Abstract
Background
Cachexia is a systemic syndrome leading to body wasting, systemic inflammation, and to metabolic chaos. It is a progressive condition, and little is known about its dynamics. Detection of the early signs of the disease may lead to the attenuation of the associated symptoms. The white adipose tissue is an organ with endocrine functions, capable of synthesising and secreting a plethora of proteins, including cytokines, chemokines, and adipokines. It is well established that different adipose tissue depots demonstrate heterogeneous responses to physiological and pathological stimuli. The present study aimed at providing insight into adipocyte involvement in inflammation along the progression of cachexia.
Methods
Eight‐weeks‐old male rats were subcutaneously inoculated with a Walker 256 carcinosarcoma cell suspension (2 × 107 cells in 1.0 mL; tumour‐bearing, T) or Phosphate‐buffered saline (control, C). The retroperitoneal, epididymal, and mesenteric adipose pads were excised on Days 0, 7, and 14 post‐tumour cell injection, and the adipocytes were isolated.
Results
Mesenteric and epididymal adipocytes showed up‐regulation of IL‐1β protein expression and activation of the inflammasome pathway, contributing for whole tissue inflammation. The stromal vascular fraction of the retroperitoneal adipose tissue, on the other hand, seems to be the major contributor for the inflammation in this specific pad.
Conclusion
Adipocytes seem to play a relevant role in the establishment of white adipose tissue inflammation, through the activation of the NF‐κB and inflammasome pathways. In epididymal adipocytes, induction of the inflammasome may be detected already on Day 7 post‐tumour cell inoculation.
Keywords: Cancer cachexia, Adipocyte, Inflammasome, Inflammation
Background
Cancer cachexia is a syndrome with complex aetiology,1, 2 which affects up to 80% of patients with advanced cancer.3, 4, 5 It is associated with diminished expected effects of chemotherapy and radiotherapy treatment, compromised life quality, and decreased survival.3, 5, 6, 7, 8 The main symptoms include marked decrease in body weight, with depletion of both white adipose tissue (WAT) and skeletal muscle, profound metabolic disruption, reduction of appetite, hypoalbuminemia and systemic inflammation.9, 10 This syndrome has been recently proposed to comprise at least three stages: the first is associated with metabolic change and with weight loss (<5%), while in the second stage, a 5% decrease of body mass is consistently reported, along with systemic inflammation and anorexia. In the last stage, patients are no longer responsive to anticancer treatment, and the expected survival does not exceed 3 months.10
There is current evidence pointing out WAT as an important contributor to cachexia.11, 12 WAT biology is impaired both in human cachexia and in animal models, and it is suggested that WAT alterations precede muscle wasting.11, 13 Therefore, WAT is not only a ‘victim’ of the syndrome, it is also a relevant player, actively synthesizing a plethora of pro‐inflammatory cytokines,14 which take part in contributing to systemic inflammation.
White adipose tissue is composed of different cellular elements, among which resident and infiltrated immune cells.15 In addition, the proportion of the stromal vascular fraction, as well as the biology of the adipocyte, are subject to diversity according to anatomical variation, regarding vascularization and innervation of the pad.16, 17, 18, 19 This implies that the response of different depots is heterogeneous under different physiopathological conditions,20, 21 as reported in obesity.17, 22 Previous studies of our group (Seelaender et al., 199623) have reported differences in visceral WAT depots in concern to the morphology and biochemical characteristics of adipocytes in the Walker 256 animal model of cancer cachexia.
Given these considerations, we presently sought to examine whether the adipose cell participates in the establishment of whole adipose tissue inflammation and to address the hypothesis that adipocytes obtained from different anatomical fat depots behave heterogeneously during the progression of cachexia. For that purpose, we studied three pads at three time points [prior to tumour cell injection‐T0; and 7 (T7) or 14 days (T14) post‐inoculation]. These time points, although not necessarily bearing correspondence with the three stages proposed for cachectic patients (Fearon et al., 201110), were chosen as to provide insight on the dynamics of inflammation in WAT.
