Abstract
Background
Human ageing is a process characterized by loss of muscle mass, strength, and bone mass. We aimed to examine the efficacy of low‐dose creatine supplementation associated with resistance training on lean mass, strength, and bone mass in the elderly.
Methods
This was a 12‐week, parallel‐group, double‐blind, randomized, placebo‐controlled trial. The individuals were randomly allocated into one of the following groups: placebo plus resistance training (PL + RT) and creatine supplementation plus resistance training (CR + RT) . The participants were assessed at baseline and after 12 weeks. The primary outcomes were lean mass and strength, assessed by dual energy X‐ray absorptiometry (DXA) and ten‐repetition maximal tests (10 RM), respectively. Secondary outcomes included the lumbar spine, right and left femoral neck, both femur and whole body bone mineral density (BMD), and whole body bone mineral content (BMC), assessed by DXA.
Results
The CR + RT group had superior gains in lean mass when compared with the PL + RT group (P = 0.02). Changes in the 10 RM tests in bench press and leg press exercises, body composition, BMD, and BMC of all assessed sites did not significantly differ between the groups (P > 0.05).
Conclusions
Twelve weeks of low‐dose creatine supplementation associated with resistance training resulted in increases in lean mass in the elderly.
Keywords: Ageing, Body composition, Bone mass, Creatine, Resistance training, Strength
Introduction
In the last few years, demographic transition has drastically changed population growth rates across geographies. This phenomenon has been driven by decreased birth rates and increased life expectancy. In 2010, there were, approximately 524 million people aged over 65 years, representing 8% of the world population. It is estimated to triple by 2050, reaching about 1.5 billion people or 16% of the world population. Between 2010 and 2050, the number of the elderly will increase by 250% in the developed countries when compared with 71% in the lesser developed ones.1 In 2014, the Brazilian Institute of Geography and Statistics announced that the elderly population would quadruple by 2060, which in turn would represent 26.7% of the Brazilian population.2
Human ageing is an irreversible and inexorable process characterized by morphological, functional, and biochemical changes in the human body, including the musculoskeletal system. This system gradually modifies and acquires specific structural and morphological characteristics, which mainly include loss of muscle mass, strength, and bone mass.3 Sarcopenia, a syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength, currently affects over 50 million people and for the next 40 years could affect more than 200 million people worldwide. This condition increases the risk of adverse outcomes such as physical disability, poor quality of life, and death.4
Therefore, because of the huge impact caused by ageing on health and quality of life of the elderly, various strategies have been proposed to attenuate the progression of muscle mass, strength, and bone mass losses. Among these strategies, creatine supplementation and resistance training stand out.
Creatine (α‐methyl guanidine‐acetic acid), a guanidine‐derived compound, is a natural amine endogenously synthesized by the liver, kidney, and pancreas from the amino acids arginine, glycine, and methionine, or consumed in the diet from red meat, seafood, and dairy products.5 Creatine plays an important role in providing rapid energy and is also known to be utilized by the brain to boost mental performance. It is stored mostly in the skeletal muscles (90%) as phosphocreatine, a high‐energy phosphate involved in the rapid resynthesis of adenosine triphosphate during muscle contraction. Muscle and bone tissues demand high‐energy levels to initiate rapid movement and particularly benefit from creatine supplementation.6 Rawson et al.7 showed that creatine supplementation increased intramuscular creatine and phosphorylcreatine content in the elderly. This augmented storage may lead to enhanced mitochondrial energy provision. Therefore, when associated with resistance training, training volume is increased, thereby enhancing strength and muscle mass.8 Besides that, increased muscle mass may result in greater muscle pull on the bone during training, which the strain induced on the bone and muscle mass formation would increase.9
Recent findings have confirmed the potential therapeutic effects of creatine supplementation and have unequivocally demonstrated that in the elderly, it improves the quality of life by increasing muscle strength and resistance to fatigue, improving performance in daily activities, and preventing bone loss.6, 10, 11, 12, 13, 14, 15, 16 Similarly, resistance training has been known as one of the most important pillars in the management of disabilities and comorbidities associated with ageing, improving overall physical function, increasing muscle mass, and preventing bone loss.17, 18, 19, 20
Thus, the aim of this study was to examine the efficacy of low‐dose creatine supplementation associated with resistance training on lean mass, strength, and bone mass in the elderly.
To the best of our knowledge, this is the first study to evaluate creatine supplementation combined with resistance training on body composition, strength, bone mineral density (BMD), and bone mineral content (BMC) in the elderly, using a continuous low‐dosage supplementation protocol for a period of 3 months.
Methods
Study design
A 12‐week, double‐blind, randomized, parallel‐group, placebo‐controlled trial was conducted between June 2014 and November 2014 in Goiânia, Goiás (Brazil). The participants were randomly assigned (1:1) to either one of the following groups: (i) placebo supplementation combined with resistance training (PL + RT; n = 14) or (ii) creatine supplementation combined with resistance training (CR + RT; n = 13). Both groups undertook a supervised exercise training programme for 12 weeks.
The participants were assessed at baseline (Pre) and after 12 weeks (Post). The primary outcomes were lean mass and strength measures, and the secondary outcomes included bone mass measures.
