Abstract
Human heart failure due to myocardial infarction is a major health concern. The paucity of organs for transplantation limits curative approaches for the diseased and failing adult heart. Human induced pluripotent stem cell-derived cardiac myocytes (hiPSC-CMs) have the potential to provide a long-term, viable, regenerative-medicine alternative. Significant progress has been made with regard to efficient cardiac myocyte generation from hiPSCs. However, directing hiPSC-CMs to acquire the physiological structure, gene expression profile and function akin to mature cardiac tissue remains a major obstacle. Thus, hiPSC-CMs have several hurdles to overcome before they find their way into translational medicine. In this review, we address the progress that has been made, the void in knowledge and the challenges that remain.
Keywords: Maturation, Troponin, Heart, Differentiation, Stem cells
Introduction
Human heart failure is a major health and economic burden. Recent evidence suggests that the adult myocardium may be able to generate some new cardiac myocytes after injury; however, this is insufficient to lead to meaningful functional recovery [1, 2]. Furthermore, the paucity of organs for transplantation limits curative approaches for the diseased adult heart. As an alternative approach, stem cell-based therapies have been investigated for their capacity to repair the damaged heart. For example, non-cardiac cells obtained from bone marrow-derived mesenchymal stem cells (MSCs) have been studied with limited success [3, 4]. More recently, human induced pluripotent stem cell-derived cardiac myocytes (hiPSC-CMs) have been gaining attention as a potentially viable alternative. While exciting in principle, hiPSC-CMs do have several hurdles to overcome before meaningful translational lab-to-clinic advances can be made. It is generally viewed that hiPSC-CMs are immature as they do not display the requisite sub-cellular, cellular and tissue-level adult myocyte morphology and sarcomeric protein content and organization [5–7]. Maturation of hiPSC-CMs is indispensable because it underlies the required physiological functions of the cell, including intracellular Ca2+ handling, contractile force, and relaxation [8, 9]. In order to realize the potential applications of mature hiPSC-CMs to the emerging field of personalized medicine as well as for disease modeling and drug discovery, further testing and refinement is paramount.
Focus and rationale
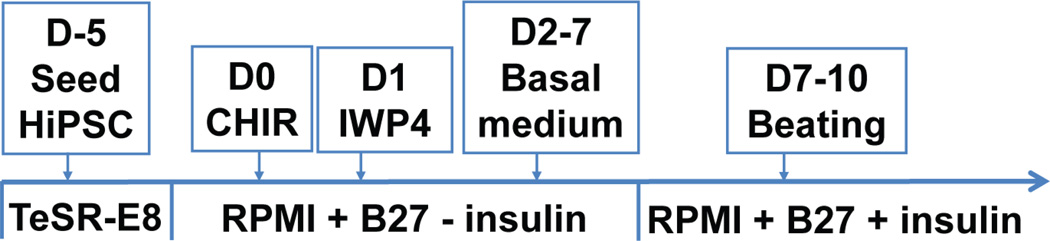
Although efficient cardiac myocytes can be generated from hiPSCs after differentiation using small molecules [7] (Fig. 1), directing these cells to acquire the physiological structure, gene expression profile, and function akin to mature cardiac tissue remains a major obstacle. This review will focus on the developmental progression of fetal cardiac myocytes (maturation), growth and nuclear kinetics (ploidy), and gene expression, with emphasis on disease-unaltered isoforms, such as TnI isoforms and chamber specific isoforms, as well as diseased-altered isoforms. Finally, factors that promote maturation of hiPSC-CMs will be reviewed together with an assessment of efforts to characterize the functional properties of hiPSC-CMs, culminating in a discussion of practical applications of hiPSC-CMs.
Figure 1. Differentiation of hiPSC-CMs.
Schematic of the differentiation protocol for hiPSC-CMs as described recently [7, 13].
Developmental progression of fetal cardiac myocytes
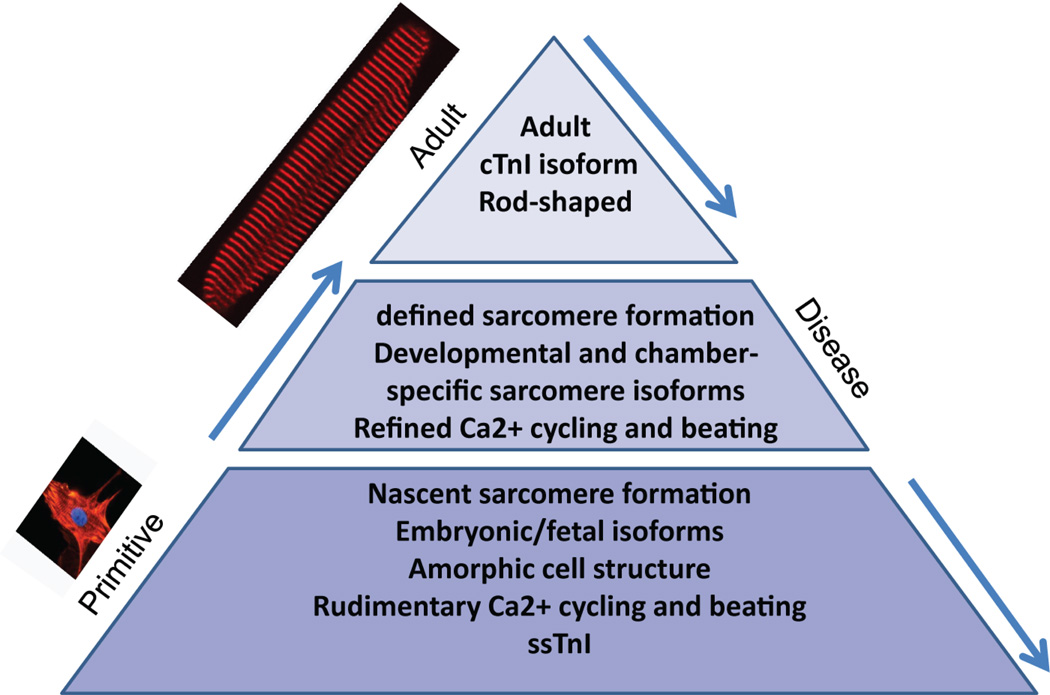
Fetal cardiac myocytes are characterized by amorphous cell morphology, nascent sarcomere formation, embryonic/fetal gene expression, rudimentary Ca2+ cycling, and spontaneous beating (Fig. 2) [7, 10, 11]. As development progresses, enhanced sarcomere formation ensues with expression of developmental and chamber-specific sarcomeric isoforms, refined Ca2+ cycling, and synchronized beating [7, 10, 11]. Further developmental maturation results in typical rod-shaped morphology with defined sarcomeres and, importantly, key signatures of adult isoform expression, such as expression of cTnI, which confers robust mechanical performance, as well as expression of the ventricular marker MLC2v [12, 13]. Interestingly, during cardiac disease the adult myocardium resorts to expression of a fetal sarcomeric gene program by up-regulation of key fetal isoforms [7]. In this review we assess hiPSC-CMs maturation in the context of the fetal-to-adult developmental transition obtained during normal heart development.
Figure 2. Developmental progression of cardiac myocytes.
The pyramid depicts the developmental transition from fetal stages to fully mature rod-shaped adult myocytes, and the recurrence of the fetal program during heart disease.
Physiological growth and nuclear kinetics
The proliferation of cardiac myocytes is the major factor for the increase of the size of the heart during development [14]. The increasing circulatory demand of the developing embryo is matched with an increase in heart mass via cell proliferation. Ultimately, cardiac myocyte turnover, in concert with cell migration and programmed cell death, is an essential element in the developing heart [14]. Although fetal hearts from both humans and rodents grow through the proliferation of mono-nucleated cardiac myocytes with diploid nuclei, variation exists in the timing of cell cycle withdrawal [15]. Rodent cardiac myocytes withdraw from the cell cycle shortly after birth, but human cardiac myocytes continue to proliferate for several months after birth, although this proliferation slows with time [15, 16]. This complete cell division process, where karyokinesis is coupled with cytokinesis, is evident in rodent and human myocytes as they undergo a period of physiological growth (hypertrophy) to increase overall heart mass [11, 15]. This physiological growth is different from pathological hypertrophic growth, where postnatal human and rodent cardiac myocytes exhibit different nuclear kinetics. For example, in rodents, more than 75% of cardiac myocytes are bi-nucleated [11, 15]. This bi-nucleation occurs via incomplete cell divisions that involve DNA replication and nuclear division without cytokinesis (acytokinetic mitosis) with each nucleus having normal diploid (2n) DNA content [15–17]. In comparison, most adult cardiac myocytes are mono-nucleated and tetraploid in humans and primates [11, 15, 18]. Human cardiac myocytes attain mono-nucleation and polyploidy by undergoing DNA replication without nuclear division or cytokinesis, [15, 18], culminating in tetraploidy (4n) or polyploidy [15, 19]. Olivetti et al. demonstrated that the proportion of mono-nucleated human cardiac myocytes in the adult heart is 74% and that of bi-nucleated cardiac myocytes is 25.5 % [18]. Paralleling the increase of myocardial mass, the percentage of diploid cardiac myocyte nuclei decreases in the human heart [15]. Polyploidization occurs in the pre-adolescent growth phase with tetraploid nuclei (4n) being the most common ploidy state in the adult human heart [11, 15]. Collectively, bi-nucleation (DNA replication with karyokinesis but without cytokinesis) in postnatal rodent myocytes and polyploidization (DNA replication without karyokinesis or cytokinesis) in postnatal human myocytes are examples of incomplete cell division or nonproductive cell cycling that do not necessarily contribute to cardiac myocyte turnover or new cardiac myocyte formation. This is a hallmark of the postnatal mammalian heart [15].
