism (GraphPad Software, La Jolla, CA). Statistical significance was p<0.05.
Results
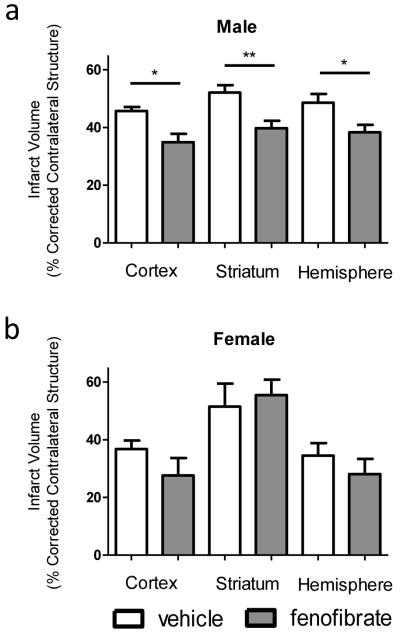
Numerous studies have reported resolution of infarct volume and neuroinflammatory factors with PPARα agonist pretreatment in males (Ouk et al. 2013). However, the response that female mice have to PPARα agonist therapy during stroke has yet to be determined. Thus we examine the response to PPARα agonist therapy during stroke in female mice. Additionally, we examined the effects of a lower dose of fenofibrate than previously tested in males and females. Male and female mice received 300ug (about 15mg/kg) of fenofibrate 30 minutes prior to MCAO and then again each day until 96 hours post-reperfusion when infarct volume was examined. Even with a lower concentration of fenofibrate, male mice were protected with PPARα agonist pretreatment, exhibiting significantly smaller infarct volumes in the cortex, striatum and hemisphere (Fig. 1a). Female mice, on the other hand, showed no change in infarct volumes (Fig. 1b). These results indicate that low-dose PPARα agonist therapy protects males, but not females, during ischemic stroke injury.
Figure 1.
Fenofibrate resolves infarct volume in male but not female mice. Male and female mice were treated with fenofibrate or vehicle 30 minutes prior to, and once a day following transient MCAO (60 min). Brains were harvested 96 h after MCAO and brain slices were stained with 2,3,5- TTC. Infarct volumes of male (a) and female (b) mice were measured as percentage of contralateral structure. Values represent mean numbers (±SEM). n=7 for male vehicle, 10 for male fenofibrate, 7 for female vehicle and 5 for female fenofibrate. * indicates p<0.05. ** indicates p<0.01.
Next, we looked at the frequency of leukocytes in the ischemic hemisphere to determine if leukocyte infiltration was effected by PPARα agonist therapy. CD11b+CD45+ cells are activated microglia/infiltrating monocytes and CD11b+Ly6G+ cells are infiltrating neutrophils. Both cell types perpetuate inflammatory conditions after ischemia. We did not observe any significant differences in CD11b+CD45+ or CD11b+Ly6G+ frequency between vehicle and fenofibrate in either male (Fig. 2a) or female (Fig.2b) mice. CD19+ B cells can be potentially beneficial for stroke outcome (Bodhankar et al. 2014). Again, there were no significant differences in B cell frequency with vehicle vs. fenofibrate treated male or female mice in the ischemic hemisphere (Fig. 2a and 2b, respectively). There was, however, a significant decrease in the frequency of 7-aminoactinomycin D (7AAD+) staining of dead cells with fenofibrate in both male (Fig. 2a) and female (Fig. 2b) mice, indicating that PPARα agonist therapy reduces cell death after ischemia.
Figure 2.
Immune subsets in the ischemic hemisphere with fenofibrate treatment. Brains were harvested 96 hours after MCAO from vehicle or fenofibrate treated mice, processed and immunophenotyped by flow cytometry. Infiltrating and activated monocytes/microglia was determined by gating on CD11b+ cells and examining CD45 high cells. Averages of CD11b+CD45+ activated monocytes/microglia, CD11b+Ly6G+ neutrophils, CD19+ B cells and 7AAD+ dead cells of male (a) and female (b) mice are represented. Values represent mean numbers (±SEM) of 2-4 male mice per group and 3 female mice per group.