Participants
Participants were recruited via flyer advertisements put into their mailbox from a district near the campus of the Federal University of Goiás. Of the 42 participants who were recruited, 32 eligible healthy, nonathletic men and women, between 60 and 80 years, were randomized into two groups (PL + RT; CR + RT). The inclusion criteria were as follows: age 60–80 years, a non‐vegetarian diet, not having consumed any ergogenic supplement for the previous 6 months before the start of the study, not having consumed any medication that could affect muscle growth or the ability to train intensely during the study (e.g. statins, muscle relaxants, and anti‐inflammatory drugs), not be involved in the practice of systematized physical activity, not be involved in food restriction programs, not have any kidney, liver, and/or heart disease, and not having undergone any radioactive procedure in the last year.
The study was approved by the Human Research Ethics Committee of Federal University of Goiás (number 840.317). The participants were informed of the risks and purpose of the study before written consent was obtained. The study complied with the World Medical Association Declaration of Helsinki–Ethical Principles for Medical Research Involving Human Subjects. This trial was registered in ensaiosclinicos.gov.br (clinical trial RBR‐7r7t52).
Creatine supplementation protocol and blinding procedure
The participants from CR + RT group received 5 g/day of creatine monohydrate (supplied by MEDNUTRITION INC., Brazil), and the participants from the PL + RT group were administered the same dose of maltodextrin (supplied by MEDNUTRITION INC., Brazil). Supplements were in the form of powder and presented in packages. Participants were asked to dissolve the supplements in one glass of liquid. The creatine dose of 5 g/day was selected to ensure the saturation of muscle compound.21 In order to verify the purity of the creatine used, a sample was analysed by high‐performance liquid chromatography and was determined to be 99.7% pure.
The supplement packages were coded so that neither the investigators nor the participants were aware of the contents until the completion of the intervention. The supplements were provided by a staff member of our research team who did not have any participation in the data acquisition, analyses, and interpretation. Participants were instructed to consume the supplement packages on non‐training days immediately after lunch in order to increase the muscle creatine concentration.22, 23 On training days, to achieve optimal muscle adaptation, the participants consumed it immediately after resistance training sessions dissolved in a beverage comprising 100 g of lemon‐flavoured maltodextrin prepared by our research team.24
Exercise training
The participants from the CR + RT and the PL + RT groups engaged in a 12‐week supervised resistance training programme. Both groups trained under the same protocol. The exercise sessions occurred three times a week (on Mondays, Wednesdays, and Fridays, at the same time of day to avoid circadian variation, i.e. between 3 and 4 p.m.) and were monitored by fitness professionals. The duration of one training session for each participant was approximately 60 min and remained the same throughout the 12‐week training programme. The resistance training programme was performed in a laboratory (Laboratory of Resistance Training, Faculty of Physical Education, Federal University of Goiás), comprised mono‐articular and multi‐articular exercises, and progressed with increased training volume in (weight × sets × repetitions) each session on an individual basis.
Before the 12‐week resistance training programme, all participants underwent a week of familiarization training, when the dynamics and details of the exercises were explained, and the participants were required to perform two sets of 15 repetition maximal (RM) of the following exercises: leg press, free squat, bench press, lat‐pull down, abdominal floor crunch, and calf raise.
In the next 12 weeks, each training session comprised (i) a warm‐up phase characterized by 5–7 min of a light run on a stationary bicycle or 12 repetitions with 30% RM of the first exercise, (ii) a main phase characterized by resistance exercises for the major muscle groups (three sets of 13–15 RM with 60 s of rest between the sets and exercises for abdominal and lumbar regions and three sets of 10–13 RM with 60 s of rest between the sets and exercises for the other muscle groups), and (iii) a final phase characterized by stretching exercises for the major muscle groups. On alternate days, the participants underwent training either for the upper limbs and abdomen [bench press, lat‐pull down, seated row, biceps curl, triceps extension (presses), shoulder press, and abdominal floor crunch exercises] or for the lower limbs and lumbar region [hip abduction, adduction, squat, hamstrings curl, quadriceps (knee) extension, ankle dorsi‐flexion and plantar flexion, and back extension exercises], according to the American College of Sports Medicine suggestion.25
Food intake assessment
All participants were instructed by nutritionists to fill out a 3‐day dietary intake record (including one weekend day) at baseline and after the 12‐week training programme to calculate the total energy intake, macronutrients, and micronutrients. Dietary data from household measures were converted to gramme and millilitre to enable the chemical analysis of food consumption by the software NutriQuanti (São Paulo, Brazil).26 All participants were instructed to maintain a habitual diet, water intake ad libitum, and avoid caffeinated products because earlier studies had demonstrated that caffeine could eliminate the effects of creatine.27
Muscle strength assessments
At baseline and after the 12‐week resistance training programme, the participants underwent a 10‐repetition maximal (10 RM) test. A recognized 10 RM testing protocol and exercise execution guidelines were followed, as previously documented.28 10 RM tests were selected to avoid excessive cardiovascular stress.29 The tests were conducted for the bench press and leg press exercises. Prior to the 10 RM test, two light warm‐up sets of exercises comprised 15 repetitions so that the participants could get used to the technical movements of the exercises. Subsequently, the participants had two more attempts to achieve the 10 RM load, with a 3–5 min interval between attempts and exercises. Before the beginning of the test, participants were kept at rest while receiving guidance from the instructors, and verbal encouragement was provided during all 10 RM attempts.
Anthropometric assessment
At baseline and after 12 weeks of resistance training programme, the participants were weighed and measured. A recognized protocol was followed, as previously documented.30 Weighing was performed on a digital scale, accurate to 0.1 kg, installed on a flat, firm, and smooth surface, and away from the wall. The measure of stature was held in a wall‐fixed stadiometer with a graduation of 0.1 cm. The values were recorded immediately after the measures, without rounding off the values. All measurements were collected with the participants' bare feet and wearing light clothing.
