Abstract
Cardiac transverse tubules (t-tubules) are specific membrane organelles critical in calcium signaling and excitation-contraction coupling required for beat-to-beat heart contraction. T-tubules are highly branched and form an interconnected network that penetrates the myocyte interior to form junctions with the sarcoplasmic reticulum. T-tubules are selectively enriched with specific ion channels and proteins crucial in calcium transient development necessary in excitation-contraction coupling, thus t-tubules are a key component of cardiac myocyte function. In this review, we focus primarily on two proteins concentrated within the t-tubular network, the L-type calcium channel (LTCC) and associated membrane anchor protein, bridging integrator 1 (BIN1). Here, we provide an overview of current knowledge in t-tubule morphology, composition, microdomains, as well as the dynamics of the t-tubule network. Secondly, we highlight multiple aspects of BIN1-dependent t-tubule function, which includes forward trafficking of LTCCs to t-tubules, LTCC clustering at t-tubule surface, microdomain organization and regulation at t-tubule membrane, and the formation of a slow diffusion barrier within t-tubules. Lastly, we describe progress in characterizing how acquired human heart failure can be attributed to abnormal BIN1 transcription and associated t-tubule remodeling. Understanding BIN1-regulated cardiac t-tubule biology in human heart failure management has the dual benefit of promoting progress in both biomarker development and therapeutic target identification.
1. Introduction
Each beat-to-beat heart contraction is a result of the synchronous contraction of millions of cardiac muscle cells initiated by a single action potential. To generate a coordinated and effective contraction of the heart chambers, a rapid and efficient intracellular conversion of the electrical signal to mechanical contractile force is required at the individual myocyte level. This process is known as cardiac excitation-contraction (EC) coupling, which involves a sequence of intracellular signaling events [1]. Efficient cardiac EC coupling requires development of normal calcium transients for healthy heart activity. In contrast, a weakened calcium transient and the resultant impaired EC uncoupling are the key pathological conditions of human heart failure, an end stage cardiac syndrome affecting more than 40 millions of people worldwide.
In mammalian ventricular cardiomyocytes, the functional unit of the working myocardium, the production of a healthy calcium transient relies on a well-developed complex membrane network of transverse tubules (t-tubules). Specific to striated muscle cells, t-tubules are sarcolemmal invaginations penetrating into the intracellular space of myocytes, allowing fast propagation of action potentials into the cell interior. Cardiac t-tubules are enriched in L-type calcium channels (LTCC), which mediate the initial action potential-triggered calcium entry into myocytes. A rise in local calcium concentration subsequently activates nearby ryanodine receptors (RyR), located at the sarcoplasmic reticulum (SR), resulting in a large release of calcium from SR stores to the cytoplasm. By concentrating LTCCs and bringing them in proximity to RyRs at the SR membrane, t-tubules serve as the structural foundation for healthy calcium transient production necessary for efficient EC coupling. The essential function of t-tubules is highlighted by dynamic changes in their structure during disease progression, whereby t-tubules undergo substantial remodeling. For instance, t-tubule remodeling in heart failure not only causes impaired contractile function, but also alters local electrophysiological properties and increases ventricular arrhythmia susceptibility.
In this review, we will first explore the current views of how t-tubules organize, function, and turnover in normal and diseased hearts, with a focus on mechanisms related to calcium signaling. Second, we will address in detail a crucial cardiac t-tubule membrane adaptor protein, bridging integrator 1 (BIN1, alternatively known as amphiphysin 2), which facilitates microtubule-dependent LTCC trafficking and clustering, as well as formation of t-tubule microdomains. Only within the past five years has BIN1 emerged as an essential multifunctional regulator of calcium signaling at cardiac t-tubules. Finally, we will discuss a role of BIN1 and t-tubule regulation in heart failure.
2. Cardiac t-tubules
Cardiac t-tubules are critical in controlling the calcium transient and EC coupling, which ultimately determines the strength of each heartbeat. As a membrane organelle, t-tubules also serve as a signaling hub by selectively compartmentalizing membrane proteins and signaling molecules. In this section, we will address the nature of t-tubule morphology and composition, followed by a discussion of t-tubule dyad microdomains and dynamic changes in cardiac t-tubule structure.
2.1. T-tubule morphology and composition
T-tubules are continuous membrane invaginations extended from sarcolemmal membrane. Originally discovered by Dr. Lindner, the primary element of cardiac t-tubules runs transverse to the long axis of myocytes [2]. However, the longitudinal element and branched tubular component can comprise up to 40% of t-tubule volume [3], thereby forming a complex tubular network. This tubular network significantly expands onto the sarcolemmal surface area and is estimated to constitute approximately 21% to 64% [3–6] of the total membrane. This type of complex network allows extracellular signals such as an action potential to propagate rapidly and simultaneously to activate all myofilaments [7–9]. Extracellular ions and circulating hormones can also reach deep inside of cardiomyocytes through t-tubules and activate receptors and transporters to initiate various cellular processes.
Present in mature ventricular cardiomyocytes across all mammalian species studied [10], cardiac t-tubules have unique characteristics distinct from skeletal muscle t-tubules that allow for precise cardiac EC coupling. In cardiomyocytes, t-tubules occur periodically at ~1.8 µm intervals along the longitudinal axis coinciding with the Z-disc (see Figure 1 for schematic illustration). Along a Z-line, t-tubule invaginations are spaced out at an interval of ~1.2 µm [11]. The diameters of cardiac t-tubules are much more heterogeneous compared to those of skeletal muscle. For example, within a single rat cardiomyocyte, t-tubule diameter can range from 20 nm to 450 nm, with an overall average of 200–300 nm [3], which is almost ten times wider than that of skeletal muscle [12, 13]. The size and density of cardiac t-tubules also vary across mammalian species and correlates to their respective heart rate. Small murine like rodents [3, 14] with higher heart rates that require fast action potential penetration into the cell interior have t-tubules that are much deeper and denser. In contrast, large mammals [15] and humans [16, 17] with heart rates less than 100 beats per minute have larger and more scarce t-tubules.
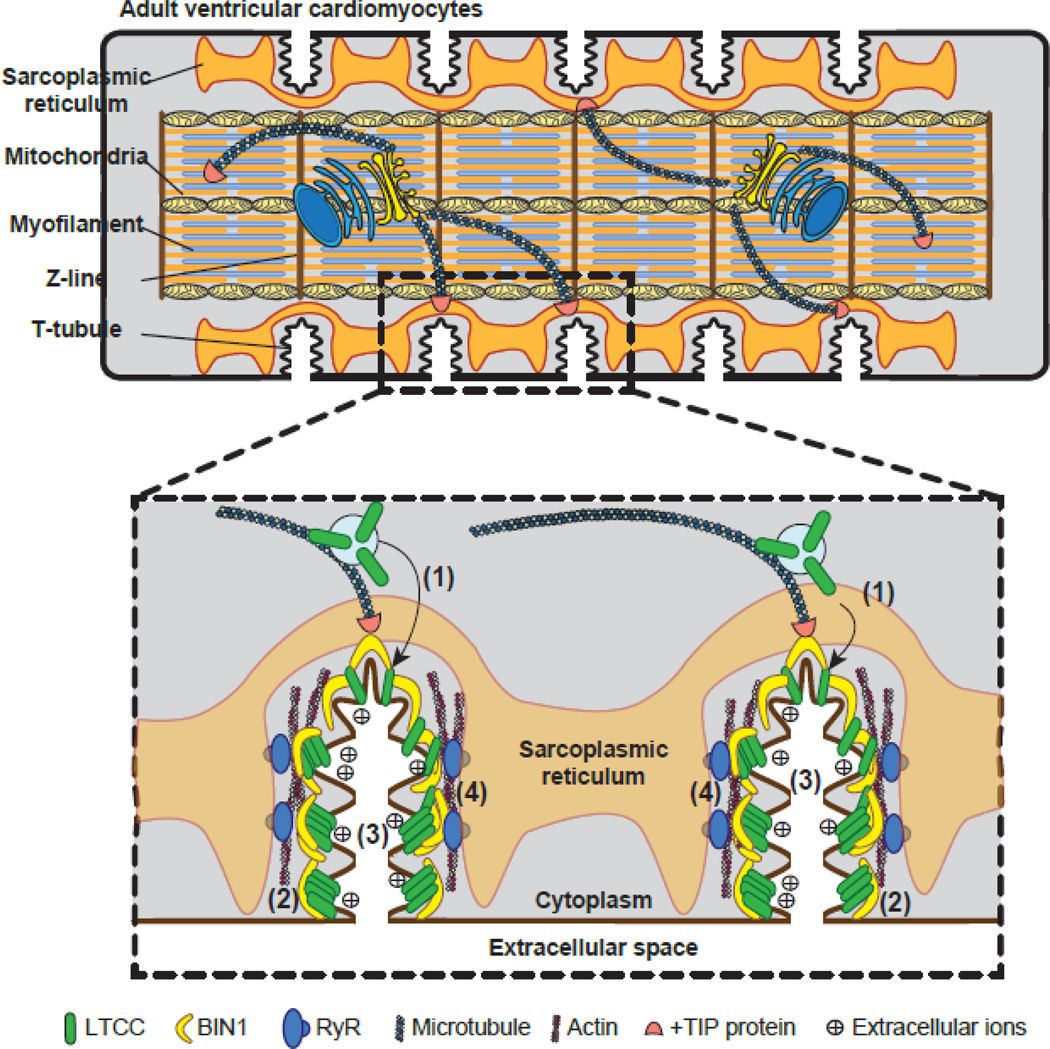
Figure 1.
Schematic illustration of BIN1-regulated cardiac t-tubule function. Top: cartoon of mammalian adult ventricular cardiomyocytes, which are rod-shaped striated muscle cells with t-tubule invagination occurring periodically at the Z-lines of myofilament. Bottom: The “banana” molecule BIN1 regulates cardiac t-tubule function through: (1) facilitating microtubule-dependent targeted delivery of LTCCs to t-tubules; (2) clustering LTCCs at the t-tubule surface; (3) creating a slow diffusion zone within t-tubule lumen for extracellular ions; (4) organizing microdomains critical for dyad formation and functions.
