Abstract
Background
Previous studies have demonstrated that high estimated glomerular filtration rate (eGFR) is paradoxically associated with an increased risk of mortality, and the association becomes more predominant in older people. However, the role of malnutrition–inflammation–cachexia syndrome (MICS) in the association between eGFR and mortality has never been explored.
Methods
We conducted a community‐based cohort study using data from the Taipei City Elderly Health Examination Database, collected during the period 2001–10. All participants aged ≥65 years were included and stratified by the absence or presence of MICS, which is defined as the presence of at least one of the following markers: body mass index <22 kg/m2, serum albumin <3.0 mg/dL, or Geriatric Nutritional Risk Index (GNRI) <98. The study endpoints were all‐cause and cardiovascular mortality.
Results
A total of 131 354 participants were identified and categorized according to the chronic kidney disease stage based on eGFR. Compared with the reference eGFR of 60–89 mL/min/1.73 m2, the overall and cardiovascular mortality risks were markedly high in the groups with eGFR of <30 mL/min/1.73 m2 [overall: adjusted hazard ratio (aHR), 1.86; 95% confidence interval (CI), 1.72–2.00; cardiovascular: aHR, 1.87; 95% CI, 1.60–2.19] and ≥90 mL/min/1.73 m2 (overall: aHR, 1.23; 95% CI, 1.13–1.34; cardiovascular: aHR, 1.28; 95% CI, 1.06–1.54). In the absence of MICS, high eGFR was associated with lower mortality risk (aHR, 0.71; 95% CI, 0.62–0.80), and the U‐shaped relationship disappeared. Subgroup analyses produced consistent results.
Conclusions
MICS could influence the association observed between high eGFR and mortality in older people, particularly in those with low body mass index, albumin level, GNRI, and very low serum creatinine level.
Keywords: Cardiovascular death, Estimated glomerular filtration rate, Malnutrition–inflammation–cachexia syndrome, Mortality, Sarcopenia
Introduction
Cumulative evidence has shown inverse relationships between estimated glomerular filtration rate (eGFR) and overall and cardiovascular mortality.1, 2 Recent studies have shown a U‐shaped relationship between eGFR and mortality. Intriguingly, high eGFRs are paradoxically associated with increased mortality, particularly in the elderly population.3, 4 However, the reason for this paradox has not been explored.
Experimental and clinical studies have suggested that glomerular hyperfiltration, an early phase of diabetes, is associated with subsequent higher mortality risk.5, 6, 7 However, not all people included in previous studies had diabetes mellitus,3, 4 and the interval between hyperfiltration and the development of catastrophic outcomes is typically long. Therefore, this paradoxical relationship cannot be fully explained by glomerular hyperfiltration. In contrast, low serum creatinine levels in older people with loss of muscle mass may contribute to high eGFR. Malnutrition, chronic inflammation, and cachexia tend to occur concurrently in elderly individuals, which is often termed as ‘malnutrition–inflammation–cachexia syndrome’ (MICS) or protein‐energy wasting.8, 9, 10 Chronic disease accompanied by MICS could accelerate muscle mass wasting in older people, thereby affecting survival outcomes. Moreover, protein‐energy wasting, a systematically defined condition that reflects malnutrition and wasting resulting from inflammatory and non‐inflammatory conditions, is associated with increased mortality in older patients.11, 12, 13 Several nutritional assessment tools are not only able to measure malnutrition but also detect the presence and severity of inflammation. Therefore, the existence of an overlap between malnutrition and inflammation at both overlapping causes and diagnosis is increasingly acknowledged.8 The Geriatric Nutritional Risk Index (GNRI) has been developed and validated as a simplified assessment tool for evaluating the nutritional status complications in hemodialysis patients and at‐risk elderly individuals.14, 15 It was regarded as a good prognostic predictor of morbidity and mortality for grading the nutritional risk and identifying elderly individuals at risk for MICS. Based on this background, we tested the hypothesis that high eGFR and MICS may be an interactive factor for mortality in a large, well‐characterized cohort of patients using data from the Taipei City Elderly Health Examination Database.
Materials and Methods
Data source
In 2001, the Taipei City Government launched an annual Geriatric Health Examination Program for citizens aged ≥65 years. This programme provides health examinations that are performed according to an identical protocol and medical services with no formal referral requirement. Older citizens participate on a voluntary basis, are encouraged to undergo health examination annually, and are free to choose any hospital contracted with the Taipei City Government.16, 17 All participants provide written informed consent authorizing the Taipei City Government Institution to process health examination data and release data for research purposes. This database is linked to the national death registry system. Detailed data are centrally stored in the Taipei City Elderly Health Examination Database and are adequately de‐identified before being released. The institutional review board of the Taipei City Hospital approved this study (TCHIRB‐1010323‐E; TCHIRB‐1030601‐W).