Body composition and bone mass assessments
Body composition, BMD, and BMC were measured at baseline and after 12 weeks of intervention by dual energy X‐ray absorptiometry (DXA), using a DPX NT densitometry equipment (General Electric Medical Systems Lunar, Madison, EUA). BMD was determined at the lumbar spine, right, and left femoral neck, both femur bones, and the whole body. BMC was determined for the whole body only. The coefficient of variation for the DXA tests of right femoral neck was 0.69%, left femoral neck was 0.67%, both femur bones was 0.55%, and lumbar spine was 1.66%.
Participants were scanned at the same time of the day (between 7 a.m. and 12 noon), while they were bare feet and wearing light clothing. The same operator performed all the measures and calibrations. The performance of the equipment was evaluated by calibration block on a daily basis and by spine phantom on a weekly basis. The coefficient of variation for the DXA tests of muscle and fat mass were 0.75% and 1.03%, respectively.
Diagnosis of sarcopenia
At baseline and after 12 weeks of resistance training, the participants were diagnosed with one of the three stages of sarcopenia (pre‐sarcopenia, sarcopenia, and severe sarcopenia), as defined previously.4
The diagnosis of pre‐sarcopenia was confirmed when the skeletal muscle mass index (SMI), defined as the appendicular skeletal muscle mass/height2, was under 7.26 kg/m2 for men and 5.45 kg/m2 for women.31 Sarcopenia was confirmed when SMI was under 7.26 kg/m2 for men and 5.45 kg/m2 for women,31 associated with a handgrip strength under 30 kg for men and 20 kg for women,32 or a gait speed under 0.8 m/s.32, 33 Severe sarcopenia was confirmed when the SMI was under 7.26 kg/m2 for men and 5.45 kg/m2 for women,31 associated with a handgrip strength under 30 kg for men and 20 kg for women32 and a gait speed under 0.8 m/s.32, 33
The appendicular skeletal muscle mass was calculated as the sum of arms and legs lean soft‐tissue mass, assumed as skeletal muscle mass of all non‐fat and non‐bone tissue. The same operator performed all these measures.
Adverse events and adherence to supplementation protocol
Adverse events and adherence to supplementation protocol were recorded throughout the trial and were checked each time the researcher had contact with the participants (three times a week for 12 weeks). Besides, participants were free to call or send messages and e‐mails to the researcher at any time if they felt any discomfort during the intervention period.
Statistical analysis
The achieved power (1–β) of the analysis was calculated with the assistance of the G‐Power® software (version 3.1.7). An effect size of 0.99 for the lean mass (one of the primary outcomes) was calculated by the difference between two independent means and was entered in the analysis with a Type I error probability of 0.05. The estimated achieved power was 80%.
Data distributions were evaluated by the Shapiro–Wilk W‐test. Variables were converted into delta scores (i.e. post–pre values). The statistical significance of the effects of the intervention was assessed by the Student's t‐test or by the Mann–Whitney test, according to data distribution. Fisher's test was applied to assess the possible differences between groups in the proportion of participants who correctly guessed their supplements. All analyses were performed using Statistica for Windows software (version 10.0; Statsoft, Tulsa, OK, USA), considering a critical significance value of 5%. The sarcopenia incidence was reported descriptively, and all data are presented as mean ± standard deviation.
When there were significant correlations between values of nutrients and energy intake, the residual method was applied in order to describe the relationship between aspects of food intake and biochemical characteristics independent of energy intake.34
Results
Participants
The participant flow, along with losses and exclusions, is displayed in Figure 1. A total of 42 participants were screened for participation, and 32 met the inclusion criteria. These individuals were randomly assigned into the PL + RT (n = 16) and CR + RT (n = 16) groups. Five participants withdrew because of personal reasons (n = 2 and n = 3 from the PL + RT and CR + RT groups, respectively). Therefore, 14 participants from the PL + RT group and 13 from CR + RT group completed the trial and were analysed.
Figure 1.
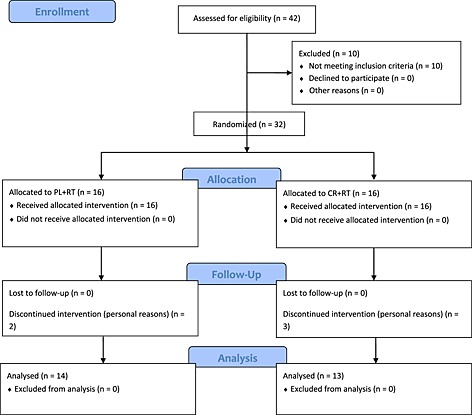
Participant flow through the study.
Medications used by the participants during intervention was assessed: eight participants from PL + RT and five from CR + RT were using anti‐hypertensive medications; three from PL + RT and two from CR + RT were using oral hypoglycemic medications; one of each group was using long‐acting insulin; two from PL + RT and one from CR + RT were using thyroid medication; and one of each group was using hormone replacement therapy.
Baseline data are presented in Table 1. There were no differences between groups for any baseline measurements (P > 0.05).
Table 1.