T-tubules are lipid bilayers embedded with specific transmembrane and lipid-associated proteins [18]. The protein components of t-tubules include: regulatory membrane scaffolding proteins, structural proteins, transmembrane ion channels, ion handling proteins, and signaling molecules. Specific membrane scaffolding proteins and cytoskeletal structural proteins are necessary for the organization and regulation of the t-tubule network and structure. Membrane scaffolding proteins caveolin-3 [19, 20], junctophilin-2 [21], and BIN1 [22–24] have been suggested to be important in the maintenance of t-tubule morphology. Moreover, caveolin-3 [25], ankyrin-B [26], and BIN1 [23] are critical in the organization and regulation of distinct subdomains at the t-tubule membrane. Ion channels and transporters, which are important in regulation of EC coupling and membrane excitability, have been well characterized in the cardiac t-tubule system (see reviews in [10, 27]). Important signaling molecules such as G-protein coupled β-adrenergic receptors (β–ARs) are also found to be present [28] or enriched [29] in t-tubules. Recent studies further suggest that the β2 subtype of the β–AR family is mainly localized within t-tubules [30], and redistribution of β2–ARs out of t-tubules is a pathophysiological phenomenon of heart failure [30, 31]. By differentially compartmentalizing proteins involved in ion handling and signaling, t-tubules serve as a signaling hub-like organelle to regulate myocyte function. The following subsections will focus on some critical subdomains within t-tubule membrane including dyads.
2.2. Dyad and L-type calcium channels
The primary function of a cardiac t-tubule is its role in initiating calcium transients for efficient EC coupling. The current accepted model of intracellular calcium transient development is calcium-induced-calcium-release (CICR) [32]. In response to action potential-triggered inward sodium current, sarcolemmal membrane depolarization activates voltage-gated LTCCs. This initial calcium influx subsequently induces a massive calcium release from SR stores. After its original proposal in early 1980s, the CICR model was supplemented by the identification of the calcium release channels on the SR membrane, the ryanodine receptors [33, 34]. In 1993, the Lederer lab further identified and described calcium sparks from the SR as the “elementary units” of the calcium transient [35]. The complexes consisting of t-tubule LTCCs together with junctional SR membrane RyRs (approximately 1:4 ratio) are known as dyads [36, 37]. Optimal CICR requires close physical association of RyRs, the calcium sensing and releasing channel, at junctional SR membrane with sarcolemma LTCCs [32–35], which is achieved by LTCC localization in t-tubules [1, 38]. Upon membrane depolarization, the initial calcium influx through LTCCs and the close association between LTCCs and RyRs (~15 nm) at dyads permit efficient CICR and subsequent sarcomeric contraction [39].
LTCCs, which initiate the calcium transient, are formed by a large pore-forming α subunit, together with auxiliary β, α2δ1, and γ subunits. The auxiliary subunits are critical in regulating the trafficking and gating properties of the pore-forming α subunit [40]. The splice variant of the LTCC α-subunit expressed in the heart is CaV1.2 [41], which is critical in cardiac development and function [42]. Global deletion of CaV1.2 induces abnormal cardiac morphogenesis with embryonic lethality [42], whereas cardiac specific deletion in adult mice causes reduced contractility and increased susceptibility to stress-induced heart failure [43]. As the t-tubule component of dyads, LTCCs need to be enriched at t-tubules for normal intracellular calcium transient development. Immunocytochemistry and electrophysiological data indicate that 80% of the sarcolemma LTCCs are concentrated to the t-tubule surface [18]. The precise t-tubule localization of LTCCs requires the membrane scaffolding protein BIN1. Previously, we have shown that BIN1 facilitates microtubule-dependent targeted delivery of CaV1.2 channels to t-tubules [44], as discussed later in Section 3.2. More recently, we demonstrated that BIN1 also creates microfolds within t-tubules [23] (details in Section 3.3), which may serve as the structural base of dyad microdomains anchoring CaV1.2 channels at t-tubules.
2.3. Other microdomains in t-tubules
In addition to dyad subdomains, the most well studied membrane microdomain is caveolae. Caveolae are flask-shaped structures enriched with cholesterol and sphingolipids formed by the cholesterol-binding scaffolding protein Caveolin-3. Biochemical fractionation and electron microscopy studies have identified a subpopulation of many ion channels at caveolae, and loss of caveolae is associated with arrhythmogenesis [25]. Caveolae exist in both t-tubule and lateral sarcolemma of ventricular cardiomyocytes [5, 45]. Within t-tubules, caveolae are often found at sub-sarcolemmal areas connecting t-tubules to extracellular space [46]. The mechanism of caveolae localization and regulation at t-tubules remains unclear, although a recent study indicates BIN1 may play a role in caveolae distribution within t-tubules [47]. Furthermore, studies show that a subset of CaV1.2 channels are localized within caveolae and are perhaps involved in regulating calcium signaling [25]. Likewise, β2–ARs are also enriched in caveolae [48]. Localization of CaV1.2 channels and β2–ARs to the small caveolae microdomain indicates that caveolae may facilitate β–AR-mediated regulation of LTCC function. The precise role of caveolae on ion channel regulation and its significance still awaits further investigation.
Moreover, a different membrane scaffolding protein from the ankyrin family, ankyrin B, has been identified as essential in clustering the macromolecular complex of the sodium calcium exchanger (NCX) / sodium potassium ATPase (NKA) / inositol trisphosphate receptor (InsP3) to t-tubule membrane subdomains that are distinct from the dyads [26]. Integrity of the NCX/NKA/InsP3 complex is critical in removing calcium from the clefts between the t-tubule surface and SR membrane, facilitating calcium decline during relaxation. Therefore, intracellular calcium equilibrium can be achieved in two ways: one, by balancing the calcium entry through the LTCCs clustered with BIN1 at the membrane, and two, by calcium removal through NCX anchored at ankyrin B-induced membrane.
2.4. T-tubules are dynamic membrane organelles
Despite structural complexity, t-tubules are extremely labile [49]. T-tubules are absent in embryonic and neonatal cardiomyocytes [50] and only start to develop after birth [50, 51]. In cultured myocytes, t-tubules dedifferentiate rapidly [52–54] but can be slowed by actin stabilization [52, 55]. Interestingly, t-tubule loss and remodeling are also associated with cardiac hypertrophy and heart failure [31, 54, 56, 57], and can improve during functional recovery of hearts [31]. However, very little is known about the biogenesis and dedifferentiation processes of t-tubules. Several membrane scaffolding proteins, like caveolin-3, junctophilin-2, and BIN1, have been implicated in t-tubule biogenesis. Follow up studies using transgenic mice with deletion of an individual gene, i.e. caveolin-3, junctophilin-2 or BIN1 reveal that the primary t-tubule invaginations still exist, indicating that a single protein alone is not responsible for tubulogenesis. Meanwhile, intracellular cytoskeleton proteins including cortical actin, microtubules, and costameres are actively regulated to control membrane integrity and dynamics at t-tubules. Future studies to elucidate the mechanisms of cardiac tubulogenesis will require a systemic approach that incorporates intracellular signaling molecules, costamere structural proteins, membrane scaffolding proteins together with cytoskeleton, and extracellular matrix as well. Understanding tubulogenesis and degeneration mechanisms will identify important steps necessary to help induced pluripotent stem cells differentiated cardiomyocytes mature into adult phenotype with developed t-tubules and efficient EC coupling machinery.
Membrane microdomains within t-tubules can also be extremely dynamic as mentioned previously. For example, we recently identified that the cardiac t-tubule membrane is extensively folded and is induced by a cardiac isoform of BIN1. These BIN1-microfolds are lost in isolated cardiomyocytes during extended in vitro culture [23]. Loss of BIN1 microfolds can be rescued by replenishment of the microfolds-forming BIN1 isoform and stabilization of filamentous actin cytoskeleton. Furthermore, BIN1 is blood available and plasma BIN1 correlates with cardiac function [58]. It is possible that plasma BIN1 originates from cardiac t-tubules as a result of BIN1-microfold turnover and release. Whether and how these t-tubules turnover membrane microdomains are the focus of future studies.
Additional t-tubule components including transmembrane ion channels and receptors are also extremely dynamic. These channels often contain multiple signaling sequences that govern the rate of their synthesis, trafficking through endoplasmic reticulum to their insertion into the plasma membrane [59–61]. These processes taken together are referred to as forward trafficking. The life span of ion channels on the plasma membrane is also limited by internalization, recycling and degradation. Therefore, the turnover of these proteins is tightly regulated by both forward trafficking and recycling, to maintain a steady-state and continuously replenished t-tubule pool of ion channels and signaling molecules for functional homeostasis. The half-lives of the calcium and potassium channels, and sodium-calcium exchangers, are considered all in the range of 2–12 hours, with the exception of the Nav1.5 (measured in ventricular cardiomyocytes) half-life of ~35 hours [62]. However, it is important to mention that the majority of these studies focusing on life cycles of ion channels were conducted in cell lines or in cultured neurons, with a few exceptions in neonatal and adult cardiomyocytes. Therefore, the results should be interpreted with caution. Nevertheless, it is fair to conclude that the bulk of ion channels in each cardiomyocyte are regenerated and positioned to the t-tubule membrane during the course of a day. This dynamic turnover is essential for normal function of cardiomyocytes.