We also examined pro- and anti-inflammatory gene expression in the ischemic hemisphere after PPARα agonist therapy. There were no significant differences in expression of the pro-inflammatory cytokines IL-6, TNFα and IL-1β in the ischemic tissue between vehicle and fenofibrate with males (Fig. 3a). However, females treated with fenifibrate during experimental stroke significantly upregulated expression of TNFα and exhibited a trend upregulation of IL-6 in ischemic tissue compared to their vehicle controls (Fig 3b). There was no difference in the ischemic tissue gene expression of inducible nitric oxide synthase (iNOS) in either males or females (Fig. 3a and 3b, respectively). As opposed to females, males treated with fenofibrate during stroke show a significant increase in matrix metallopeptidase 9 (MMP-9) and M2 macrophage marker, CD206 (Fig. 3a). Neither males nor females exhibited any significant differences in ischemic tissue with adhesion molecules (ICAM-1 and VCAM-1) or p-selectin between vehicle and PPARα agonist treatment during experimental stroke.
Figure 3.
Gene expression profile from ischemic brain. Relative expression of genes in the ischemic hemisphere 96 hours after MCAO was analyzed by real-time PCR. Brain tissue from male (a) and female (b) mice was examined and results are relative to the vehicle group which was normalized to 1. Values represent mean numbers (±SEM) of 2-3 mice per group. * indicates p<0.05. ** indicates p<0.01.
Additionally, we compared PPARα expression in the ischemic hemisphere 96 hours after experimental stroke between males and females. We found a trend decrease in PPARα expression in vehicle female mice compared to males after MCAO (Fig. 4a). Moreover, there was a significant decrease in PPARα expression in female compared to male ischemic tissue 96 hours after stroke (Fig. 4b) suggesting a lower baseline expression of PPARα expression in females after stroke that is perpetuated with PPARα agonist. These data also suggest a mechanism of differential response to fenofibrate.
Figure 4.
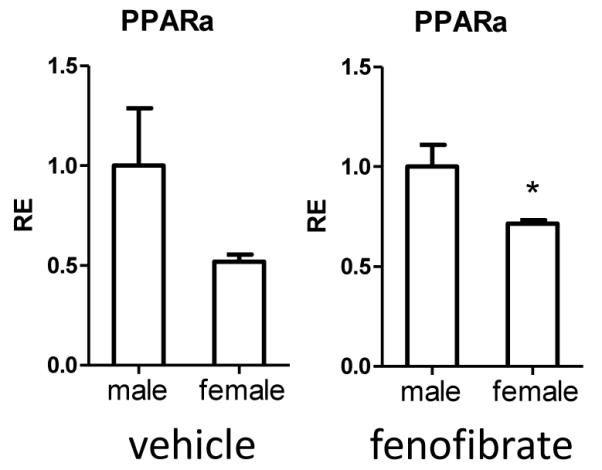
PPARα expression in the ischemic brain. Relative expression of PPARα in the ischemic hemisphere 96 hours after MCAO was analyzed by real-time PCR. Brain tissue from male and female mice treated with vehicle or fenofibrate was examined. Results are relative to the male group which was normalized to 1. Values represent mean numbers (±SEM) of 2-3 mice per group. * indicates p<0.05.
To get an idea of how peripheral immune responses could be affecting brain-specific outcomes after stroke, we studied the effect that PPARα agonist had on cells in the spleen. Overall, we observed no significant differences between PPARα agonist and vehicle control with either males or females in the frequency of T cells, monocytes/macrophages and B cells in the spleen (Table 1). There was a significant increase in dendritic cells in males treated with fenofibrate compared to vehicle control after stroke. We observed a trend decrease in CD4+CD69+ early activated cells in the spleen of males treated with fenofibrate (Table 1) but again, there were no major differences between treatment or control in activated (CD69+) or effector (CD44+) T cells in the spleens of males and females after experimental stroke (Table 1).
Table 1.
