Abstract
Cystic fibrosis (CF) patients battle life-long pulmonary infections with the respiratory pathogen Pseudomonas aeruginosa (PA). An overabundance of mucus in CF airways provides a favorable niche for PA growth. When compared to that of non-CF individuals, mucus of CF airways is enriched in sialyl-Lewisx, a preferred binding receptor for PA. Notably, the levels of sialyl-Lewisx directly correlate with infection severity in CF patients. However, the mechanism by which PA causes increased sialylation remains uncharacterized. In this study, we examined the ability of PA virulence factors to modulate sialyl-Lewisx modification in airway mucins. We found pyocyanin (PCN) to be a potent inducer of sialyl-Lewisx in both mouse airways and in primary and immortalized CF and non-CF human airway epithelial cells. PCN increased the expression of C2/4GnT and ST3Gal-IV, two of the glycosyltransferases responsible for the stepwise biosynthesis of sialyl-Lewisx, through a TNF-α-mediated phosphoinositol-specific phospholipase C (PI-PLC) dependent pathway. Furthermore, PA bound more efficiently to airway epithelial cells pre-exposed to PCN through a flagellar cap-dependent manner. Importantly, antibodies against sialyl-Lewisx and anti-TNF-α attenuated PA binding. These results indicate that PCN secretes PCN to induce a favorable environment for chronic colonization of CF lungs by increasing the glycosylation of airway mucins with sialyl-Lewisx.
INTRODUCTION
Pulmonary infections with Pseudomonas aeruginosa (PA) are a critical clinical concern for patients with cystic fibrosis (CF),1,2 with 95% of individuals colonized with the pathogen by the age of three.3 Pulmonary failure, a sequela of acute exacerbations and tissue scarring in chronic infections, results in high morbidity and mortality in CF patients.1,2 Previously understood factors contributing to PA colonization in the CF airways include overproduction of hyperviscous mucus and impeded mucocilliary clearance of trapped microbes.1 Mucin glycoproteins are major components of airway mucus that contain on their structure a diverse population of carbohydrate chains that have been shown to be receptors for bacteria. Their intraluminal location in the airway serves as a first line of interaction with microbes in the lung.4-8 Mucins recovered from CF airways are enriched with the tetracarbohydrate moiety sialyl-Lewisx.9-11 Through its flagellar cap, PA binds sialyl-Lewisx-glycosylated CF mucins with a higher affinity than other carbohydrate moieties over control lung tissues.4,7,12,13 The enzymes core 2/core 4 beta-1,6-N-acetylglucosaminyltransferase (C2/4GnT) and α2,3-sialyltransferase IV (ST3Gal-IV), which are crucial for sialyl-Lewisx synthesis, are upregulated during pulmonary inflammation, especially in CF.6,8,14-16 Specifically, exposure to TNF-α, IL-6 and IL-8 increases the level of sialyl-Lewisx on mucins.13-17 Although controversy remains, increasing evidence suggests that CF epithelium is proinflammatory primed, and chronic bacterial infection causes a prolonged inflammatory response when compared to other diseased airways.18,19 The further finding of a direct correlation between severity of CF infection and the levels of sialyl-Lewisx glycosylation on airway mucins11 underscores the importance of bacterial etiology as an inciting factor in the modification of these mucins. Together, these findings warrant further investigation on the effects of PA virulence in relation to changes in sialyl-Lewisx levels.
RESULTS
Pyocyanin is a potent inducer of sialomucins
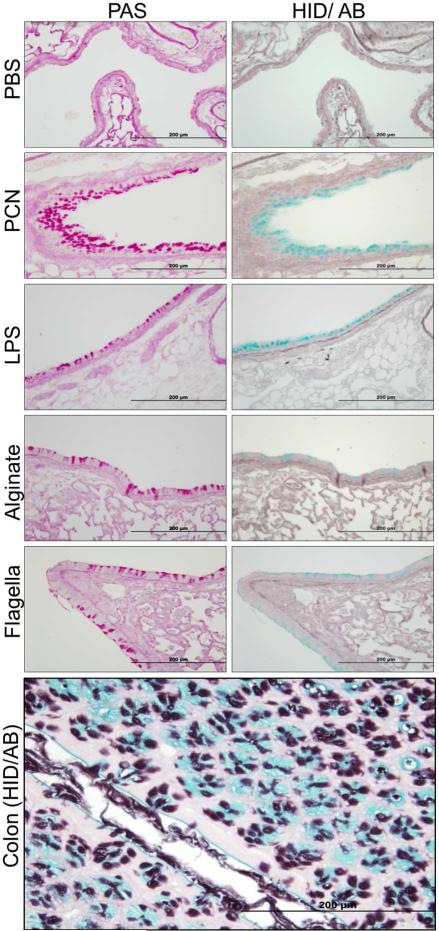
We evaluated the ability of various purified PA components to induce changes in mucin glycosylation during chronic exposure in mouse lungs. Recovered lung sections were stained with Periodic acid-Schiff (PAS) to determine the presence of goblet cell hyperplasia and metaplasia (GCHM) and mucin hypersecretion, and by the high iron diamine-alcian blue (HID-AB) to detect sialomucins (blue) and sulfomucins (brown). Although all PA components were able to induce higher expression of sialomucins when compared to the PBS, PCN caused the most dramatic increase (Figure 1). Interestingly, no sulfomucins were detected in mouse airways, despite their prominent presence in colon sections from the same animals (Figure 1).
Figure 1.
PCN is a potent inducer of sialomucins. Serial sections of paraffin-embedded lungs from mice (n=10) exposed to PBS or various purified PA components were stained using PAS to detect goblet cells and high iron diamine/Alcian blue (HID/AB) to detect sialo- and sulfomucins. Sections of mouse colon were used as positive control tissues for the HID/ AB staining. PAS-stained goblet cells are pink. Sialomucins in colon stained blue. Sulfomucins stained brown.
Pyocyanin induces sialyl-Lewisx epitopes in mouse airway epithelium
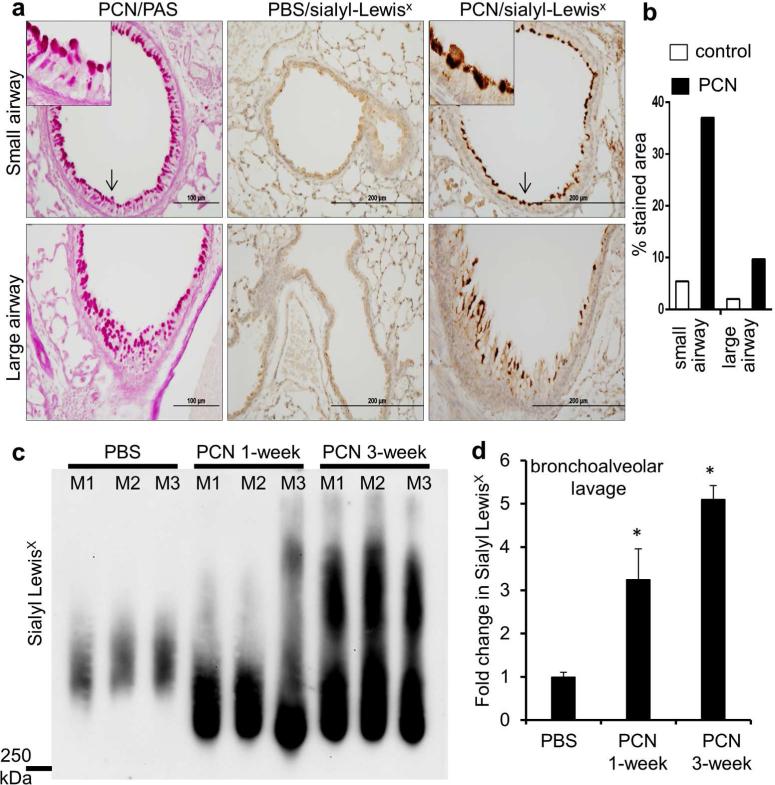
PCN is a redox-active tricyclic toxin that has been recovered in varying concentrations from trace quantities to 100 μM (27 μg/ml sputum) in pulmonary secretions of CF and non-CF bronchiectatic patients infected by PA, and its concentrations are inversely correlated with the lung function of CF patients.20,21 We and others have shown that PCN is a potent inducer of GCHM and mucus hypersecretion,22-25 by inactivating FOXA2, a key transcription repressor of GCHM and mucus biosynthesis.23-24 Because PCN also induces mucin sialylation, the remainder of this study focused on PCN-mediated mucin sialylation. We examined the effect of chronic PCN exposure on the levels of sialyl-Lewisx epitopes on mucins secreted by mouse bronchial mucosa. PAS staining indicated that PCN induced GCHM and mucin hypersecretion in mouse airways (Figure 2a). Immunohistochemical (IHC) analyses demonstrated that chronic PCN administration significantly increased the expression of mucins harboring sialyl-Lewisx epitopes in both large and small airways by 10 and 35-fold, respectively, when compared to control lungs (Figure 2a and b). To examine whether secreted mucins were sialylated, we performed bronchoalveolar lavage, and examined mucin sialylation in recovered BAL fluid (BALF). At 1-week and 3-week post exposure, PCN increased the amounts of secreted sialomucins by 3.3 and 5.1-fold, respectively, in comparison to control mice exposed to PBS (Figure 2c and d). These results indicate that PCN induces an increase in the amount of sialyl-Lewisx present on cell-associated and secreted mucins in bronchial mucosa in vivo.