Markers of maturation
Quantitative methods to track hiPSC-CM maturation are critically important and currently limited. One of the great challenges of an effective maturation marker rests in its "reliability" as an adult marker. Many potential cardiac markers of the adult mature state are susceptible to change in that they can "revert" to a fetal signature during stress, thus compromising their function as an adult marker. Recently, we have demonstrated the usefulness of troponin I isoforms as a valid quantitative marker of maturation that overcomes this key limitation [7]. The TnI isoforms are part of the thin-filament protein network and are central to cardiac muscle function. In striated muscle, there are three isoforms which are transcribed from three separate genes in a muscle-fiber type-specific manner [20–22]. These isoforms are fast skeletal troponin I (fsTnI/TNNI2), expressed in fast skeletal muscle fibers; slow skeletal troponin I (ssTnI/TNNI1), expressed in slow skeletal muscle fibers and fetal cardiac muscle; and cardiac troponin I (cTnI/TNNI3), expressed only in adult cardiac muscle. In this review, we focus on ssTnI and cTnI because these two key myofilament isoform proteins are antithetically expressed during the transition from fetal to adult life [22, 23]. The ssTnI isoform is expressed during neonatal or fetal life and is then stoichiometrically replaced by the cTnI isoform, which is exclusively expressed in mature adult cardiac myocytes [22–27]. The cTnI isoform has a unique N-terminal extension containing serine residues that are phosphorylated in response to adrenergic stimulation of the heart, making it PKA-responsive. This PKA responsiveness is critically required for the robust relaxation that is ultimately required during adult cardiac function [28–30]. Thus, acquisition of this key adult signature maturation marker by hiPSC-CMs is indispensable.
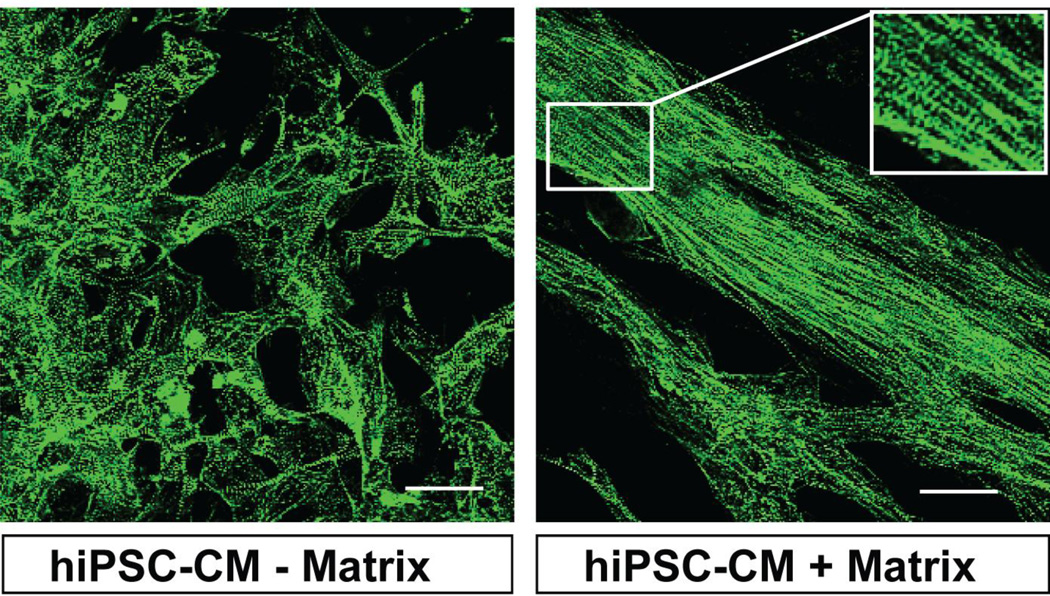
The unique advantage of this isoform switch as a quantitative marker of maturation is that this isoform transition is not altered by disease conditions such as hypertrophy or heart failure [23, 24, 31]. This is distinct from other studied genes and isoforms which have been used as markers of maturation in prior studies [32, 33]. The transition of TnI isoforms during development has been understudied in addressing stem cell-derived cardiac maturation. Currently, it is not entirely clear what factors regulate the transition from ssTnI to cTnI. To address this, our recent work evaluated developmental progression of hiPSC-CMs and rodent-CMs after culturing in a de-cellularized cardiac matrix [7]. Results show matrix-induced cell alignment and sarcomere organization of hiPSC-CMs (Fig. 3). Assessment of a mature signature, such as the switch from ssTnI to cTnI, showed stalled developmental maturation of hiPSC-CMs, with cTnI accounting for only 2% of all TnI expression after 9.5 months in culture [7]. Although hiPSC-CMs expressed detectable cTnI after 2 months in culture, this did not increase after as much as 6 to 9.5 months in culture. On the whole, the acquisition of a mature phenotype for hiPSC-CMs in terms of the cTnI:ssTnI isoform ratio is extremely delayed compared to the developmental acquisition in rodent pups or in cultured rodent CMs [7]. In order to use hiPSC-CMs for disease modeling or drug studies, it is important to investigate this delayed maturation of hiPSC-CMs. To this end, it is critical to incorporate the transition from the ssTnI to the cTnI isoform, along with other key markers such as MLC2v, as a readout of developmental progression.
Figure 3. Structural organization of hiPSC-CMs.
Schematic of hiPSC-CMs repopulating a biological matrix aligned and displaying sarcomere organization (right panel) compared with those cultured in the absence of biological matrix (left panel). Calibration bar is 50 µm.
Chamber specific isoforms in hiPSC-CMs
Cardiac myosin consists of two cardiac myosin light chain 2 (MLC2) isoforms, MLC2a and MLC2v. In the developing human and mouse heart, MLC2a expression is detected in all chambers [34]. In the postnatal heart, MLC2v is confined to the ventricle, and this chamber specificity persists to adulthood [34, 35]. MLC2v expression is also considered to be a marker of cardiac myocyte maturity [34, 35]. Thus, we and others have used the expression patterns of MLC2a and MLC2v to define cardiac myocyte identity and stages of development. Recently, we reported that hiPSC-CMs primarily express MLC2a at early time points. At later time points, expression of MLC2v increases and expression of MLC2a decreases, with the majority of hiPSC-CMs co-expressing both MLC2a and MLC2v [7]. Here, hiPSC-CMs expressing MLC2v most likely represent ventricular-like cells, while those expressing MLC2a may represent a range of immature cardiac myocytes, including atrial-like cells. Earlier reports demonstrated prevalent MLC2v/MLC2a double-positive cardiac myocytes, with disorganized sarcomeres and weak hERG channel responses, to be immature, resembling human fetal cardiac myocytes [36]. The ultimate goal is to one day to produce ventricular hiPSC-CMs that fully express cTnI and MLC2v, and that have zero ssTnI expression, as in adult myocardium.
Stress-altered markers of maturation
A large number of cardiac isoforms encoding different contractile protein exist in the sarcomere. Many genes that encode for contractile proteins have a characteristic pattern of expression during development, and many of these are also altered by disease conditions [37]. The failing heart reactivates fetal genes and reverts to a fetal pattern of energy substrate metabolism [37, 38]. For instance, levels of fetal genes such as ANP, BNP, βMHC, skeletal actin, and metabolic genes such as GLUT1 are reactivated in the failing adult heart [32, 37, 38]. Moreover, αMHC, SERCA2a, ion channels, and metabolic genes such as GLUT4 are reduced during heart failure [32, 39]. Because the failing adult heart reverts to a fetal-like structural, hormonal and metabolic gene profile by down-regulating adult gene transcripts, the utility of these reversible markers, that are prone to changes during disease and culture conditions, has to be interpreted with caution. Thus, we recommend incorporation of isoforms that are not prone to such alteration, such as ssTnI and cTnI, and MLC2a and MLC2v [23, 24, 40], into studies involving hiPSC-CM maturation.
Structural maturation of hiPSC-CMs on a biological matrix
Sarcomere maturation in hiPSC-CMs is indispensable for the study of disease modeling. Sarcomere structure maturation in cardiac myocytes derived from human pluripotent stem cells could be achieved by cues from extracellular matrix proteins (ECMs) [41]. Our recent work has shown that hiPSC-CMs and rodent CMs repopulated into a biological cardiac matrix display sarcomere organization, as revealed by α-actinin at the Z-line [7]. In contrast, the myofibrils in these cell types are oriented in multiple directions within the myocytes in the absence of such a biological matrix (Fig. 3).
Molecular maturation of hiPSC-CMs with thyroid hormone (T3)
The role of T3 in regulating cardiac genes and isoforms is well known [42, 43]. Studies have shown an increase in the expression of mRNA for αMHC, cardiac actin, skeletal actin and calcium handling genes such as SERCA2a following T3 treatment, suggesting an important role of T3 in the regulation of cardiac genes [42, 43]. Previous work demonstrated that changes in myosin heavy chain (MHC) isoforms that occur in failing human hearts resemble the pattern produced in rodent myocardium in response to hypothyroidism. Furthermore, failing or hypertrophied human myocardium may have a defect in thyroid hormone signaling due to alterations in expression of thyroid hormone receptors (TRs) [38]. Contrary to recent work [44], our work has shown that hiPSC-CMs do not respond to T3 treatment, despite the fact that rodent CMs respond readily to T3 treatment [7].