Immune subsets in the spleen 96 hours after MCAO
| Splenocyte frequency |
male vehicle | male fenofibrate | female vehicle | female fenofibrate |
|---|---|---|---|---|
| Splenocyte # | 28.6×106 ± 4.52×106 | 14.6×106 ± 3.48×106* | 31.7×106 ± 6.76×106 | 17.2×106 ± 4.79×106 |
| CD3+ (%) | 39.16 ± 3.52 | 37.16 ± 4.35 | 36.55 ± 3.12 | 35.94 ± 6.35 |
| CD4+ (%) | 20.36 ± 1.72 | 20.69 ± 2.24 | 21.31 ± 1.75 | 19.34 ± 3.03 |
| CD8+(%) | 15.54 ± 1.73 | 14.32 ± 2.11 | 15.65 ± 2.15 | 14.55 ± 2.56 |
| CD4+CD69+ (%) | 18.07 ± 2.087 | 12.76 ± 1.131a | 17.39 ± 1.74 | 17.38 ± 1.83 |
| CD8+CD69+ (%) | 5.73 ± 0.54 | 6.83 ± 1.06 | 9.71 ± 1.7 | 5.86 ± 0.61 |
| CD4+CD44+ (%) | 27.49 ± 2.13 | 24.93 ± 1.46 | 25.18 ± 2.55 | 25.68 ± 2.43 |
| CD8+CD44+ (%) | 16.51 ± 2.31 | 23.2 ± 2.21 | 18.34 ± 2.1 | 16.35 ± 2.72 |
| CD11b+ (%) | 11.76 ± 2.89 | 6.88 ± 1.46 | 5.92 ± 0.65 | 7.37 ± 0.81 |
| CD11c+ (%) | 0.88 ± 0.2 | 1.73 ± 0.14** | 1.01 ± 0.07 | 0.74 ± 0.13 |
| CD19+ (%) | 37.25 ± 5.43 | 46.0 ± 3.8 | 48.34 ± 2.69 | 47.32 ± 6.91 |
indicates p<0.05,
indicates p <0.01
indicates p=0.057 by t-test.
Splenocyte #: n=7 for both male and 12 for both female groups
Immune subsets: n=7 for male vehicle, 6 for male fenofibrate, 9 for female vehicle and 7 for female fenofibrate
Although there were no major differences in immune subset frequencies in the spleen after stroke and PPARα agonist therapy, we checked whether the effector activity of those cells was altered by treatment. Both sexes exhibited a trend of significant decrease in TNFα production by CD11b+ cells with PPARα agonist therapy during stroke (Fig. 5a and 5b) but only males showed a sizable and significant decrease in TNFα produced by CD3+ T cells with fenofibrate treatment (Fig. 5a). PPARα agonist did not significantly affect the frequency of CD11b+CD206 M2 macrophages in the spleen after stroke in either sex (Fig. 5a, 5b). When we examined the frequency of regulatory cells we noticed a trending increase in splenic CD122+CD8+ regulatory T cells and IL-10 produced by the CD8+ Tregs in males treated with fenofibrate during experimental stroke (Fig. 5a). Additionally, when females were given PPARα agonist during stroke, the frequency of CD122+CD8+ Tregs and CD4+Foxp3+ Tregs were significantly or nearly significantly reduced, respectively (Fig. 5b).
Figure 5.
Effector and regulatory cells in the spleen after MCAO. Spleens were harvested 96 hours after MCAO and immunophenotyped by flow cytometry in male (a) and female (b) mice treated with or without fenofibrate. TNFα production was determined by gating on CD11b+ or CD3+ subsets and measuring TNFα positive cells compared to isotype. CD206 expression was determined by gating on CD11b+ cells and measuring CD206 positive cells compared to isotype. The frequency of CD8+ regulatory T cells was determined by gating on CD3+ and CD8+ cells before measuring CD122 and IL-10 expression. The frequency of CD4+ regulatory T cells was determined by gating on CD4+ cells before measuring Foxp3 positive cells. Values represent mean numbers (±SEM) of 4-6 mice per group for TNFα+, CD206+, CD122 and IL-10+ cells and 3-8 mice per group for CD4+ Tregs. * indicates p<0.05.
Discussion
PPARα agonist, fenofibrate, has been a promising stroke therapy in males. Fibrates are a class of amphipathic carboxylic acids that are lipid lowering drugs used to treat hypercholesterolemia and hypertriglyceridemia (Staels et al. 1998). In males, fenofibrate has been shown to improve initial stroke outcomes by reducing infarct volume and enhancing cerebral blood flow recovery after reperfusion (Guo et al. 2010). Additionally, treatment with fenofibrate before stroke reduces the expression of ICAM-1 and VCAM-1, reduces adherent leukocytes, infiltrating neutrophils and regulates oxidative stress in the brain (Deplanque et al. 2003; Ouk et al. 2014a). Pre-treatment with fenofibrate also reduces neutrophils and gene expression of CXCL10, CXCL1 and SAA-1 in the liver after stroke (Losey et al. 2015). PPARα agonist, WY14643, exhibits a similar reduction of adhesion molecule expression, oxidative stress and iNOS expression when used as a pretreatment to stroke (Collino et al. 2006).