One of the main inflammatory pathways in mammalian cells is that of the nuclear factor of kappa light polypeptide gene enhancer in B‐cells (NF‐κB). NF‐κB is composed of subunits p50, p105, p52, p100, and p65, which induce gene expression by binding to DNA target sites.18, 24, 25 Studies with obese patients showed an increased activity of the NF‐κB pathway in the adipose tissue and, consequently, of local inflammation.26 The inflammasome pathway has also been similarly described as having an important role in the inflammation of adipose tissue in obesity.22, 27, 28 Its major role is to activate the pro‐inflammatory cytokines IL‐1β and IL‐18, which are synthesized as immature proteins and require the inflammasome pathway for cleavage and release, yielding the mature, biologically active cytokine forms.29, 30, 31 The pathway comprises two compartments: the (nucleotide‐binding oligomerization domain) (NOD)‐like receptor (NLR) family (NLRP1, NLRP2, NLRP3, NLRP6, NLRC4, and NLRP12), and the pyrin and HIN200 (haematopoietic interferon‐inducible nuclear antigens) domain‐containing protein (PYHIN) family.27, 32 To the best of our knowledge, there is no study in the literature reporting a possible contribution of this pathway to the enhanced inflammatory cytokine secretion by the adipose tissue in cachexia.
Therefore, a third aim of the present study was to investigate the pathways associated with adipocyte inflammation and their activation along the aggravation of the syndrome.
Materials and methods
Animals
Male adult Wistar rats (160–250 g), obtained from the Institute of Biomedical Sciences, University of São Paulo, were maintained in metabolic cages, under a 12 h light/12 h dark cycle (lights on at 7:00 a.m.), and controlled temperature conditions (23 ± 1°C), receiving water and food (commercial chow; Nuvilab, Nuvital, Brazil) ad libitum. The Biomedical Sciences Institute/USP Ethical Committee for Animal Research approved all the adopted procedures (050/102 ECAR), which were carried out in accordance with the ethical principles stated by the Brazilian College of Animal Experimentation. The experiments were performed as a time course study, and the rats were killed by decapitation on Days 0, 7, and 14 following subcutaneous injection of a suspension of Walker 256 tumour (2 × 107) cells,33 or of the same volume of Phosphate‐buffered saline (n = 5 group); after 12 h fast, always in the interval between 8:00 and 11:00 a.m. The following groups were studied: T0 (rats euthanized on the day on which T7 and T14 were injected with the tumour cells), T7 (rats euthanized 7 days after tumour cell injection), and T14 (rats euthanized 14 days after tumour cell injection). Additional groups of non‐tumour‐bearing animals (C) were included, as a control for body and tissue weight on Days 7 (C7) and 14 (C14).
Blood samples and adipose tissue collection
Approximately 8 mL of blood were collected. In brief, samples were centrifuged at 500 g at 4°C for 15 min, and serum was stored at −80°C. Epididymal adipose tissue (EAT), retroperitoneal adipose tissue, (RPAT), and mesenteric adipose tissue (MEAT) (after careful removal of adjacent lymph nodes) were removed, weighed, snap frozen in liquid nitrogen, and stored at −80°C.
Serum analysis
All serum analyses were performed with commercial kits: total cholesterol, triglycerides (TG), and high‐density lipoprotein (Labtest®, Brazil); free glycerol (Sigma–Aldrich, Switzerland); and non‐esterified fatty acids (Wako Chemicals, USA).
Adipose cell isolation
The different visceral adipose tissue pads were excised and washed in sterile saline (0.9% NaCl). The samples (0.8 g) were placed immediately in digestion buffer (DMEM Sigma‐D5671), 5% BSA, and 2 mg/mL type I collagenase (Life‐technologies 17100‐017), and digested for 60 min, at 37°C. The resulting suspension was then filtered through a sterile 250 µm nylon mesh and the mature adipocytes [mesenteric adipocytes (MEa), retroperitoneal adipocytes (RPa), and epididymal adipocytes (Ea)], obtained from the supernatant. The cells were washed twice with sterile 0.9% NaCl and stored at −80°C for mRNA and protein analysis.
Real‐time PCR
Total RNA was isolated from the tissues and cells, with Trizol® (Invitrogen, CA, USA), following the manufacturer's recommendations. The first strand of cDNA was generated from 2 µg of total RNA, employing a commercial kit (High Capacity cDNA Reverse Transcription Kit, Invitrogen). Polymerase chain reaction (PCR) amplification was performed in duplicates, with SYBR Green PCR Master Mix (Applied Biosystems, CA, USA) in the QuantStudio™ 12 K Flex Real Time PCR (Applied Biosystems, CA, USA), employing the primers listed in Table 1. Gene expression was normalized to the Rpl19 and Rpl27 reference genes. Data were calculated with the 2‐ΔΔCT method and are presented as the fold change in gene expression relative to the control sample.
Table 1.