Directed maturation of hiPSC-CMs with stoichiometric replacement of TnI
Adult cardiac muscles are highly organized contractile machines. Because the cardiac myofilament proteins are undergoing dynamic protein synthesis and turnover, stringent stoichiometry is required for the assembly of myofilament proteins. For instance, postnatal expression of ssTnI is significantly prolonged in the hearts of cTnI knockout (cTnI-KO) mice [45], suggesting cTnI accelerates ssTnI isoform turnover. Because of the apparent developmental block in the acquisition of the mature molecular signature marker (cTnI) in hiPSC-CMs, we sought to complement hiPSC-CMs with exogenous adenoviral mediated gene transfer of human cTnI. Stoichiometric gene replacement with exogenous human cTnI significantly and stoichiometrically increases cTnI and reduces content of ssTnI in hiPSC-CMs [7]. The developmentally stalled acquisition of a mature signature in hiPSC-CMs could thus be accelerated with gene transfer of cTnI.
Functional Characterization
Many researchers have devoted substantial effort to characterizing pluripotent stem cell-derived cardiac myocytes in terms of gene and protein expression. Over the course of differentiation, the cells are thought to act much like early embryonic pluripotent and precursor cells [46]. Here, the transition from pluripotency through mesodermal and cardiac progenitor-like cells, culminating in a cardiac myocyte phenotype over the course of 7–10 days, can be easily tracked through gene profile studies. Gene and protein expression profiles also allow characterization of changes in differentiated cells, including limited phenotypic maturation, and isoform switching of adrenergic receptors, ion channels, and sarcomeric proteins, as reviewed above. However, as a myocyte’s usefulness inherently depends on more than simply gene expression, it is crucial to evaluate pluripotent stem cell-derived cardiac myocytes in terms of properties necessary for functionality of a contracting cell. These include electrical conductance, Ca2+ handling, and contractility.
Electrical Function
Due to the essential role of ionic currents and electrical conduction in the functionality of cardiac muscle, deciphering electrical function of iPSC-CMs allows researchers to characterize mature versus immature, healthy versus diseased cells, and ventricular versus atrial cell types. However, at such an immature state, the last categorization may be difficult to distinguish, and attempting to do so may not provide realistically useful information [47]. Generally, electrical function is measured using extracellular field potential recordings, sharp electrode recordings, or patch clamp recordings.
Perhaps the easiest electrical assay to perform is the recording of extracellular field potentials using a multielectrode array (MEA). MEA recording does not require recording from a single cell, but rather measures the voltage from a population of cells in a dish [48] In cardiac myocytes, the field potential duration (FPD) corresponds to the action potential duration (APD) of a single cell, which in turn corresponds to the QT interval on the electrocardiogram, an important parameter for researchers using iPSC-CMs to model arrhythmic diseases or as a platform for drug discovery. This technique has been well validated in hESC-CM models [48–50]. It has recently been further validated in iPSC-CMs, demonstrating that it can be used to reliably detect drug-induced arrhythmias and repolarization delay, even across distinct facilities [51]. Recent work characterized and optimized FP recordings, including in response to a drug, and included some limitations of the method that should be taken into consideration when performing experiments and data analysis [52].
In order to dissect out the role of specific currents and ions, single cell electrophysiology techniques are useful, including patch clamp and sharp electrode recording. A patch-clamp pipette can be attached to the cell in several configurations, including cell-attached, whole-cell, and perforated-patch modes. Patch clamp has been used to evaluate currents in hiPSC-CMs derived from patients with long QT syndrome type 2. This technique was employed to examine the effects of various drugs on those cells, demonstrating its value in studying models of arrhythmia [53]. Patch clamp electrophysiology has also been used to evaluate individual currents of iPSC-CMs, including INa, ICa, IKr, and IKs, as well as their ability to be blocked by known channel blockers [54]. It was concluded that use of iPSC-CMs for electrophysiology studies is feasible. Similar experiments using an automated patch clamp technology came to similar conclusions [55]. However, a separate group, using patch clamp to study APD, AP frequency, AP shape, INa, and ICa, as well as effects of channel blockers TTX and lidocaine, noted significant variability between cells from different sources, and suggest utilizing these techniques with caution [56].
Sharp electrode electrophysiology has been used in several published iPSC-CM studies. Intracellular recordings of APs of murine iPSC-CMs [57] and human iPSC-CMs [58] have been made to examine whether the cells differentiated into atrial, ventricular, or nodal types. However, due to the immature nature of these cells, cell types can be difficult to distinguish based on AP shape alone, and caution should be used when drawing conclusions from these types of experiments. Overall, these studies show that iPSC-CMs express appropriate ion channels and have electrical activity similar to human cardiac myocytes, and can be reliably tested for action potential duration, ion currents, and drug interactions if appropriate cautions are taken when drawing conclusions.
Ca2+ handling in hiPSC-CMs
The adult ventricular cardiac myocyte displays a well-defined sequence of events with regard to Ca2+ cycling. Ca2+ influx via L-type Ca2+ channels serves as an initial trigger, initiating Ca2+ release from the sarcoplasmic reticulum by activating Ryanodine receptor 2 (RyR2) via a process called calcium-induced calcium release (CICR) [59, 60]. Phosphorylation of these channels increases calcium flux, increasing contractility. In general, little is known about the excitation contraction coupling (ECC) and Ca2+ handling properties of hiPSC-CMs. Gene expression and immunostaining studies showed that key Ca2+ handling proteins are expressed in hiPSC-CMs [61]. Furthermore, hiPSC-CMs are dependent on both trans-sarcolemmal Ca2+ entry via L-type Ca2+ channels and on RYR2-regulated SR Ca2+ release and functional SERCA2a pump-based Ca2+ reuptake [61].
The majority of Ca2+ in the cytoplasm during systole is released from and then taken back up into the sarcoplasmic reticulum (SR) [62]. Release is triggered by depolarization of the cell membrane, and, as such, is intricately connected to AP activity of the cell as well as sarcomere contraction. However, it can be uncoupled from both, and it is necessary to measure Ca2+ activity separately. Calcium transients in myocytes are typically measured using fluorescent Ca2+-binding dyes on a fluorescent microscope [62]. Satin et al. laid the groundwork using hESC-CMs [63]. They loaded cells with the dye Fluo-4 AM and recorded intracellular Ca2+ transients with a confocal microscope. Using this technique, they observed both entire-cell AP-driven transients, as well as localized SR Ca2+ release events (sparks). They then used caffeine to mobilize Ca2+ release from the SR, as well as ryanodine to inhibit release from the ryanodine receptor RyR. They were able to provide evidence that hESC-CMs have large stores of Ca2+ in the SR, a hallmark of mature cardiac myocytes. Moving to iPSC-CMs, several labs [64] [61] have imaged Ca2+ transients and sparks in healthy cells by loading them with fluo-4 under a confocal microscope, and used caffeine to probe SR stores. Fura-4F, a ratiometric calcium dye, has been used to observe the propagation of Ca2+ transients through iPSC-CM monolayers, while simultaneously measuring intracellular voltage with the voltage-sensitive fluorescent dye, di-8-ANEPPS [65].
Using hiPSC-CMs derived from patients with catecholaminergic polymorphic ventricular tachycardia (CPVT), a genetic arrhythmic defect, differences was shown in Ca2+ handling in diseased versus healthy cells based on Fluo-4 fluorescent imaging [66]. Furthermore, they used voltage clamping to directly measure the L-type Ca2+ current (ICa) and the Na+-Ca2+ exchanger (INCX). A combined fluorescent Ca2+ imaging with voltage-clamped ICa measurement can also demonstrate changes in how iPSC-CMs handle Ca2+ as they mature [67]. Recently, Ca2+ handling characteristics across cells derived in different laboratories have been compared [68], using the ratiometric Ca2+ dye Fura-2 AM and recording transients while the cells were electrically stimulated. Using caffeine, SR Ca2+ stores were estimated, and ICa was measured by whole-cell patch clamp. Overall, there were comparable results between these laboratories. To summarize, iPSC-CMs evidently have functional Ca2+ stores and release and reuptake Ca2+ into the cytosol or SR, which can be detected by Ca2+-sensitive fluorescent dyes such as a Fura or a Fluo derivative.
Contractility
The most important parameter of a myocyte’s function is its force production and motion, as this determines how effectively the organ can circulate blood. An adult myocyte, with its well-organized sarcomeres and rectangular shape, can relatively easily be studied using video microscopy or force transducers [69]. In comparison, the morphology of a stem cell-derived myocyte presents some distinct challenges. Early on, groups used video edge detection to track movement of stimulated beating EBs as a percent of baseline length [70]. They were able to show increased contractility in response to β-adrenergic stimulation, a hallmark feature of cardiac myocytes. iPSC-CMs derived by a monolayer method can also be monitored by video microscopy. Motion tracking software can be used to assess beating frequency, amplitude, and kinetics of a 3D tissue-like construct formed by seeding iPSC-CMs onto a filamentous polymer matrix [71].
While video-based edge detection of contractility is highly useful, the ability to measure force production against a load provides a more direct measure of myocyte function, and several researchers have sought out ways of doing so, by seeding beating clusters from iPSC-derived EBs onto 300 µm-thin strips of neonatal murine ventricular myocardium, and then attached the myocardial strip to force transducers for measurement [57]. Sun et al., using single cell-dispersed iPSC-CMs derived from a patient with dilated cardiomyopathy, and then attempted to assess single-cell force production using atomic force microscopy. This approach relies on the microscope for detecting changes in force at the cell surface caused by changes in stiffness within the cell, presumably due to rearrangement of sarcomeric and cytostructural proteins during contraction [72]. However, it should be noted that this is a technique that has not been validated in pluripotent cell-derived cardiac myocytes, neonatal cardiac myocytes, or adult myocytes. In the paper, the authors cite only a paper exploring use of the technique on embryonic chicken cardiac myocytes, where the authors themselves note that parameters such as beat period and pulse amplitude were observed to be unstable [73].