For the first time, we demonstrate that males are protected from stroke by low-dose fenofibrate, but females are not. Male mice had significantly smaller infarct volumes after stroke when treated with fenofibrate while females exhibited no change compared to vehicle (Fig. 1). Although other groups have observed decreases in infiltrating macrophages and neutrophils after stroke with fenofibrate treatment, we did not observe any changes in the frequency of immune subsets in males or females treated with either fenofibrate or vehicle (Fig. 2). One hypotheses for the difference compared to the literature is that we were looking at a 96 hour post-stroke time point while previous studies are looking at early times after stroke, most being 24 hours post-stroke. We also started fenofibrate treatment only 30 minutes prior to MCAO compared to many studies that pre-treat for 14 days before stroke onset. Additionally, low-dose, i.p. treatment may not exhibit the widespread effects of long-term, high-dose fenofibrate treatment. Collino, et al. did a low dose study with PPARα agonist, WY14643, 30 minutes prior to MCAO (Collino et al. 2006). The differences they observed in oxidative stress, MAP kinase, NF-κB, iNOS and ICAM-1 expression after stroke with and without PPARα agonist were lost by 24 hours of reperfusion (Collino et al. 2006), suggesting that many of the PPARα mechanisms happen early after stroke and may be completely undetectable by 96 hours.
Fenofibrate pretreatment does, however, effect gene expression in the ischemic hemisphere. Figure 3 illustrates that while males exhibit an increase in CD206 gene expression, females significantly increase TNFα expression with fenofibrate treatment prior to stroke. These data indicate that fenofibrate treatment of stroke pushes males to more of an M2 macrophage phenotype, and females an M1 macrophage phenotype. Moreover, fenofibrate caused an increase gene expression of proinflammatory cytokine, IL-6, in the ischemic hemisphere of females. It was interesting that pretreatment of stroke with fenofibrate increased MMP-9 expression in brains of male mice. Although matrix metalloproteinases are known to contribute to stroke damage by degrading the cellular matrix, they are also part of the recovery and rebuilding process days after stroke by promoting brain tissue restructuring, remodeling and movement of neurons (Lee et al. 2006; Zhao et al. 2006). Finally, we observed no difference in adhesion molecule or selectin gene expression after stroke between fenofibrate and vehicle in either sex. Again, we speculate that any differences in adhesion molecule expression would have occurred early after stroke and may be undetectable by 96 hours of reperfusion.
The effect of PPARα agonist treatment on the peripheral immune response prior to stroke had largely been unexamined before this study. Fenofibric acid does not cross the blood-brain at a very efficient rate (Deplanque et al. 2003). Therefore, it is likely that its effects take place predominantly in the periphery. Although both males and females exhibited a decrease in TNFα secretion by immune cells in the spleen with fenofibrate treatment of stroke (Fig. 5), the sex differences were mostly observed in regulatory cell frequency. CD8 regulatory T cells express CD122 and regulate effector cell function with perforin and IL-10 (Wang and Alexander 2009). CD8 Tregs have been directly linked with a better stroke outcome after MCAO (Banerjee et al. 2013; Bodhankar et al. 2013). There are conflicting views on whether CD4+ regulatory T cells help or hurt stroke related neuronal damage (Schabitz 2013; Xu et al. 2013) but in general, CD4+ Tregs regulate the peripheral immune response using regulatory immunosuppressive cytokines (Li et al. 2013; Liesz et al. 2009; Planas and Chamorro 2009). The trending increase of CD8 Tregs in males and decrease of CD4 and CD8 Tregs in females treated with fenofibrate prior to stroke suggests that modulation of regulatory immune subsets in the periphery has important neuroinflammatory effects.