Furthermore, it should be noted that the overall protein turnover is distinct from the functional protein turnover at the plasma membrane. For example, a discrepancy was observed with the CaV1.2 protein, where the functional half-life of the channel is approximately 3 hours, but the total protein turnover occurs at a much slower rate of 25 hours [63], indicating a fairly short life span and dynamic turnover of CaV1.2 proteins in the plasma membrane. Given that 80% of plasma membrane LTCCs is localized with t-tubules, it is likely that Cav1.2 protein turnover at t-tubule membrane is relatively faster than intracellular protein turnover. The recruitment and assembly of LTCC auxiliary subunits and regulatory proteins through BIN1-microdomains may play a crucial role in the fast turnover of Cav1.2 proteins at the t-tubule membrane. The precise measurement of channel life cycles at t-tubules requires a combination of pulse-chase experiments with biochemical fractionation and exhaustive life cell imaging. Similarly, the turnover rate of the NCX measured using metabolic labeling in lysates from neonatal cardiomyocytes yields a long half-life of 33 hours [64]. Whereas the functional half-life of NCX in the plasma membrane as indicated by channel activity is likely to be much faster at under 12 hours [65, 66], indicating regulation by channel binding partners at the local environment. The importance of protein-protein interaction on channel half-life is further supported by the fact that coexpression of Kv1.3 with their regulators in neurons significantly changes its half-life at the plasma membrane [67]. Furthermore, mutations in ion channels affecting their interaction with microdomain-organizing proteins such as caveolin-3 and ankyrin B can result in serious diseases (see review [68]), likely due to altered channel life-cycle at the plasma membrane.
The unique dynamic and flexible element of t-tubules allows these membrane organelles to serve as a critical signaling hub in cardiomyocytes under both normal and stressed physiological conditions. Better understanding of the life cycles of t-tubule membranes and membrane associated proteins will help to further elucidate the function of cardiac t-tubules.
3. Multilayer regulation of t-tubules by BIN1
Since the initial discovery of its expression at cardiac t-tubules, membrane deformation protein BIN1 has emerged as a crucial cardiac t-tubule adaptor protein. In this section, an overview of the BIN1 gene and protein will be discussed, followed by a summary of recent studies revealing the multiple functions of BIN1 in regulating cardiac t-tubule function, from the role of BIN1 in regulation of LTCC trafficking and clustering, to t-tubule ionic diffusion and microdomain formation (Figure 1). We propose that BIN1 could be a master organizer of t-tubule membrane and its associated proteins.
3.1. BIN1, a cardiac t-tubule adaptor protein
BIN1, also known as amphiphysin 2, belongs to the Bin1-Amphiphysin-Rvs (BAR) domain containing protein superfamily. BIN1 shares 49% sequence homology to its closest cousin amphiphysin 1 [69]. BIN1 was first identified through its interaction with the MYC oncoprotein [70, 71] and proposed to be a tumor suppressor [72]. The BAR domain protein superfamily consists of membrane deformation proteins involved in multiple cellular processes across different tissues and organs. This family of proteins has a wide range of cellular functions, including membrane trafficking and remodeling, cytoskeleton regulation, DNA repair, cell cycle progression and apoptosis [73]. The most well studied function of amphiphysin proteins is their involvement in endocytosis [74] and membrane trafficking.
Greater than ten BIN1 protein isoforms are encoded by a single gene with 20 exons, which undergo extensive alternative splicing in a tissue and disease specific manner. BIN1 contains the signature BAR-domain at the N-terminus encoded by exons 1–9. The crystal structure of the amphiphysin BAR domain revealed a dimeric interaction between helixes from each monomer to form a 6-helix bundle [75]. The structure of this dimer has an elongated banana shape [75]. Therefore, based on the BAR domain shape and its general function as membrane curvature protein, BIN1 has been referred to as the “banana” molecule [76–78]. The interface between the monomers is largely hydrophobic and the curvature of the dimer results from how the monomers intersect and the kinks in the helixes that heavily depend on the proline residues. These helix kinks are highly conserved [76, 79] and reside at the end of the 6-helix bundle. A recent study shows that proline isomerization represents a rate-limiting step in proper dimer formation [78]. The N-terminal amphipatheic helix is also important for lipid binding. Furthermore, the concave surface of the dimer contains several positively charged patches that may be crucial for interactions with membrane phospholipids. The BAR domain is immediately followed by a phosphoinositide (PI)-binding motif encoded by skeletal spliced exon 11 [22], the clathrin and AP2 (CLAP) binding domain encoded by four consecutive exons 13–16 spliced together in the neuronal system [80, 81], the Myc-binding domain encoded by exons 17–18 [80–85], and the constitutive Src homology3 (SH3) domain encoded by the C-terminal exons 19–20. The C-terminal SH3 domain is critical for interaction with cytoskeleton and other intracellular protein partners [86, 87]. In different tissues, the middle coiled-coil regions are regulated by alternative splicing. In 1992, Dr. De Camilli’s group showed that a PI-binding domain, encoded by skeletal specifically expressed exon 11, increases the ability of BIN1 lipid binding and t-tubule membrane curvature formation [22]. During mouse myoblast differentiation, a switch of splicing to include the PI-domain encoding exon 11 is necessary for tubulogenesis in skeletal muscle. Along with altered Bin1 splicing, subcellular localization of BIN1 changes from exclusive localization in the nucleus of myoblasts to diffuse distribution in cytoplasm of differentiated myotubes. Mis-splicing of exon 7 and 11 has been linked to myotonic dystrophy [88, 89]. Mutations in other sequences encoding the BAR and SH3 domains are also shown to induce centronuclear myopathies and myotonic dystrophy [90]. In addition to its membrane scaffolding property, BIN1 also regulates cell proliferation through inhibition of c-Myc-mediated cell transformation, and a defect in splicing of exon 13 is often associated with cancer due to displaced binding of c-MYC from BIN1 [81].
The role of BIN1 as a cardiac adaptor protein was first observed when global deletion of the Bin1 gene in mice caused perinatal lethality due to cardiomyopathy [91]. We later found that BIN1 localizes to cardiac t-tubules, facilitating microtubule-dependent forward delivery of CaV1.2 channels to t-tubules [44], as detailed in Section 3.2. Follow up studies from our group and others have shown that BIN1 level decrease affects LTCC surface expression at the t-tubules in human [92] and animal models of heart failure [24, 93]. In zebrafish, morpholino mediated knockdown of the Bin1 gene induces significant contractile dysfunction and cardiomyopathy [92]. More recently, mice with cardiac conditional Bin1 knockout after birth have increased tendency of aging and pressure overload-induced cardiomyopathy [47]. We further identified four Bin1 splice variants in adult mouse cardiomyocytes that encode four BIN1 protein isoforms [23]. In addition to the ubiquitously expressed smallest BIN1 and BIN1+17, we also cloned two cardiac spliced and expressed isoforms BIN1+13 and BIN1+13+17. Of the four BIN1 isoforms, cardiac specific BIN1+13+17 with inclusion of exons 13 and 17 is the primary BIN1 isoform localized to cardiac t-tubules and responsible for cardiac t-tubule membrane ultrastructure formation [23]. Whether aberrant splicing and mutation in cardiac spliced exons plays a role in cardiomyopathy development remains to be observed.
3.2. BIN1 regulates LTCC trafficking and localization at t-tubules
As discussed earlier, enrichment of CaV1.2 channels at the t-tubules is essential for the efficient contractile function of the myocardium. Interestingly, we identified that BIN1 is crucial in CaV1.2 enrichment at t-tubules. Exploring the trafficking machinery of CaV1.2 channels, we found that trafficking of CaV1.2 vesicles from the trans-Golgi network to t-tubules follows microtubule-dependent Targeted Delivery [44], a model of channel delivery initially reported for connexin 43 trafficking to intercalated discs in a series of studies [44, 92, 94, 95]. In Targeted Delivery, once the channels are formed and exit the Golgi they can be rapidly directed across the cytoplasm to their respective specific membrane subdomains. The highways for transport are microtubules whose positive ends are growing outward and can be captured at the plasma membrane by membrane anchor protein complexes. Specificity of delivery requires a combination of the individual channel and the plus-end-tracking proteins at the positive ends of microtubules, which guide microtubule growth and capture. The membrane-bound anchor complex captures the microtubule and completes the highway for channel delivery.
In the case of CaV1.2 channels, BIN1 serves as the critical membrane anchor protein to attract growing microtubules [44], which preferentially interact with the specific membrane anchor BIN1 in order to facilitate targeted delivery of CaV1.2 channels. BIN1 contains a membrane curvature BAR-domain (which confers the ability to form membrane curvature), the middle coiled-coil regions, and an SH3 protein-protein interaction domain. Perhaps most compelling for BIN1 utilizing the cytoskeleton is the finding that deletion of the coiled-coil and SH3 domains does not affect membrane invagination, but abrogates CaV1.2 localization to these structures. Therefore, target delivery of CaV1.2 is achieved through interaction with the BIN1 protein specifically, not t-tubule structures. The involvement of BIN1 in CaV1.2 trafficking to t-tubules is further confirmed in the subsequent studies using shRNA mediated knockdown of Bin1 in isolated adult mouse cardiomyocytes [92], as well as adult mouse cardiomyocytes isolated from mice with cardiac specific deletion of the Bin1 allele [23], where CaV1.2 surface expression dramatically reduced [23].
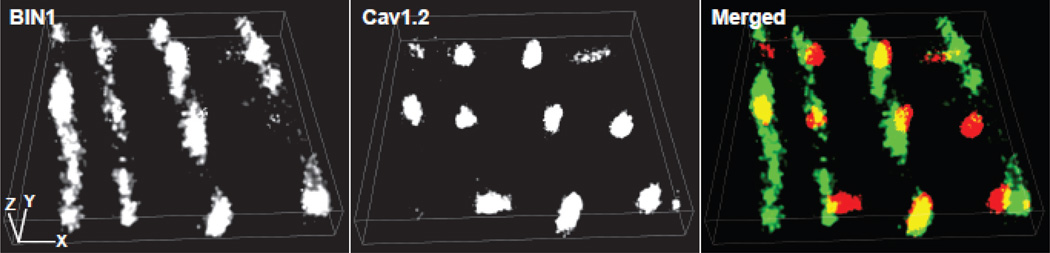
Clustering of CaV1.2 channels at the t-tubule membrane is also critical for the regulation of ion channel kinetic properties, for example gating and open probability [96, 97]. Given that microtubules deliver CaV1.2 channels to BIN1 proteins at t-tubules and that BIN1 proteins shape the t-tubule membrane to form microfolds, it is possible that CaV1.2 channels at the t-tubule surface form clusters at these BIN1-microfolds induced microdomains. Using super-resolution stochastic optical reconstruction microscopy (STORM) imaging, we are able to image CaV1.2 and BIN1 proteins at the nanoscale resolution (XY-resolution at 10–20 nm, Z-resolution at 50 nm). As indicated in the cardiomyocyte STORM image presented in Figure 2, BIN1 (green) is distributed along t-tubules forming microfolds holding CaV1.2 clusters (red). We have also observed smaller CaV1.2 clusters in Bin1 heterozygous adult mouse ventricular cardiomyocytes using both spinning disc confocal [23] and super-resolution STORM imaging. These results reveal that CaV1.2 channels cluster to BIN1-microdomains within t-tubules. How CaV1.2 clustering to BIN1-microdomains regulates ion channel properties awaits future studies from the electrophysiology laboratories interested in LTCC channel clustering and coupled-gating.