Study design and data collection
The study cohort comprised participants aged ≥65 years who had undergone at least one serum creatinine measurement between 2001 and 2010. We excluded individuals with histories of end‐stage renal disease or kidney transplant. Sociodemographic and lifestyle‐related data (i.e. age, gender, marital status, education level, alcohol consumption, smoking status, and exercise habit) were collected using a self‐administered questionnaire through the Geriatric Health Examination Program. We also recorded the body height, body weight, systolic and diastolic blood pressure measured at each visit, and participants' past medical histories of hypertension, diabetes mellitus, dyslipidaemia, coronary artery disease, and cerebrovascular disease. Body mass index (BMI) was calculated using the formula weight (in kilogrammes) / height (in square metres). The following laboratory data were also included in the analysis: white blood cell (WBC) count, dipstick urinalysis results, fasting glucose, and total cholesterol levels, and concentrations of haemoglobin, blood urea nitrogen, creatinine, albumin, uric acid, and triglycerides.
Assessment of malnutrition–inflammation–cachexia syndrome
The GNRI formula used is as follows: GNRI = (1.489 × albumin in grammes per litre) + (41.7 × present/ideal body weight). The ideal body weight was calculated according to the Lorentz formula, which takes into account a patient's height and sex: for men, height − 100 − [(height − 150)/4]; for women, height − 100 − [(height − 150)/2.5]. The presence of MICS in elderly individuals was defined as the presence of at least one of the following conditions: BMI <22 kg/m2, serum albumin <3.0 mg/dL, or GNRI <98.15 The participants were categorized into groups according to the presence or absence of MICS to examine the impact of this condition on the outcomes.
Assessment of kidney function
After an overnight fast, blood was collected, and serum creatinine was analysed using the uncompensated Jaffe method and alkaline picrate kinetic test.18 The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equation or the Modification of Diet in Renal Disease (MDRD) Study equation.19 The participants were further classified by the stage of CKD based on their eGFR.
Study outcomes
Mortality data were obtained from the national death registry system and coded from death certificates according to the International Classification of Diseases (ICD)‐9 or ICD‐10. The primary outcome was all‐cause mortality (ICD‐9 001.x–999.x or ICD‐10 A00.x–Z99.x). The secondary outcome was cardiovascular‐related death (ICD‐9 393.x–459.x; ICD‐10 I07.x–I77.x, I99.x, J00.x, and J04.x). All participants were followed up until death or 31 December 2010.
Statistical analysis
Descriptive statistics were used to describe the baseline characteristics of the study cohort. Baseline characteristics of the two groups were compared using the Pearson χ2 test for categorical variables and the independent t‐test for parametric continuous variables. A Cox proportional hazard model was used to calculate adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) for the risk of mortality in each group. We further used eGFR linear splines (knots at intervals of 15 mL/min/1.73 m2 between 30 and 105 mL/min/1.73 m2) in Cox proportional hazard models, providing HRs with eGFR of 80 mL/min/1.73 m2 selected as a stable reference for all age categories, based on the research by Hallan et al.20 Reference ranges are important for statistical testing, but they do not alter the shape of the association across the full range of exposure. Other covariates included in the models are listed in Table 1. In addition, we conducted further subgroup analysis stratified by sex, age, smoking, diabetes mellitus, hypertension, and dyslipidaemia to explore the interaction between eGFR and mortality in the presence or absence of MICS. We considered an interaction term to be significant by Bonferroni‐corrected alpha levels of 0.008 (0.05/6). All statistical analyses were conducted using STATA statistical software (version 13.0; StataCorp, College Station, TX, USA). A P value of <0.05 was considered to be statistically significant.
Table 1.