The micropost array, a technique that has been well validated in other myocyte cell types, has been used to measure contractile force of iPSC-CMs [74]. In this assay, an array of microposts is fabricated from polydimethylsiloxane (PDMS) using photolithography; the tops of the posts are coated with ECM proteins, and cells are seeded on top of that. As cells contract, the posts deflect with the cell, and video microscopy with subsequent analysis is able to convert movement into force needed to move the microposts. The authors were able to examine different force production of cells that had been seeded on different types of ECM protein. In a similar vein, Sheehy et al. utilized the muscular thin film (MTF) assay, wherein thin strips of PDMS are fabricated and coated with ECM proteins. The iPSC-CMs are then seeded and stimulated [75]. As cells contract, the MTFs bend and curvature is analyzed with video microscopy, and force production is extrapolated from MTF displacement. These studies show that iPSC-CMs produce a force that is able to be measured; however, the techniques for force measurement are not well developed, and there is a strong need for improved methods.
Applications
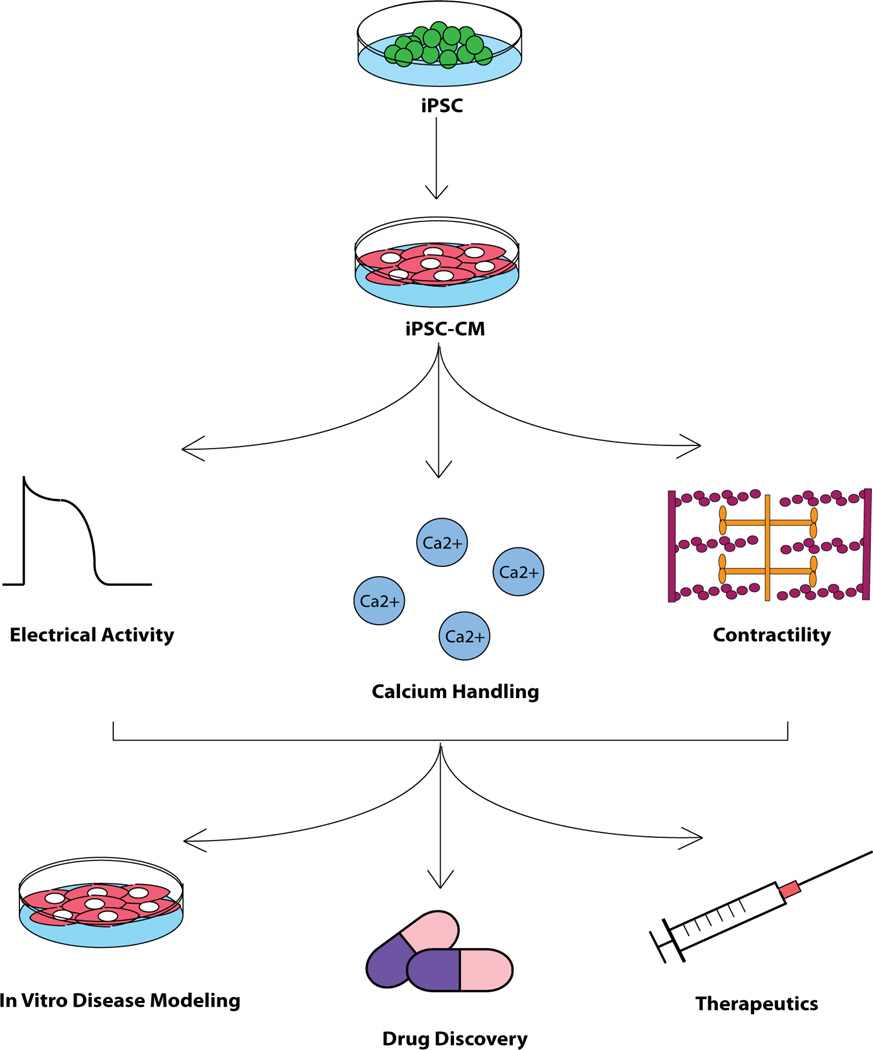
The technologies allowing dedifferentiation of cells into a pluripotent state, and subsequent differentiation to cardiac phenotypes, have been adopted by laboratories and research groups around the world with remarkable speed. The study of iPSC-CMs has become its own field of research over the span of a few short years, and new discoveries are made at a steady and impressive pace. An explanation for this rapidity lies in the wealth of potential uses and applications envisioned for these cells (Figure 4).
Figure 4. Overview of experimental flow utilizing hiPSC-CMs.
Functionality of hiPSCs, having been differentiated into cardiac myocytes, is characterized in terms of electrical activity, calcium cycling, and contractility. Fully characterized hiPSC-CMs are used for diverse applications including disease modeling, drug discovery, and therapeutic applications.
Disease Models
Pluripotent stem cells derived from individuals with genetic diseases can be used to illuminate disease phenotypes in an in vitro model. Here, iPSC-CMs derived from patients with known genetic mutations, especially monogenic mutations, that lead to cardiac phenotypes can be used to probe gene function and potential therapies. With the electrical assays readily available as mentioned above, an early target for disease modeling with iPSC-CMs was Long QT syndrome, a genetic disorder characterized by delayed repolarization of cardiac myocytes, and which puts patients at risk of deadly arrhythmias including Torsade de Pointes and ventricular fibrillation [76]. In hiPSC-CMs from patients with Long QT Syndrome Type 3 (LQTS-3)(caused by mutations in the sodium channel SCN5A) there is faster recovery from inactivation of Na+ current in mutant cells versus wild-type cells, and a larger tetrodotoxin (TTX)-sensitive current in the mutant cells [76]. Both of these findings are consistent with data from adult mouse myocyte studies [77]. The group also found prolonged AP duration in SCN5A mutant cells. Similar observations were made using iPSC-CMs from LQTS-3 patients, however with different mutations in the SCN5A gene [78]. Using patch clamp to study Na+ and Ca2+ currents, as well as whole-cell current clamp to measure AP, they found that LQTS cells have tendencies toward prolonged AP duration, smaller Na+ current density, slower time to inactivation, and increased time to peak. However, they failed to reach statistical significance in many of these comparisons, likely due to either small sample sizes or overall immature phenotypes of derived cells.
At least three groups have studied LQTS using pluripotent cells from patients with LQTS Type 2, caused by mutations in KCNH2 (hERG channel, responsible for inwardly rectifying potassium current IKr). The first group, using cells from a single patient, utilized whole-cell patch clamp and found AP duration prolongation and reduced amplitude of peak IKr activation and tail currents [79]. Using MEA, they reported prolonged field potential duration (FPD). Importantly, by looking at single cell AP as well as MEA, they found significant arrhythmogenicity in the form of EADs and ectopic activity. The second [53] and third groups [80] found similar prolongation of APD and FPD in KCNH2 mutant iPSC-CMs. Furthermore, these studies demonstrated significant reduction in IKr density and enhanced arrhythmogenic potential. In hiPSC-CMs from a patient with Timothy syndrome [caused by a mutation in the L-type Ca2+ channel (CaV1.2)] the QT interval was prolonged [81]. This study reported prolonged APD, arrhythmic activity, and abnormal Ca2+ transients in the patient-derived cells compared to control cells. Other models of arrhythmia have been studied using patient-derived cells. Catecholaminergic polymorphic ventricular tachycardia (CPVT), caused by a mutation in the ryanodine receptor (RYR2), is characterized by aberrant Ca2+ release from the SR and ventricular arrhythmia. Cells derived from patients with this disease indeed show elevated diastolic Ca2+ concentrations, reduced SR Ca2+ content, and susceptibility to DAD in patient derived cells compared to control [82].
Apart from arrhythmic disease models, other hereditary conditions have been studied in patient-derived iPSC-CMs. Using cells derived from a patient with Barth Syndrome, a mitochondrial disorder, it was observed that the cells closely recapitulated several hallmarks of the disease, including irregular sarcomere formation, irregular mitochondria, and weak contractility [83]. Furthermore, they were able to elucidate unknown mechanisms of pathophysiology of the disease involving excess reactive oxygen species. Others have studied cells from a patient with Pompe disease, a glycogen storage disorder caused by mutations in acid alpha-glucosidase (GAA) [84]. Here the patient-derived hiPSC-CMs showed abnormally high levels of glycogen and mitochondrial dysfunction, and ultrastructural abnormalities, consistent with findings from myocytes. In LEOPARD syndrome, a developmental disorder characterized by a cluster of abnormal findings caused by mutations in the Ras-MAPK signaling genes, a common abnormality is hypertrophic cardiomyopathy. Differentiated iPSC-CMs derived from patients with a mutation in a protein tyrosine phosphatase encoded by PTPN11, display abnormal Ras-MAPK signaling, cell hypertrophy and abnormal sarcomere organization [85].
Models of cardiomyopathies, while more difficult to study due to the immature phenotype of iPSC-CMs, have also shown some progress. For example, cells were taken from a patient with arrhythmogenic right ventricular cardiomyopathy (ARVC), a poorly-characterized disease associated with arrhythmia and sudden cardiac death, as well as fibrofatty replacement of the right ventricular myocardium [86]. This patient had a mutation in PKP2, encoding plakophilin 2, a desmosomal protein. The hiPSC-CMs from this patient showed decreased expression of desmosomal proteins and high levels of lipid storage. However, while electrophysiological studies were done on patient-derived cells, they were not compared to control cells, proving to be a limitation of this study. A separate in vitro model of dilated cardiomyopathy (DCM) [72], characterized by eccentric ventricular hypertrophy, decreased Ca2+ sensitivity, and impaired force production, was derived iPSCs from patients carrying a mutation in cardiac troponin T (TNNT2) and their unaffected family members. Mutant iPSC-CMs showed comparable cell size to control, but had deranged sarcomere organization. They also had smaller Ca2+ transient amplitudes and smaller SR Ca2+ stores, and lower force production based on an atomic force microscopy assay. A limitation to this study was the immaturity of the iPSC-CMs, inhibiting the ability to fully assess a disease phenotype normally associated with mature myocytes. For example, the researchers saw deleterious effects of norepinephrine on DCM iPSC-CMs, including inhibition of beating and sarcomere derangement, both of which are outside the norm for mature myocytes.