Figure 2.
PCN induces the expression of sialyl-Lewisx epitopes in mouse airways. (a) IHC analyses of mouse lung sections exposed to PCN or PBS stained with an anti-sialyl-Lewisx antibody. Serial PCN-exposed sections were stained with PAS for the presence of goblet cell hyperplasia and metaplasia. Magnified areas are indicated by arrows. (b) Sialyl-Lewisx stained areas in control versus PCN-treated large and small airways in C57BL6 mice were quantified using the ImageJ software. Average values large and small airways from 3 mice from each treatment, respectively, are shown. (c) Western blot analysis of sialyl-Lewisx in the BALF (15 μg total protein per lane) of mice exposed to PBS (once daily, 3 weeks) and or to 25 μg PCN (once daily, 1 week or 3 weeks. (d) Densitometry analysis of sialyl-LewisX expression in (c). Statistical significance comparisons among various time points were determined by using the one-way ANOVA analysis (p < 0.05). *p < 0.05 when compared against PBS control by using the Tukey's test.
Pyocyanin induces sialyl-Lewisx in time and concentration-dependent manners
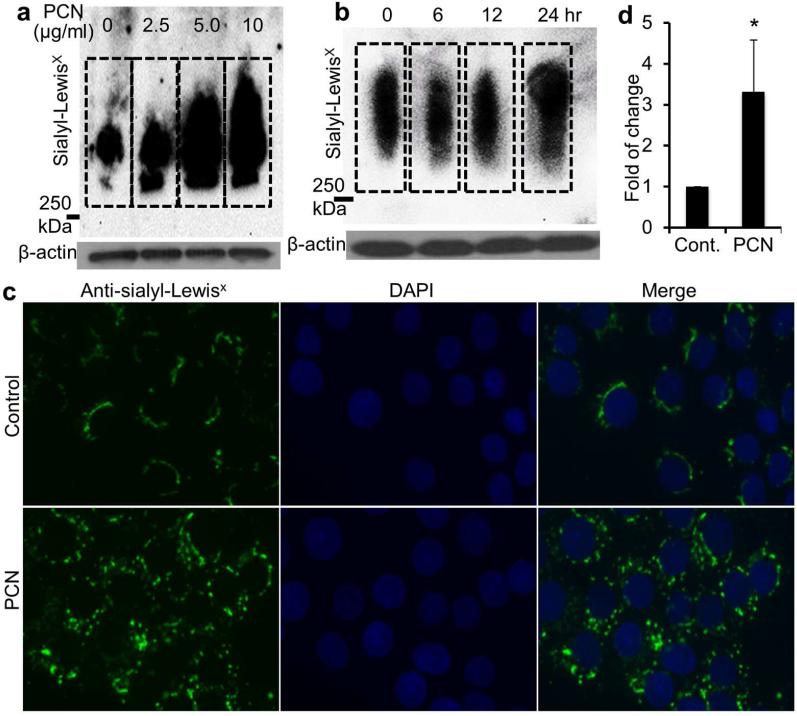
To examine the mechanism of PCN-induced mucin sialylation, we tested the amenability of NCI-H292 cells on the expression of sialyl-Lewisx in vitro during exposure to the toxin. After 24 hr, PCN at 2.5, 5.0 and 10 μg/ml concentrations significantly increased the expression of sialyl-Lewisx by 2.7, 5.6 and 6.4-fold, respectively (Figure 3a, Figure S1a). Furthermore, PCN (5.0 μg/ml) induced an increase in sialyl-Lewisx at 24 hr post-exposure (Figure 3b, Figure S1b), a time point where significant amounts of synthesized mucins are secreted. To further confirm these findings, an immunofluorescence assay (IFA) was used to examine the induction of sialyl-Lewisx by PCN. In the PCN (5 μg/ml, 24 hr) treated NCI-H292 cells, 3.3-fold higher sialyl-Lewisx epitopes were seen, along with a change in cellular localization from predominantly perinuclear to diffused cytoplasmic (Figure 3c and d). Collectively, these results indicate that PCN induces the expression of sialyl-Lewisx in NCI-H292 cells in concentration and time-dependent manners.
Figure 3.
PCN upregulates the expression of sialyl-Lewisx in time and concentration-dependent manners. (a and b) NCI-H292 cells were exposed to PBS (control) or indicated concentrations of PCN or at predetermined time intervals (with 5 μg/ml PCN). Total proteins were separated on an agarose-acrylamide gel. The expression of sialyl-Lewisx was analyzed using specific antibody by Western blots. β-actin was used as loading controls. Densitometry analysis of the sialyl-LewisX expression can be found in Figure S1. (c) Separate sets of cells from above were stained for sialyl-LewisX with antibodies and visualized with Alexa Fluor®488-conjugated secondary antibody (green color). (d) Quantification of total fluorescence of control and PCN treated cells. The mean ± standard error of total florescence in 10 representative high power fields from each treatment group in one typical experiment is shown. *p < 0.05 when compared against PBS control using the parametric Student's t test.
PCN induces the sialyl-LewisX glycosylation on MUC5AC mucin in primary human bronchial epithelial cells
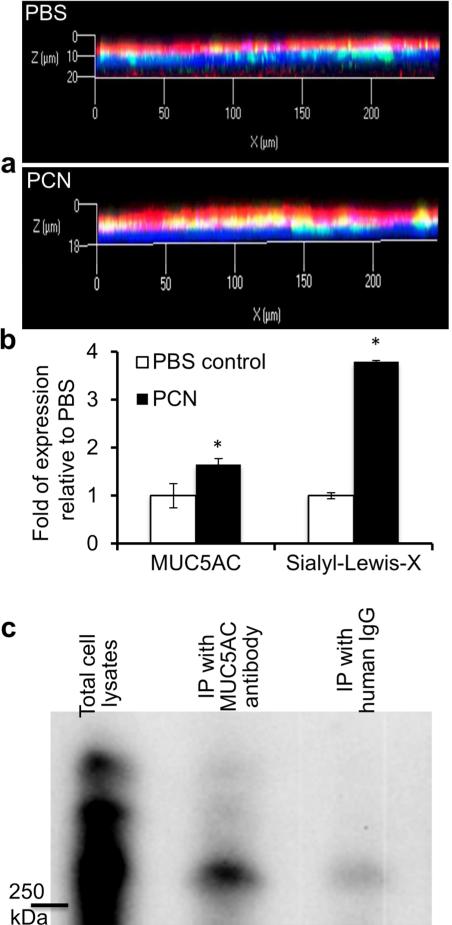
Normal primary human bronchial epithelial (NHBE) cells cultured in the air-liquid interface (ALI) have emerged as a powerful tool for the study of airway biology. We have shown that PCN induces the secretion of major airway mucins, MUC5B and MUC5AC in NHBE cells.23,24 Thus, we determined if clinically relevant concentration of PCN (12.5 μg/ml) could induce the modification of MUC5AC with sialyl-LewisX in ALI culture of NHBE cells. The amount of MUC5AC increased by 65% within 24 hr (Figure 4a and b). Significantly, PCN induced a 380% increase in the sialylation of MUC5AC when compared to PBS (Figure 4 and b).