Demographic and clinical characteristics of the study population
| Characteristic | Overall | With MICS | Without MICS | P |
|---|---|---|---|---|
| Number of patients, N (%) | 131 354 (100) | 38 151 (29.0) | 93 203 (71.0) | |
| Demographic characteristics | ||||
| Age (years) | 72.6 ± 6.3 | 73.5 ± 6.3 | 71.6 ± 6.1 | <0.001 |
| Sex (male), N (%) | 68 533 (52.1) | 19 290 (50.6) | 49 243 (52.8) | 0.147 |
| Hypertension, N (%) | 72 159 (54.9) | 16 978 (44.5) | 55 181 (59.2) | <0.001 |
| Diabetes mellitus, N (%) | 15 536 (11.8) | 3858 (10.1) | 11 678 (12.5) | <0.001 |
| Dyslipidaemia, N (%) | 68 408 (52.0) | 18 451 (48.4) | 49 957 (53.6) | <0.001 |
| Coronary artery disease, N (%) | 15 277 (11.6) | 3879 (10.2) | 11 398 (12.2) | 0.679 |
| Cerebrovascular disease, N (%) | 1212 (0.9) | 452 (1.2) | 760 (0.8) | <0.001 |
| Smoking, N (%) | 13 332 (10.1) | 4265 (11.2) | 9067 (9.7) | <0.001 |
| Alcohol use, N (%) | 19 468 (14.8) | 4642 (12.2) | 14 826 (15.9) | <0.001 |
| Systolic blood pressure (mm Hg) | 135.7 ± 20.3 | 132.4 ± 21.0 | 137.1 ± 19.5 | <0.001 |
| Diastolic blood pressure (mm Hg) | 77.1 ± 12.0 | 74.7 ± 12.1 | 78.1 ± 11.7 | <0.001 |
| eGFR (mL/min/1.73 m2) | <0.001 | |||
| ≥90, N (%) | 13 187 (10.0) | 4254 (11.2) | 8933 (9.6) | |
| 60–89, N (%) | 72 631 (55.3) | 21 312 (55.9) | 51 319 (55.1) | |
| 30–59, N (%) | 42 633 (32.5) | 11 444 (30.0) | 31 189 (33.5) | |
| 15–29, N (%) | 2199 (1.7) | 799 (2.1) | 1400 (1.5) | |
| <15, N (%) | 704 (0.5) | 342 (0.9) | 362 (0.4) | |
| MICS indicators | ||||
| Albumin (g/dL) | 4.3 ± 0.3 | 4.2 ± 0.4 | 4.4 ± 0.3 | <0.001 |
| BMI (kg/m2) | 24.3 ± 3.5 | 21.0 ± 2.8 | 25.6 ± 2.7 | <0.001 |
| GNRI | 112.7 ± 11.0 | 104.2 ± 8.4 | 116.3 ± 10.0 | <0.001 |
| Other laboratory data | ||||
| WBC count (/mm3) | 6100 ± 1,834 | 5955 ± 2018 | 6159 ± 1748 | <0.001 |
| Total cholesterol (mg/dL) | 200.1 ± 37.6 | 197.0 ± 38.6 | 201.4 ± 37.2 | <0.001 |
| Triglyceride (mg/dL) | 130.0 ± 81.7 | 122.6 ± 48.0 | 133.0 ± 55.3 | <0.001 |
| HDL cholesterol (mg/dL) | 53.5 ± 14.7 | 55.0 ± 10.4 | 52.9 ± 8.3 | <0.001 |
| Haemoglobin (g/dL) | 13.5 ± 1.4 | 13.1 ± 1.5 | 13.7 ± 1.4 | <0.001 |
| Uric acid (mg/dL) | 6.1 ± 1.7 | 5.8 ± 1.7 | 6.3 ± 1.7 | <0.001 |
| BUN (mg/dL) | 18.0 ± 6.8 | 18.1 ± 7.8 | 17.9 ± 6.3 | <0.001 |
| Fasting glucose (mg/dL) | 107.3 ± 31.1 | 103.2 ± 29.8 | 108.9 ± 31.4 | <0.001 |
Data are presented as n (%) or mean ± standard deviation.
BMI, body mass index; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; GNRI, Geriatric Nutritional Risk Index; HDL, high‐density lipoprotein; MICS, malnutrition–inflammation–cachexia syndrome; WBC, white blood cell.
Results
Clinical characteristics of the study population
During the 10‐year study period, a total of 131 354 older participants were identified. Their baseline characteristics are shown in Table 1. The study participants were predominantly male (52.1%), and the mean age was 72.6 years. The most common comorbidities were hypertension (54.9%) and dyslipidaemia (52%). The prevalence rates of smoking and alcohol consumption were 10.1% and 14.8%, respectively. Of the total participants, 38 151 (29.0%) had MICS, and 93 203 (71.0%) did not have MICS. Compared with the participants with MICS, those without MICS were younger and more likely to have diabetes and/or cerebrovascular disease. Participants without MICS also had higher systolic blood pressure, WBC count, total cholesterol level, serum albumin, triglyceride, and haemoglobin concentrations. The detailed numbers of patients with low levels of albumin, BMI, or GNRI were listed in eTable 1 (see Supporting Information).
Malnutrition–inflammation–cachexia syndrome modified the U‐shaped relationship between estimated glomerular filtration rate and mortality
The U‐shaped relationship between eGFR and mortality, representing a gradually increased risk of all‐cause mortality, was observed in older participants with eGFR <60 and ≥90 mL/min/1.73 m2 in comparison with those with eGFR of 80 mL/min/1.73 m2 (Figure 1A).
Figure 1.