A recent area of great research interest involves the use of genome editing techniques. These include such technologies as transcription activator-like effector nucleases (TALENs) and CRISPR/Cas9. Both cleave DNA in a site-specific manner, which may be repaired by non-homologous end-joining (NHEJ), potentially disrupting the gene, or, if a template is provided, by homology-directed repair (HDR) [87]. These can be used to introduce disease-specific mutations into an otherwise healthy cell line, reducing noise from genetic variability between lines. It may also be used to correct disease-causing mutations. Hematopoietic diseases such as Fanconi Anemia, β-thalassemia, and myelodysplastic syndrome have been modeled and corrected [88], but the technology has not yet been extensively applied to cardiovascular disease.
Together, these studies demonstrate the feasibility and wide-reaching capacity for modeling genetic diseases using iPSC-CMs. Emerging and advancing technologies will allow this capacity to increase even further, and significant advances may come from these studies that may not be found in rodent and animal models.
Drug Discovery
An iPSC-CM expresses ion channels, including sodium channels, potassium channels, the hERG (Kv1.1) channel, and the hyperpolarization-activated cyclic nucleotide-gated (HCN) channel (funny current), that have the potential to interact with many pharmaceutical compounds [46]. Pharmaceutical researchers have a strong interest in identifying interactions between these channels and potential clinical drugs due to the severe risks that such interactions entail. As such, iPSC-CMs present an attractive alternative for other models, such as isolated adult myocytes, or human embryonic kidney cells or Chinese hamster ovary cells that have been used to force expression of hERG channels. These earlier techniques, where a single channel in a non-myocyte is probed, can miss a potential drug interaction. A better approach is where a cell expressing many types of ion channels is used to more accurately assess all potential interactions. One group, using MEA, tested the effects of eleven reference compounds on electrical activity of iPSC-CMs [89]. Of these, 5 were hERG blockers, 2 were Ca2+ channel blockers, 1 was a nonselective Ca2+ channel/hERG blocker, 1 was a KATP-channel blocker, and 2 were IKs blockers. The hERG blockers all prolonged the FPD, as expected, and the Ca2+ blockers shortened the FPD, as expected. The IKs blockers had only minor effects on FPD, but there was no expected response for either, and the authors were able to use the iPSC-CMs to evaluate the potential role of these drugs as well as the role of the IKs current in cardiac myocyte function.
Furthermore, as mentioned above, iPSC-CMs can be useful models for channelopathies, including Long QT syndrome, where the risk of a fatal drug interaction is much higher than in a healthy individual. Using cells from a LQTS-2 patient, effects of three compounds including nifedipine, a Ca2+ channel blocker; pinacidil, a KATP-channel opener; and ranolazine, a Na+ channel blocker was also examined [79]. All three drugs had predictable effects on LQTS iPSC-CMs, namely anti-arrhythmic effects, demonstrating the ability of these cells to recapitulate the in vivo effects of anti-arrhythmic drugs in an in vitro system. A second group, used another LQTS-2 iPSC line, and showed the ability of an IKr blocker, E4031, to prolong APD/FPD, and of a KATP-channel opener, nicorandil, to shorten APD and abolish EADs [53]. Furthermore, they demonstrated counteracting effects of isoprenaline, a β-adrenergic receptor agonist, and nadolol or propranolol, both β-adrenergic antagonists. On the other hand, the arrhythmogenic effects of the β antagonist sotalol was demonstrated at high concentrations when applied to LQTS iPSC-CMs, but not control iPSC-CMs [80]. Moreover, research groups [82] have been able to show positive effects of dantrolene on aberrant Ca2+ handling in cells from a patient with CPVT. Taken together, these studies in healthy as well as diseased cells show that iPSC-CMs can robustly examine the effects of current-affecting drugs on myocytes, and may be able to predict effects in future studies. Additionally, as shown above [89], iPSC-CMs can be useful for examining the mechanism of action of untested compounds. In this context, there is a report of altered hypertrophic signaling via the α- adrenergic pathway in hiPSC-CMs that should be addressed by detailed further study when pursuing iPSC lines for drug discovery [90].
Cell Therapy
Pluripotent stem cells and derived cardiac myocytes have long excited researchers with the prospect of being used as a clinical therapy. In the field of cardiology, there is great interest in using these cells as a potential treatment for heart failure and post-infarction cardiomyopathy. Pluripotent stem cell-derived cardiac myocytes injected directly into the myocardium are an obvious starting point. If these cells can engraft and electrically couple to existing myocardium they might provide sufficient contractile force to improve cardiac output. On the other hand, the cells may not survive the procedure, or may fail to couple to native myocytes, or may even be detrimental, as the procedure may cause deadly arrhythmia. These potential complications warrant extensive basic research before translation to human studies.
Several groups have published results from experiments injecting stem cells into animal models. It was demonstrated that hESC-CMs transplanted into immunocompromised NOD-SCID mice that had undergone myocardial infarction, engrafted and transiently improved cardiac function [91]. Transplanted cells were tracked by GFP and anti-human protein antibodies and the GFP+ cells were detected in the heart at least 12 weeks after transplant. The authors proposed functional coupling of transplanted and native cells based on connexin-43 and desmoplakin staining. Four weeks after transplant, transplanted mice had greater EF than non-transplanted controls; however, this difference in EF was lost 12 weeks after transplant. At least two important caveats to these results should be considered: first, that the population of transplanted cells, taken from beating EBs, was only 20–25% CMs. Second, connexin and desmoplakin staining are insufficient evidence of functional coupling in the absence of electrical or ionic data, especially since, as the authors noted, gap junctions and desmosomes were seen between hESC-CMs and themselves, but not between hESC-CMs and native myocytes. The same group later published results after injecting 3 times as many cells as previously, but noting, again, no functional improvement 12 weeks post-transplant [92].
In a similar study, EB-derived hESC-CMs (71% expressing cardiac TnI) were transplanted into male Sprague-Dawley rats that had undergone LAD and were treated with cyclosporine A and methylprednisolone to prevent rejection [93]. They reported evidence of engraftment of cells by imaging GFP or by staining for human markers, noting engraftment took place mostly in the border zone of the infarct region. Echocardiography showed decreased LV dilatation, greater fractional shortening (FS), and decreased pulmonary congestion in animals transplanted with hESC-CMs.
In an effort to improve engraftment, human ES-CMs was transplanted into infarcted rat hearts along with a “prosurvival cocktail (PSC)” that included Matrigel, a peptide from Bcl-XL, cyclosporine A, pinacidil, IGF-1, and a caspase inhibitor ZVAD-fmk [94]. This group detected engraftment based on human specific immunostaining and qPCR. Based on echocardiography, hearts transplanted with hESC-CMs showed decreased left ventricular end systolic diameter (LVESD) and increased FS, as well as increased thickening of the left ventricular wall in the infarct region. Later, they published a similar study [95], this time injecting cells 1 month after infarction. This treatment improved cardiac function, but did not alter dimensions or geometry of the myocardium. In 2012, this group published a study [96] using transplanted cells in immunosuppressed guinea pigs, wherein they attempted to demonstrate electrical coupling between hESC-CMs and host myocytes using the genetically encoded calcium sensor GCaMP3. To do this, they correlated fluorescent transients to ECG to determine synchrony. They also showed fewer arrhythmias in the form of premature ventricular contractions (PVCs) and ventricular tachycardia (VT) in transplanted animals versus controls. However, in their isolated heart studies, they demonstrated heterogeneous calcium transients and incomplete coupling, as well as a loss of coupling at higher pacing frequencies. In a technical tour de force, this group transplanted hESC-CMs into a non-human primate model of infarction [97]. The hESC-CM transplanted animals responded differently to treatment; while some had improved EF after transplant, some had no improvement. Perhaps most concerning, all hESC-CM transplanted animals displayed increased arrhythmias following transplant. This important study represents the current state of stem cell engraftment. It will be important to see follow-up studies in coming years as the issues of stable, physiological engraftment and functional restitution are established.
Concluding Remarks
We have discussed a large number of characteristics of pluripotent stem cell-derived cardiac myocytes, including differentiation methods, gene and protein expression, functionality, as well as potential applications. We have drawn on the work of many outstanding and diverse research groups to do so. Over the past decade, as our understanding of pluripotency and cardiac development has expanded, and our ability to engineer desired cell lineages continues to improve. Critical, additional study will allow us to improve and refine differentiation protocols, maturation protocols, and functionality assays. Our ability to model genetic diseases, and, potentially, acquired diseases quickly and efficiently, and without the use of animals, will allow for a shift in methods of drug and therapy innovation. Finally, continued work in the field may, someday, make iPSC-CM transplant an effective and safe therapy for cardiovascular disease.
Highlights.