Figure 4.
PCN induces sialylation of MUC5AC mucin in ALI culture of NHBE cells and in immortalized airway epithelial cells. (a) Differentiated NHBE cells were exposed to PBS or PCN (12.5 μg/ml) for 24 hr. MUC5AC mucin and sialyl-LewisX were stained with specific primary antibodies, and visualized with the Alexa Fluor®488-conjugated secondary antibody (green color, MUC5AC) or Alexa Fluor®647-conjugated secondary antibody (red color, sialyl-Lewisx). Nuclei were stained with DAPI (blue color). Three independent experiments showed similar results. Typical strips of cells are shown. (b) Expression of MUC5AC and sialyl-Lewisx from 10 representative strips were quantified using the AxioVision Rel. 4.8 software. *p < 0.05 when compared against PBS control using the parametric Student's t test. (c) Sialylation of MUC5AC in NCI-H292 cells. After exposure to PCN (24 hr, 5 μg/ml), total protein extracts (600 μg/ml) were immunoprecipitated with anti-MUC5AC antibody, or with human IgG as negative controls. Proteins were separated on an agarose-acrylamide gel and the expression of sialyl-Lewisx was analyzed using specific antibody by Western blotting. Experiments were repeated independently three times with similar results. A typical Western blot is shown.
To determine whether PCN-induced specific sialylation on MUC5AC, we immunoprecipitated MUC5AC from NCI-H292 cells previously exposed to PCN, and examined the sialylation of the mucin with sialyl-LewisX-specific antibodies. Sialylated MUC5AC was detected (Figure 4c) on the Western blot. Because MUC5AC is only one of the multiple mucins (e.g., MUC5B, MUC2, etc) secreted by airway cells, not surprisingly, the amount of sialylated MUC5AC was less when compared to total amounts of sialylated mucins in total cell extracts. Collectively, these results indicate that PCN is a potent inducer of mucin sialylation in airway epithelial cells.
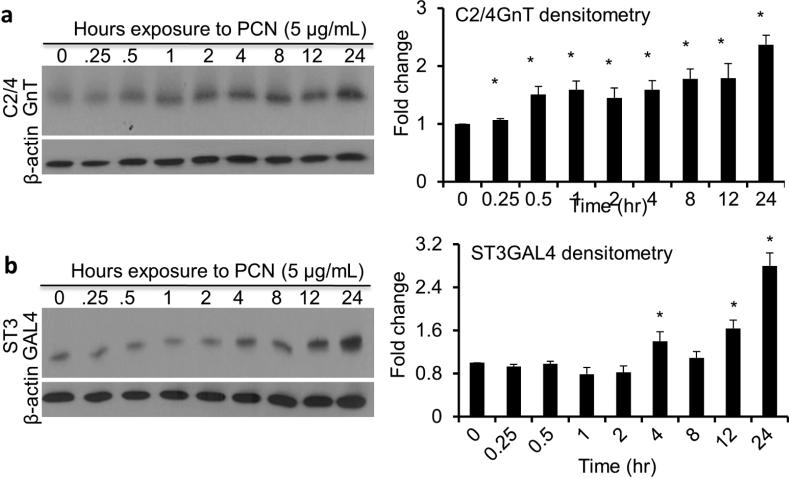
Pyocyanin induces upregulation of C2/4GnT and ST3Gal-IV
Sialyl-Lewisx is synthesized through stepwise processes involving many enzymes.8 Previous work has shown that levels of sialyl-Lewisx biosynthesis glycosyltransferases C2/4GnT and ST3Gal-IV are upregulated in response to inflammation.6,8,14,15 We examined whether PCN could directly upregulate these enzymes in the NCI-H292 cells in a time dependent manner. PCN significantly increased the amounts of both C2/4GnT and ST3Gal-IV by 2.4 and 3.0-fold, respectively, at 24 hr post-exposure (Figure 5a and b). These results indicate that PCN is capable of upregulating the expression of enzymes crucial for the biosynthesis of sialyl-Lewisx.
Figure 5.
PCN induces the expression of sialyl-Lewisx glycosyltransferases. NCI-H292 cells were exposed to 5 μg/ml PCN for the indicated time intervals. (a, b) The expression of C2/4GnT and ST3Gal-IV was analyzed by western blots. β-actin was used as loading controls. Representative western blots from one of the three independent experiments are shown. Densitometry analyses of C2/4GnT and ST3Gal-IV represent the mean ± standard error from three independent western blot experiments. Statistical significance comparisons among various time points were determined by using the one-way ANOVA analysis (p < 0.05). *p < 0.05 when compared against Time 0 hr by using the using the Tukey's test.
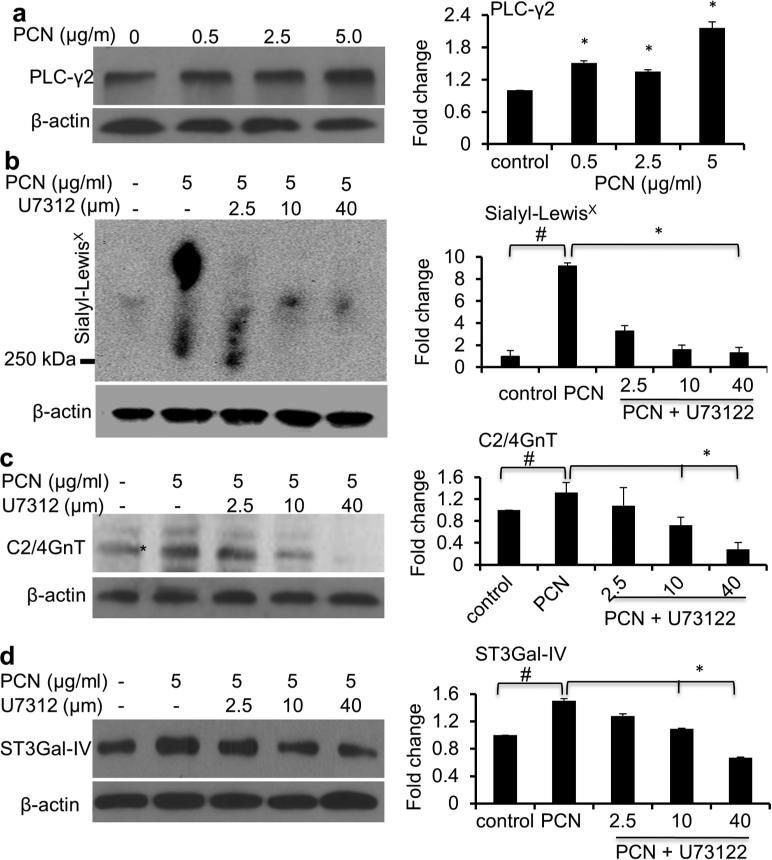
PCN induces an increase in sialyl-Lewisx through the PI-PLC pathway
Previously, it has been shown that TNF-α could increase the expression of C2/4GnT and ST3Gal-IV through the induction of PI-PLC signaling pathway.6,8,15,16 In addition, IL-6 and IL-8 may also play a role.17 In contrast, EGFR signaling negatively regulates C2/4GnT and ST3Gal-IV.15,26 Importantly, PCN induces production and release of TNF-α, IL-6 and IL-8 from bronchial airway epithelial cells.27 Furthermore, we and others have shown that PCN induces the expression of dominant airway mucins MUC5B and MUC5AC through activation of EGFR.23,25 We used two complementary approaches to examine the upregulation of PI-PLC-dependency C2/4GnT and ST3Gal-IV induced by PCN. First, we showed that PCN could upregulate the expression of PLC-γ2, a component of the PI-PLC signaling pathway (Figure 6a). As expected, TNF-α induced the expression of sialyl-LewisX (Figure S2). Next, we examined whether U-73122, a PI-PLC pathway inhibitor, could disrupt the induction of sialyl-LewisX biosynthesis by PCN. In the absence of U-73122, PCN significantly increased the levels of sialyl-LewisX, C2/4GnT and ST3Gal-IV (Figure 6b-d). In contrast, addition of U-73122 caused a dose-dependent decrease in PCN-mediated upregulation in sialyl-Lewisx, C2/4GnT, ST3Gal-IV (Figure 6b-d). Collectively, these results demonstrate that upregulation of sialyl-Lewisx biosynthesis by PCN is dependent upon the PI-PLC signaling pathway.