Primer list
| Gene (species) | Sequence 5′ 3′ |
|---|---|
|
Il‐6 (Rattus norvegicus) (NM 012589.2) |
Fw: GAA GCT GAA GAC CCT CTG GA Rev: TAA GCC TCG GAC TTG TGA AGT GGT |
|
Il‐10 (R. norvegicus) (NM 012854.2) |
Fw: AAG GCA TTC TTC ACC TGC TC Rev: CCA AGC TGA GAA CCA AGA CC |
|
Tnf‐α (R. norvegicus) (NM 012675.3) |
Fw: ATC ACT CCA AAG TGC AGC AG Rev: CTC TCT CCC CTG GAA AGG AC |
|
p50 (R. norvegicus) (NM 001276711.1) |
Fw: AGG TCC AGA AAG ATG ACA TCC A Rev: CAA TGG CAA ACT GTC TGT GAA |
|
p65 (R. norvegicus) (NM 199267.2) |
Fw: ATG CAT CCA CAG CTT CCA G Rev: TGC TCC TCT ATG GGA ACT TGA |
|
Tlr4 (R. norvegicus) (NM 019178.1) |
Fw: TCT AAA TGC CAA CTG GAA CAG A Rev: ATG GGA TGG ATC CAG AAA CA |
|
Myd88 (R. norvegicus) (NM 198130.1) |
Fw: GCG AGC TCA TTG AGA AAA GG Rev: ACA CCT GGA GAC AGG CTG A |
|
Traf6 (R. norvegicus) (NM 001107754.2) |
Fw: AAG TCC ATA AGG GAT GCA GGT Rev: TCG CTT TGC AAA ATT GTC AG |
|
Ikk‐α (R. norvegicus) (NM 001107588.1) |
Fw: TCA AGA TGT TGG TGG GAA GAT A Rev: CTC TGG GGC CAA ATA CTG TAA |
|
NLRP1 (R. norvegicus) (NM 001145755.2) |
Fw: GCT TCA GCC CCC AAA GAT Rev: TTG TCC AAG AGA GGG TCC AC |
|
NLRP3 (R. norvegicus) (NM 001191642.1) |
Fw: GCT GAA CTT GAG CAA CAA CG Rev: CAC CCA ACT GTA GGC TCT GC |
|
Tlr2 (R. norvegicus) (NM 198769.2) |
Fw: TTT GAT CAC TGC ACC CTC AA Rev: ATG TGC AGG CTC CGT ATT GT |
|
Hif‐1α (R. norvegicus) (NM 024359.1) |
Fw: CAA CTG CCA CCA CTG ATG A Rev: GGG TAG AAG GTG GAG ATG C |
|
Caspase 1 (R. norvegicus) (NM 012762.2) |
Fw: ACA TCT TTC TCC GAG GGT TG Rev: CAC CTC TTT CAC CAT CTC CAG |
|
Rpl19 (R. norvegicus) (NM 031103.1) |
Fw: GAG GGA CGC TTC ATT TCT TG Rev: CAT GGA GCA CAT CCA CAA AC |
|
Rpl27 (R. norvegicus) (NM 022514.1) |
Fw: CCT CAT GCC CAC AAG GTA CT Rev: CTG TCT TGT ATC GCT CCT CAA A |
White adipose tissue adipokine levels
Rat WAT (0.4–0.7 g) was homogenized in RIPA buffer (0.625% Nonidet P‐40, 0.625% sodium deoxycholate, 6.25 mm sodium phosphate, and 1 mm ethylene‐diaminetetraacetic acid, at pH 7.4), containing a protease inhibitor‐cocktail (Roche®, Brazil). Homogenates were centrifuged at 12 000 g for 30 min at 4°C; the supernatant was collected, and protein concentration was determined with a commercial kit (Bio‐Rad, CA, USA). Total adipose tissue protein extracts were employed for the quantitative assessment by enzyme‐linked immunosorbent assay (DuoSet ELISA, R&D Systems, MN, USA) of IL‐6 (DY506), TNF‐α (DY510), IL‐10 (DY522), and IL‐1β (DY501). The IL‐6 assay sensitivity was found to be 8.000 pg/mL in the range of 125–8000 pg/mL. For TNF‐α, IL‐10, and IL‐1β, the assay sensitivity was found to be 4.000 pg/mL in the range of 62.5–4000 pg/mL. All samples were assayed as duplicates, and the mean value was reported. The results were equalized to total protein.