-
♦
Maturation of hiPSC-CMs is essential to achieve full physiological function
-
♦
Contractile protein content and isoform profile are critical milestones in tracking hiPSC-CMs
-
♦
Facilitating the maturation of hiPSC-CMs is required realize the potential of hiPSC-CMs
Acknowledgments
This work is supported by NIH HL 1000407
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None
References
- 1.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malliaras K, Zhang Y, Seinfeld J, Galang G, Tseliou E, Cheng K, Sun B, Aminzadeh M, Marban E. Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO molecular medicine. 2013;5:191–209. doi: 10.1002/emmm.201201737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abraham MR, Henrikson CA, Tung L, Chang MG, Aon M, Xue T, Li RA, B OR, Marban E. Antiarrhythmic engineering of skeletal myoblasts for cardiac transplantation. Circulation research. 2005;97:159–167. doi: 10.1161/01.RES.0000174794.22491.a0. [DOI] [PubMed] [Google Scholar]
- 4.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 5.Kong CW, Akar FG, Li RA. Translational potential of human embryonic and induced pluripotent stem cells for myocardial repair: insights from experimental models. Thrombosis and haemostasis. 2010;104:30–38. doi: 10.1160/TH10-03-0189. [DOI] [PubMed] [Google Scholar]
- 6.Poon E, Kong CW, Li RA. Human pluripotent stem cell-based approaches for myocardial repair: from the electrophysiological perspective. Molecular pharmaceutics. 2011;8:1495–1504. doi: 10.1021/mp2002363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedada FB, Chan SS, Metzger SK, Zhang L, Zhang J, Garry DJ, Kamp TJ, Kyba M, Metzger JM. Acquisition of a quantitative, stoichiometrically conserved ratiometric marker of maturation status in stem cell-derived cardiac myocytes. Stem cell reports. 2014;3:594–605. doi: 10.1016/j.stemcr.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brette F, Orchard C. T-tubule function in mammalian cardiac myocytes. Circulation research. 2003;92:1182–1192. doi: 10.1161/01.RES.0000074908.17214.FD. [DOI] [PubMed] [Google Scholar]
- 9.Louch WE, Bito V, Heinzel FR, Macianskiene R, Vanhaecke J, Flameng W, Mubagwa K, Sipido KR. Reduced synchrony of Ca2+ release with loss of T-tubules-a comparison to Ca2+ release in human failing cardiomyocytes. Cardiovascular research. 2004;62:63–73. doi: 10.1016/j.cardiores.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 10.Karakikes I, Ameen M, Termglinchan V, Wu JC. Human induced pluripotent stem cell-derived cardiomyocytes: insights into molecular, cellular, and functional phenotypes. Circulation research. 2015;117:80–88. doi: 10.1161/CIRCRESAHA.117.305365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang X, Pabon L, Murry CE. Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circulation research. 2014;114:511–523. doi: 10.1161/CIRCRESAHA.114.300558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Klos M, Wilson GF, Herman AM, Lian X, Raval KK, Barron MR, Hou L, Soerens AG, Yu J, Palecek SP, Lyons GE, Thomson JA, Herron TJ, Jalife J, Kamp TJ. Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: the matrix sandwich method. Circulation research. 2012;111:1125–1136. doi: 10.1161/CIRCRESAHA.112.273144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, Raval KK, Zhang J, Kamp TJ, Palecek SP. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E1848–E1857. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sedmera D, Thompson RP. Myocyte proliferation in the developing heart. Developmental dynamics : an official publication of the American Association of Anatomists. 2011;240:1322–1334. doi: 10.1002/dvdy.22650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kikuchi K, Poss KD. Cardiac regenerative capacity and mechanisms. Annual review of cell and developmental biology. 2012;28:719–741. doi: 10.1146/annurev-cellbio-101011-155739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li F, Wang X, Capasso JM, Gerdes AM. Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development. Journal of molecular and cellular cardiology. 1996;28:1737–1746. doi: 10.1006/jmcc.1996.0163. [DOI] [PubMed] [Google Scholar]
- 18.Olivetti G, Cigola E, Maestri R, Corradi D, Lagrasta C, Gambert SR, Anversa P. Aging, cardiac hypertrophy and ischemic cardiomyopathy do not affect the proportion of mononucleated and multinucleated myocytes in the human heart. Journal of molecular and cellular cardiology. 1996;28:1463–1477. doi: 10.1006/jmcc.1996.0137. [DOI] [PubMed] [Google Scholar]
- 19.Adler CP, Costabel U. Cell number in human heart in atrophy, hypertrophy, and under the influence of cytostatics. Recent advances in studies on cardiac structure and metabolism. 1975;6:343–355. [PubMed] [Google Scholar]
- 20.Parmacek MS, Solaro RJ. Biology of the troponin complex in cardiac myocytes. Progress in cardiovascular diseases. 2004;47:159–176. doi: 10.1016/j.pcad.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 21.MacGeoch C, Barton PJ, Vallins WJ, Bhavsar P, Spurr NK. The human cardiac troponin I locus: assignment to chromosome 19p13.2–19q13.2. Human genetics. 1991;88:101–104. doi: 10.1007/BF00204938. [DOI] [PubMed] [Google Scholar]
- 22.Hunkeler NM, Kullman J, Murphy AM. Troponin I isoform expression in human heart. Circulation research. 1991;69:1409–1414. doi: 10.1161/01.res.69.5.1409. [DOI] [PubMed] [Google Scholar]
- 23.Sasse S, Brand NJ, Kyprianou P, Dhoot GK, Wade R, Arai M, Periasamy M, Yacoub MH, Barton PJ. Troponin I gene expression during human cardiac development and in end-stage heart failure. Circulation research. 1993;72:932–938. doi: 10.1161/01.res.72.5.932. [DOI] [PubMed] [Google Scholar]
- 24.Averyhart-Fullard V, Fraker LD, Murphy AM, Solaro RJ. Differential regulation of slow-skeletal and cardiac troponin I mRNA during development and by thyroid hormone in rat heart. Journal of molecular and cellular cardiology. 1994;26:609–616. doi: 10.1006/jmcc.1994.1073. [DOI] [PubMed] [Google Scholar]
- 25.Schiaffino S, Gorza L, Ausoni S. Troponin isoform switching in the developing heart and its functional consequences. Trends in cardiovascular medicine. 1993;3:12–17. doi: 10.1016/1050-1738(93)90022-X. [DOI] [PubMed] [Google Scholar]
- 26.Westfall MV, Samuelson LC, Metzger JM. Troponin I isoform expression is developmentally regulated in differentiating embryonic stem cell-derived cardiac myocytes. Developmental dynamics : an official publication of the American Association of Anatomists. 1996;206:24–38. doi: 10.1002/(SICI)1097-0177(199605)206:1<24::AID-AJA3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 27.Metzger JM, Westfall MV. Covalent and noncovalent modification of thin filament action: the essential role of troponin in cardiac muscle regulation. Circulation research. 2004;94:146–158. doi: 10.1161/01.RES.0000110083.17024.60. [DOI] [PubMed] [Google Scholar]
- 28.Kentish JC, McCloskey DT, Layland J, Palmer S, Leiden JM, Martin AF, Solaro RJ. Phosphorylation of troponin I by protein kinase A accelerates relaxation and crossbridge cycle kinetics in mouse ventricular muscle. Circulation research. 2001;88:1059–1065. doi: 10.1161/hh1001.091640. [DOI] [PubMed] [Google Scholar]
- 29.Layland J, Solaro RJ, Shah AM. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovascular research. 2005;66:12–21. doi: 10.1016/j.cardiores.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 30.Yasuda S, Coutu P, Sadayappan S, Robbins J, Metzger JM. Cardiac transgenic and gene transfer strategies converge to support an important role for troponin I in regulating relaxation in cardiac myocytes. Circulation research. 2007;101:377–386. doi: 10.1161/CIRCRESAHA.106.145557. [DOI] [PubMed] [Google Scholar]
- 31.Huang X, Lee KJ, Riedel B, Zhang C, Lemanski LF, Walker JW. Thyroid hormone regulates slow skeletal troponin I gene inactivation in cardiac troponin I null mouse hearts. Journal of molecular and cellular cardiology. 2000;32:2221–2228. doi: 10.1006/jmcc.2000.1249. [DOI] [PubMed] [Google Scholar]
- 32.Razeghi P, Young ME, Alcorn JL, Moravec CS, Frazier OH, Taegtmeyer H. Metabolic gene expression in fetal and failing human heart. Circulation. 2001;104:2923–2931. doi: 10.1161/hc4901.100526. [DOI] [PubMed] [Google Scholar]
- 33.Lundy SD, Zhu WZ, Regnier M, Laflamme MA. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem cells and development. 2013;22:1991–2002. doi: 10.1089/scd.2012.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kubalak SW, Miller-Hance WC, O'Brien TX, Dyson E, Chien KR. Chamber specification of atrial myosin light chain-2 expression precedes septation during murine cardiogenesis. The Journal of biological chemistry. 1994;269:16961–16970. [PubMed] [Google Scholar]