Figure 6.
PCN upregulates sialyl-Lewisx through the PI-PLC pathway. (a) PCN induced the expression of PI-PLC signaling pathway effector PLC-γ2 in NCI-H292 cells exposed to increasing concentrations of PCN. (b-d) NCI-H292 cells were pre-exposed to PI-PLC inhibitor U73122 for 40 minutes before the addition of PBS or PCN (5 μg/ml) for 24 hr. Total cell lysates were used in Western blot analysis using antibodies against (a) PLC-γ2, (b) sialyl-LewisX, (c) C2/4GnT or (d) ST3Gal-IV. For sialyl-LewisX western blot (b), proteins were separated on an agarose-acrylamide gel. β-actin was used for loading controls. Experiments were repeated three times. Representative western blots are shown. Densitometry analyses represent the mean ± standard error of western blots from three independent experiments. *p < 0.05 against PBS control, or in the presence or absence of U73122 by using the one-way ANOVA analysis. #p < 0.05 against PBS control by using the Tukey's test.
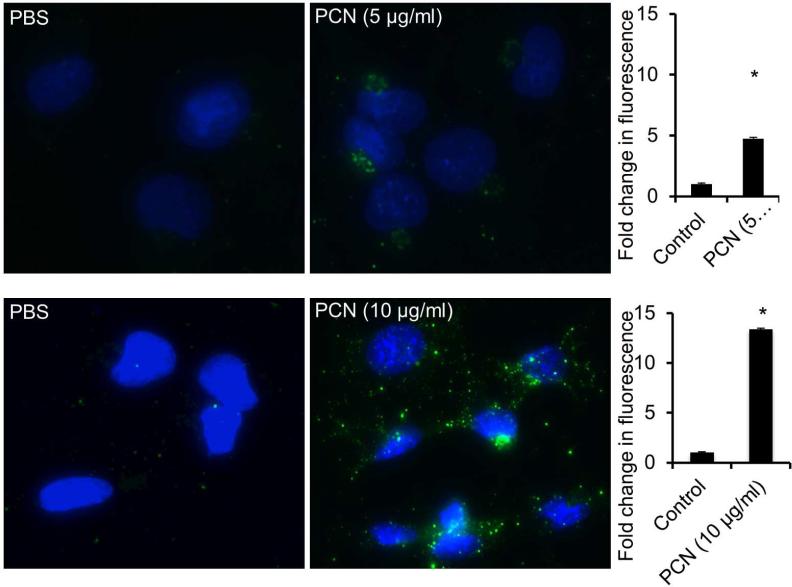
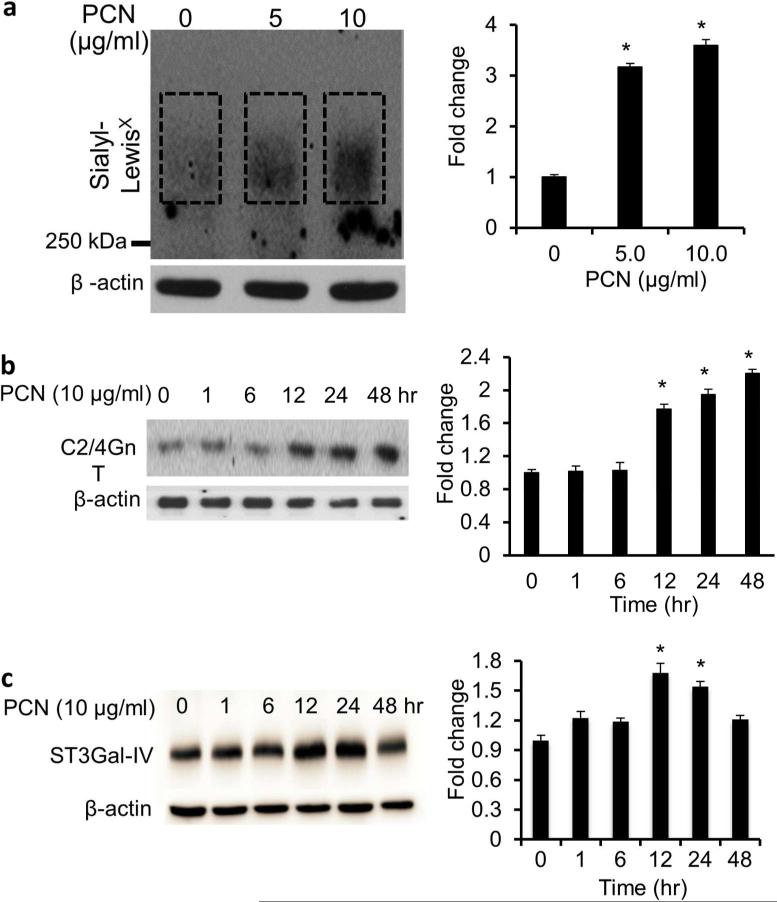
Pyocyanin induces the expression of sialyl-Lewisx in the immortalized CF airway epithelial IB3-1 cells
Mucins recovered from CF airways are enriched with the tetracarbohydrate moiety sialyl-Lewisx.9-11 We examined whether PCN could induce sialyl-Lewisx glycosylation on mucins produced by the CF IB3-1 cells. In contrast to NCI-H292 cells, we found that PCN at the concentration of 10 μg/ml and at the time point of 48 hr was most optimal in inducing the glycosylation of mucins with sialyl-Lewisx. IFA analyses indicated that at 5.0 μg/ml and 10.0 μg/ml PCN concentrations, the expression of sialyl-Lewisx was increased by 4.8 and 13.4-fold, respectively, when compared against the PBS controls (Figure 7). The IFA results were corroborated by western blot analysis, which showed an increased expression of sialyl-Lewisx (Figure 8a), as well as the glycosyltransferases C2/4GnT (Figure 8b) and ST3Gal-IV (Figure 8c). Collectively, these results confirmed the ability of PCN to induce sialyl-Lewisx in the CFTR-deficient IB3-1 cells.
Figure 7.
PCN upregulates the expression of sialyl-Lewisx in immortalized CF epithelial cells. IB3-1 cells were exposed to PBS (control) or indicated concentrations of PCN for 48 hr. Sialyl-LewisX was stained with antibodies and visualized with Alexa Fluor®488-conjugated secondary antibody (green color). Quantification of total fluorescence of control and PCN treated cells. The mean ± standard error of total florescence in 10 representative high power fields from each treatment group in one typical experiment is shown. *p < 0.05 when compared against PBS control by parametric Student's t-test.
Figure 8.
PCN upregulates the expression of sialyl-Lewisx in CF airway epithelial cells. IB3-1 cells were exposed to PBS (control) or indicated concentrations of PCN or time intervals (with 5 μg/ml PCN). (a) The expression of sialyl-Lewisx was analyzed using specific antibody by Western blots. Total protein extracts were separated on an agarose-acrylamide gel. (b and c) The expression of C2/4GnT (b) and ST3Gal-IV (c) was analyzed by western blots. β-actin was used as loading controls. Representative western blots from one of the three independent experiments are shown. Densitometry analyses represent the mean ± standard error from three independent western blot experiments. *p < 0.05 when compared sialyl-Lewisx (a) and C2/4GnT (b) and ST3Gal-IV (c) expression among the group by using the one-way ANOVA analysis. *p < 0.05 when compared each sample against PBS control (a) or Time 0 hr (b) by using the Tukey's test.