Baseline data and characteristics of the participants
| CR + RT (n = 13) | PL + RT (n = 14) | P | |
|---|---|---|---|
| Age (year) | 67.4 ± 4.7 | 67.1 ± 6.3 | 0.877 |
| Height (cm) | 158.3 ± 7.6 | 159.5 ± 8.9 | 0.706 |
| Body mass (kg) | 68.1 ± 13.7 | 70.1 ± 17.2 | 0.747 |
| Lean mass (kg) | 38.3 ± 8 | 40.4 ± 8.6 | 0.512 |
| Body fat (%) | 39.6 ± 10.4 | 39.2 ± 7.8 | 0.930 |
| Android fat (%) | 45.1 ± 11.2 | 46.4 ± 6.4 | 0.865 |
| Gynoid fat (%) | 44.1 ± 11.8 | 43.6 ± 9.2 | 0.910 |
| SMI (kg/m2) | 6.4 ± 0.9 | 6.6 ± 1.1 | 0.596 |
| 10 RM bench press (kg) | 14.7 ± 7.1 | 16 ± 5.5 | 0.616 |
| 10 RM leg press (kg) | 60.4 ± 35.5 | 57.6 ± 26.0 | 0.819 |
| Whole body BMC (kg) | 2.4 ± 0.5 | 2.3 ± 0.4 | 0.584 |
| Whole body BMD (g/cm2) | 1.1 ± 0.1 | 1.1 ± 0.1 | 0.884 |
| Lumbar spine BMD (g/cm2) | 1.1 ± 0.2 | 1.1 ± 0.2 | 0.607 |
| Dual femur BMD (g/cm2) | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.272 |
| Right femoral neck BMD (g/cm2) | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.281 |
| Left femoral neck BMD (g/cm2) | 0.9 ± 0.1 | 0.9 ± 0.2 | 0.273 |
Data are expressed as mean ± standard deviation. No significant between‐group differences were observed.
BMC, bone mineral content; BMD, bone mineral density; CR + RT, creatine plus resistance training; PL + RT, placebo plus resistance training; SMI, skeletal muscle mass index; 10 RM, ten‐repetition maximal.
Assessment of blinding, adherence to the exercise programme and food intake
At the conclusion of the intervention, four (30.8%) of the CR + RT group participants and two (14.3%) of the PL + RT group participants correctly identified which supplement they had received. No significant differences between the groups were noticed (P = 0.286).
The adherence to the exercise programme was comparable between the groups (89.1 ± 10.3% and 92.9 ± 7.7% for the CR + RT and the PL + RT groups, respectively; P = 0.274).
Total energy, macronutrients, and calcium intake did not significantly differ between and intra groups (P > 0.05; Table 2).
Table 2.
Daily food intake at baseline (pre) and after 12 weeks of intervention (post)
| CR + RT (n = 13) | PL + RT (n = 14) | P | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Δ | Pre | Post | Δ | ||
| Energy (kcal) | 1589.6 ± 395.2 | 1578.7 ± 372.8 | −10.9 ± 302.8 | 1472.3 ± 531.8 | 1460.7 ± 508.4 | −11.6 ± 331.5 | 0.865 |
| Protein (g) | 62.6 ± 19.1 | 68.8 ± 14.5 | 6.2 ± 14.6 | 65.5 ± 20.8 | 66.4 ± 23.6 | 0.9 ± 28.3 | 0.512 |
| Fat (g) | 51.9 ± 10.7 | 55.8 ± 11.8 | 3.9 ± 11.3 | 52.1 ± 5.9 | 49.7 ± 5.3 | −2.4 ± 5.8 | 0.078 |
| Carbohydrate (g) | 226.1 ± 35.8 | 212.2 ± 27.8 | −13.9 ± 30.8 | 193.2 ± 28.1 | 190.5 ± 34.3 | −2.7 ± 30.2 | 0.274 |
| Calcium (mg) | 576.8 ± 166.8 | 635.4 ± 198.4 | 58.6 ± 198.0 | 472.2 ± 165.7 | 482.1 ± 204.9 | 9.9 ± 175.2 | 0.504 |
Data are expressed as mean ± standard deviation. No significant differences intra and between groups were observed.
CR + RT, creatine plus resistance training; PL + RT, placebo plus resistance training.
Muscle strength assessments
There were no significant differences in 10 RM bench press and leg press changes between the groups (P > 0.05; Table 3). Both groups had a significant increase in the 10 RM test in bench press (CR + RT: +11.5 ± 5.0 kg; P < 0.0001 and PL + RT: +10.7 ± 6.2 kg; P < 0.0001, respectively) and leg press (CR + RT: +54.2 ± 46.8 kg; P < 0.0001 and PL + RT: +70.9 ± 33.4 kg; P < 0.0001, respectively) exercises.
Table 3.