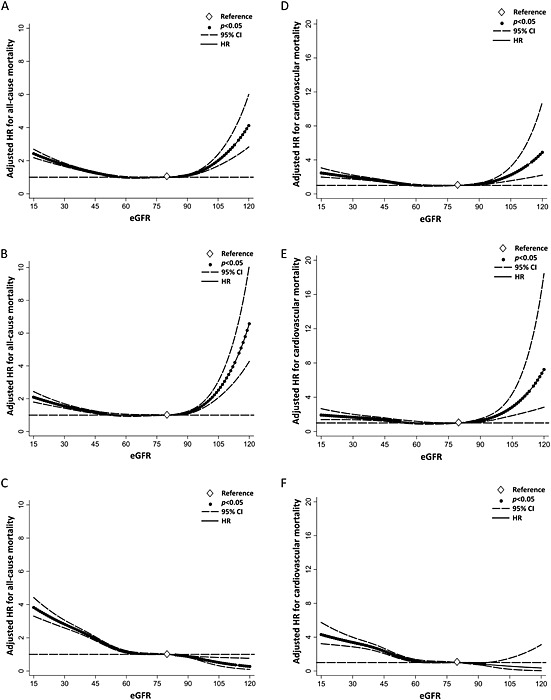
Adjusted hazard ratios for all‐cause mortality and cardiovascular mortality according to estimated glomerular filtration rate in all participants (A and D) in those with (B and E) and without (C and F) malnutrition–inflammation–cachexia status. CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio.
In the presence of MICS, the association of eGFR with all‐cause mortality was similar (Figure 1B). The exclusion of participants with MICS resulted in the disappearance of the U‐shaped curve between eGFR and mortality and an exponential increase in the risk of all‐cause mortality amongst those with low, but not high, eGFR (Figure 1C). Similar associations of eGFR with cardiovascular mortality were noted (Figure 1D–F). In the subgroup analyses stratified by MICS parameters, sex and diabetes mellitus, the interaction between MICS and the relationship between eGFR and mortality remained consistent (see Supporting Information, eFigures 1–5). The Taipei City Government included the urine dipstick testing in the Geriatric Health Examination Program in 2005. In a stratified analysis of the cohort from 2005 to 2010 that included proteinuria as a categorical variable (negative, trace, 1+, and >2+), the interaction between MICS and the association between eGFR and mortality remained consistent (see Supporting Information, eFigure 6).
The risk of all‐cause and cardiovascular mortality amongst older adults
Using the CKD‐EPI equation, the risk of all‐cause mortality was higher in the groups with eGFR of <30 mL/min/1.73 m2 (aHR, 1.86; 95% CI, 1.72–2.00) and > 90 mL/min/1.73 m2 (aHR, 1.23; 95% CI, 1.13–1.34) than in those with eGFR of 60–89 mL/min/1.73 m2 as the reference group (Table 2). The risk of cardiovascular mortality was also higher in the groups with eGFR of <30 mL/min/1.73 m2 (aHR, 1.87; 95% CI, 1.60–2.19) and ≥90 mL/min/1.73 m2 (aHR, 1.28; 95% CI, 1.06–1.54) than in the reference group.
Table 2.
Risk of all‐cause and cardiovascular mortality amongst older participants
| Overall | Presence of MICS | Absence of MICS | P interaction c | ||||
|---|---|---|---|---|---|---|---|
| eGFRa (mL/min/1.73 m2) | HR (95% CI)b | P | HR (95% CI)b | P | HR (95% CI)b | P | |
| All‐cause mortality | |||||||
| ≥90 | 1.23 (1.13–1.34) | <0.001 | 1.36 (1.21–1.53) | <0.001 | 0.71 (0.62–0.80) | <0.001 | <0.001 |
| 60–89 | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| 30–59 | 1.17 (1.13–1.21) | <0.001 | 1.09 (1.03–1.15) | 0.001 | 1.55 (1.48–1.61) | <0.001 | |
| <30 | 1.86 (1.72–2.00) | <0.001 | 1.73 (1.55–1.94) | <0.001 | 2.53 (2.28–2.81) | <0.001 | |
| Cardiovascular mortality | |||||||
| ≥90 | 1.28 (1.06–1.54) | 0.010 | 1.52 (1.18–1.96) | 0.001 | 0.65 (0.49–0.85) | 0.002 | <0.001 |
| 60–89 | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| 30–59 | 1.30 (1.21–1.39) | <0.001 | 1.23 (1.11–1.38) | <0.001 | 1.72 (1.58–1.88) | 0.094 | |
| <30 | 1.87 (1.60–2.19) | <0.001 | 1.66 (1.30–2.12) | <0.001 | 2.71 (2.21–3.33) | <0.001 | |
eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equation.
Adjusted for age, sex, smoking and alcohol habits, systolic and diastolic blood pressure, hypertension, diabetes mellitus, dyslipidaemia, coronary artery disease, cerebrovascular disease, body mass index, white blood cell count, and total cholesterol, triglyceride, high‐density lipoprotein, haemoglobin, uric acid, blood urea nitrogen, fasting glucose, and albumin levels.
P interaction values represent the modifying effect of malnutrition–inflammation–cachexia status on the risk relationship between baseline eGFR and mortality. Values were obtained using Cox models with eGFR serving as a categorical variable.
CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; MICS, malnutrition–inflammation–cachexia syndrome.