Figure 2.
Super-resolution STORM images of BIN1 and CaV1.2 at the surface of adult mouse ventricular cardiomyocytes. Merged image indicates that CaV1.2 channels (red) form clusters at the microdomains formed by BIN1 (green). Scale bar: 1 µm.
3.3. BIN1 regulates t-tubule ion diffusion
One of the functional attributes of t-tubules is the presence of slow diffusion regions near the t-tubule sarcolemma [98–100]. Restricted diffusion occurs both at extracellular and intracellular spaces near the t-tubule membrane, readily known as “fuzzy space” with accumulated ions like calcium [101]. Growing evidence indicates slow diffusion for extracellular ions within the t-tubule lumen, with t-tubule ion diffusion coefficients normally five to ten times slower than those in the extracellular bulk environment [99, 100]. As a result, there is a lag phase for fluctuations in extracellular ion concentration sensed by channels at the t-tubule membrane. For example, in guinea pig myocytes, the change of extracellular calcium is delayed by up to 2.3 seconds in the t-tubules [102]. This delay period due to restricted diffusion within the t-tubule lumen can have a significant effect on the susceptibility of the muscle cells to changes in the extracellular environment, particularly during a stress response. In stress-induced increased heart rate, quick transmembrane ion flux with limited diffusion can quickly accumulate outward current ions like potassium [100] and deplete inward current ions like calcium [98, 103], affecting the driving force of ion channels at t-tubule membrane and shortening the action potential duration [104]. Slow diffusion at t-tubules therefore serves as a crucial protective mechanism to maintain the electrical stability of cardiomyocytes preventing detrimental arrhythmias.
Although t-tubules are highly branched and inter-connected [3], this network feature does not explain the highly restricted diffusion near the t-tubule sarcolemma. As early as 1970, the cardiac t-tubule configuration was described not as a straight smooth tubule, but rather making torturous frequent hairpin bends [9]. The structural foundation of tortuous t-tubules was not revealed until our recent discovery of the t-tubule membrane microfolds created by BIN1. In wild type adult mouse ventricular cardiomyocytes, we identified that BIN1-microfolds create highly tortuous t-tubules [23]. When the Bin1 gene is deleted in cardiomyocytes, these microfolds are lost and tortuous t-tubules become straight and dilated. We recently cloned a cardiac specific BIN1 isoform, BIN1+13+17, which is the largest cardiac isoform and includes both exons 13 and 17. This specific isoform localizes to cardiac t-tubules and is responsible for formation of membrane microfolds at t-tubules. By promoting actin polymerization through N-WASP activation, BIN1+13+17 anchors and maintains these t-tubule microfolds to myofilament Z-disc structural protein α-actinin. Using transmission electron microscopy imaging, Dr. Franzini-Armstrong’s laboratory also observed t-tubule membrane infolding to lumen at t-tubule cross sections from mouse hearts [105], similar to the t-tubule microfolds reported in our study. These microfolds help trap extracellular ions, which create a diffusion barrier inside the t-tubule network. Loss of cardiac BIN1-organized microfolds removes the protective ion diffusion barrier and increases membrane excitability and arrhythmias [23]. Therefore, BIN1 plays a pivotal role in maintaining and organizing t-tubule microfolds and ultrastructure that are essential for electrical stability of the cardiomyocytes. Recent studies in zebrafish skeletal muscle indicate that skeletal BIN1 may also have a similar function in maintaining t-tubule ultrastructure [106].
3.4. BIN1 regulates t-tubule membrane microdomains
Other than creating a protective ionic diffusion barrier, BIN1-microfolds hold LTCC clusters (Figure 2). These data indicate that BIN1 not only traffics CaV1.2 channels, but also forms membrane ultrastructure to support these channels. It is possible that BIN1-microfolds can be the structural base of dyad microdomains at t-tubules. The calcium channel-enriched BIN1-microfolds are very likely the t-tubule membrane components contacting junctional SR membrane for dyad formation. As a result, BIN1 may also control the synchrony of CICR through microdomain formation. Whether and how BIN1 regulates the dyad organization and function needs to be investigated. On the other hand, BIN1 is blood available and plasma BIN1 correlates with cardiac function [58], indicating cardiac release of BIN1 protein. The blood availability of BIN1 with an internal cardiac message suggests a likely turnover and release of BIN1-microfolds from cardiomyocytes. Understanding the dynamics of BIN1-microfolds and folds-organized microdomains at t-tubules may reveal important new aspects of cardiomyocyte biology.
In addition to BIN1-microfolds, BIN1 may be involved in the organization and regulation of other membrane subdomains at t-tubules as well. A recent study identified that intracellular localization of caveolae structure protein caveolin-3 is altered in cardiomyocytes missing the Bin1 allele [47], indicating a role of BIN1 in regulation of caveolae microdomains. Given that BIN1 is a membrane curvature formation protein, it is likely that BIN1 can interact with caveolin-3 and redistribute caveolae at sarcolemma surface. Interestingly, a subpopulation of CaV1.2 channels is delivered to caveolae through interaction between subunits of the LTCC channel complex and caveolin-3 [107]. Therefore, by altering caveolin-3 localization, BIN1 may also alter CaV1.2 trafficking to caveolae. The mechanisms affecting ion channel enrichment at caveolae are unknown, but close interactions between caveolae and the cytoskeleton present an appealing possibility of targeted LTCC delivery to these sarcolemmal microdomains [108], a process that may be facilitated by BIN1. This may have broader implications as many CaV1.2 channel regulating molecules such as β2-ARs are primarily localized in caveolae [48]. Therefore, as versatile regulator of t-tubules, BIN1 may affect cell signaling both at the local level and ubiquitously. Nonetheless, the role and potential mechanism of BIN1 in regulation of caveolae localization and function remain to be tested.
4. Implications in human diseases
Heart failure is the fastest growing cardiovascular disorder affecting more than six million Americans and 40 million patients worldwide [109–111]. The primary mortality causes of heart failure are loss of pump function due to impaired cardiac contractility and increased sudden cardiac death due to severe ventricular arrhythmias. The current best therapy for end-stage heart failure is a heart transplant, which is a severely limited option due to poor organ availability [112]. The only alternative destination therapy is implantation of left ventricular assist devices (LVAD) [113, 114]. For ventricular arrhythmias management, implantable cardioverter defibrillators (ICDs) can defibrillate life threatening arrhythmias in heart failure patients [115]. However, the cost of LVAD and ICD implantation, occurrence of needless implantation, and morbidity associated with implanted devices are enormous. Lack of an accurate understanding of cardiomyocyte health and recovery potential limits the ability in making critical decisions with regard to heart transplant priorities and criteria for LVAD and ICD implantation [116, 117]. New diagnostic and prognostic tests that accurately evaluate myocardial health and arrhythmogenic potential at the cellular level could significantly improve decision making for LVAD and ICD implantation with improvement in patient care. New therapeutic tools with the ability to break pathophysiological cycling of heart failure progression are deeply desired to help postpone and rescue disease development in millions of patients with heart failure.
Better understanding of the biology of failing cardiomyocytes is a necessity for efficient development of new diagnostic, prognostic, and therapeutic tools of human acquired heart failure. The pathophysiologic hallmark of failing cardiomyocytes is impaired calcium transients [118–120]. A normal transient requires optimal CICR at dyads where t-tubule LTCCs [1, 38] are closely associated with SR RyRs [32–35]. Disturbed calcium transients in failing myocytes are also attributed to t-tubule remodeling [31, 56, 57] which uncouples LTCC-RyR dyads [57, 118, 119, 121–123]. In the past decade, t-tubule remodeling has gained substantial appreciation as marking the transition from hypertrophy to failure [56], and as a contributing mechanism of heart failure progression (see reviews in [10, 27, 57, 124–126]). In addition to the observed loss and diminishment of a gross t-tubule network, protein components at t-tubules including ion channels and signaling molecules are also reported to be either reduced at t-tubule surface or redistributed out to non-t-tubule sarcolemma. Loss of complex t-tubules in heart failure not only causes impaired contractile function due to desynchronized CICR, but also alters local electrophysiological properties and increases ventricular arrhythmia susceptibility. The molecular and cellular mechanisms of t-tubule remodeling in failing cardiomyocytes, in particular the alterations in t-tubule membrane ultrastructure and subdomains, await further investigation.
BIN1 has emerged as a critical regulator of t-tubule function and calcium signaling in cardiomyocytes. In healthy cardiomyocytes, BIN1 facilitates microtubule-dependent LTCC trafficking to t-tubules [44], shapes actin dependent t-tubule membrane microdomains [23] to regulate ionic flux, and facilitates CaV1.2 channel cluster formation (Figure 2). More importantly, BIN1 is transcriptionally decreased in acquired human heart failure [92], contributing to the pathophysiology of diseased calcium transients and electrical instability in failing hearts. The emergence of BIN1-microdomain based regulation of calcium transients has brought a new perspective to traditional dyad research, and follow up studies by independent research groups have further identified that cardiac BIN1 decreases in multiple animal models of acquired heart failure [24, 93]. BIN1 expression also improves during functional myocardium recovery [93] in a rat model of cardiomyopathy. The detailed mechanisms of how BIN1-microdomains regulate dyad function and how BIN1 is reduced in human acquired heart failure remain to be tested. Preservation of BIN1-microdomains may be a new method to block the worsening cycle of heart failure progression, postponing or rescuing pump function in millions of patients with acquired heart failure.