Body composition, strength, and bone mass at baseline (pre) and after 12 weeks of intervention (post)
| CR + RT (n = 13) | PL + RT (n = 14) | Creatine main effect | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Δ | P | Pre | Post | Δ | P | ||
| Body mass (kg) | 68.1 ± 13.7 | 69.1 ± 14.1 | 0.9 ± 1.2 | 0.014 | 70.1 ± 17.2 | 70.2 ± 17.9 | 0.1 ± 1.5 | 0.757 | 0.122 |
| Lean mass (kg) | 38.3 ± 8 | 40.1 ± 8.7 | 1.8 ± 1.3 | 0.001 | 40.4 ± 8.6 | 40.9 ± 9.3 | 0.6 ± 1.3 | 0.143 | 0.023 |
| Body fat (%) | 39.6 ± 10.4 | 38.3 ± 10.1 | −1.2 ± 2.0 | 0.053 | 39.2 ± 7.8 | 38.6 ± 7.9 | −0.7 ± 1.8 | 0.179 | 0.461 |
| Android fat (%) | 45.1 ± 11.2 | 44.1 ± 10 | −1.0 ± 2.4 | 0.028 | 46.4 ± 6.4 | 46.2 ± 7.4 | −0.2 ± 2.7 | 0.802 | 0.482 |
| Gynoid fat (%) | 44.1 ± 11.8 | 42.5 ± 12.2 | −1.6 ± 2.4 | 0.035 | 43.6 ± 9.2 | 43.0 ± 9.0 | −0.6 ± 1.9 | 0.290 | 0.251 |
| SMI (kg/m2) | 6.4 ± 0.98 | 6.7 ± 1.1 | 0.4 ± 0.2 | 0.001 | 6.6 ± 1.1 | 6.8 ± 1.1 | 0.2 ± 0.3 | 0.007 | 0.145 |
| 10 RM bench press (kg) | 14.7 ± 7.1 | 26.2 ± 8.7 | 11.5 ± 5.0 | 0.001 | 16.0 ± 5.5 | 26.7 ± 7.7 | 10.7 ± 6.2 | 0.001 | 0.966 |
| 10 RM leg press (kg) | 60.4 ± 35.5 | 114.6 ± 41.3 | 54.2 ± 46.8 | 0.001 | 57.6 ± 26 | 128.6 ± 53.4 | 70.9 ± 33.5 | 0.001 | 0.294 |
| Whole body BMC (kg) | 2.4 ± 0.5 | 2.4 ± 0.5 | −0.02 ± 0.07 | 0.177 | 2.3 ± 0.4 | 2.3 ± 0.3 | −0.02 ± 0.1 | 0.331 | 0.912 |
| Whole body BMD (g/cm2) | 1.1 ± 0.1 | 1.2 ± 0.1 | 0.01 ± 0.03 | 0.090 | 1.1 ± 0.1 | 1.1 ± 0.1 | 0.004 ± 0.02 | 0.427 | 0.350 |
| Lumbar spine BMD (g/cm2) | 1.1 ± 0.2 | 1.1 ± 0.2 | −0.009 ± 0.04 | 0.377 | 1.1 ± 0.18 | 1.1 ± 0.2 | −0.01 ± 0.04 | 0.295 | 0.928 |
| Dual femur BMD (g/cm2) | 0.9 ± 0.1 | 0.9 ± 0.1 | −0.008 ± 0.03 | 0.308 | 0.91 ± 0.1 | 0.9 ± 0.1 | 0.006 ± 0.01 | 0.046 | 0.079 |
| Right femoral neck BMD (g/cm2) | 0.9 ± 0.1 | 0.9 ± 0.1 | −0.007 ± 0.03 | 0.345 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.01 ± 0.02 | 0.068 | 0.063 |
| Left femoral neck BMD (g/cm2) | 0.9 ± 0.1 | 0.9 ± 0.1 | −0.009 ± 0.03 | 0.295 | 0.9 ± 0.2 | 0.9 ± 0.1 | 0.002 ± 0.01 | 0.354 | 0.175 |
Data are expressed as mean ± standard deviation.
BMC, bone mineral content; BMD, bone mineral density; CR + RT, creatine plus resistance training; PL + RT, placebo plus resistance training; SMI, skeletal muscle mass index; 10 RM, ten‐repetition maximal.
Anthropometric assessment
CR + RT group had a significant increase in body mass after the intervention (+0.9 ± 1.2 kg; P = 0.014) intra group. Changes in body mass (Table 3) did not significantly differ between the groups (P > 0.05).
Body composition and bone mass assessments
The CR + RT group had superior gains in lean mass than the PL + RT group (P = 0.02), displayed in Figure 2. CR + RT group had a significant increase in SMI and muscle (+0.36 ± 0.23 kg/m2; P < 0.0001 and +1.79 ± 1.29 kg; P < 0.0001, respectively), a significant decrease in android and gynoid fat (−1.02 ± 2.39%; P = 0.028 and −1.56 ± 2.37%; P = 0.035, respectively) and a tendency to decrease body fat (−1.22 ± 2.05%; P = 0.053) after the intervention. PL + RT only had a significant increase in SMI (0.21 ± 0.25 kg/m2; P = 0.007) (Table 3).
Figure 2.
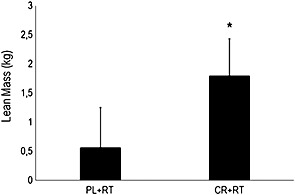
Effects of resistance training combined or not with creatine supplementation on lean mass in the elderly. CR + RT = creatine + resistance training; PL + RT = placebo + resistance training. *P < 0.05. Data are expressed as delta score ± standard deviation.
Changes in BMD and BMC (Table 3) did not significantly differ between groups in any assessed sites. The CR + RT group had a tendency to increase whole body BMD (+0.01 ± 0.02 g/cm2; P = 0.090) while PL + RT group had a significant increase in dual femur BMD (+0.01 ± 0.01 g/cm2; P = 0.046) and a tendency to increase right femoral neck (+0.01 ± 0.01 g/cm2; P = 0.068).
Diagnosis of sarcopenia
In the PL + RT group, three participants were classified as pre‐sarcopenic, none was classified as sarcopenic, and one was classified as severe sarcopenic. In the CR + RT group, three participants were also classified as pre‐sarcopenic, one was classified as sarcopenic, and one was classified as severe sarcopenic.
Following the intervention, pre‐sarcopenia incidence remained unchanged in the PL + RT group (n = 3) and decreased in the CR + RT group (n = 1). Sarcopenia incidence remained the same in both groups (PL + RT, n = 0; CR + RT, n = 1), while severe sarcopenia decreased in CR + RT (n = 0) and remained unchanged in the PL + RT group (n = 1).