Malnutrition–inflammation–cachexia syndrome and its correlates in older adults
In the presence of MICS, eGFR of >90 mL/min/1.73 m2 remained associated with an increased risk of all‐cause mortality (aHR, 1.36; 95% CI, 1.21–1.53) and cardiovascular mortality (aHR, 1.52; 95% CI, 1.18–1.96) compared with the reference eGFR of 60–89 mL/min/1.73 m2 (Table 2). In the absence of MICS, however, the association of high eGFR with an increased risk of mortality disappeared. In contrast, eGFR of >90 mL/min/1.73 m2 was associated with a lower risk of all‐cause mortality (aHR, 0.71; 95% CI, 0.62–0.80). These results remained similar after adjusting for BMI alone (see Supporting information, eTable 2). We performed another analysis using MDRD equation to calculate eGFR, and the results remain similar (see Supporting Information, eTable 3). In the subgroup analyses, the risk of mortality was increased in older subjects with high eGFR in the presence of MICS, and the results were almost entirely consistent across all subgroups (Figure 2). Conversely, in the absence of MICS, the risk of mortality was lower in the high eGFR group than in the reference eGFR group.
Figure 2.
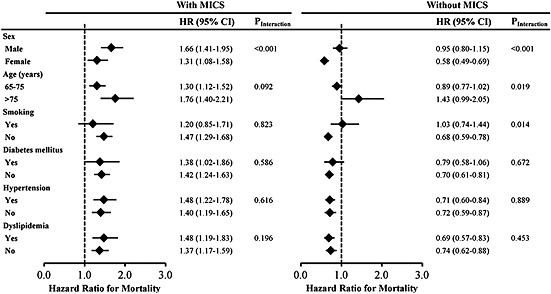
Subgroup analysis of the association between high estimated glomerular filtration rate (>90 mL/min/1.73 m2) and risk of all‐cause mortality as compared with the median estimated glomerular filtration rate (60–89 mL/min/1.73 m2) in older participants with malnutrition–inflammation–cachexia status (left panel) and without malnutrition–inflammation–cachexia status (right panel). CI, confidence interval; HR, hazard ratio; MICS, malnutrition–inflammation–cachexia status.
Discussion
Previous large collaborative meta‐analyses have shown that the association between eGFR and mortality forms a U‐shaped curve with an increased risk associated with lower and higher eGFRs, particularly in older people.4, 20, 21 A pooled analysis of community‐based studies found that patients with CKD and eGFR of <60 mL/min/1.73 m2 had a greater risk of all‐cause mortality.22 Several studies have further shown an exponential increase in the mortality rate with declining eGFR, with a sharp increase in eGFR to <45 mL/min/1.73 m2.1 However, the reason underlying this paradoxical association of higher eGFR with mortality has not been thoroughly explored. Cox et al.3 have noted that respiratory disease and cancer, rather than cardiovascular disease, were the main causes of death in subjects with high eGFR. Similarly, Tonelli et al.4 have suggested that cardiovascular mortality is not the only explanation for death in these subjects. Our study revealed that the increased risk of mortality in subjects with high eGFR was primarily attributable to the presence of MICS, regardless of respiratory disease, cancer, or cardiovascular disease. In the absence of MICS, the U‐shaped relationship between eGFR and mortality disappeared. To our knowledge, these results demonstrate for the first time that MICS could modify the relationship between high eGFR and mortality in older people and that the all‐cause mortality risk did not increase exponentially in subjects with high eGFR in the absence of MICS.
A multicentre trial including 8941 patients with a history of atherosclerotic vascular disease found that high eGFR was associated with an increased risk of adverse cardiovascular outcomes.23 In contrast, another population‐based study found no association between myocardial infarction and higher eGFR, except in subjects with severe proteinuria.4 However, the latter study may be limited by an unbalanced distribution of age, as subjects in the low eGFR group were much older than those in the high eGFR group (mean age, 74.7 vs. 34.1 years). In the present study, all participants were aged ≥65 years. We found that high eGFR was associated with an increased risk of cardiovascular death in the presence but not absence of MICS. Thus, we suggest that older people with high eGFR and MICS remain at risk for adverse cardiovascular outcomes.