Sex differences in response to stroke are becoming better documented. For instance, the spleen and IL-4 are needed to observe sex difference in infarct size after stroke, and without either the spleen or IL-4, infarct sex difference is lost (Dotson et al. 2015; Xiong et al. 2015). Additionally, higher levels of IL-10 after stroke have been associated with poor acute and long-term outcomes in females (Conway et al. 2015). The difference in stroke protection with fenofibrate in males but not females is likely due to the lower levels of PPARα expression in females. We observed a lower gene expression of PPARα in the ischemic hemisphere in females treated with fenofibrate compared to males treated with fenofibrate (Fig. 4). Males also express higher PPARα levels in the liver and in lymphocytes than females (Dunn et al. 2007; Jalouli et al. 2003). Male but not female PPARα−/− knockout mice develop more severe symptoms in experimental autoimmune encephalomyelitis (EAE) suggesting that PPARα expression in T cells of males leads them less prone to Th-1 based autoimmunity (Dunn et al. 2007). It is also likely that lower PPARα expression in lymphocytes is partially responsible for the poor, long-term stroke outcome observed in females.
Taken together, these data illustrate that the pre-treatment of stroke with PPARα agonist, fenofibrate, improves stroke outcome in males but not females. We observed a reduction in infarct volume in male mice 96 hours post-stroke with low-dose fenofibrate pre-treatment that was due to an increase of M2 phenotype in the brain and an increase in regulatory cells in the periphery. These findings were not replicated in females, likely due to the lower PPARα expression in cells and tissues. In addition to the failure of PPARα agonist pretreatment to protect against stroke in females, a previous study reports that fenofibrate does not reduce cerebral infarct volume when administered one hour after stroke onset (Deplanque et al. 2003). Therefore, it could be concluded that PPARα agonists may not translate well to a clinical stroke therapy. Finally, this study confirms the importance for representation of females in stroke therapy research and indicates PPARα as a probable mechanism of sex differences in stroke.
Acknowledgements
The authors wish to thank Gail Kent for assistance with manuscript submission. This work was supported by NIH/NINDS 5R01NS076013 (HO, JAS). This material is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
Abbreviations
- PPARα
Peroxisome proliferator-activated receptor alpha
- MCAO
middle cerebral artery occlusion
- Treg
regulatory T cell
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical approval: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of Oregon Health & Science University's Institutional Animal Care and Use Committee.
This article does not contain any studies with human participants performed by any of the authors.
References
- Banerjee A, Wang J, Bodhankar S, Vandenbark AA, Murphy SJ, Offner H. Phenotypic changes in immune cell subsets reflect increased infarct volume in male vs. female mice. Transl Stroke Res. 2013;4:554–563. doi: 10.1007/s12975-013-0268-z. doi:10.1007/s12975-013-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop-Bailey D. Peroxisome proliferator-activated receptors in the cardiovascular system. British journal of pharmacology. 2000;129:823–834. doi: 10.1038/sj.bjp.0703149. doi:10.1038/sj.bjp.0703149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhankar S, Chen Y, Vandenbark AA, Murphy SJ, Offner H. PD-L1 enhances CNS inflammation and infarct volume following experimental stroke in mice in opposition to PD-1. Journal of neuroinflammation. 2013;10:111. doi: 10.1186/1742-2094-10-111. doi:10.1186/1742-2094-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhankar S, Chen Y, Vandenbark AA, Murphy SJ, Offner H. Treatment of experimental stroke with IL-10-producing B-cells reduces infarct size and peripheral and CNS inflammation in wild-type B-cell-sufficient mice. Metabolic brain disease. 2014;29:59–73. doi: 10.1007/s11011-013-9474-3. doi:10.1007/s11011-013-9474-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Differential expression of peroxisome proliferator-activated receptors, (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. doi:10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- Chinetti G, et al. Activation of proliferator-activated receptors alpha and gamma induces apoptosis of human monocyte-derived macrophages. The Journal of biological chemistry. 1998;273:25573–25580. doi: 10.1074/jbc.273.40.25573. [DOI] [PubMed] [Google Scholar]