Pyocyanin increases binding affinity of P. aeruginosa to immortalized CF and non-CF airway epithelial cells
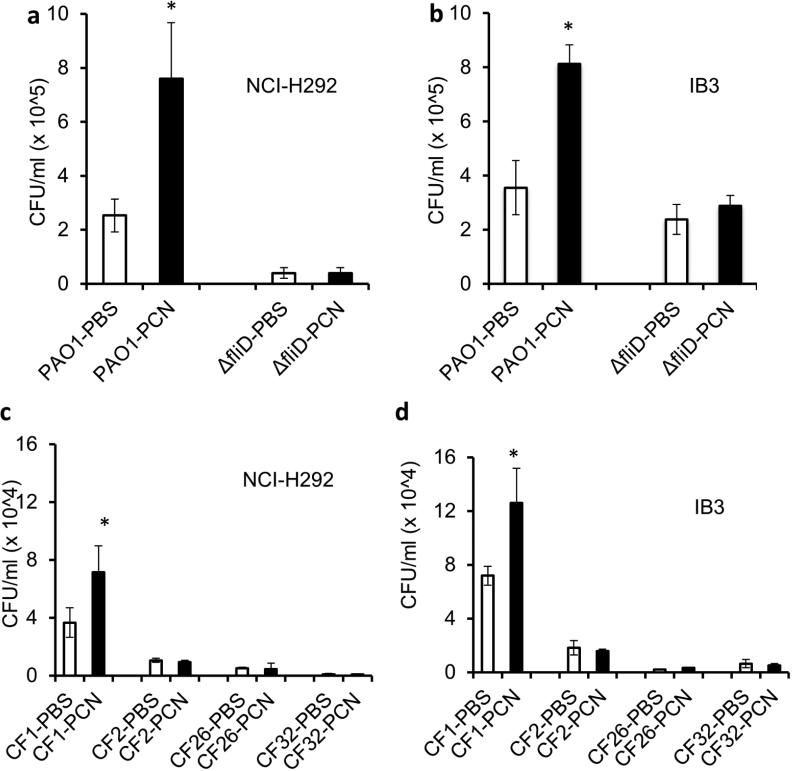
Because sialyl-Lewisx is a preferred binding receptor for PA.7 Because our results demonstrated that PCN increases mucin glycosylation with sialyl-Lewisx, we examined whether PA would be better able to bind to NCI-H292 and IB3-1 cells previously-treated with PCN. The binding of PA strain PAO1 to PCN-treated NCI-H292 and IB3-1 cells was 3-fold and 2.3-fold higher than the control cells (Figure 9a and b). In addition, the binding was partially dependent on the expression of flagellar cap protein FliD. PCN-mediated increase in binding affinity to NCI-H292 and IB3-1 cells was abolished in the flagellar cap deficient ΔfliD mutant (Figure 9a and b). In addition, the basal levels of PAO1 and ΔfliD cells bound to IB3-1 cells were higher than NCI-H292 cells in the absence of PCN induction.
Figure 9.
Pyocyanin increases P. aeruginosa binding efficiency to NCI-H292 cells. PA strain PAO1 was added in a 1:1 ratio to NCI-H292 cells (n = 12 wells) pretreated with PBS or PCN (5. μg/ml) for 24 hr. After 1 hr, cells were washed to remove unattached PAO1, lysed, serially diluted, and plated on Pseudomonas isolation agar. Data represents CFU recovered from each group. *p < 0.05 when compared bacterial binding among various treatments by using the one-way ANOVA analysis. *p < 0.05 when compared bacterial binding in each sample against PBS control by using the Tukey's test.
Next, we examined the binding of PA CF clinical isolates to NCI-H292 and IB3-1 cells. CF1 is non-mucoid, motile and produces PCN. CF2 is non-mucoid, non-motile and PCN-deficient. CF26 is mucoid, non-motile and produces low levels of PCN. CF32 is mucoid, non-motile and PCN-deficient (Figure S3a, b). Binding experiments showed that only CF1 was able to bind to airways cells, consistent with the requirement of flagellum for attachment to sialylated mucins. In addition, binding to NCI-H292 and IB3-1 cells pretreated with PCN increased by 1.95 and 1.75-fold, respectively (Figure 9c and d). Collectively, these results indicate that the elevated mucin sialylation induced by PCN facilitates the binding of PA in a more efficient manner.
Blocking of sialyl-LewisX and TNF-α with antibodies attenuates the binding of P. aeruginosa to airway epithelial cells pre-exposed to PCN
Blocking the binding of PA sialylated mucins may serve as an adjunctive therapy against chronic colonization and infection within CF airways. We examined whether treatment of NCI-H292 and IB3-1 cells with antibodies against sialyl-LewisX and anti-TNF-α could reduce the binding of PA to NCI-H292 and IB3-1 cells. Blocking with anti-sialyl-LewisX antibodies decreased the binding of PAO1 and CF1 to NCI-H292 cells by 2.88 and 3.01-fold respectively (Figure 10a); and by 3.26 and 4.2-fold respectively to IB3-1 cells (Figure 10b), when compared against cells pretreated with an irrelevant human IgG. Blocking with anti-sialyl-TNF-α antibodies decreased the binding of PAO1 and CF1 to NCI-H292 cells by 1.80 and 1.78-fold respectively (Figure 10c); and by 1.70 and 1.68-fold respectively to IB3-1 cells (Figure 10d). Collectively, these results suggest that blocking of sialylated mucins as well as signaling pathway regulating mucin sialylation may inhibit PA binding and reduce infection in CF airways.
Figure 10.
Antibodies against sialyl-LewisX and TNF-α attenuate the binding of P. aeruginosa to airway epithelial cells pre-exposed to PCN. NCI-H292 and IB3-1 cells were serum-starved for 24 hr before exposure to PCN (5 μg/ml for 24 hr or 10 μg/ml for 48 hr, respectively). To block sialomucins, anti-sialyl-LewisX antibodies (15 μg/well) were added 2 hr before the binding assay. To block TNF-α, anti-TNF-α antibodies (15 μg/well) were added simultaneously with PCN for the same duration. Human IgG (15 μg/well) was used as controls. PA strains PAO1 or CF1 were added in a 1:1 ratio to NCI-H292 or IB3-1 cells (n = 12 wells). After 1 hr, cells were washed to remove unattached PAO1, lysed, serially diluted, and plated on Pseudomonas isolation agar. Data represents CFU recovered from each group. *p < 0.05 when compared bacterial binding among various treatments by using the one-way ANOVA analysis. *p < 0.05 when compared bacterial binding in each sample against human IgG controls by using the Tukey's test. PAO1-IgG and CF1-IgG: binding of bacteria to airway cells pre-blocked with human IgG. PAO1-α-SLX and CF1-α-SLX: binding of bacteria to airway cells pre-blocked with anti-sialyl-LewisX antibodies. PAO1-α-TNF-α and CF1-α-TNF-α: binding of bacteria to airway cells pre-blocked with anti- TNF-α antibodies.
DISCUSSION
PCN is a redox-active phenazine toxin found to be excreted in levels up to 100 μM in PA-infected bronchiectatic airways.20,21 PCN is important for chronic lung infection.22 Chronic instillation of PCN causes GCHM, fibrosis, and airspace destruction, pathological features mirroring those found in CF lungs chronically infected with PA.22 Levels of sialyl-Lewisx, which acts as a binding receptor for PA,7,13 are also upregulated in CF mucins.5,9-12,28,29 Historically, this has been attributed to increased inflammation in the lung.5 Evidence suggesting the importance of bacterial infection in modulating levels of sialyl-Lewisx include observations of correlation between severity of infection and increased sialyl-Lewisx expression,11,28 as well as studies demonstrating an initial bacterial component required for inflammation in CF lungs.18,19 We postulated that virulence factors of PA may be the initial stimuli leading to increased sialyl-Lewisx. In this study, we show that chronic PCN administration increases the expression of sialomucins in mouse airways through a TNF-α-PI-PLC pathway. Little or no sulfomucins were detected in mouse airways, despite strong expression in colon tissues from the same animals. This is not surprising because mucin sulfation in cystic fibrosis has been directly linked to the loss of CFTR function,30 which is absent in our mouse model and may be independent from the bacterial-mediated inflammation. Our study suggests a causal link between chronic PCN administration in mouse airways and increased levels of sialyl-Lewisx. Furthermore, increased expression of sialyl-Lewisx was also seen in ALI culture of NHBE cells, and in immortalized mucoepidermoid NCI-H292 cells. Moreover, PCN also increased the expression of sialyl-Lewisx in the CF cell line IB3-1, which was accompanied by elevated binding of PA. Importantly, antibody-based blocking of TNF-α and sialylated mucins reduces the binding of PA to airway cells exposed to PCN. Collectively, these results demonstrate the ability of PCN to augment sialyl-Lewisx expression in the CF airway epithelial cells.