- 35.O'Brien TX, Lee KJ, Chien KR. Positional specification of ventricular myosin light chain 2 expression in the primitive murine heart tube. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:5157–5161. doi: 10.1073/pnas.90.11.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mummery CL, Zhang J, Ng ES, Elliott DA, Elefanty AG, Kamp TJ. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circulation research. 2012;111:344–358. doi: 10.1161/CIRCRESAHA.110.227512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parker TG, Packer SE, Schneider MD. Peptide growth factors can provoke "fetal" contractile protein gene expression in rat cardiac myocytes. The Journal of clinical investigation. 1990;85:507–514. doi: 10.1172/JCI114466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kinugawa K, Minobe WA, Wood WM, Ridgway EC, Baxter JD, Ribeiro RC, Tawadrous MF, Lowes BA, Long CS, Bristow MR. Signaling pathways responsible for fetal gene induction in the failing human heart: evidence for altered thyroid hormone receptor gene expression. Circulation. 2001;103:1089–1094. doi: 10.1161/01.cir.103.8.1089. [DOI] [PubMed] [Google Scholar]
- 39.Reiser PJ, Portman MA, Ning XH, Schomisch Moravec C. Human cardiac myosin heavy chain isoforms in fetal and failing adult atria and ventricles, American journal of physiology. Heart and circulatory physiology. 2001;280:H1814–H1820. doi: 10.1152/ajpheart.2001.280.4.H1814. [DOI] [PubMed] [Google Scholar]
- 40.Chuva de Sousa Lopes SM, Hassink RJ, Feijen A, van Rooijen MA, Doevendans PA, Tertoolen L, Brutel de la Riviere A, Mummery CL. Patterning the heart, a template for human cardiomyocyte development. Developmental dynamics : an official publication of the American Association of Anatomists. 2006;235:1994–2002. doi: 10.1002/dvdy.20830. [DOI] [PubMed] [Google Scholar]
- 41.Lockhart M, Wirrig E, Phelps A, Wessels A. Extracellular matrix and heart development, Birth defects research, Part A. Clinical and molecular teratology. 2011;91:535–550. doi: 10.1002/bdra.20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morkin E. Regulation of myosin heavy chain genes in the heart. Circulation. 1993;87:1451–1460. doi: 10.1161/01.cir.87.5.1451. [DOI] [PubMed] [Google Scholar]
- 43.Morkin E. Control of cardiac myosin heavy chain gene expression. Microscopy research and technique. 2000;50:522–531. doi: 10.1002/1097-0029(20000915)50:6<522::AID-JEMT9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 44.Yang X, Rodriguez M, Pabon L, Fischer KA, Reinecke H, Regnier M, Sniadecki NJ, Ruohola-Baker H, Murry CE. Tri-iodo-l-thyronine promotes the maturation of human cardiomyocytes-derived from induced pluripotent stem cells. Journal of molecular and cellular cardiology. 2014;72:296–304. doi: 10.1016/j.yjmcc.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng HZ, Hossain MM, Huang XP, Jin JP. Myofilament incorporation determines the stoichiometry of troponin I in transgenic expression and the rescue of a null mutation. Archives of biochemistry and biophysics. 2009;487:36–41. doi: 10.1016/j.abb.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Priori SG, Napolitano C, Di Pasquale E, Condorelli G. Induced pluripotent stem cell-derived cardiomyocytes in studies of inherited arrhythmias. The Journal of clinical investigation. 2013;123:84–91. doi: 10.1172/JCI62838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng S, Lacerda AE, Kirsch GE, Brown AM, Bruening-Wright A. The action potential and comparative pharmacology of stem cell-derived human cardiomyocytes. Journal of pharmacological and toxicological methods. 2010;61:277–286. doi: 10.1016/j.vascn.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 48.Stett A, Egert U, Guenther E, Hofmann F, Meyer T, Nisch W, Haemmerle H. Biological application of microelectrode arrays in drug discovery and basic research. Analytical and bioanalytical chemistry. 2003;377:486–495. doi: 10.1007/s00216-003-2149-x. [DOI] [PubMed] [Google Scholar]
- 49.Yamazaki K, Hihara T, Taniguchi T, Kohmura N, Yoshinaga T, Ito M, Sawada K. A novel method of selecting human embryonic stem cell-derived cardiomyocyte clusters for assessment of potential to influence QT interval. Toxicology in vitro : an international journal published in association with BIBRA. 2012;26:335–342. doi: 10.1016/j.tiv.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 50.Kehat I, Gepstein A, Spira A, Itskovitz-Eldor J, Gepstein L. High-resolution electrophysiological assessment of human embryonic stem cell-derived cardiomyocytes: a novel in vitro model for the study of conduction. Circulation research. 2002;91:659–661. doi: 10.1161/01.res.0000039084.30342.9b. [DOI] [PubMed] [Google Scholar]
- 51.Nakamura Y, Matsuo J, Miyamoto N, Ojima A, Ando K, Kanda Y, Sawada K, Sugiyama A, Sekino Y. Assessment of testing methods for drug-induced repolarization delay and arrhythmias in an iPS cell-derived cardiomyocyte sheet: multi-site validation study. Journal of pharmacological sciences. 2014;124:494–501. doi: 10.1254/jphs.13248fp. [DOI] [PubMed] [Google Scholar]
- 52.Asakura K, Hayashi S, Ojima A, Taniguchi T, Miyamoto N, Nakamori C, Nagasawa C, Kitamura T, Osada T, Honda Y, Kasai C, Ando H, Kanda Y, Sekino Y, Sawada K. Improvement of acquisition and analysis methods in multi-electrode array experiments with iPS cell-derived cardiomyocytes. Journal of pharmacological and toxicological methods. 2015 doi: 10.1016/j.vascn.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 53.Matsa E, Rajamohan D, Dick E, Young L, Mellor I, Staniforth A, Denning C. Drug evaluation in cardiomyocytes derived from human induced pluripotent stem cells carrying a long QT syndrome type 2 mutation. European heart journal. 2011;32:952–962. doi: 10.1093/eurheartj/ehr073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Honda M, Kiyokawa J, Tabo M, Inoue T. Electrophysiological characterization of cardiomyocytes derived from human induced pluripotent stem cells. Journal of pharmacological sciences. 2011;117:149–159. doi: 10.1254/jphs.11038fp. [DOI] [PubMed] [Google Scholar]
- 55.Scheel O, Frech S, Amuzescu B, Eisfeld J, Lin KH, Knott T. Action potential characterization of human induced pluripotent stem cell-derived cardiomyocytes using automated patch-clamp technology. Assay and drug development technologies. 2014;12:457–469. doi: 10.1089/adt.2014.601. [DOI] [PubMed] [Google Scholar]
- 56.Sheng X, Reppel M, Nguemo F, Mohammad FI, Kuzmenkin A, Hescheler J, Pfannkuche K. Human pluripotent stem cell-derived cardiomyocytes: response to TTX and lidocain reveals strong cell to cell variability. PloS one. 2012;7:e45963. doi: 10.1371/journal.pone.0045963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pfannkuche K, Liang H, Hannes T, Xi J, Fatima A, Nguemo F, Matzkies M, Wernig M, Jaenisch R, Pillekamp F, Halbach M, Schunkert H, Saric T, Hescheler J, Reppel M. Cardiac myocytes derived from murine reprogrammed fibroblasts: intact hormonal regulation, cardiac ion channel expression and development of contractility. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2009;24:73–86. doi: 10.1159/000227815. [DOI] [PubMed] [Google Scholar]
- 58.Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, Thomson JA, Kamp TJ. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circulation research. 2009;104:e30–e41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 60.Cannell MB, Cheng H, Lederer WJ. The control of calcium release in heart muscle. Science. 1995;268:1045–1049. doi: 10.1126/science.7754384. [DOI] [PubMed] [Google Scholar]
- 61.Itzhaki I, Rapoport S, Huber I, Mizrahi I, Zwi-Dantsis L, Arbel G, Schiller J, Gepstein L. Calcium handling in human induced pluripotent stem cell derived cardiomyocytes. PloS one. 2011;6:e18037. doi: 10.1371/journal.pone.0018037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gorski PA, Ceholski DK, Hajjar RJ. Altered myocardial calcium cycling and energetics in heart failure-a rational approach for disease treatment. Cell metabolism. 2015;21:183–194. doi: 10.1016/j.cmet.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Satin J, Itzhaki I, Rapoport S, Schroder EA, Izu L, Arbel G, Beyar R, Balke CW, Schiller J, Gepstein L. Calcium handling in human embryonic stem cell-derived cardiomyocytes. Stem Cells. 2008;26:1961–1972. doi: 10.1634/stemcells.2007-0591. [DOI] [PubMed] [Google Scholar]
- 64.Mauritz C, Schwanke K, Reppel M, Neef S, Katsirntaki K, Maier LS, Nguemo F, Menke S, Haustein M, Hescheler J, Hasenfuss G, Martin U. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation. 2008;118:507–517. doi: 10.1161/CIRCULATIONAHA.108.778795. [DOI] [PubMed] [Google Scholar]
- 65.Lee P, Klos M, Bollensdorff C, Hou L, Ewart P, Kamp TJ, Zhang J, Bizy A, Guerrero-Serna G, Kohl P, Jalife J, Herron TJ. Simultaneous voltage and calcium mapping of genetically purified human induced pluripotent stem cell-derived cardiac myocyte monolayers. Circulation research. 2012;110:1556–1563. doi: 10.1161/CIRCRESAHA.111.262535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang XH, Haviland S, Wei H, Saric T, Fatima A, Hescheler J, Cleemann L, Morad M. Ca2+ signaling in human induced pluripotent stem cell-derived cardiomyocytes (iPS-CM) from normal and catecholaminergic polymorphic ventricular tachycardia (CPVT)-afflicted subjects. Cell calcium. 2013;54:57–70. doi: 10.1016/j.ceca.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ivashchenko CY, Pipes GC, Lozinskaya IM, Lin Z, Xiaoping X, Needle S, Grygielko ET, Hu E, Toomey JR, Lepore JJ, Willette RN. Human-induced pluripotent stem cell-derived cardiomyocytes exhibit temporal changes in phenotype, American journal of physiology. Heart and circulatory physiology. 2013;305:H913–H922. doi: 10.1152/ajpheart.00819.2012. [DOI] [PubMed] [Google Scholar]