Previous studies found that diabetic hyperfiltration was associated with an increased risk of mortality amongst patients with type 1 and type 2 diabetes.5, 6, 7 However, in our study, high eGFR was associated with increased risks of overall and cardiovascular mortality, even in the absence of diabetes mellitus. Thus, glomerular hyperfiltration may not be the primary explanation for the paradoxical association between high eGFR and mortality. The mechanisms underlying the effect of MICS on mortality in older people with high eGFR may include sarcopenia (age‐related loss of muscle mass and strength), which contributes to increase the mortality and illness;24 underweight status, which can affect the immune responses, leading to long‐lasting inflammatory response and increased vulnerability to disease development;25 and malnutrition, which also profoundly affects the immune response and increases frailty, enforcing the vicious cycle of muscle wasting and negatively impacting survival.26
This study has strengths as follows. First, data for the cohort of 131 354 older participants covering the 10‐year study period were retrieved from the Taipei Geriatric Health Examination Database, providing adequate statistical power for the analysis of long‐term outcomes, including all‐cause and cardiovascular mortality. Second, as the impact of the U‐shaped relationship between eGFR and mortality seems to be more apparent in older than in younger patients,4, 20 we targeted a general population of older individuals who underwent routine health examinations. Some potential limitations of the present study should be acknowledged. First, we did not consider proinflammatory biomarkers, such as C‐reactive protein, interleukins, and cytokines, because their xmeasurement requires sophisticated and expensive techniques that are not feasible in the context of routine health examinations or in a large epidemiological study. Second, sarcopenia has been associated with increased mortality and numerous adverse outcomes,27, 28 but it cannot be accurately evaluated using BMI because fat and muscle mass cannot be readily distinguished. Dual energy X‐ray absorptiometry and bioimpedance analysis may be better for the evaluation of sarcopenia in older people with MICS, but further research is warranted.29, 30
Our study demonstrated that the paradoxical U‐shaped relationship between high eGFR and mortality was due to the presence of MICS in older people. In the absence of MICS, low (rather than high) eGFR was strongly associated with increased risks of overall and cardiovascular mortality. We thus recommend that physicians closely monitor older people with high eGFR and screen for MICS, particularly in subjects with very low serum creatinine and high eGFR levels.
Funding
This study was supported by grants to Dr D‐C.T. from the National Science Council (NSC 102‐2314‐B‐010‐004‐MY3), Taipei Veterans General Hospital (V102C‐129, V103C‐024), and Taipei City Hospital (10102‐62‐083), Foundation for Poison Control, and the Ministry of Education's Aim for the Top University Plan. This study was based in part on data from the Taipei City Public Health Database, provided by the Department of Health of the Taipei City Government and managed by the Databank for Public Health Analysis. The interpretations and conclusions contained herein do not represent those of the Taipei City Government.
Ethical statements
All the authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle (von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7–8).
Conflict of interest
S‐M.O., Y‐T.C., S‐C.H., C‐J.S., C‐H.L., C‐K.C. and D‐C.T. declare that they have no conflict of interest.
Supporting information
eTable 1. Patients and Levels of Indicators for Malnutrition‐Inflammation‐Cachexia Syndrome.
eTable 2. Risk of All‐cause and Cardiovascular Mortality among Older Participants.
eTable 3. Risk of All‐cause and Cardiovascular Mortality among Older Participants According to Modification of Diet in Renal Disease (MDRD) Equation.
eFigure 1. Adjusted Hazard Ratios for All‐Cause Mortality According to Estimated Glomerular Filtration Rate in Older Participants in Those with Geriatric Nutritional Risk Index <98 (A), with Albumin <3.8mg/dL (B), and with Body Mass Index <22kg/m2 (C).Abbreviation: HR, hazard ratio.
eFigure 2. Adjusted Hazard Ratios for All‐Cause Mortality According to Estimated Glomerular Filtration Rate in Older Men (A), in Those with (B) and without (C) Malnutrition‐Inflammation‐Cachexia Status Syndrome. Abbreviation: HR, hazard ratio.
eFigure 3. Adjusted Hazard Ratios for All‐Cause Mortality According to Estimated Glomerular Filtration Rate in Older Women (A), in Those with (B) and without (C) Malnutrition‐Inflammation‐Cachexia Status Syndrome. Abbreviation: HR, hazard ratio.
eFigure 4. Adjusted Hazard Ratios for All‐Cause Mortality According to Estimated Glomerular Filtration Rate in Older Participants with Diabetes Mellitus (A), in Those with (B) and without (C) Malnutrition‐Inflammation‐Cachexia Status Syndrome. Abbreviation: HR, hazard ratio.
eFigure 5. Adjusted Hazard Ratios for All‐Cause Mortality Rates According to Estimated Glomerular Filtration Rate in Older Participants without Diabetes Mellitus (A), in Those with (B) and without (C) Malnutrition‐Inflammation‐Cachexia Status Syndrome. Abbreviation: HR, hazard ratio.
eFigure 6. Adjusted Hazard Ratios for All‐Cause Mortality Rates According to Estimated Glomerular Filtration Rate in Older Participants with Proteinuria (A), in Those with (B) and without (C) Malnutrition‐Inflammation‐Cachexia Status Syndrome. Abbreviation: HR, hazard ratio.
Supporting info item
Ou, S‐M. , Chen, Y‐T. , Hung, S‐C. , Shih, C‐J. , Lin, C‐H. , Chiang, C‐K. , Tarng, D‐C. , and the Taiwan Geriatric Kidney Disease (TGKD) Research Group (2016) Association of estimated glomerular filtration rate with all‐cause and cardiovascular mortality: the role of malnutrition–inflammation–cachexia syndrome. Journal of Cachexia, Sarcopenia and Muscle, 7: 144–151. doi: 10.1002/jcsm.12053.