The expression of sialyl-Lewisx increases significantly in response to stimulation by TNF-α, IL-6 and IL-8 in both immortalized airway cells and bronchial explants.6,8, 15,16 In agreement, PCN induces the production of TNF-α, IL-6 and IL-8 from airway epithelial cells.27 Taken together, this suggests that PCN may induce the secretion of TNF-α to increase sialyl-Lewisx in the airways. Previous studies involving the effects of inflammatory cytokines on glycosyltransferases responsible for the synthesis of sialyl-Lewisx demonstrate, in addition to C2/4GnT and ST3Gal-IV, an upregulation of the α1-3 fucosyltransferases FucT-III/IV/VII.8,15,16 However, increased expression of FucT-III/IV/VII was not observed in response to PCN administration (data not shown). We hypothesize this may be due to higher sensitivity of FucT-III/IV/VII than C2/4GnT and ST3Gal-IV to repression by the PCN-activated EGFR signaling,23,25 which is inhibitory to these glycosyltransferases.15,26 It is likely that the amount of sialyl-Lewisx glycosylation is the net result of PCN-mediated induction of the positively acting TNF-α-PI-PLC signaling versus the negatively acting EGFR signaling. Because anti-TNF-α antibodies were less efficient than anti-sialyl-LewisX antibodies in reducing the binding of PA (Figure 10), it is likely that additional signaling pathways also may be involved in upregulating mucin sialylation. As we have mentioned earlier, FOXA2 is a key transcriptional repressor of mucin biosynthesis that maintains the airway mucus at healthy baseline levels23,24,31, by inhibiting the function of SPDEF, a IL-4/IL-13-STAT6 dependent transcriptional activator of mucus biosynthesis and glycosyltransferases involved in mucin modification. Thus far, the sole evidence of SPDEF activating sialyl/sulfo/fucosyltransferases was derived from transcriptome analysis.32 Previously, we have shown that PCN induces GCHM and mucin biosynthesis by inducing the IL-4/IL-13-STAT6 signaling. Thus, blocking both TNF-α-PI-PLC and IL-4/IL-13-STAT6 signaling may yield more robust inhibition of PA binding. Further studies will elucidate the aforementioned issues. Also, it will be of interest to determine whether the levels of PCN within CF sputa positively correlate with the levels of sialomucins.
Sialyl-Lewisx has been previously shown to be a preferential binding receptor for PA. Because PCN upregulates the amount of sialyl-Lewisx, this suggests PA is able to modulate a favorable host environment to facilitate chronic lung infection by secreting PCN. Our observation may have clinical implications. For example, after aggressive antibiotic therapy that eradicates most PA cells, the airway mucins rich in sialyl-Lewisx would be well suited for binding by the residual PA or new bacteria that entered the airways. It is also possible that the production of sialomucins may be continued after the removal of the majority of PA. In support of this idea, Muhlebach et al33 described increased and prolonged inflammatory responses in PA and other bacterial-infected CF lungs compared to non-CF lungs, suggesting that an inflammatory response in CF epithelium is not only pronounced, but delayed in cessation. Thus, adjunctive treatment involving blocking the binding of remainder PA to sialylated mucins may help to decrease recurring infection in CF.
In summary, PCN modulates airway mucin glycosylation by upregulating the levels of sialyl-Lewisx. The importance of this carbohydrate moiety as a binding receptor for PA underscores the importance of increasing our knowledge of the effects of PCN on airway mucins during PA-mediated chronic pulmonary infection in CF.
Methods
Chemicals and purified bacterial components
Chemically synthesized PCN (#R9532) and PA LPS (#L7018) were purchased from Sigma. Alginate was purified from the mucoid PA strain FDR132 and quantified as previously described.34 Flagella were purified from PA strain PAO1 as previously described.35 U73122 was purchased from Sigma Aldrich (#U6756). TNF-α was purchased from R&D systems (#210-TA-010).
P. aeruginosa cultures
PA strains PAO1 and its isogenic ΔfliD mutant were generous gifts from Professor Reuben Ramphal (University of Florida). CF clinical PA isolates CF1, CF2, CF26 and CF32 were collected while the Dr. Lau was on staff at the University of Cincinnati College of Medicine under the IRB # 04-7-16-2. All PA strains were cultured in plain Luria-Bertani (LB) broth (Fisher Scientific) with the exception of ΔfliD, which was grown in LB containing 75 μg/ml gentamicin (Life Technologies), at 37°C overnight. They were then stored at −80°C in 25% glycerol (Sigma-Aldrich). Before each experiment, bacteria were cultured from frozen stocks in 5 ml LB broth to stationary phase to OD at 600 nm ~ 3.0 by using a spectrophotometer, Genesys 10 UV (Thermo Scientific). Bacteria were then washed 3x with sterile PBS, diluted to appropriate concentrations for binding experiments.
Mucoidy phenotypes of CF clinical PA strains were determined by streaking the frozen stocks onto the Pseudomonas Isolation Agar plates. Motility of PA strains were determined by inoculating 1 μl of overnight bacterial culture (OD 600nm 3.0) onto the Brain Heart Infusion broth supplemented with 0.4% agar. Bacterial plates were incubated at 37°C with 5% CO2 for 48 - 72 hr for observation.
Mouse lung exposure, tissue analyses and BAL
Animal studies were carried out in strict accordance to the protocol approved by the Institutional Animal Care and Use Committee at the University of Illinois at Urbana Champaign. C57BL6 mice (6-week old, n = 10) were housed in positively-ventilated microisolator cages with automatic recirculating water, located in a room with laminar, high efficiency particle accumulation-filtered air. The animals received autoclaved food, water and bedding. PCN (25 μg), LPS (2 μg), alginate (90 μg), and flagella (2 μg) were intranasally inoculated into the mice anaesthetized with isoflurane once daily for 3 weeks (PCN) or 7 days (LPS, alginate and flagella). Control mice were exposed to the same volume (50 μL) of PBS for 3 weeks. Time points were based on our previous studies demonstrating PCN and LPS-induced GCHM in C57BL6 mice, where clear differences in lung pathology, cytokine, and immune cell profiles can be detected between the treated and control mice, as well as the health status of mice. Paraffin embedded lung sections (4-5 μm thickness) were stained with HID-AB or PAS, or for IHC and IFA analyses with the primary antibodies against sialyl-Lewisx (BD Pharmingen #551344) or MUC5AC (Santa Cruz Biotechnology #sc-21701).
To further confirm the expression of sialyl-LewisX in mouse airways, the lungs of PCN-exposed mice (n=3) were BAL. The trachea was exposed and intubated with a 1.7-mm outer diameter polyethylene catheter, followed by instillation of PBS in three successive 1 ml aliquots. Proteins from the first lavages (15 μg) were used for Western blotting to determine the glycosylation of mucins with sialyl-LewisX by using anti-sialyl-LewisX antibodies.
Cell cultures
The human lung mucoepidermoid carcinoma cell line NCI-H292 (ATCC®CRL-1848™) were cultured in RPMI-1640 supplemented with 10% FBS (Sigma) in 5% CO2. Epithelial cells that reached 70% confluency were serum starved for 24 hr before exposure to PCN, TNF-α or U73122. The CF cell line IB3-1 (ΔF508/W1282X) was purchased from ATCC (CRL-2777). IB3-1 cells were cultured in LHC-8 medium (Gibco) on culture plates coated with a solution containing 35 μg/ml bovine collagen (Advanced-BioMatrix), 1 μg/ml human fibronectin (Advanced-BioMatrix) and 1 μg/ml BSA (Sigma-Aldrich) supplemented with 10% FBS in 5% CO2. For IFA analysis, NCI-H292 cells were exposed to PCN (5 μg/ml) for 24 hr. IB3-1 cells were exposed to PCN (10 μg/ml) for 48 hr. IFA analyses were performed using a primary anti-sialyl-Lewisx, followed by Alexa Flour®488-labeled secondary antibodies (Invitrogen). Slides were mounted using DAPI, and the subcellular localization of sialyl-Lewisx was observed using a fluorescence microscope.