Adverse events
There were no self‐reported side effects induced by creatine supplementation. However, discomforts related to muscle damage induced by the execution of resistance exercise training, such as muscle pain, swelling of the limbs, and reduced range of motion, were reported by the participants in both groups.
Discussion
In the present study, we examined the efficacy of low‐dose creatine supplementation associated with resistance training on lean mass, strength, and bone mass in the elderly. The main finding of this clinical trial indicated that creatine supplementation combined with resistance training increased lean mass in our elderly cohort in a greater magnitude than with isolated resistance training. The number of participants diagnosed with one of the three stages of sarcopenia at baseline decreased in the creatine supplemented group in comparison with the placebo group. To the best of our knowledge, this was the first study to evaluate creatine supplementation combined with resistance training on BMD and BMC in the elderly population, using a continuous low‐dosage supplementation protocol (i.e. 5 g/day during 12 weeks).
The results of the current study indicate that, in the elderly of both sexes, a continuous low‐dosage creatine supplementation protocol associated with resistance training is able not only to neutralize ageing‐induced loss of lean mass but also to augment it (+1.8 kg), when compared with resistance training alone (+0.6 kg). The increase in lean mass following creatine supplementation has been demonstrated in some but not all previous studies, as shown recently by a meta‐analysis16 and a review.15 Chrusch et al.35 demonstrated significant increases in lean mass in older men (+3.3 kg) after 3 months of creatine supplementation (0.3 g/kg of body weight/day for the first 5 days and 0.07 g/kg of body weight/day until completion of the final test period) associated with resistance training, when compared with placebo. Thus, it is noticeable that both loading phase followed by maintenance phase and continuous low‐dosage creatine supplementation are able to augment lean mass in the elderly after 3 months of intervention.
Although creatine supplementation was able to augment lean mass, this increase was not translated in an increase in muscle strength. Some studies has shown that maintenance, or gain of lean mass was not associated with increases in or maintenance of muscle strength in older adults, demonstrating that muscle strength is not entirely determined by muscle mass.36, 37, 38 Goodpaster et al.36 suggests that progressive muscle weakness in the elderly may be related to age‐related neurological changes, the hormonal and metabolic milieu, pro‐inflammatory cytokines, and fat infiltration. However, further studies are needed to better elucidate these relations. Furthermore, both CR + RT and PL + RT groups showed increased muscle strength, but it was effect of resistance training itself. Corroborating with this finding, studies has demonstrated that creatine supplementation alone was not able to improve muscle strength in healthy older adults.39, 40, 41, 42 It is well‐known that the mechanisms by which creatine improves muscle strength is by increasing muscle phosphocreatine stores, speeding its resynthesis, and decreasing recovery time, suggesting greater training volume.43 Nonetheless, both CR + RT and PL + RT groups trained under the same training protocol, without differences in the volume of training, making it unlikely to have different response from the supplement itself.
Both in younger44 and older45 individuals, creatine supplementation is able to increase intramuscular creatine and phosphorylcreatine content. However, Rawson et al.45 demonstrated that these increases are smaller in the elderly than in young people. Thus, in the current study, to maximize muscle creatine retention, participants were instructed to consume the supplement immediately after lunch on non‐training days or immediately after resistance training sessions dissolved in a beverage comprising 100 g of maltodextrin on training days. As shown by the studies of Green et al.22, 23 and concluded by a meta‐analysis,16 although creatine monohydrate is highly bioavailable, when large amounts of high glycemic index carbohydrate (approximately 95 g) are ingested with this amine, muscle retention is enhanced. It is speculated that this response is generated because of the increased plasma insulin concentration, which would stimulate the transport of creatine by its transporter (CreaT; sodium‐dependent) into muscle cells. Moreover, Robinson et al.46 demonstrated that a submaximal exercise session is able to enhance the accumulation of creatine induced by supplementation and that this effect is restricted to the muscle groups exercised. The explanation comes from an increased blood flow induced by exercise in an exercised member, which generates most of creatine offered to the exercised muscle.
In addition, this amine did not generate statistically significant changes in BMC and BMD between the groups in any of the assessed sites. Until now, only a single study43 has assessed BMD and BMC in different sites, using a continuous low‐dosage creatine supplementation protocol associated with resistance training for 12 months wherein it was demonstrated that the femoral neck BMD was only preserved and did not increase in post‐menopausal women. Collectively, these data led to conclude that creatine supplementation is unable to cause changes in BMC and BMD in the elderly or that supplementation protocol and/or intervention time was insufficient to ensure an increase in these variables.
Following the intervention, in our study, the number of participants diagnosed with one of the three stages of sarcopenia at baseline decreased in the creatine supplemented group in comparison with the placebo group (CR + RT, n = −3; PL + RT, n = 0). Devries and Phillips16 conducted a meta‐analysis to determine whether the addition of creatine to resistance training improved body composition, increased strength, and functional performance in older adults and concluded that these strategies together are able to attenuate sarcopenic changes in this group. The results of this study, although had no statistical significance, demonstrates the clinical relevance and the necessity for future studies addressing the same intervention on a population diagnosed with one of the three stages of sarcopenia.
Although, in our study, we were unable to detect significant changes in body mass, body fat, android and gynoid fat, SMI, strength, BMC, and BMD between groups, intra‐group analyses showed significant differences in the CR + RT group. There was a significant increase in body mass, SMI, and strength and a significant decrease in android and gynoid fat after the intervention. Furthermore, the group had a tendency to decrease body fat. Possible explanations may involve the limitations of this trial, which includes the absence of a control group, low sample size, and lack of muscle creatine and phosphorylcreatine content measurement. Existing literature has already demonstrated that creatine supplementation in older and younger individuals can increase muscle creatine and phosphorylcreatine content.7, 44 However, Rawson45 demonstrated that these increases are smaller in the elderly than in young people. Thus, future studies must assess this information to make sure muscle creatine and phosphorylcreatine content significantly increased in the elderly.