The benefit of BIN1 in heart failure management is two-fold. One, it may serve as a target for therapeutic development. Second, BIN1 is a potential biomarker of myocardial health and reserve. BIN1 is blood available and plasma BIN1 carries a message of cardiac health [58]. The first report in a cohort of arrhythmogenic right ventricular cardiomyopathy patients indicates that plasma BIN1 decreases in patients developing symptomatic heart failure [58]. Low plasma level of BIN1 not only diagnoses cardiac function, but also predicts future arrhythmia incidences [58]. The recent discovery of the functional significant cardiac specific BIN1 isoform, BIN1+13+17 [23], provides an opportunity to develop a cardiac specific BIN1 blood test. Current studies are focused on identifying the mechanisms of BIN1 release into blood, as well as developing a blood assay of cardiac specific BIN1 to be tested for biomarker potential in a large cohort of dilated cardiomyopathy patients.
5. Concluding remarks
In this review we highlight the current knowledge of the organization and function of the cardiac t-tubule system, which is important in maintaining cardiac EC coupling, electrical stability, and membrane excitability. Membrane deformation protein BIN1 has recently surfaced as a crucial regulator of cardiac t-tubule function. This review provides insights into the recent discoveries of BIN1 as a t-tubule adaptor protein, with functions ranging from calcium channel trafficking to shaping membrane microdomains. Future explorations of BIN1-based cardiac t-tubule remodeling mechanisms during heart failure progression will provide new insights and targets to detect and correct contractile dysfunction and arrhythmia potentials in diseases.
Highlights.
T-tubules are dynamic membrane organelles central to excitation-contraction coupling
BIN1 (amphiphysin II) facilitates LTCC forward trafficking and clustering at t-tubules
BIN1-sculpted t-tubule microfolds create extracellular ionic diffusion barrier important for membrane electrical stability
BIN1-microdomains within T-tubules are critical for normal dyad formation and function
BIN1 reduction in heart failure contributes to dyad dysfunction and impaired excitation-contraction coupling
Acknowledgments
We thank our funding sources American Heart Association (Fu, 12SDG12080084) and National Institute of Health / National Heart, Lung, and Blood Institute (NIH/NHLBI, Hong, R00-HL109075).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 2.Lindner E. Submicroscopic morphology of the cardiac muscle. Zeitschrift fur Zellforschung und mikroskopische Anatomie. 1957;45:702–746. [PubMed] [Google Scholar]
- 3.Soeller C, Cannell MB. Examination of the transverse tubular system in living cardiac rat myocytes by 2-photon microscopy and digital image-processing techniques. Circulation research. 1999;84:266–275. doi: 10.1161/01.res.84.3.266. [DOI] [PubMed] [Google Scholar]
- 4.Page E, McCallister LP, Power B. Sterological measurements of cardiac ultrastructures implicated in excitation-contraction coupling. Proceedings of the National Academy of Sciences of the United States of America. 1971;68:1465–1466. doi: 10.1073/pnas.68.7.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Page E. Quantitative ultrastructural analysis in cardiac membrane physiology. The American journal of physiology. 1978;235:C147–C158. doi: 10.1152/ajpcell.1978.235.5.C147. [DOI] [PubMed] [Google Scholar]
- 6.Page E, Surdyk-Droske M. Distribution, surface density, and membrane area of diadic junctional contacts between plasma membrane and terminal cisterns in mammalian ventricle. Circulation research. 1979;45:260–267. doi: 10.1161/01.res.45.2.260. [DOI] [PubMed] [Google Scholar]
- 7.Bastian J, Nakajima S. Action potential in the transverse tubules and its role in the activation of skeletal muscle. The Journal of general physiology. 1974;63:257–278. doi: 10.1085/jgp.63.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baylor SM, Oetliker H. Birefringence experiments on isolated skeletal muscle fibres suggest a possible signal from the sarcoplasmic reticulum. Nature. 1975;253:97–101. doi: 10.1038/253097a0. [DOI] [PubMed] [Google Scholar]
- 9.Forssmann WG, Girardier L. A study of the T system in rat heart. The Journal of cell biology. 1970;44:1–19. doi: 10.1083/jcb.44.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brette F, Orchard C. T-tubule function in mammalian cardiac myocytes. Circulation research. 2003;92:1182–1192. doi: 10.1161/01.RES.0000074908.17214.FD. [DOI] [PubMed] [Google Scholar]
- 11.Kieval RS, Bloch RJ, Lindenmayer GE, Ambesi A, Lederer WJ. Immunofluorescence localization of the Na-Ca exchanger in heart cells. The American journal of physiology. 1992;263:C545–C550. doi: 10.1152/ajpcell.1992.263.2.C545. [DOI] [PubMed] [Google Scholar]
- 12.Franzini-Armstrong C, Landmesser L, Pilar G. Size and shape of transverse tubule openings in frog twitch muscle fibers. The Journal of cell biology. 1975;64:493–497. doi: 10.1083/jcb.64.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandow A. Excitation-contraction coupling in skeletal muscle. Pharmacological reviews. 1965;17:265–320. [PubMed] [Google Scholar]
- 14.Wagner E, Lauterbach MA, Kohl T, Westphal V, Williams GS, Steinbrecher JH, Streich JH, Korff B, Tuan HT, Hagen B, Luther S, Hasenfuss G, Parlitz U, Jafri MS, Hell SW, Lederer WJ, Lehnart SE. Stimulated emission depletion live-cell super-resolution imaging shows proliferative remodeling of T-tubule membrane structures after myocardial infarction. Circulation research. 2012;111:402–414. doi: 10.1161/CIRCRESAHA.112.274530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savio-Galimberti E, Frank J, Inoue M, Goldhaber JI, Cannell MB, Bridge JH, Sachse FB. Novel features of the rabbit transverse tubular system revealed by quantitative analysis of three-dimensional reconstructions from confocal images. Biophysical journal. 2008;95:2053–2062. doi: 10.1529/biophysj.108.130617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cannell MB, Crossman DJ, Soeller C. Effect of changes in action potential spike configuration, junctional sarcoplasmic reticulum micro-architecture and altered t-tubule structure in human heart failure. Journal of muscle research and cell motility. 2006;27:297–306. doi: 10.1007/s10974-006-9089-y. [DOI] [PubMed] [Google Scholar]
- 17.Jayasinghe I, Crossman D, Soeller C, Cannell M. Comparison of the organization of T-tubules, sarcoplasmic reticulum and ryanodine receptors in rat and human ventricular myocardium. Clinical and experimental pharmacology & physiology. 2012;39:469–476. doi: 10.1111/j.1440-1681.2011.05578.x. [DOI] [PubMed] [Google Scholar]
- 18.Pasek M, Brette F, Nelson A, Pearce C, Qaiser A, Christe G, Orchard CH. Quantification of t-tubule area and protein distribution in rat cardiac ventricular myocytes. Progress in biophysics and molecular biology. 2008;96:244–257. doi: 10.1016/j.pbiomolbio.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Galbiati F, Engelman JA, Volonte D, Zhang XL, Minetti C, Li M, Hou H, Jr, Kneitz B, Edelmann W, Lisanti MP. Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and t-tubule abnormalities. The Journal of biological chemistry. 2001;276:21425–21433. doi: 10.1074/jbc.M100828200. [DOI] [PubMed] [Google Scholar]
- 20.Al-Qusairi L, Laporte J. T-tubule biogenesis and triad formation in skeletal muscle and implication in human diseases. Skeletal muscle. 2011;1:26. doi: 10.1186/2044-5040-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beavers DL, Landstrom AP, Chiang DY, Wehrens XH. Emerging roles of junctophilin-2 in the heart and implications for cardiac diseases. Cardiovascular research. 2014;103:198–205. doi: 10.1093/cvr/cvu151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee E, Marcucci M, Daniell L, Pypaert M, Weisz OA, Ochoa GC, Farsad K, Wenk MR, De Camilli P. Amphiphysin 2 (Bin1) and T-tubule biogenesis in muscle. Science. 2002;297:1193–1196. doi: 10.1126/science.1071362. [DOI] [PubMed] [Google Scholar]
- 23.Hong T, Yang H, Zhang SS, Cho HC, Kalashnikova M, Sun B, Zhang H, Bhargava A, Grabe M, Olgin J, Gorelik J, Marban E, Jan LY, Shaw RM. Cardiac BIN1 folds T-tubule membrane, controlling ion flux and limiting arrhythmia. Nature medicine. 2014 doi: 10.1038/nm.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caldwell JL, Smith CE, Taylor RF, Kitmitto A, Eisner DA, Dibb KM, Trafford AW. Dependence of cardiac transverse tubules on the BAR domain protein amphiphysin II (BIN-1) Circulation research. 2014;115:986–996. doi: 10.1161/CIRCRESAHA.116.303448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balijepalli RC, Kamp TJ. Caveolae, ion channels and cardiac arrhythmias. Progress in biophysics and molecular biology. 2008;98:149–160. doi: 10.1016/j.pbiomolbio.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohler PJ, Davis JQ, Bennett V. Ankyrin-B coordinates the Na/K ATPase, Na/Ca exchanger, and InsP3 receptor in a cardiac T-tubule/SR microdomain. PLoS biology. 2005;3:e423. doi: 10.1371/journal.pbio.0030423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brette F, Orchard C. Resurgence of cardiac t-tubule research. Physiology. 2007;22:167–173. doi: 10.1152/physiol.00005.2007. [DOI] [PubMed] [Google Scholar]