References
- 1. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305. [DOI] [PubMed] [Google Scholar]
- 2. Chronic Kidney Disease Prognosis C , Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all‐cause and cardiovascular mortality in general population cohorts: a collaborative meta‐analysis. Lancet 2010; 375: 2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cox HJ, Bhandari S, Rigby AS, Kilpatrick ES. Mortality at low and high estimated glomerular filtration rate values: a ‘U’ shaped curve. Nephron Clin Pract 2008; 110: c67–72. [DOI] [PubMed] [Google Scholar]
- 4. Tonelli M, Klarenbach SW, Lloyd AM, James MT, Bello AK, Manns BJ, Hemmelgarn BR. Higher estimated glomerular filtration rates may be associated with increased risk of adverse outcomes, especially with concomitant proteinuria. Kidney Int 2011; 80: 1306–1314. [DOI] [PubMed] [Google Scholar]
- 5. Amin R, Turner C, van Aken S, Bahu TK, Watts A, Lindsell DR, Dalton RN, Dunger DB. The relationship between microalbuminuria and glomerular filtration rate in young type 1 diabetic subjects: the Oxford Regional Prospective Study. Kidney Int 2005; 68: 1740–1749. [DOI] [PubMed] [Google Scholar]
- 6. Silveiro SP, Friedman R, de Azevedo MJ, Canani LH, Gross JL. Five‐year prospective study of glomerular filtration rate and albumin excretion rate in normofiltering and hyperfiltering normoalbuminuric NIDDM patients. Diabetes Care 1996; 19: 171–174. [DOI] [PubMed] [Google Scholar]
- 7. Vedel P, Obel J, Nielsen FS, Bang LE, Svendsen TL, Pedersen OB, Parving HH. Glomerular hyperfiltration in microalbuminuric NIDDM patients. Diabetologia 1996; 39: 1584–1589. [DOI] [PubMed] [Google Scholar]
- 8. Kalantar‐Zadeh K, Ikizler TA, Block G, Avram MM, Kopple JD. Malnutrition‐inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis: Official J National Kidney Foundation 2003; 42: 864–881. [DOI] [PubMed] [Google Scholar]
- 9. Young P, Lombi F, Finn BC, Forrester M, Campolo‐Girard V, Pomeranz V, Iriarte R, Bruetman JE, Trimarchi H. “Malnutrition‐inflammation complex syndrome” in chronic hemodialysis. Medicina 2011; 71: 66–72. [PubMed] [Google Scholar]
- 10. Molnar MZ, Czira ME, Rudas A, Ujszaszi A, Lindner A, Fornadi K, et al. Association of the malnutrition‐inflammation score with clinical outcomes in kidney transplant recipients. Am J Kidney Dis: Official J National Kidney Foundation 2011; 58: 101–108. [DOI] [PubMed] [Google Scholar]
- 11. Dukkipati R, Kopple JD. Causes and prevention of protein‐energy wasting in chronic kidney failure. Semin Nephrol 2009; 29: 39–49. [DOI] [PubMed] [Google Scholar]
- 12. Kim JC, Kalantar‐Zadeh K, Kopple JD. Frailty and protein‐energy wasting in elderly patients with end stage kidney disease. JASN 2013; 24: 337–351. [DOI] [PubMed] [Google Scholar]
- 13. Riella MC. Nutritional evaluation of patients receiving dialysis for the management of protein‐energy wasting: what is old and what is new? J Renal Nut: Official J Council Renal Nut National Kidney Foundation 2013; 23: 195–198. [DOI] [PubMed] [Google Scholar]
- 14. Beberashvili I, Azar A, Sinuani I, Kadoshi H, Shapiro G, Feldman L, Averbukh Z, Weissgarten J. Comparison analysis of nutritional scores for serial monitoring of nutritional status in hemodialysis patients. CJASN 2013; 8: 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, Benazeth S, Cynober L, Aussel C. Geriatric Nutritional Risk Index: a new index for evaluating at‐risk elderly medical patients. Am J Clin Nutr 2005; 82: 777–783. [DOI] [PubMed] [Google Scholar]
- 16. Department of Health, Taipei City Government. (2014, June 1). Retrieved from http://health.gov.taipei/Default.aspx?tabid=673.