In summary, 12 weeks of a low‐dosage creatine supplementation associated with resistance training resulted in an increase on lean mass in the elderly. Future long‐term research should investigate the effects of these interventions in sarcopenic elderly.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgments
We would like to thank Fundação de Amparo à Pesquisa do Estado de Goiás (FAPEG) for the support and MedNutrition for providing the supplements.
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia, and Muscle, 2010;1:7–8 (von Haehling S, Morley JE, Coats AJS, Anker SD.
Pinto, C. L. , Botelho, P. B. , Carneiro, J. A. , and Mota, J. F. (2016) Impact of creatine supplementation in combination with resistance training on lean mass in the elderly. Journal of Cachexia, Sarcopenia and Muscle, 7: 413–421. doi: 10.1002/jcsm.12094.
References
- 1. World Health Organization . Global health and ageing. National Institute on Aging (NIA), National Institutes of Health (NIH), 2011. 32 p. Available from: U.S. Department of Health and Human Services, Washington.
- 2. Brazilian Geography and Statistics Institute Web site [Internet] . Brazilian Geography and Statistics Institute, 2013. [cited 2014 Sep 9]. Available from: http://www.ibge.gov.br/home/estatistica/populacao/projecao_da_populacao/2013/default.shtm.
- 3. Araújo AP, Bertolini SMMG, Junior JM. Morphophysiologial alterations resulted from the process of musculoskeletal system aging and its consequences for the human body. Perspectivas: biológicas e saúde 2014;4:22–34. [Google Scholar]
- 4. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al Sarcopenia: European consensus on definition and diagnosis: report of the european working group on sarcopenia in older people. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wyss M, Kaddurah‐Daouk R. Creatine and creatinine metabolism. Physiol Rev 2000;80:1107–1213. [DOI] [PubMed] [Google Scholar]
- 6. Gualano B, Artioli GG, Poortmans JR, Lancha Junior AH. Exploring the therapeutic role of creatine supplementation. Amino Acids 2010;38:31–44. [DOI] [PubMed] [Google Scholar]
- 7. Rawson ES, Lieberman HR, Walsh TM, Zuber SM, Harhart JM, Matthews TC. Creatine supplementation does not improve cognitive function in young adults. Physiol Behav 2008;95:130–134. [DOI] [PubMed] [Google Scholar]
- 8. Gualano B, Roschel H, Lancha‐Jr AH, Brightbill CE, Rawson ES. In sickness and in health: the widespread application of creatine supplementation. Amino Acids 2012;43:519–529. [DOI] [PubMed] [Google Scholar]
- 9. Chilibeck PD, Sale DG, Webber CE. Exercise and bone mineral density. Sports Med 1995;19:103–122. [DOI] [PubMed] [Google Scholar]
- 10. Rawson ES, Wehnert ML, Clarkson PM. Effects of 30 days of creatine ingestion in older men. Eur J Appl Physiol Occup Physiol 1999;80:139–144. [DOI] [PubMed] [Google Scholar]
- 11. Rawson ES, Clarkson PM. Acute creatine supplementation in older men. Int J Sports Med 2000;21:71–75. [DOI] [PubMed] [Google Scholar]
- 12. Gotshalk LA, Volek JS, Staron RS, Denegar CR, Hagerman FC, Kraemer WJ. Creatine supplementation improves muscular performance in older men. Med Sci Sports Exerc 2002;34:537–543. [DOI] [PubMed] [Google Scholar]
- 13. Stout JR, Sue Graves B, Cramer JT, Goldstein ER, Costa PB, Smith AE, et al Effects of creatine supplementation on the onset of neuromuscular fatigue threshold and muscle strength in elderly men and women (64–86 years). J Nutr Health Aging 2007;11:459–464. [PubMed] [Google Scholar]
- 14. Gotshalk LA, Kraemer WJ, Mendonca MA, Vingren JL, Kenny AM, Spiering BA, et al Creatine supplementation improves muscular performance in older women. Eur J Appl Physiol 2008;102:223–231. [DOI] [PubMed] [Google Scholar]
- 15. Candow DG, Chilibeck PD, Forbes SC. Creatine supplementation and aging musculoskeletal health. Endocrine 2014;45:354–361. [DOI] [PubMed] [Google Scholar]
- 16. Devries MC, Phillips SM. Creatine supplementation during resistance training in older adults‐a meta‐analysis. Med Sci Sports Exerc 2014;46:1194–1203. [DOI] [PubMed] [Google Scholar]
- 17. Ferri A, Scaglioni G, Pousson M, Capodaglio P, Van Hoecke J, Narici MV. Strength and power changes of the human plantar flexors and knee extensors in response to resistance training in old age. Acta Physiol Scand 2003;177:69–78. [DOI] [PubMed] [Google Scholar]
- 18. Hunter GR, McCarthy JP, Bamman MM. Effects of resistance training on older adults. Sports Med 2004;34:329–348. [DOI] [PubMed] [Google Scholar]
- 19. Candow DG, Chilibeck PD. Potential of creatine supplementation for improving aging bone health. J Nutr Health Aging 2010;14:149–153. [DOI] [PubMed] [Google Scholar]
- 20. DiVasta AD, Gordon CM. Exercise and bone: where do we stand? Metabolism 2013;62:1714–1717. [DOI] [PubMed] [Google Scholar]
- 21. Hultman E, Söderlund K, Timmons JA, Cederblad G, Greenhaff PL. Muscle creatine loading in men. J Appl Physiol 1996;81:232–237. [DOI] [PubMed] [Google Scholar]