- 28.Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 2002;295:1711–1715. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
- 29.Balijepalli RC, Lokuta AJ, Maertz NA, Buck JM, Haworth RA, Valdivia HH, Kamp TJ. Depletion of T-tubules and specific subcellular changes in sarcolemmal proteins in tachycardia-induced heart failure. Cardiovascular research. 2003;59:67–77. doi: 10.1016/s0008-6363(03)00325-0. [DOI] [PubMed] [Google Scholar]
- 30.Nikolaev VO, Moshkov A, Lyon AR, Miragoli M, Novak P, Paur H, Lohse MJ, Korchev YE, Harding SE, Gorelik J. Beta2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science. 2010;327:1653–1657. doi: 10.1126/science.1185988. [DOI] [PubMed] [Google Scholar]
- 31.Lyon AR, MacLeod KT, Zhang Y, Garcia E, Kanda GK, Lab MJ, Korchev YE, Harding SE, Gorelik J. Loss of T-tubules and other changes to surface topography in ventricular myocytes from failing human and rat heart. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:6854–6859. doi: 10.1073/pnas.0809777106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. The American journal of physiology. 1983;245:C1–C14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- 33.Pessah IN, Waterhouse AL, Casida JE. The calcium-ryanodine receptor complex of skeletal and cardiac muscle. Biochemical and biophysical research communications. 1985;128:449–456. doi: 10.1016/0006-291x(85)91699-7. [DOI] [PubMed] [Google Scholar]
- 34.Inui M, Saito A, Fleischer S. Isolation of the ryanodine receptor from cardiac sarcoplasmic reticulum and identity with the feet structures. The Journal of biological chemistry. 1987;262:15637–15642. [PubMed] [Google Scholar]
- 35.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 36.Bers DM, Stiffel VM. Ratio of ryanodine to dihydropyridine receptors in cardiac and skeletal muscle and implications for E-C coupling. The American journal of physiology. 1993;264:C1587–C1593. doi: 10.1152/ajpcell.1993.264.6.C1587. [DOI] [PubMed] [Google Scholar]
- 37.Cannell MB, Cheng H, Lederer WJ. Spatial non-uniformities in [Ca2+]i during excitation-contraction coupling in cardiac myocytes. Biophysical journal. 1994;67:1942–1956. doi: 10.1016/S0006-3495(94)80677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scriven DR, Dan P, Moore ED. Distribution of proteins implicated in excitation-contraction coupling in rat ventricular myocytes. Biophysical journal. 2000;79:2682–2691. doi: 10.1016/S0006-3495(00)76506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orchard C, Brette F. t-Tubules and sarcoplasmic reticulum function in cardiac ventricular myocytes. Cardiovascular research. 2008;77:237–244. doi: 10.1093/cvr/cvm002. [DOI] [PubMed] [Google Scholar]
- 40.Dai S, Hall DD, Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiological reviews. 2009;89:411–452. doi: 10.1152/physrev.00029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takimoto K, Li D, Nerbonne JM, Levitan ES. Distribution, splicing and glucocorticoid-induced expression of cardiac alpha 1C and alpha 1D voltage-gated Ca2+ channel mRNAs. Journal of molecular and cellular cardiology. 1997;29:3035–3042. doi: 10.1006/jmcc.1997.0532. [DOI] [PubMed] [Google Scholar]
- 42.Seisenberger C, Specht V, Welling A, Platzer J, Pfeifer A, Kuhbandner S, Striessnig J, Klugbauer N, Feil R, Hofmann F. Functional embryonic cardiomyocytes after disruption of the L-type alpha1C (Cav1.2) calcium channel gene in the mouse. The Journal of biological chemistry. 2000;275:39193–39199. doi: 10.1074/jbc.M006467200. [DOI] [PubMed] [Google Scholar]
- 43.Goonasekera SA, Hammer K, Auger-Messier M, Bodi I, Chen X, Zhang H, Reiken S, Elrod JW, Correll RN, York AJ, Sargent MA, Hofmann F, Moosmang S, Marks AR, Houser SR, Bers DM, Molkentin JD. Decreased cardiac L-type Ca(2)(+) channel activity induces hypertrophy and heart failure in mice. The Journal of clinical investigation. 2012;122:280–290. doi: 10.1172/JCI58227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong TT, Smyth JW, Gao D, Chu KY, Vogan JM, Fong TS, Jensen BC, Colecraft HM, Shaw RM. BIN1 localizes the L-type calcium channel to cardiac T-tubules. PLoS biology. 2010;8:e1000312. doi: 10.1371/journal.pbio.1000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levin KR, Page E. Quantitative studies on plasmalemmal folds and caveolae of rabbit ventricular myocardial cells. Circulation research. 1980;46:244–255. doi: 10.1161/01.res.46.2.244. [DOI] [PubMed] [Google Scholar]
- 46.Zampighi G, Vergara J, Ramon F. On the connection between the transverse tubules and the plasma membrane in frog semitendinosus skeletal muscle. Are caveolae the mouths of the transverse tubule system? The Journal of cell biology. 1975;64:734–740. doi: 10.1083/jcb.64.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laury-Kleintop LD, Mulgrew JR, Heletz I, Nedelcoviciu RA, Chang MY, Harris DM, Koch WJ, Schneider MD, Muller AJ, Prendergast GC. Cardiac-Specific Disruption of Bin1 in Mice Enables a Model of Stress- and Age-Associated Dilated Cardiomyopathy. Journal of cellular biochemistry. 2015 doi: 10.1002/jcb.25198. [DOI] [PubMed] [Google Scholar]
- 48.Wright PT, Nikolaev VO, O'Hara T, Diakonov I, Bhargava A, Tokar S, Schobesberger S, Shevchuk AI, Sikkel MB, Wilkinson R, Trayanova NA, Lyon AR, Harding SE, Gorelik J. Caveolin-3 regulates compartmentation of cardiomyocyte beta2-adrenergic receptor-mediated cAMP signaling. Journal of molecular and cellular cardiology. 2014;67:38–48. doi: 10.1016/j.yjmcc.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kohl T, Lehnart SE. Imaging T-tubules: dynamic membrane structures for deep functions. Cardiovascular research. 2013;98:162–164. doi: 10.1093/cvr/cvt058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seki S, Nagashima M, Yamada Y, Tsutsuura M, Kobayashi T, Namiki A, Tohse N. Fetal and postnatal development of Ca2+ transients and Ca2+ sparks in rat cardiomyocytes. Cardiovascular research. 2003;58:535–548. doi: 10.1016/s0008-6363(03)00255-4. [DOI] [PubMed] [Google Scholar]
- 51.Haddock PS, Coetzee WA, Cho E, Porter L, Katoh H, Bers DM, Jafri MS, Artman M. Subcellular [Ca2+]i gradients during excitation-contraction coupling in newborn rabbit ventricular myocytes. Circulation research. 1999;85:415–427. doi: 10.1161/01.res.85.5.415. [DOI] [PubMed] [Google Scholar]
- 52.Leach RN, Desai JC, Orchard CH. Effect of cytoskeleton disruptors on L-type Ca channel distribution in rat ventricular myocytes. Cell calcium. 2005;38:515–526. doi: 10.1016/j.ceca.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 53.Mitcheson JS, Hancox JC, Levi AJ. Action potentials, ion channel currents and transverse tubule density in adult rabbit ventricular myocytes maintained for 6 days in cell culture. Pflugers Archiv : European journal of physiology. 1996;431:814–827. doi: 10.1007/s004240050073. [DOI] [PubMed] [Google Scholar]
- 54.Louch WE, Bito V, Heinzel FR, Macianskiene R, Vanhaecke J, Flameng W, Mubagwa K, Sipido KR. Reduced synchrony of Ca2+ release with loss of T-tubules-a comparison to Ca2+ release in human failing cardiomyocytes. Cardiovascular research. 2004;62:63–73. doi: 10.1016/j.cardiores.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 55.Tian Q, Pahlavan S, Oleinikow K, Jung J, Ruppenthal S, Scholz A, Schumann C, Kraegeloh A, Oberhofer M, Lipp P, Kaestner L. Functional and morphological preservation of adult ventricular myocytes in culture by sub-micromolar cytochalasin D supplement. Journal of molecular and cellular cardiology. 2012;52:113–124. doi: 10.1016/j.yjmcc.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 56.Wei S, Guo A, Chen B, Kutschke W, Xie YP, Zimmerman K, Weiss RM, Anderson ME, Cheng H, Song LS. T-tubule remodeling during transition from hypertrophy to heart failure. Circulation research. 2010;107:520–531. doi: 10.1161/CIRCRESAHA.109.212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Louch WE, Sejersted OM, Swift F. There goes the neighborhood: pathological alterations in T-tubule morphology and consequences for cardiomyocyte Ca2+ handling. Journal of biomedicine & biotechnology. 2010;2010:503906. doi: 10.1155/2010/503906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hong TT, Cogswell R, James CA, Kang G, Pullinger CR, Malloy MJ, Kane JP, Wojciak J, Calkins H, Scheinman MM, Tseng ZH, Ganz P, De Marco T, Judge DP, Shaw RM. Plasma BIN1 correlates with heart failure and predicts arrhythmia in patients with arrhythmogenic right ventricular cardiomyopathy. Heart rhythm : the official journal of the Heart Rhythm Society. 2012;9:961–967. doi: 10.1016/j.hrthm.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu J, Watanabe I, Gomez B, Thornhill WB. Heteromeric Kv1 potassium channel expression: amino acid determinants involved in processing and trafficking to the cell surface. The Journal of biological chemistry. 2003;278:25558–25567. doi: 10.1074/jbc.M207984200. [DOI] [PubMed] [Google Scholar]
- 60.Sun XX, Hodge JJ, Zhou Y, Nguyen M, Griffith LC. The eag potassium channel binds and locally activates calcium/calmodulin-dependent protein kinase II. The Journal of biological chemistry. 2004;279:10206–10214. doi: 10.1074/jbc.M310728200. [DOI] [PubMed] [Google Scholar]