Human primary bronchial epithelial (NHBE) cells were purchased from Lonza (Walkersville, MD) and cultured as we have previously described.23,24 Briefly, cells were thawed and passaged in 5% CO2 at 37°C using the bronchial epithelial growth medium (BEGM) supplemented with growth factors supplied in the SingleQuot® kit (Lonza). Cells at passage 3 were trypsinized and seeded onto the Costar Transwells® inserts with 0.4μm pore size (Corning) at a density of 1.5 × 105 cells/cm2 in media comprised of 50% BEBM and 50% DMEM-F12 low glucose (Invitrogen) supplemented with the growth factors provided in the SingleQuot® kits and retinoic acid (50 nM). Once the cells reached confluency (approximately seven days after seeding, examined by transepithelial electrical resistance (TEER) measurements (data not shown), they were switched to an air-liquid interface for additional 2 weeks to achieve mucociliary differentiation. NHBE cells were exposed to PCN (12.5 μg/ml) for 24 hr and stained with anti-MUC5AC and anti-sialyl-LewisX antibodies, and visualized with Alexa Fluor®488-conjugated secondary antibody (green color) and Alexa Fluor®647-conjugated secondary antibody (red color), respectively, under a confocal microscope. Nuclei were stained with DAPI (blue color). The percentage of MUC5AC and sialyl-LewisX expression was calculated based on the fluorescence signal of MUC5AC or sialyl-LewisX divided by the total signal of DAPI.
Protein extraction, immunoprecipitation and Western blotting analysis
NCI-H292 cells were stimulated with PCN (0.5, 2.5 and 5.0 μg/ml) for the indicated time intervals. Total protein was extracted with M-PER Mammalian Protein Extraction Reagent (Thermo Scientific #78501), quantified with the Pierce BCA Protein Assay Kit (Thermo Scientific # 23227) and used in western blotting analysis. For inhibitor studies, NCI-H292 cells were serum starved for 24 hr and then exposed to the PI-PLC inhibitor U73122 at concentrations of 2.5, 10 or 40 μM for 40 min before addition of PCN (5 μg/ml) or the same volume of PBS. These cells were harvested after 24 hr. For immunoprecipitation, 600 μg of total protein extract was incubated with 2 μg of anti-TNF-α antibodies (Santa Cruz Biotechnology #sc-20118). For the analysis of sialyl-Lewisx, total protein was separated on 0.5%-6% agarose-acrylamide gradient gels as previously described.36 For the analysis of C2/4GnT, St3Gal-IV and PLC-y2 expression, total protein was separated on 8% polyacrylamide gels. Western blotting analyses were performed with antibodies against sialyl-Lewisx, C2/4GnT, St3Gal-IV and PLC-y2 (Santa Cruz Biotechnology #sc-161625, #sc-134041, #sc-407). The immune complexes were visualized using the ECL Western Blotting Detection System (Amersham Biosciences) and Hyblot CL (Denville Scientific) autoradiography films.
Image Analysis
Densitometry analysis of Western blots, total fluorescence levels of IFA images and threshold analysis of IHC staining area was accomplished using the ImageJ software from NIH (http://rsbweb.nih.gov/ij/). Protocol for threshold analysis was as described.37 Quantitative analyses of IFA images on ALI cultures of NHBE cells were performed with AxioVision Rel. 4.8 software (Carl Zeiss MicroImaging, LLC). The percentage of positively stained cells was calculated against the total number of cells (DAPI stained) within individual sections in the stained area.
PA binding assay
After reaching 70% confluency, NCI-H292 and IB3-1 cells (~ 5 × 106/well, n = 12 wells) were serum-starved for 24 hr before stimulation with PCN or equal volume of PBS. NCI-H292 cells were exposed to 5 μg/ml PCN for 24 hr whereas IB3-1 cells were exposed to 10 μg/ml PCN for 48 hr. After exposure, the cells were washed with PBS three times, and incubated in sterile PBS and infected with PA strain PAO1, the isogenic flagellar cap-deficient mutant ΔfliD (MOI = 1:1), or the clinical isolates CF1, CF2, CF26 and CF32. After 1 hr of incubation, these wells were washed vigorously with 3 changes of 1 ml PBS. The epithelial cells were collected in 1 ml PBS, serially diluted, and plated onto Pseudomonas Isolation Agar for colonies enumeration. Similar results were obtained from three independent experiments. Results from a typical experiment are shown.
For the antibody-mediated blocking of sialomucins, following PCN exposure, NCI-H292 and IB3-1 cells were treated with anti-sialyl-LewisX antibodies (15 μg/well) for 2 hr before the binding assay. For blocking TNF-α, anti-TNF-α antibodies (Santa Cruz Biotechnology #sc-366359, 15 μg/well) were added simultaneously with PCN for the same duration. Human IgG (15 μg/well) was used as controls. Cells were washed with PBS three times before the PA binding assays.
Statistical analyses
Quantitative data were expressed as the mean ± standard error. Statistical significance comparisons for samples with equal variances were determined using the parametric Student's t test for two unpaired samples. For comparing the means of groups of three or more, data were analyzed for statistical significance by the one-way ANOVA followed by Tukey's tests for comparison between the means. A significant difference was considered to be p < 0.05.
Supplementary Material
Acknowledgments
We thank Ms. Jennifer Ida for critical reading of the manuscript. We thank Professor Reuben Ramphal (University of Florida) for the provision of PA strains PAO1 and its isogenic ΔfliD mutant.
This work was supported by the American Lung Association DeSouza Research Award (DS-192835-N) and NIH (HL090699) to GW Lau. The funders had no role in study design, data collection and analysis of this publication.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
REFERENCES
- 1.Boucher RC. An overview of the pathogenesis of cystic fibrosis lung disease. Adv. Drug Deliver. Rev. 2002;54:1359–1371. doi: 10.1016/s0169-409x(02)00144-8. [DOI] [PubMed] [Google Scholar]
- 2.Lyczak JB, Cannon CL, Pier GB. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 2002;15:194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burns JL, et al. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J. Infect. Dis. 2001;183:444–452. doi: 10.1086/318075. [DOI] [PubMed] [Google Scholar]
- 4.Carnoy C, Scharfman A, Van Brussel E, Lamblin G, Ramphal R, Roussel P. Pseudomonas aeruginosa outer membrane adhesins for human respiratory mucus glycoproteins. Infect. Immun. 1994;62:1896–1900. doi: 10.1128/iai.62.5.1896-1900.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamblin G, et al. Human airway mucin glycosylation: a combinatory of carbohydrate determinants which vary in cystic fibrosis. Glycoconjugate J. 2001;18:661–684. doi: 10.1023/a:1020867221861. [DOI] [PubMed] [Google Scholar]
- 6.Colomb F, et al. TNF regulates sialyl-Lewisx and 6-sulfo-sialyl-Lewisx expression in human lung through up-regulation of ST3Gal-IV transcript isoform BX. Biochimie. 2012;94:2045–2053. doi: 10.1016/j.biochi.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 7.Scharfman A, Degroote S, Beau J, Lamblin G, Roussel P, Mazurier J. Pseudomonas aeruginosa binds to neoglycoconjugates bearing mucin carbohydrate determinants and predominantly to sialyl-Lewis x conjugates. Glycobiology. 1999;9:757–764. doi: 10.1093/glycob/9.8.757. [DOI] [PubMed] [Google Scholar]
- 8.Delmotte P, Degroote S, Lafitte JJ, Lamblin G, Perini JM, Roussel P. Tumor necrosis factor alpha increases the expression of glycosyltransferases and sulfotransferases responsible for the biosynthesis of sialylated and/or sulfated Lewis x epitopes in the human bronchial mucosa. J. Biol. Chem. 2002;277:424–431. doi: 10.1074/jbc.M109958200. [DOI] [PubMed] [Google Scholar]
- 9.Lo-Guidice JM, Wieruszeski JM, Lemoine J, Verbert A, Roussel P, Lamblin G. Sialylation and sulfation of the carbohydrate chains in respiratory mucins from a patient with cystic fibrosis. J. Biol. Chem. 1994;269:18794–18813. [PubMed] [Google Scholar]