Western blotting
Frozen adipose tissue and adipocytes were homogenized in Radioimmunoprecipitation assay (RIPA) buffer (0.625% Nonidet P‐40, 0.625% sodium deoxycholate, 6.25 mm sodium phosphate, and 1 mm ethylene‐diaminetetraacetic acid at pH 7.4 with proteinase) and phosphatase inhibitors (Roche®, Brazil) and centrifuged at 15 000 g for 30 min at 4°C. The protein‐rich fraction (intermediary layer) was collected and centrifuged at 12 000 g for 10 min at 4°C, the supernatant (fatty layer) was discarded, and the infranatant was collected. Protein concentration was determined with a BCA protein quantification kit (Pierce, IL, USA), with Bovine serum albumin (BSA) as a reference. Samples containing 30 µg protein were separated by electrophoresis in 10% Tricine SDS–PAGE. Proteins were then transferred to PVDF membranes at 25 V for 40 min (Trans‐Blot Turbo Blotting System, Bio‐Rad) in transfer buffer, consisting of 20 mm Tris, 150 mm Glycine, and 10% Methanol. PVDF membranes were then blocked in TBS containing 0.1% Tween 20 and 5% skimmed milk, for 1 h. After three washes with TBS plus 0.1% Tween 20, the PVDF membranes were incubated with primary antibodies against IL‐1β (Santa Cruz, SC‐1251, Goat), p65 (Cell signalling, C22B4, Rabbit), Myd88 (Santa Cruz, SC‐11356), TNFr1 (Santa Cruz, SC‐1070, Mouse), p50 (Santa Cruz, SC‐8414), GAPDH (Sigma, G9545, Rabbit). HRP‐conjugated secondary antibody anti‐rabbit and anti‐mouse (Cell signalling, MA, USA), and anti‐goat (Abcam ab 6885‐ UK) for 2 h at room temperature. The resulting bands were detected by ECL (Amersham, UK). GAPDH was employed as loading control, and the blots were stripped and incubated with a GAPDH antibody (Sigma, G9545, Rabbit). Quantification of antigen–antibody complexes was performed with the Image J Analysis Software (http://rsb.info.nih.gov/ij/). Optical density units were obtained as pixels for fold target protein/control protein.
Statistical analysis
Data are expressed as mean values and standard errors of the means. Differences concerning the studied groups (T0, T7, and T14) were analysed with GraphPad5 software (GraphPad, San Diego, CA, USA), and statistical significance was determined performing one‐way ANOVA with post hoc Tukey's test, for comparison among groups, for each different adipose pad. Comparison among pads was not performed. For the results regarding body and tissue mass in Cachectic (T0, T7, and T14) and control (C7 and C14) animals, we employed ANOVA two‐way, followed by Tukey's post‐test. P < 0.05 was considered significant.
Results
Fourteen days after tumour cell injection, experimental cancer cachexia induced a reduction in body weight (10.6%; P < 0.05) and in WAT mass (42.5% in MEAT and 40.2% in RPAT; P < 0.05) (Table 2), compared with animals of the control group. Terminal cachexia (T14) was accompanied by increased concentrations of non‐esterified fatty acid, high‐density lipoprotein, and triglycerides in the plasma (Table 3).
Table 2.
Body and tissue mass in control (C) and cachectic (T) animals
| Initial | Two‐way ANOVA P‐values | |||||||
|---|---|---|---|---|---|---|---|---|
| T0 | C7 | T7 | C14 | T14 | Time | Tumour | Time/tumour | |
| BM‐T (g) | 220.8 ± 18.4 | 264.4 ± 21.4 | 247.8 ± 17.8 | 285.4 ± 19.7* * | 255.1 ± 21.4* | ns | * P < 0.05 | ** P < 0.01 |
| TM (g) | 4.4 ± 2.53 | 12.05 ± 6.43 | ||||||
| MEAT (g) | 1.03 ± 0.33 | 1.82 ± 0.47 | 1.37 ± 0.32 | 2.54 ± 0.43**# | 1.46 ± 0.22&& | # P < 0.05 | && P < 0.01 | ** P < 0.01 |
| EAT (g) | 1.52 ± 0.38 | 2.15 ± 0.34 | 2.04 ± 0.41 | 3.46 ± 0.40 | 2.83 ± 0.45 | ns | ns | ns |
| RPAT (g) | 0.87 ± 0.38 | 2.12 ± 0.35 | 1.39 ± 0.40 | 3.45 ± 0.48** | 2.06 ± 0.46*# | # P < 0.05 | * P < 0.05 | ** P < 0.01 |
Values are expressed as mean ± standard error of the mean n = 5.