- 61.Misonou H, Trimmer JS. Determinants of voltage-gated potassium channel surface expression and localization in Mammalian neurons. Critical reviews in biochemistry and molecular biology. 2004;39:125–145. doi: 10.1080/10409230490475417. [DOI] [PubMed] [Google Scholar]
- 62.Maltsev VA, Kyle JW, Mishra S, Undrovinas A. Molecular identity of the late sodium current in adult dog cardiomyocytes identified by Nav1.5 antisense inhibition. American journal of physiology. Heart and circulatory physiology. 2008;295:H667–H676. doi: 10.1152/ajpheart.00111.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Catalucci D, Zhang DH, DeSantiago J, Aimond F, Barbara G, Chemin J, Bonci D, Picht E, Rusconi F, Dalton ND, Peterson KL, Richard S, Bers DM, Brown JH, Condorelli G. Akt regulates L-type Ca2+ channel activity by modulating Cavalpha1 protein stability. The Journal of cell biology. 2009;184:923–933. doi: 10.1083/jcb.200805063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Slodzinski MK, Blaustein MP. Na+/Ca2+ exchange in neonatal rat heart cells: antisense inhibition and protein half-life. The American journal of physiology. 1998;275:C459–C467. doi: 10.1152/ajpcell.1998.275.2.C459. [DOI] [PubMed] [Google Scholar]
- 65.Egger M, Porzig H, Niggli E, Schwaller B. Rapid turnover of the "functional" Na(+)-Ca2+ exchanger in cardiac myocytes revealed by an antisense oligodeoxynucleotide approach. Cell calcium. 2005;37:233–243. doi: 10.1016/j.ceca.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 66.Lipp P, Schwaller B, Niggli E. Specific inhibition of Na-Ca exchange function by antisense oligodeoxynucleotides. FEBS letters. 1995;364:198–202. doi: 10.1016/0014-5793(95)00391-l. [DOI] [PubMed] [Google Scholar]
- 67.Colley BS, Biju KC, Visegrady A, Campbell S, Fadool DA. Neurotrophin B receptor kinase increases Kv subfamily member 1.3 (Kv1.3) ion channel half-life and surface expression. Neuroscience. 2007;144:531–546. doi: 10.1016/j.neuroscience.2006.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Curran J, Mohler PJ. Alternative paradigms for ion channelopathies: disorders of ion channel membrane trafficking and posttranslational modification. Annual review of physiology. 2015;77:505–524. doi: 10.1146/annurev-physiol-021014-071838. [DOI] [PubMed] [Google Scholar]
- 69.Frost A, Unger VM, De Camilli P. The BAR domain superfamily: membrane-molding macromolecules. Cell. 2009;137:191–196. doi: 10.1016/j.cell.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cole MD. The myc oncogene: its role in transformation and differentiation. Annual review of genetics. 1986;20:361–384. doi: 10.1146/annurev.ge.20.120186.002045. [DOI] [PubMed] [Google Scholar]
- 71.DePinho RA, Schreiber-Agus N, Alt FW. myc family oncogenes in the development of normal and neoplastic cells. Advances in cancer research. 1991;57:1–46. doi: 10.1016/s0065-230x(08)60994-x. [DOI] [PubMed] [Google Scholar]
- 72.Wechsler-Reya R, Elliott K, Herlyn M, Prendergast GC. The putative tumor suppressor BIN1 is a short-lived nuclear phosphoprotein, the localization of which is altered in malignant cells. Cancer research. 1997;57:3258–3263. [PubMed] [Google Scholar]
- 73.Prokic I, Cowling BS, Laporte J. Amphiphysin 2 (BIN1) in physiology and diseases. Journal of molecular medicine. 2014;92:453–463. doi: 10.1007/s00109-014-1138-1. [DOI] [PubMed] [Google Scholar]
- 74.Bauerfeind R, Takei K, De Camilli P. Amphiphysin I is associated with coated endocytic intermediates and undergoes stimulation-dependent dephosphorylation in nerve terminals. The Journal of biological chemistry. 1997;272:30984–30992. doi: 10.1074/jbc.272.49.30984. [DOI] [PubMed] [Google Scholar]
- 75.Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303:495–499. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- 76.Casal E, Federici L, Zhang W, Fernandez-Recio J, Priego EM, Miguel RN, DuHadaway JB, Prendergast GC, Luisi BF, Laue ED. The crystal structure of the BAR domain from human Bin1/amphiphysin II and its implications for molecular recognition. Biochemistry. 2006;45:12917–12928. doi: 10.1021/bi060717k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qualmann B, Koch D, Kessels MM. Let's go bananas: revisiting the endocytic BAR code. The EMBO journal. 2011;30:3501–3515. doi: 10.1038/emboj.2011.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gruber T, Balbach J. Protein Folding Mechanism of the Dimeric AmphiphysinII/Bin1 N-BAR Domain. PloS one. 2015;10:e0136922. doi: 10.1371/journal.pone.0136922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weissenhorn W. Crystal structure of the endophilin-A1 BAR domain. Journal of molecular biology. 2005;351:653–661. doi: 10.1016/j.jmb.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 80.Butler MH, David C, Ochoa GC, Freyberg Z, Daniell L, Grabs D, Cremona O, De Camilli P. Amphiphysin II (SH3P9; BIN1), a member of the amphiphysin/Rvs family, is concentrated in the cortical cytomatrix of axon initial segments and nodes of ranvier in brain and around T tubules in skeletal muscle. The Journal of cell biology. 1997;137:1355–1367. doi: 10.1083/jcb.137.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ge K, DuHadaway J, Du W, Herlyn M, Rodeck U, Prendergast GC. Mechanism for elimination of a tumor suppressor: aberrant splicing of a brain-specific exon causes loss of function of Bin1 in melanoma. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:9689–9694. doi: 10.1073/pnas.96.17.9689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ramjaun AR, Micheva KD, Bouchelet I, McPherson PS. Identification and characterization of a nerve terminal-enriched amphiphysin isoform. The Journal of biological chemistry. 1997;272:16700–16706. doi: 10.1074/jbc.272.26.16700. [DOI] [PubMed] [Google Scholar]
- 83.Leprince C, Romero F, Cussac D, Vayssiere B, Berger R, Tavitian A, Camonis JH. A new member of the amphiphysin family connecting endocytosis and signal transduction pathways. The Journal of biological chemistry. 1997;272:15101–15105. doi: 10.1074/jbc.272.24.15101. [DOI] [PubMed] [Google Scholar]
- 84.Tsutsui K, Maeda Y, Tsutsui K, Seki S, Tokunaga A. cDNA cloning of a novel amphiphysin isoform and tissue-specific expression of its multiple splice variants. Biochemical and biophysical research communications. 1997;236:178–183. doi: 10.1006/bbrc.1997.6927. [DOI] [PubMed] [Google Scholar]
- 85.Sakamuro D, Elliott KJ, Wechsler-Reya R, Prendergast GC. BIN1 is a novel MYC-interacting protein with features of a tumour suppressor. Nature genetics. 1996;14:69–77. doi: 10.1038/ng0996-69. [DOI] [PubMed] [Google Scholar]
- 86.Wigge P, Kohler K, Vallis Y, Doyle CA, Owen D, Hunt SP, McMahon HT. Amphiphysin heterodimers: potential role in clathrin-mediated endocytosis. Molecular biology of the cell. 1997;8:2003–2015. doi: 10.1091/mbc.8.10.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fernando P, Sandoz JS, Ding W, de Repentigny Y, Brunette S, Kelly JF, Kothary R, Megeney LA. Bin1 SRC homology 3 domain acts as a scaffold for myofiber sarcomere assembly. The Journal of biological chemistry. 2009;284:27674–27686. doi: 10.1074/jbc.M109.029538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Toussaint A, Cowling BS, Hnia K, Mohr M, Oldfors A, Schwab Y, Yis U, Maisonobe T, Stojkovic T, Wallgren-Pettersson C, Laugel V, Echaniz-Laguna A, Mandel JL, Nishino I, Laporte J. Defects in amphiphysin 2 (BIN1) and triads in several forms of centronuclear myopathies. Acta neuropathologica. 2011;121:253–266. doi: 10.1007/s00401-010-0754-2. [DOI] [PubMed] [Google Scholar]
- 89.Fugier C, Klein AF, Hammer C, Vassilopoulos S, Ivarsson Y, Toussaint A, Tosch V, Vignaud A, Ferry A, Messaddeq N, Kokunai Y, Tsuburaya R, de la Grange P, Dembele D, Francois V, Precigout G, Boulade-Ladame C, Hummel MC, Lopez de Munain A, Sergeant N, Laquerriere A, Thibault C, Deryckere F, Auboeuf D, Garcia L, Zimmermann P, Udd B, Schoser B, Takahashi MP, Nishino I, Bassez G, Laporte J, Furling D, Charlet-Berguerand N. Misregulated alternative splicing of BIN1 is associated with T tubule alterations and muscle weakness in myotonic dystrophy. Nature medicine. 2011;17:720–725. doi: 10.1038/nm.2374. [DOI] [PubMed] [Google Scholar]
- 90.Nicot AS, Toussaint A, Tosch V, Kretz C, Wallgren-Pettersson C, Iwarsson E, Kingston H, Garnier JM, Biancalana V, Oldfors A, Mandel JL, Laporte J. Mutations in amphiphysin 2 (BIN1) disrupt interaction with dynamin 2 and cause autosomal recessive centronuclear myopathy. Nature genetics. 2007;39:1134–1139. doi: 10.1038/ng2086. [DOI] [PubMed] [Google Scholar]