- 10.Lamblin G, Boersma A, Klein A, Roussel P, van Halbeek H, Vliegenthart JF. Primary structure determination of five sialylated oligosaccharides derived from bronchial mucus glycoproteins of patients suffering from cystic fibrosis. The occurrence of the NeuAc alpha(2----3)Gal beta(1----4)[Fuc alpha(1----3)] GlcNAc beta(1----.) structural element revealed by 500-MHz 1H NMR spectroscopy. J. Biol. Chem. 1984;259:9051–9058. [PubMed] [Google Scholar]
- 11.Davril M, et al. The sialylation of bronchial mucins secreted by patients suffering from cystic fibrosis or from chronic bronchitis is related to the severity of airway infection. Glycobiology. 1999;9:311–321. doi: 10.1093/glycob/9.3.311. [DOI] [PubMed] [Google Scholar]
- 12.Carnoy C, et al. Altered carbohydrate composition of salivary mucins from patients with cystic fibrosis and the adhesion of Pseudomonas aeruginosa. Am. J. Respir. Cell. Mol. Biol. 1993;9:323–334. doi: 10.1165/ajrcmb/9.3.323. [DOI] [PubMed] [Google Scholar]
- 13.Colomb F, et al. TNF induces the expression of the sialyltransferase ST3Gal IV in human bronchial mucosa via MSK1/2 protein kinases and increases FliD/sialyl-Lewis(x)-mediated adhesion of Pseudomonas aeruginosa. Biochem. J. 2014;457:79–87. doi: 10.1042/BJ20130989. [DOI] [PubMed] [Google Scholar]
- 14.Delmotte P, et al. Influence of TNFalpha on the sialylation of mucins produced by a transformed cell line MM-39 derived from human tracheal gland cells. Glycoconjugate J. 2001;18:487–497. doi: 10.1023/a:1016038219183. [DOI] [PubMed] [Google Scholar]
- 15.Ishibashi Y, Inouye Y, Okano T, Taniguchi A. Regulation of sialyl-Lewis x epitope expression by TNF-alpha and EGF in an airway carcinoma cell line. Glycoconjugate J. 2005;22:53–62. doi: 10.1007/s10719-005-0292-7. [DOI] [PubMed] [Google Scholar]
- 16.Muhlebach MS, Noah TL. Endotoxin activity and inflammatory markers in the airways of young patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2002;165:911–915. doi: 10.1164/ajrccm.165.7.2107114. [DOI] [PubMed] [Google Scholar]
- 17.Groux-Degroote S, et al. IL-6 and IL-8 increase the expression of glycosyltransferases and sulfotransferases involved in the biosynthesis of sialylated and/or sulfated Lewis-x epitopes in the human bronchial mucosa. Biochem. J. 2008;410:213–223. doi: 10.1042/BJ20070958. [DOI] [PubMed] [Google Scholar]
- 18.Berger M. Lung inflammation early in cystic fibrosis: bugs are indicted, but the defense is guilty. Am. J. Respir. Crit. Care Med. 2002;165:857–858. doi: 10.1164/ajrccm.165.7.2202030a. [DOI] [PubMed] [Google Scholar]
- 19.Dakin CJ, Numa AH, Wang H, Morton JR, Vertzyas CC, Henry RL. Inflammation, infection, and pulmonary function in infants and young children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2002;165:904–910. doi: 10.1164/ajrccm.165.7.2010139. [DOI] [PubMed] [Google Scholar]
- 20.Wilson R, Sykes DA, Watson D, Rutman A, Taylor GW, Cole PJ. Measurement of Pseudomonas aeruginosa phenazine pigments in sputum and assessment of their contribution to sputum sol toxicity for respiratory epithelium. Infect. Immun. 1988;56:2515–2517. doi: 10.1128/iai.56.9.2515-2517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunter RC, Klepac-Ceraj V, Lorenzi MM, Grotzinger H, Martin TR, Newman DK. Phenazine content in the cystic fibrosis respiratory tract negatively correlates with lung function and microbial complexity. Am. J. Respir. Cell Mol. Biol. 2012;47:738–745. doi: 10.1165/rcmb.2012-0088OC. [DOI] [PubMed] [Google Scholar]
- 22.Caldwell CC, et al. Pseudomonas aeruginosa exotoxin pyocyanin causes cystic fibrosis airway pathogenesis. Am. J. Pathol. 2009;175:2473–2488. doi: 10.2353/ajpath.2009.090166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hao Y, et al. Pseudomonas aeruginosa pyocyanin causes airway goblet cell hyperplasia and metaplasia and mucus hypersecretion by inactivating the transcriptional factor FoxA2. Cell. Microbiol. 2012;14:401–415. doi: 10.1111/j.1462-5822.2011.01727.x. [DOI] [PubMed] [Google Scholar]
- 24.Hao Y, Kuang Z, Xu Y, Walling BE, Lau GW. Pyocyanin-induced mucin production is associated with redox modification of FOXA2. Respir. Res. 2013;14:82. doi: 10.1186/1465-9921-14-82. doi: 10.1186/1465-9921-14-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rada B, Gardina P, Myers TG, Leto TL. Reactive oxygen species mediate inflammatory cytokine release and EGFR-dependent mucin secretion in airway epithelial cells exposed to Pseudomonas pyocyanin. Mucosal Immunol. 2011;4:158–171. doi: 10.1038/mi.2010.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beum PV, Bastola DR, Cheng PW. Mucin biosynthesis: epidermal growth factor downregulates core 2 enzymes in a human airway adenocarcinoma cell line. Am. J. Respir. Cell Mol. Biol. 2003;29:48–56. doi: 10.1165/rcmb.2002-0147OC. [DOI] [PubMed] [Google Scholar]
- 27.Denning GM, Wollenweber LA, Railsback MA, Cox CD, Stoll LL, Britigan BE. Pseudomonas pyocyanin increases interleukin-8 expression by human airway epithelial cells. Infect. Immun. 1998;66:5777–5784. doi: 10.1128/iai.66.12.5777-5784.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morelle W, Sutton-Smith M, Morris HR, Davril M, Roussel P, Dell A. FAB-MS characterization of sialyl Lewis x determinants on polylactosamine chains of human airway mucins secreted by patients suffering from cystic fibrosis or chronic bronchitis. Glycoconjugate J. 2001;18:699–708. doi: 10.1023/a:1020871322769. [DOI] [PubMed] [Google Scholar]
- 29.Shori DK, et al. Altered sialyl- and fucosyl-linkage on mucins in cystic fibrosis patients promotes formation of the sialyl-Lewis X determinant on salivary MUC-5B and MUC-7. Pflugers Arch. 2001;443(Suppl 1):S55–61. doi: 10.1007/s004240100645. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Doranz B, Yankaskas JR, Engelhardt JF. Genotypic analysis of respiratory mucous sulfation defects in cystic fibrosis. J. Clin. Invest. 1995;96:2997–3004. doi: 10.1172/JCI118372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wan H, et al. Foxa2 regulates alveolarization and goblet cell hyperplasia. Development. 2004;131:953–964. doi: 10.1242/dev.00966. [DOI] [PubMed] [Google Scholar]
- 32.Chen G, et al. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J. Clin. Invest. 2009;119:2914–2924. doi: 10.1172/JCI39731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muhlebach MS, Stewart PW, Leigh MW, Noah TL. Quantitation of inflammatory responses to bacteria in young cystic fibrosis and control patients. Am. J. Respir. Crit. Care Med. 1999;160:186–191. doi: 10.1164/ajrccm.160.1.9808096. [DOI] [PubMed] [Google Scholar]
- 34.Ohman DE, Chakrabarty AM. Genetic mapping of chromosomal determinants for the production of the exopolysaccharide alginate in a Pseudomonas aeruginosa cystic fibrosis isolate. Infect. Immun. 1981;33:142–148. doi: 10.1128/iai.33.1.142-148.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffmann N, et al. Novel mouse model of chronic Pseudomonas aeruginosa lung infection mimicking cystic fibrosis. Infect. Immun. 2005;73:2504–2514. doi: 10.1128/IAI.73.4.2504-2514.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schulz BL, Packer NH, Karlsson NG. Small-scale analysis of O-linked oligosaccharides from glycoproteins and mucins separated by gel electrophoresis. Anal. Chem. 2002;74:6088–6097. doi: 10.1021/ac025890a. [DOI] [PubMed] [Google Scholar]
- 37.Väyrynen JP, Vornanen JO, Sajanti S, Böhm JP, Tuomisto A, Mäkinen MJ. An improved image analysis method for cell counting lends credibility to the prognostic significance of T cells in colorectal cancer. Virchows Arch. 2012;460:455–465. doi: 10.1007/s00428-012-1232-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.