Note: BM‐T, body mass‐tumour mass; C, control; EAT, epididymal adipose tissue; MEAT, mesenteric adipose tissue; ns, not significant; RPAT, retroperitoneal adipose tissue; T, cancer cachexia; TM, tumour mass.
Table 3.
Plasma lipid profile along cachexia progression
| T0 | T7 | T14 | |
|---|---|---|---|
| Glycerol (mg/mL) | 0.14 ± 0.09 | 0.09 ± 0.02 | 0.11 ± 0.04 |
| NEFA (mE/mL) | 0.75 ± 0.03 | 0.80 ± 0.03 | 1.25 ± 0.22* |
| HDL (mg/dL) | 43.73 ± 2.24 | 46.80 ± 4.54 | 52.12 ± 1.15# |
| TG (mg/dL) | 82.95 ± 5.38 | 114.81 ± 29.00 | 151.30 ± 50.18* |
Values are expressed as mean ± SEM n = 5.
HDL, high‐density lipoprotein; NEFA, non‐esterified fatty acid; TG, triglycerides.
P < 0.05 vs. all groups.
P < 0.05 vs. T0.
White adipose tissue inflammation in experimental cancer cachexia
Mesenteric adipose tissue
In MEAT, increased tumour necrosis factor alpha (TNF‐α) gene expression was found in T7 and in T14 (23.7 and 16.5‐fold, respectively; P < 0.05) (Figure 1 B), as compared with T0. Interleukin 1b (IL‐1β) protein content was increased (5.7‐fold; P < 0.05) in T14 (Figure 2 G) in relation to T0.
Figure 1.
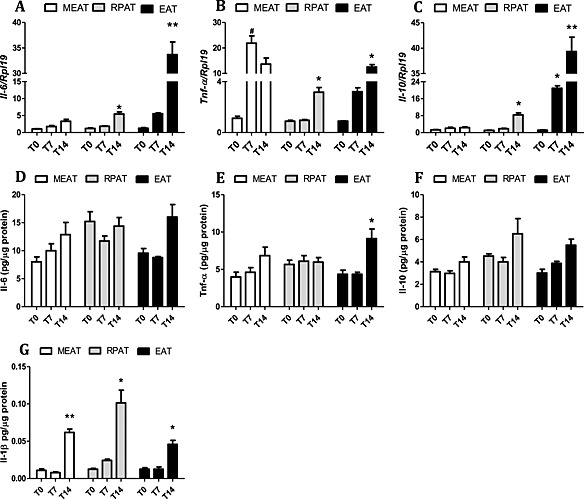
Cytokine gene and protein expression in adipose tissue along cachexia progression. (A–C) gene expression (D–G) protein expression. Results are expressed as mean (n = 5) ± SEM. Adipose tissue was obtained from animals 0, 7, and 14 days after inoculation of tumour cells. *P < 0.05 difference between groups, for the same tissue; **P < 0.01 different from all the groups for the same tissue; # P < 0.05 vs. control. Mesenteric adipose tissue (MEAT), retroperitoneal adipose tissue (RPAT), and epididymal adipose tissue (EAT). (A) gene expression of Il‐6, (B) gene expression of Tnf‐α, (C) gene expression of Il‐10, (D) protein expression of Il‐6, (E) protein expression of Tnf‐α, (F) protein expression of Il‐10, and (G) protein expression of Il‐1β.
Figure 2.
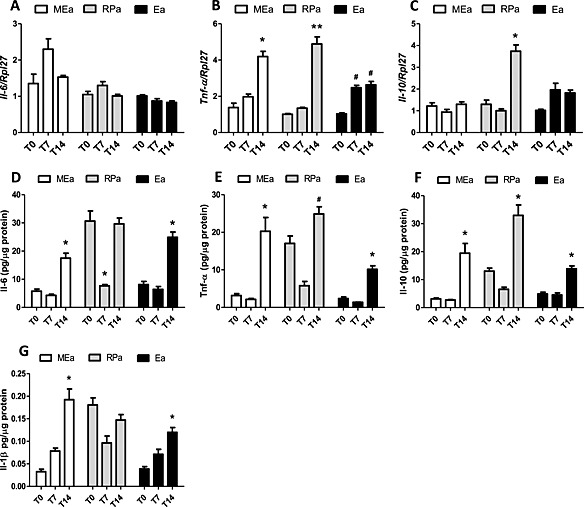
Cytokine gene and protein expression in isolated adipocytes along cachexia progression. (A–C) gene expression and (D–G) protein expression. Results are expressed as mean ± standard error of the mean (n = 4). Adipocytes were isolated from adipose tissue obtained from animals 0, 7, and 14 days after inoculation of tumour cells: mesenteric adipose tissue (MEAT), retroperitoneal adipose tissue (RPAT), and epididymal adipose tissue (EAT) cells. *P < 0.05 vs. all the groups for the same tissue; # P < 0.05 vs. Day 7. (A) gene expression of Il‐6, (B) gene expression of Tnf‐α, (C) gene expression of Il‐10, (D) protein expression of Il‐6, (E) protein expression of Tnf‐α, (F) protein expression of Il‐10, and (G) protein expression of Il‐1β.