- Collino M, et al. Oxidative stress and inflammatory response evoked by transient cerebral ischemia/reperfusion: effects of the PPAR-alpha agonist WY14643. Free radical biology & medicine. 2006;41:579–589. doi: 10.1016/j.freeradbiomed.2006.04.030. doi:10.1016/j.freeradbiomed.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Conway SE, Roy-O'Reilly M, Friedler B, Staff I, Fortunato G, McCullough LD. Sex differences and the role of IL-10 in ischemic stroke recovery. Biology of sex differences. 2015;6:17. doi: 10.1186/s13293-015-0035-9. doi:10.1186/s13293-015-0035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullingford TE, Bhakoo K, Peuchen S, Dolphin CT, Patel R, Clark JB. Distribution of mRNAs encoding the peroxisome proliferator-activated receptor alpha, beta, and gamma and the retinoid X receptor alpha, beta, and gamma in rat central nervous system. Journal of neurochemistry. 1998;70:1366–1375. doi: 10.1046/j.1471-4159.1998.70041366.x. [DOI] [PubMed] [Google Scholar]
- Delerive P, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors in inflammation control. The Journal of endocrinology. 2001;169:453–459. doi: 10.1677/joe.0.1690453. [DOI] [PubMed] [Google Scholar]
- Deplanque D, et al. Peroxisome proliferator-activated receptor-alpha activation as a mechanism of preventive neuroprotection induced by chronic fenofibrate treatment. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:6264–6271. doi: 10.1523/JNEUROSCI.23-15-06264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Carlo A, et al. Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in Europe: data from a multicenter multinational hospital-based registry. Stroke. 2003;34:1114–1119. doi: 10.1161/01.STR.0000068410.07397.D7. doi:10.1161/01.str.0000068410.07397.d7. [DOI] [PubMed] [Google Scholar]
- Dotson AL, Wang J, Saugstad J, Murphy SJ, Offner H. Splenectomy reduces infarct volume and neuroinflammation in male but not female mice in experimental stroke. J Neuroimmunol. 2015;278:289–298. doi: 10.1016/j.jneuroim.2014.11.020. doi:10.1016/j.jneuroim.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn SE, et al. Peroxisome proliferator-activated receptor, (PPAR)alpha expression in T cells mediates gender differences in development of T cell-mediated autoimmunity. The Journal of experimental medicine. 2007;204:321–330. doi: 10.1084/jem.20061839. doi:10.1084/jem.20061839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Wang G, Namura S. Fenofibrate improves cerebral blood flow after middle cerebral artery occlusion in mice. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2010;30:70–78. doi: 10.1038/jcbfm.2009.185. doi:10.1038/jcbfm.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurn PD, Subramanian S, Parker SM, Afentoulis ME, Kaler LJ, Vandenbark AA, Offner H. T- and B- cell-deficient mice with experimental stroke have reduced lesion size and inflammation. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007;27:1798–1805. doi: 10.1038/sj.jcbfm.9600482. doi:10.1038/sj.jcbfm.9600482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalouli M, et al. Sex difference in hepatic peroxisome proliferator-activated receptor alpha expression: influence of pituitary and gonadal hormones. Endocrinology. 2003;144:101–109. doi: 10.1210/en.2002-220630. doi:10.1210/en.2002-220630. [DOI] [PubMed] [Google Scholar]
- Jones DC, Ding X, Daynes RA. Nuclear receptor peroxisome proliferator-activated receptor alpha, (PPARalpha) is expressed in resting murine lymphocytes. The PPARalpha in T and B lymphocytes is both transactivation and transrepression competent. The Journal of biological chemistry. 2002;277:6838–6845. doi: 10.1074/jbc.M106908200. doi:10.1074/jbc.M106908200. [DOI] [PubMed] [Google Scholar]
- Kainu T, Wikstrom AC, Gustafsson JA, Pelto-Huikko M. Localization of the peroxisome proliferator-activated receptor in the brain. Neuroreport. 1994;5:2481–2485. doi: 10.1097/00001756-199412000-00019. [DOI] [PubMed] [Google Scholar]