- 22. Green AL, Hultman E, Macdonald IA, Sewell DA, Greenhaff PL. Carbohydrate ingestion augments skeletal muscle creatine accumulation during creatine supplementation in humans. Am J Physiol 1996;271:E821–E826. [DOI] [PubMed] [Google Scholar]
- 23. Green AL, Simpson EJ, Littlewood JJ, Macdonald IA, Greenhaff PL. Carbohydrate ingestion augments creatine retention during creatine feeding in humans. Acta Physiol Scand 1996;158:195–202. [DOI] [PubMed] [Google Scholar]
- 24. Antonio J, Ciccone V. The effects of pre versus post workout supplementation of creatine monohydrate on body composition and strength. J Int Soc Sports Nutr 2013;10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garber CE, Deschenes M. General principles of exercise prescription In Pescatello LS, Arena R, Riebe D, Thompson PD, eds. ACSM's Guidelines for Exercise Testing and Prescription. Baltimore: Lippincott Williams & Wilkins; 2014. p162–193. [Google Scholar]
- 26. Galante AP. Development and validation of a computerized method for assessment of dietary intake, filled by adults using the Web [dissertation]. São Paulo (SP): University of São Paulo; 2007. p 1–129. [Google Scholar]
- 27. Vandenberghe K, Gillis N, Van Leemputte M, Van Hecke P, Vanstapel F, Hespel P. Caffeine counteracts the ergogenic action of muscle creatine loading. J Appl Physiol 1996;80:452–457. [DOI] [PubMed] [Google Scholar]
- 28. Farinatti PT, Geraldes AA, Bottaro MF, Lima MV, Albuquerque RB, Fleck SJ. Effects of different resistance training frequencies on the muscle strength and functional performance of active women older than 60 years. J Strength Cond Res 2013;27:2225–2234. [DOI] [PubMed] [Google Scholar]
- 29. Miguel FM, Grings LA, Pereira GB, Leite RD, Vieira A, Sousa NMF, et al Different cardiovascular responses to a resistance training session in hypertensive women receiving propanolol compared with normotensive controls. Sci World J 2012; doi: 10.1100/2012/913271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual. Human Kinetics: Champaign (IL); 1988. p177. [Google Scholar]
- 31. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147:755–763. [DOI] [PubMed] [Google Scholar]
- 32. Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, et al Age‐associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol 2003;95:1851–1860. [DOI] [PubMed] [Google Scholar]
- 33. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al A short physical performance battery assessing lower extremity function: association with self‐reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 34. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;654 Suppl: 1220S–1228S discussion 1229S–1231S. [DOI] [PubMed] [Google Scholar]
- 35. Chrusch MJ, Chilibeck PD, Chad KE, Davison KS, Burke DG. Creatine supplementation combined with resistance training in older men. Med Sci Sports Exerc 2001;33:2111–2117. [DOI] [PubMed] [Google Scholar]
- 36. Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2006;61:1059–1064. [DOI] [PubMed] [Google Scholar]
- 37. Cawthon PM, Fox KM, Gandra SR, Delmonico MJ, Chiou CF, Anthony MS, et al Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J Am Geriatr Soc 2009;57:1411–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez‐Mieyer P, et al Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr 2009;90:1579–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bermon S, Venembre P, Sachet C, Valour S, Dolisi C. Effects of creatine monohydrate ingestion in sedentary and weight‐trained older adults. Acta Physiol Scand 1998;164:147–155. [DOI] [PubMed] [Google Scholar]
- 40. Eijnde B, Van Leemputte M, Goris M, et al Effects of creatine supplementation and exercise training on fitness in men 55–75 yr old. J Appl Physiol1985 2003;95:818–828. [DOI] [PubMed] [Google Scholar]
- 41. Carter JM, Bemben DA, Knehans AW, Bemben MG, Witten MS. Does nutritional supplementation influence adaptability of muscle to resistance training in men aged 48 to 72 years. J Geriatr Phys Ther 2005;28:40–47. [DOI] [PubMed] [Google Scholar]
- 42. Chilibeck PD, Candow DG, Landeryou T, Kaviani M, Paus‐Jenssen L. Effects of creatine and resistance training on bone health in postmenopausal women. Med Sci Sports Exerc 2015;47:1587–1595. [DOI] [PubMed] [Google Scholar]
- 43. Rawson ES, Venezia AC. Use of creatine in the elderly and evidence for effects on cognitive function in young and old. Amino Acids 2011;40:1349–1362. [DOI] [PubMed] [Google Scholar]
- 44. Harris RC, Söderlund K, Hultman E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin Sci (Colch) 1992;83:367–374. [DOI] [PubMed] [Google Scholar]
- 45. Rawson ES, Clarkson PM, Price TB, Miles MP. Differential response of muscle phosphocreatine to creatine supplementation in young and old subjects. Acta Physiol Scand 2002;174:57–65. [DOI] [PubMed] [Google Scholar]
- 46. Robinson TM, Sewell DA, Hultman E, Greenhaff PL. Role of submaximal exercise in promoting creatine and glycogen accumulation in human skeletal muscle. J Appl Physiol 1999;87:598–604. [DOI] [PubMed] [Google Scholar]