Retroperitoneal adipose tissue
Changes in pro‐inflammatory cytokine gene expression in this pad could only be detected at the end stage (T14) of cachexia, when Interleukin 6 (IL‐6) and TNF‐α (P < 0.05) (Figure 1 A,B) mRNA levels were increased. Protein expression, on the other hand, was similar to the patterns found in MEAT, as IL1β protein expression was higher in RPAT in T14 (10.0‐fold; P < 0.05) (Figure 2 G). Interleukin 10, the only anti‐inflammatory cytokine examined, showed increased gene expression in T14, despite no significant modification of protein content of the cytokine in this depot.
Epididymal adipose tissue
In this fat pad, pro‐inflammatory cytokine mRNA expression (IL‐6 and TNF‐α) was increased only in T14 (P < 0.05) (Figure 1 A,B). Protein expression of TNF‐α and IL‐1β was also increased in T14 (3.7‐fold and 2.03‐fold, respectively; P < 0.05) (Figure 1 E,G), when compared with T0. The gene expression of IL‐10 was found to be increased in T7 (P < 0.05), and an even more conspicuous increment was observed in T14 (P < 0.01), in relation to both T0 and T7.
White adipose tissue cells inflammation in experimental cancer cachexia
As WAT is composed of various cells types, each potentially contributing to inflammation, we investigated whether adipocytes per se would be relevant to local and, possibly, to systemic inflammation.
Mesenteric adipocytes
In the isolated adipocytes (MEa), gene expression was solely increased for TNF‐α (Figure 2 B), despite the observed increase in protein expression of all pro and anti‐inflammatory proteins in advanced cachexia (T14) in this pad (Figure 2 D–G). IL‐1β protein content increased in a similar proportion to that found in the whole mesenteric adipose depot. We thus speculate that the increase in the expression of other cytokines was insufficient to affect whole tissue values.
Retroperitoneal adipocytes
In the adipocytes isolated from the retroperitoneal pad (RPa), gene expression of TNF‐α increased in T14 in comparison with T0 (P < 0.05) (Figure 2 B). Such increase was parallel to the enhancement of gene expression levels found in whole retroperitoneal tissue in the same group (T14). It is possible that the higher values observed for IL‐10 gene expression in T14 in RPa (Figure 2 C) are a consequence of the increase of TNF‐α gene expression in these cells. IL‐1β content was found to be increased in whole RPAT, but not in RPa, and therefore, we postulate a possible contribution of the stromal vascular fraction, comprised by the non‐adipocyte cellular components of the tissue. The high IL‐6 and TNF‐α (Figure 2 D,E) protein content in adipocytes obtained from animals with terminal cachexia was not reflected in the whole tissue, even though gene expression was increased in T14, as compared with T0.
Epididymal adipocytes
In the cells isolated from this pad (Ea), we observed an increase in gene expression of TNF‐α in T7, in relation to T0 (Figure 2 B). This might be the cause for the higher expression of all analysed cytokines we report in the adipocytes in terminal cachexia (T14) (Figure 2 D–G) and bears correspondence with the enhanced content of TNF‐α and IL‐1β in the whole EAT. This tissue showed a progressive increase of IL‐10 gene expression levels between T7 and T14, maybe in an attempt to counterbalance increasing inflammation.
Pathways related with the induction of pro‐inflammatory cytokines in adipocytes
In MEa, all genes related with the studied transcription factors were increased in T14: nuclear factor kappa B subunits p50 (NF‐κB p50) and p65(NF‐κB p65) and Chuk conserved helix‐loop‐helix ubiquitous kinase (Ikk‐α) (P < 0.05) (Figure 3 A–C). Toll‐like receptor 2 (Tlr2) gene expression was up‐regulated in the same group (Figure 3 E), as well as myeloid differentiation primary response 88 (Myd88) and TNF receptor‐associated factor 6 (Traf6) (Figure 3 F,G). All genes of the Tlr/NF‐κB pathway were thus shown to be regulated by cachexia. With regard to protein expression, the results of NF‐κB p65 and Myd88 paralleled the gene expression data, being increased only in terminal cachexia (T14) (P < 0.05) (Figure 5 B,C).