- Lee SR, Kim HY, Rogowska J, Zhao BQ, Bhide P, Parent JM, Lo EH. Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:3491–3495. doi: 10.1523/JNEUROSCI.4085-05.2006. doi:10.1523/jneurosci.4085-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, et al. Adoptive regulatory T-cell therapy protects against cerebral ischemia. Ann Neurol. 2013;74:458–471. doi: 10.1002/ana.23815. doi:10.1002/ana.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesz A, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nature medicine. 2009;15:192–199. doi: 10.1038/nm.1927. doi:10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- Losey P, Ladds E, Laprais M, Geuvel B, Burns L, Bordet R, Anthony DC. The role of PPAR activation during the systemic response to brain injury. Journal of neuroinflammation. 2015;12:99. doi: 10.1186/s12974-015-0295-7. doi:10.1186/s12974-015-0295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx N, Sukhova GK, Collins T, Libby P, Plutzky J. PPARalpha activators inhibit cytokine-induced vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation. 1999;99:3125–3131. doi: 10.1161/01.cir.99.24.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Farioli-Vecchioli S, Ceru MP. Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat. CNS Neuroscience. 2004;123:131–145. doi: 10.1016/j.neuroscience.2003.08.064. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. doi:10.1161/cir.0000000000000152. [DOI] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2006a;26:654–665. doi: 10.1038/sj.jcbfm.9600217. doi:10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- Offner H, et al. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol. 2006b;176:6523–6531. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- Ouk T, et al. Effects of the PPAR-alpha agonist fenofibrate on acute and short-term consequences of brain ischemia. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2014a;34:542–551. doi: 10.1038/jcbfm.2013.233. doi:10.1038/jcbfm.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouk T, et al. PPARs: a potential target for a disease-modifying strategy in stroke. Curr Drug Targets. 2013;14:752–767. doi: 10.2174/1389450111314070005. [DOI] [PubMed] [Google Scholar]
- Planas AM, Chamorro A. Regulatory T cells protect the brain after stroke. Nature medicine. 2009;15:138–139. doi: 10.1038/nm0209-138. doi:10.1038/nm0209-138. [DOI] [PubMed] [Google Scholar]
- Poynter ME, Daynes RA. Peroxisome proliferator-activated receptor alpha activation modulates cellular redox status, represses nuclear factor-kappaB signaling, and reduces inflammatory cytokine production in aging. The Journal of biological chemistry. 1998;273:32833–32841. doi: 10.1074/jbc.273.49.32833. [DOI] [PubMed] [Google Scholar]
- Schabitz WR. Regulatory T cells in ischemic stroke: helpful or hazardous? Stroke. 2013;44:e84. doi: 10.1161/STROKEAHA.113.002228. doi:10.1161/STROKEAHA.113.002228. [DOI] [PubMed] [Google Scholar]
- Seifert HA, Hall AA, Chapman CB, Collier LA, Willing AE, Pennypacker KR. A transient decrease in spleen size following stroke corresponds to splenocyte release into systemic circulation. J Neuroimmune Pharmacol. 2012a;7:1017–1024. doi: 10.1007/s11481-012-9406-8. doi:10.1007/s11481-012-9406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert HA, et al. The spleen contributes to stroke induced neurodegeneration through interferon gamma signaling. Metabolic brain disease. 2012b;27:131–141. doi: 10.1007/s11011-012-9283-0. doi:10.1007/s11011-012-9283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98:2088–2093. doi: 10.1161/01.cir.98.19.2088. [DOI] [PubMed] [Google Scholar]
- Turner RC, Lucke-Wold B, Lucke-Wold N, Elliott AS, Logsdon AF, Rosen CL, Huber JD. Neuroprotection for ischemic stroke: moving past shortcomings and identifying promising directions. Int J Mol Sci. 2013;14:1890–1917. doi: 10.3390/ijms14011890. doi:10.3390/ijms14011890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YM, Alexander SI. CD8 regulatory T cells: what's old is now new. Immunol Cell Biol. 2009;87:192–193. doi: 10.1038/icb.2009.8. doi:10.1038/icb.2009.8. [DOI] [PubMed] [Google Scholar]
- Xiong X, Xu L, Wei L, White RE, Ouyang YB, Giffard RG. IL-4 Is Required for Sex Differences in Vulnerability to Focal Ischemia in Mice. Stroke. 2015;46:2271–2276. doi: 10.1161/STROKEAHA.115.008897. doi:10.1161/strokeaha.115.008897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Li M, Jiang Y. The paradox role of regulatory T cells in ischemic stroke. ScientificWorldJournal. 2013;2013:174373. doi: 10.1155/2013/174373. doi:10.1155/2013/174373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, et al. Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia. Stroke. 2008;39:2073–2078. doi: 10.1161/STROKEAHA.107.508325. doi:10.1161/strokeaha.107.508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao BQ, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nature medicine. 2006;12:441–445. doi: 10.1038/nm1387. doi:10.1038/nm1387. [DOI] [PubMed] [Google Scholar]