- 68.Hwang HS, Kryshtal DO, Feaster TK, Sanchez-Freire V, Zhang J, Kamp TJ, Hong CC, Wu JC, Knollmann BC. Comparable calcium handling of human iPSC-derived cardiomyocytes generated by multiple laboratories. Journal of molecular and cellular cardiology. 2015;85:79–88. doi: 10.1016/j.yjmcc.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koubassova NA, Tsaturyan AK. Molecular mechanism of actin-myosin motor in muscle. Biochemistry, Biokhimiia. 2011;76:1484–1506. doi: 10.1134/S0006297911130086. [DOI] [PubMed] [Google Scholar]
- 70.He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circulation research. 2003;93:32–39. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- 71.Ma Z, Koo S, Finnegan MA, Loskill P, Huebsch N, Marks NC, Conklin BR, Grigoropoulos CP, Healy KE. Three-dimensional filamentous human diseased cardiac tissue model. Biomaterials. 2014;35:1367–1377. doi: 10.1016/j.biomaterials.2013.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun N, Yazawa M, Liu J, Han L, Sanchez-Freire V, Abilez OJ, Navarrete EG, Hu S, Wang L, Lee A, Pavlovic A, Lin S, Chen R, Hajjar RJ, Snyder MP, Dolmetsch RE, Butte MJ, Ashley EA, Longaker MT, Robbins RC, Wu JC. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Science translational medicine. 2012;4:130ra147. doi: 10.1126/scitranslmed.3003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Domke J, Parak WJ, George M, Gaub HE, Radmacher M. Mapping the mechanical pulse of single cardiomyocytes with the atomic force microscope. European biophysics journal : EBJ. 1999;28:179–186. doi: 10.1007/s002490050198. [DOI] [PubMed] [Google Scholar]
- 74.Rodriguez ML, Graham BT, Pabon LM, Han SJ, Murry CE, Sniadecki NJ. Measuring the contractile forces of human induced pluripotent stem cell-derived cardiomyocytes with arrays of microposts. Journal of biomechanical engineering. 2014;136:051005. doi: 10.1115/1.4027145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sheehy SP, Pasqualini F, Grosberg A, Park SJ, Aratyn-Schaus Y, Parker KK. Quality metrics for stem cell-derived cardiac myocytes. Stem cell reports. 2014;2:282–294. doi: 10.1016/j.stemcr.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Malan D, Friedrichs S, Fleischmann BK, Sasse P. Cardiomyocytes obtained from induced pluripotent stem cells with long-QT syndrome 3 recapitulate typical disease-specific features in vitro. Circulation research. 2011;109:841–847. doi: 10.1161/CIRCRESAHA.111.243139. [DOI] [PubMed] [Google Scholar]
- 77.Nuyens D, Stengl M, Dugarmaa S, Rossenbacker T, Compernolle V, Rudy Y, Smits JF, Flameng W, Clancy CE, Moons L, Vos MA, Dewerchin M, Benndorf K, Collen D, Carmeliet E, Carmeliet P. Abrupt rate accelerations or premature beats cause life-threatening arrhythmias in mice with long-QT3 syndrome. Nature medicine. 2001;7:1021–1027. doi: 10.1038/nm0901-1021. [DOI] [PubMed] [Google Scholar]
- 78.Fatima A, Kaifeng S, Dittmann S, Xu G, Gupta MK, Linke M, Zechner U, Nguemo F, Milting H, Farr M, Hescheler J, Saric T. The disease-specific phenotype in cardiomyocytes derived from induced pluripotent stem cells of two long QT syndrome type 3 patients. PloS one. 2013;8:e83005. doi: 10.1371/journal.pone.0083005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Itzhaki I, Maizels L, Huber I, Zwi-Dantsis L, Caspi O, Winterstern A, Feldman O, Gepstein A, Arbel G, Hammerman H, Boulos M, Gepstein L. Modelling the long QT syndrome with induced pluripotent stem cells. Nature. 2011;471:225–229. doi: 10.1038/nature09747. [DOI] [PubMed] [Google Scholar]
- 80.Lahti AL, Kujala VJ, Chapman H, Koivisto AP, Pekkanen-Mattila M, Kerkela E, Hyttinen J, Kontula K, Swan H, Conklin BR, Yamanaka S, Silvennoinen O, Aalto-Setala K. Model for long QT syndrome type 2 using human iPS cells demonstrates arrhythmogenic characteristics in cell culture. Disease models & mechanisms. 2012;5:220–230. doi: 10.1242/dmm.008409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yazawa M, Hsueh B, Jia X, Pasca AM, Bernstein JA, Hallmayer J, Dolmetsch RE. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature. 2011;471:230–234. doi: 10.1038/nature09855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jung CB, Moretti A, Mederos y Schnitzler M, Iop L, Storch U, Bellin M, Dorn T, Ruppenthal S, Pfeiffer S, Goedel A, Dirschinger RJ, Seyfarth M, Lam JT, Sinnecker D, Gudermann T, Lipp P, Laugwitz KL. Dantrolene rescues arrhythmogenic RYR2 defect in a patient-specific stem cell model of catecholaminergic polymorphic ventricular tachycardia. EMBO molecular medicine. 2012;4:180–191. doi: 10.1002/emmm.201100194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang G, McCain ML, Yang L, He A, Pasqualini FS, Agarwal A, Yuan H, Jiang D, Zhang D, Zangi L, Geva J, Roberts AE, Ma Q, Ding J, Chen J, Wang DZ, Li K, Wang J, Wanders RJ, Kulik W, Vaz FM, Laflamme MA, Murry CE, Chien KR, Kelley RI, Church GM, Parker KK, Pu WT. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nature medicine. 2014;20:616–623. doi: 10.1038/nm.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang HP, Chen PH, Hwu WL, Chuang CY, Chien YH, Stone L, Chien CL, Li LT, Chiang SC, Chen HF, Ho HN, Chen CH, Kuo HC. Human Pompe disease-induced pluripotent stem cells for pathogenesis modeling, drug testing and disease marker identification. Human molecular genetics. 2011;20:4851–4864. doi: 10.1093/hmg/ddr424. [DOI] [PubMed] [Google Scholar]
- 85.Carvajal-Vergara X, Sevilla A, D'Souza SL, Ang YS, Schaniel C, Lee DF, Yang L, Kaplan AD, Adler ED, Rozov R, Ge Y, Cohen N, Edelmann LJ, Chang B, Waghray A, Su J, Pardo S, Lichtenbelt KD, Tartaglia M, Gelb BD, Lemischka IR. Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature. 2010;465:808–812. doi: 10.1038/nature09005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ma D, Wei H, Lu J, Ho S, Zhang G, Sun X, Oh Y, Tan SH, Ng ML, Shim W, Wong P, Liew R. Generation of patient-specific induced pluripotent stem cell-derived cardiomyocytes as a cellular model of arrhythmogenic right ventricular cardiomyopathy. European heart journal. 2013;34:1122–1133. doi: 10.1093/eurheartj/ehs226. [DOI] [PubMed] [Google Scholar]
- 87.Vasileva EA, Shuvalov OU, Garabadgiu AV, Melino G, Barlev NA. Genome-editing tools for stem cell biology. Cell death & disease. 2015;6:e1831. doi: 10.1038/cddis.2015.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arai S, Miyauchi M, Kurokawa M. Modeling of hematologic malignancies by iPS technology. Experimental hematology. 2015;43:654–660. doi: 10.1016/j.exphem.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 89.Braam SR, Tertoolen L, Casini S, Matsa E, Lu HR, Teisman A, Passier R, Denning C, Gallacher DJ, Towart R, Mummery CL. Repolarization reserve determines drug responses in human pluripotent stem cell derived cardiomyocytes. Stem cell research. 2013;10:48–56. doi: 10.1016/j.scr.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 90.Foldes G, Matsa E, Kriston-Vizi J, Leja T, Amisten S, Kolker L, Kodagoda T, Dolatshad NF, Mioulane M, Vauchez K, Aranyi T, Ketteler R, Schneider MD, Denning C, Harding SE. Aberrant alpha-adrenergic hypertrophic response in cardiomyocytes from human induced pluripotent cells. Stem cell reports. 2014;3:905–914. doi: 10.1016/j.stemcr.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Laake LW, Passier R, Monshouwer-Kloots J, Verkleij AJ, Lips DJ, Freund C, den Ouden K, Ward-van Oostwaard D, Korving J, Tertoolen LG, van Echteld CJ, Doevendans PA, Mummery CL. Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem cell research. 2007;1:9–24. doi: 10.1016/j.scr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 92.van Laake LW, Passier R, Doevendans PA, Mummery CL. Human embryonic stem cell-derived cardiomyocytes and cardiac repair in rodents. Circulation research. 2008;102:1008–1010. doi: 10.1161/CIRCRESAHA.108.175505. [DOI] [PubMed] [Google Scholar]
- 93.Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, Yankelson L, Aronson D, Beyar R, Gepstein L. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. Journal of the American College of Cardiology. 2007;50:1884–1893. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 94.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nature biotechnology. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 95.Fernandes S, Naumova AV, Zhu WZ, Laflamme MA, Gold J, Murry CE. Human embryonic stem cell-derived cardiomyocytes engraft but do not alter cardiac remodeling after chronic infarction in rats. Journal of molecular and cellular cardiology. 2010;49:941–949. doi: 10.1016/j.yjmcc.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, Palpant NJ, Gantz J, Moyes KW, Reinecke H, Van Biber B, Dardas T, Mignone JL, Izawa A, Hanna R, Viswanathan M, Gold JD, Kotlikoff MI, Sarvazyan N, Kay MW, Murry CE, Laflamme MA. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489:322–325. doi: 10.1038/nature11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, Murry CE. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]