Figure 3.
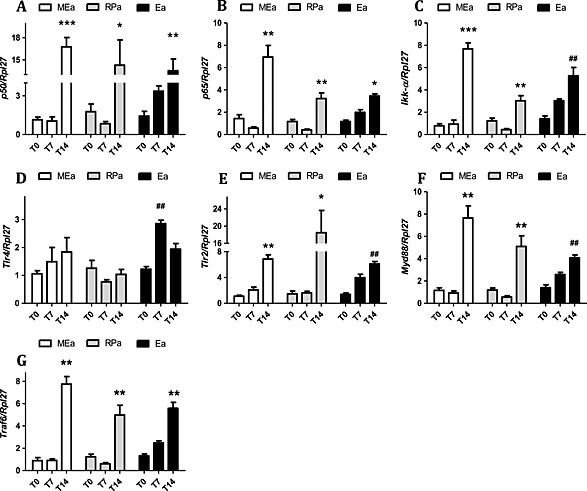
Gene expression of transcription factors involved in pro‐inflammatory pathways in isolated adipocytes along cachexia progression. Results are expressed as mean ± standard error of the mean (n = 4). Adipocytes isolated from adipose tissue were obtained from animals 0, 7, and 14 days after inoculation of tumour cells: *P < 0.01 vs. all the groups for the same tissue; # P < 0.05 vs. T0; **P < 0.01 vs. all the groups for the same tissue; ## P < 0.01 vs. T0. Mesenteric adipocytes (MEa), retroperitoneal adipocytes (RPa), and epididymal adipocytes (Ea). (A) gene expression of p50, (B) gene expression of p65, (C) gene expression of Ikk‐α, (D) gene expression of TLR4, (E) gene expression of TLR2, (F) gene expression of Myd88, and (G) gene expression of Traf6.
Figure 5.
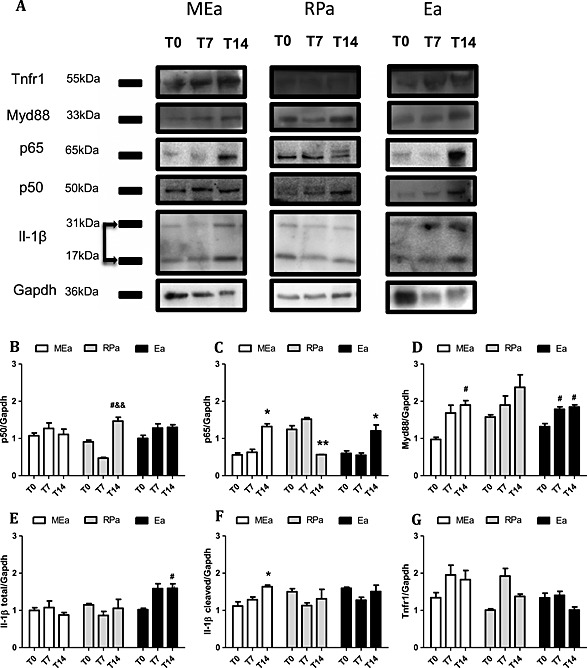
Protein expression in isolated adipocytes along cachexia progression. Results are expressed as mean (n = 3) ± standard error of the mean. Adipocytes were isolated from adipose tissue obtained from animals 0, 7, and 14 days after inoculation of tumour cells: mesenteric adipose tissue (MEa), retroperitoneal adipose tissue (RPa), and epididymal adipose tissue (Ea) cells. (A) Blots were rearranged, and representative images were selected for p50, p65, Myd88, Il‐1β (total and cleaved form), and GAPDH (normalized protein); (B–F) band intensity detection with software Image J. *P < 0.05 vs. all the groups for the same tissue; # P < 0.01 vs. T0; && P < 0.05 vs. T7.
Similarly to what was observed in MEa, the changes regarding TLRs/NF‐κB pathway in RPa occurred solely at the final stage of cachexia when p50, p65, Ikk‐α, TLR2, Myd88, and Traf6 gene expression was enhanced (P < 0.05) (Figure 3 A–D,F,G). However, in contrast with the protein expression results reported for MEa, p50 protein content increased, p65 protein content decreased, and Myd88 protein expression was unchanged in RPa in T14 (Figure 5 A–C).