- 17. Wu CY, Chou YC, Huang N, Chou YJ, Hu HY, Li CP. Association of body mass index with all‐cause and cardiovascular disease mortality in the elderly. PLoS One 2014; 9: e102589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Myers GL, Miller WG, Coresh J, Fleming J, Greenberg N, Greene T, et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem 2006; 52: 5–18. [DOI] [PubMed] [Google Scholar]
- 19. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hallan SI, Matsushita K, Sang Y, Mahmoodi BK, Black C, Ishani A, et al. Age and association of kidney measures with mortality and end‐stage renal disease. JAMA 2012; 308: 2349–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all‐cause and cardiovascular mortality. a collaborative meta‐analysis of high‐risk population cohorts. Kidney Int 2011; 79: 1341–1352. [DOI] [PubMed] [Google Scholar]
- 22. Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ. Chronic kidney disease as a risk factor for cardiovascular disease and all‐cause mortality: a pooled analysis of community‐based studies. JASN 2004; 15: 1307–1315. [DOI] [PubMed] [Google Scholar]
- 23. Inrig JK, Gillespie BS, Patel UD, Briley LP, She L, Easton JD, Topol E, Szczech LA. Risk for cardiovascular outcomes amongst subjects with atherosclerotic cardiovascular disease and greater‐than‐normal estimated glomerular filtration rate. CJASN 2007; 2: 1215–1222. [DOI] [PubMed] [Google Scholar]
- 24. Thomas DR. Loss of skeletal muscle mass in aging: examining the relationship of starvation, sarcopenia and cachexia. Clin Nutr 2007; 26: 389–399. [DOI] [PubMed] [Google Scholar]
- 25. Lesourd B. Nutritional factors and immunological ageing. Proc Nutr Soc 2006; 65: 319–325. [DOI] [PubMed] [Google Scholar]
- 26. Keusch GT. Host defense mechanisms in protein energy malnutrition. Adv Exp Med Biol 1981; 135: 183–209. [DOI] [PubMed] [Google Scholar]
- 27. Landi F, Cruz‐Jentoft AJ, Liperoti R, Russo A, Giovannini S, Tosato M, Capoluongo E, Bernabei R, Onder G. Sarcopenia and mortality risk in frail older persons aged 80 years and older: results from ilSIRENTE study. Age Ageing 2013; 42: 203–209. [DOI] [PubMed] [Google Scholar]
- 28. Bunout D, de la Maza MP, Barrera G, Leiva L, Hirsch S. Association between sarcopenia and mortality in healthy older people. Australas J Ageing 2011; 30: 89–92. [DOI] [PubMed] [Google Scholar]
- 29. Chien MY, Huang TY, Wu YT. Prevalence of sarcopenia estimated using a bioelectrical impedance analysis prediction equation in community‐dwelling elderly people in Taiwan. J Am Geriatr Soc 2008; 56: 1710–1715. [DOI] [PubMed] [Google Scholar]
- 30. Trevino‐Aguirre E, Lopez‐Teros T, Gutierrez‐Robledo L, Vandewoude M, Perez‐Zepeda M. Availability and use of dual energy X‐ray absorptiometry (DXA) and bio‐impedance analysis (BIA) for the evaluation of sarcopenia by Belgian and Latin American geriatricians. J Cachexia Sarcopenia Muscle 2014; 5: 79–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Patients and Levels of Indicators for Malnutrition‐Inflammation‐Cachexia Syndrome.
eTable 2. Risk of All‐cause and Cardiovascular Mortality among Older Participants.
eTable 3. Risk of All‐cause and Cardiovascular Mortality among Older Participants According to Modification of Diet in Renal Disease (MDRD) Equation.
eFigure 1. Adjusted Hazard Ratios for All‐Cause Mortality According to Estimated Glomerular Filtration Rate in Older Participants in Those with Geriatric Nutritional Risk Index <98 (A), with Albumin <3.8mg/dL (B), and with Body Mass Index <22kg/m2 (C).Abbreviation: HR, hazard ratio.
eFigure 2. Adjusted Hazard Ratios for All‐Cause Mortality According to Estimated Glomerular Filtration Rate in Older Men (A), in Those with (B) and without (C) Malnutrition‐Inflammation‐Cachexia Status Syndrome. Abbreviation: HR, hazard ratio.
eFigure 3. Adjusted Hazard Ratios for All‐Cause Mortality According to Estimated Glomerular Filtration Rate in Older Women (A), in Those with (B) and without (C) Malnutrition‐Inflammation‐Cachexia Status Syndrome. Abbreviation: HR, hazard ratio.
eFigure 4. Adjusted Hazard Ratios for All‐Cause Mortality According to Estimated Glomerular Filtration Rate in Older Participants with Diabetes Mellitus (A), in Those with (B) and without (C) Malnutrition‐Inflammation‐Cachexia Status Syndrome. Abbreviation: HR, hazard ratio.
eFigure 5. Adjusted Hazard Ratios for All‐Cause Mortality Rates According to Estimated Glomerular Filtration Rate in Older Participants without Diabetes Mellitus (A), in Those with (B) and without (C) Malnutrition‐Inflammation‐Cachexia Status Syndrome. Abbreviation: HR, hazard ratio.
eFigure 6. Adjusted Hazard Ratios for All‐Cause Mortality Rates According to Estimated Glomerular Filtration Rate in Older Participants with Proteinuria (A), in Those with (B) and without (C) Malnutrition‐Inflammation‐Cachexia Status Syndrome. Abbreviation: HR, hazard ratio.
Supporting info item