- 91.Muller AJ, Baker JF, DuHadaway JB, Ge K, Farmer G, Donover PS, Meade R, Reid C, Grzanna R, Roach AH, Shah N, Soler AP, Prendergast GC. Targeted disruption of the murine Bin1/Amphiphysin II gene does not disable endocytosis but results in embryonic cardiomyopathy with aberrant myofibril formation. Molecular and cellular biology. 2003;23:4295–4306. doi: 10.1128/MCB.23.12.4295-4306.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hong TT, Smyth JW, Chu KY, Vogan JM, Fong TS, Jensen BC, Fang K, Halushka MK, Russell SD, Colecraft H, Hoopes CW, Ocorr K, Chi NC, Shaw RM. BIN1 is reduced and Cav1.2 trafficking is impaired in human failing cardiomyocytes. Heart rhythm : the official journal of the Heart Rhythm Society. 2012;9:812–820. doi: 10.1016/j.hrthm.2011.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lyon AR, Nikolaev VO, Miragoli M, Sikkel MB, Paur H, Benard L, Hulot JS, Kohlbrenner E, Hajjar RJ, Peters NS, Korchev YE, Macleod KT, Harding SE, Gorelik J. Plasticity of surface structures and beta(2)-adrenergic receptor localization in failing ventricular cardiomyocytes during recovery from heart failure. Circulation. Heart failure. 2012;5:357–365. doi: 10.1161/CIRCHEARTFAILURE.111.964692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shaw RM, Fay AJ, Puthenveedu MA, von Zastrow M, Jan YN, Jan LY. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell. 2007;128:547–560. doi: 10.1016/j.cell.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Smyth JW, Hong TT, Gao D, Vogan JM, Jensen BC, Fong TS, Simpson PC, Stainier DY, Chi NC, Shaw RM. Limited forward trafficking of connexin 43 reduces cell-cell coupling in stressed human and mouse myocardium. The Journal of clinical investigation. 2010;120:266–279. doi: 10.1172/JCI39740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Navedo MF, Cheng EP, Yuan C, Votaw S, Molkentin JD, Scott JD, Santana LF. Increased coupled gating of L-type Ca2+ channels during hypertension and Timothy syndrome. Circulation research. 2010;106:748–756. doi: 10.1161/CIRCRESAHA.109.213363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dixon RE, Moreno CM, Yuan C, Opitz-Araya X, Binder MD, Navedo MF, Santana LF. Graded Ca(2)(+)/calmodulin-dependent coupling of voltage-gated CaV1.2 channels. eLife. 2015;4 doi: 10.7554/eLife.05608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pasek M, Simurda J, Christe G. The functional role of cardiac T-tubules explored in a model of rat ventricular myocytes. Philosophical transactions. Series A, Mathematical, physical, and engineering sciences. 2006;364:1187–1206. doi: 10.1098/rsta.2006.1764. [DOI] [PubMed] [Google Scholar]
- 99.Shepherd N, McDonough HB. Ionic diffusion in transverse tubules of cardiac ventricular myocytes. The American journal of physiology. 1998;275:H852–H860. doi: 10.1152/ajpheart.1998.275.3.H852. [DOI] [PubMed] [Google Scholar]
- 100.Swift F, Stromme TA, Amundsen B, Sejersted OM, Sjaastad I. Slow diffusion of K+ in the T tubules of rat cardiomyocytes. Journal of applied physiology. 2006;101:1170–1176. doi: 10.1152/japplphysiol.00297.2006. [DOI] [PubMed] [Google Scholar]
- 101.Lederer WJ, Niggli E, Hadley RW. Sodium-calcium exchange in excitable cells: fuzzy space. Science. 1990;248:283. doi: 10.1126/science.2326638. [DOI] [PubMed] [Google Scholar]
- 102.Blatter LA, Niggli E. Confocal near-membrane detection of calcium in cardiac myocytes. Cell calcium. 1998;23:269–279. doi: 10.1016/s0143-4160(98)90023-9. [DOI] [PubMed] [Google Scholar]
- 103.Pasek M, Simurda J, Orchard CH. Role of t-tubules in the control of trans-sarcolemmal ion flux and intracellular Ca2+ in a model of the rat cardiac ventricular myocyte. European biophysics journal : EBJ. 2012;41:491–503. doi: 10.1007/s00249-012-0804-x. [DOI] [PubMed] [Google Scholar]
- 104.Pasek M, Simurda J, Orchard CH, Christe G. A model of the guinea-pig ventricular cardiac myocyte incorporating a transverse-axial tubular system. Progress in biophysics and molecular biology. 2008;96:258–280. doi: 10.1016/j.pbiomolbio.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 105.Lavorato M, Huang TQ, Iyer VR, Perni S, Meissner G, Franzini-Armstrong C. Dyad content is reduced in cardiac myocytes of mice with impaired calmodulin regulation of RyR2. Journal of muscle research and cell motility. 2015;36:205–214. doi: 10.1007/s10974-015-9405-5. [DOI] [PubMed] [Google Scholar]
- 106.Smith LL, Gupta VA, Beggs AH. Bridging integrator 1 (Bin1) deficiency in zebrafish results in centronuclear myopathy. Human molecular genetics. 2014;23:3566–3578. doi: 10.1093/hmg/ddu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Balijepalli RC, Foell JD, Hall DD, Hell JW, Kamp TJ. Localization of cardiac L-type Ca(2+) channels to a caveolar macromolecular signaling complex is required for beta(2)-adrenergic regulation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:7500–7505. doi: 10.1073/pnas.0503465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Head BP, Patel HH, Roth DM, Murray F, Swaney JS, Niesman IR, Farquhar MG, Insel PA. Microtubules and actin microfilaments regulate lipid raft/caveolae localization of adenylyl cyclase signaling components. The Journal of biological chemistry. 2006;281:26391–26399. doi: 10.1074/jbc.M602577200. [DOI] [PubMed] [Google Scholar]
- 109.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics-2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 110.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y C. American Heart Association Statistics, S. Stroke Statistics. Heart disease and stroke statistics-2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 111.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J C. American Heart Association Statistics, S. Stroke Statistics. Heart disease and stroke statistics-2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Johnson MR, Meyer KH, Haft J, Kinder D, Webber SA, Dyke DB. Heart transplantation in the United States, 1999–2008. Am J Transplant. 2010;10:1035–1046. doi: 10.1111/j.1600-6143.2010.03042.x. [DOI] [PubMed] [Google Scholar]
- 113.Lietz K, Long JW, Kfoury AG, Slaughter MS, Silver MA, Milano CA, Rogers JG, Naka Y, Mancini D, Miller LW. Outcomes of left ventricular assist device implantation as destination therapy in the post-REMATCH era: implications for patient selection. Circulation. 2007;116:497–505. doi: 10.1161/CIRCULATIONAHA.107.691972. [DOI] [PubMed] [Google Scholar]
- 114.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM, 3rd, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH, HeartMate III. Advanced heart failure treated with continuous-flow left ventricular assist device. The New England journal of medicine. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 115.Tracy CM, Epstein AE, Darbar D, Dimarco JP, Dunbar SB, Estes NA, 3rd, Ferguson TB, Jr, Hammill SC, Karasik PE, Link MS, Marine JE, Schoenfeld MH, Shanker AJ, Silka MJ, Stevenson LW, Stevenson WG, Varosy PD. 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2012;60:1297–1313. doi: 10.1016/j.jacc.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 116.Braunwald E. Biomarkers in heart failure. The New England journal of medicine. 2008;358:2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 117.Pfisterer M, Buser P, Rickli H, Gutmann M, Erne P, Rickenbacher P, Vuillomenet A, Jeker U, Dubach P, Beer H, Yoon SI, Suter T, Osterhues HH, Schieber MM, Hilti P, Schindler R, Brunner-La Rocca HP T.-C. Investigators. BNP-guided vs symptom-guided heart failure therapy: the Trial of Intensified vs Standard Medical Therapy in Elderly Patients With Congestive Heart Failure (TIME-CHF) randomized trial. JAMA : the journal of the American Medical Association. 2009;301:383–392. doi: 10.1001/jama.2009.2. [DOI] [PubMed] [Google Scholar]
- 118.Gomez AM, Guatimosim S, Dilly KW, Vassort G, Lederer WJ. Heart failure after myocardial infarction: altered excitation-contraction coupling. Circulation. 2001;104:688–693. doi: 10.1161/hc3201.092285. [DOI] [PubMed] [Google Scholar]
- 119.Gomez AM, Valdivia HH, Cheng H, Lederer MR, Santana LF, Cannell MB, McCune SA, Altschuld RA, Lederer WJ. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science. 1997;276:800–806. doi: 10.1126/science.276.5313.800. [DOI] [PubMed] [Google Scholar]
- 120.Litwin SE, Zhang D, Bridge JH. Dyssynchronous Ca(2+) sparks in myocytes from infarcted hearts. Circulation research. 2000;87:1040–1047. doi: 10.1161/01.res.87.11.1040. [DOI] [PubMed] [Google Scholar]
- 121.He J, Conklin MW, Foell JD, Wolff MR, Haworth RA, Coronado R, Kamp TJ. Reduction in density of transverse tubules and L-type Ca(2+) channels in canine tachycardia-induced heart failure. Cardiovascular research. 2001;49:298–307. doi: 10.1016/s0008-6363(00)00256-x. [DOI] [PubMed] [Google Scholar]
- 122.Bito V, Heinzel FR, Biesmans L, Antoons G, Sipido KR. Crosstalk between L-type Ca2+ channels and the sarcoplasmic reticulum: alterations during cardiac remodelling. Cardiovascular research. 2008;77:315–324. doi: 10.1093/cvr/cvm063. [DOI] [PubMed] [Google Scholar]
- 123.Louch WE, Mork HK, Sexton J, Stromme TA, Laake P, Sjaastad I, Sejersted OM. T-tubule disorganization and reduced synchrony of Ca2+ release in murine cardiomyocytes following myocardial infarction. The Journal of physiology. 2006;574:519–533. doi: 10.1113/jphysiol.2006.107227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ibrahim M, Terracciano CM. Reversibility of T-tubule remodelling in heart failure: mechanical load as a dynamic regulator of the T-tubules. Cardiovascular research. 2013;98:225–232. doi: 10.1093/cvr/cvt016. [DOI] [PubMed] [Google Scholar]
- 125.Guo A, Zhang C, Wei S, Chen B, Song LS. Emerging mechanisms of T-tubule remodelling in heart failure. Cardiovascular research. 2013;98:204–215. doi: 10.1093/cvr/cvt020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ferrantini C, Crocini C, Coppini R, Vanzi F, Tesi C, Cerbai E, Poggesi C, Pavone FS, Sacconi L. The transverse-axial tubular system of cardiomyocytes. Cellular and molecular life sciences : CMLS. 2013;70:4695–4710. doi: 10.1007/s00018-013-1410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]