Epididymal adipocytes once again showed similarity with the results found for MEa: P50, p65, Ikk‐α, TLR2, Myd88, and Traf6 gene expression increased in T14 (P < 0.05) (Figure 3 A–D,F,G). TLR4 gene levels were increased already in T7 (Figure 3 E), while in T14, the levels were similar to T0. p50, p65, and Myd88 protein expression increased in terminal cachexia (T14) (Figure 5 B–D), in epididymal adipocytes.
Inflammasome pathway
In MEa, the expression of genes related with the activity of the inflammasome pathway was increased for the NLR family, pyrin domain containing 1 and 3 (NLRP1 and NLRP3), and Caspase 1 on Day 14 (Figure 4 A–C). These results were accompanied by an increased cleavage of IL‐1β to its active form (Figure 5 F). In RPa, only NLRP3 gene expression was higher in T14 (Figure 4 B). In Ea, NLRP1, and NLRP3 gene expression increased in T14, as compared with T7 (Figure 4 A,B); however, we observed no changes in the content of cleaved IL‐1β (Figure 5 F).
Figure 4.
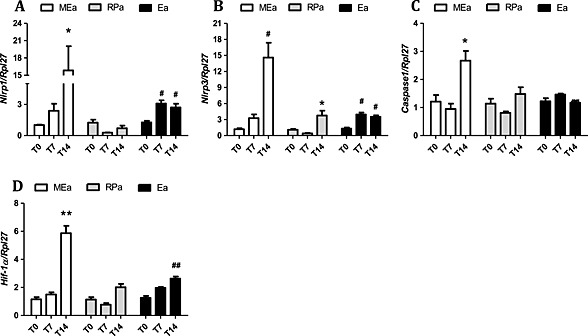
Inflammassome pathway gene expression in isolated adipocytes along cachexia progression. Results are expressed as mean ± standard error of the mean (n = 4). Adipocytes were isolated from the adipose tissue obtained from animals 0, 7, and 14 days after inoculation of tumour cells: *P < 0.05 vs. all the groups for the same tissue; # P < 0.05 vs. T0; ## P < 0.01 vs. T0. Mesenteric adipocytes (MEa), retroperitoneal adipocytes (RPa), and epididymal adipocytes (Ea). (A) gene expression of Nalp1, (B) gene expression of Nalp3, (C) gene expression of Caspase 1, and (D) gene expression of Hif‐1α.
Discussion
Cachexia is characterised by progressive loss of lean and adipose tissue,5, 8 accompanied by disruption of the biochemical profile in association with pathological increase of pro‐inflammatory cytokines in cancer patients and experimental models.3, 9, 34 Cancer cachexia‐associated chronic systemic inflammation presents a relevant contribution from the WAT, as we and others have previously shown.14, 34, 35, 36, 37, 38
Two main questions were addressed in the present study: (1) Is there heterogeneity regarding anatomical localization of WAT in concern to inflammation capacity during the progression of cachexia? and (2) What is the contribution of adipocytes per se to adipose tissue inflammation? To elucidate these aspects, we studied cachexia progression in the rodent Walker 256 carcinosarcoma model.
All the examined (mesenteric, retroperitoneal, and epididymal) fat pads showed increased gene expression of inflammatory cytokines in advanced cachexia (T14). Yet, only IL‐1β protein content was consistently augmented in all the depots. TNF‐α protein levels were shown to be higher solely in EAT of T14 animals. Adipose tissue depots from different anatomical regions show marked heterogeneity in microanatomical organization, metabolism, and physiological and molecular aspects.16, 39 One such heterogeneity can also be noticed during cachexia, as we (Batista et al.)20 have demonstrated that the subcutaneous adipose tissue of cancer patients responds in a precocious manner to the onset of the disease, with enhanced inflammatory cytokine secretion, as compared with the mesenteric depot.
Among rodent visceral depots, EAT is the one that responds more rapidly to cachexia by becoming inflamed. Therefore, it is clear that the different visceral adipose pads present distinct dynamics in response to the syndrome. Nevertheless, what is the contribution of the adipose cell per se in this scenario? WAT comprises several cell types, and studies carried out with obese patients report a great participation of infiltrating immune cells in triggering tissue inflammation.12, 13, 17
Adipocytes isolated from RPAT displayed an anti‐inflammatory profile, with a high protein content of IL‐10, and unaltered protein content of lL‐1β in terminal cachexia. Thus, it appears that RPa attempts to counterbalance local inflammation caused by the high concentration of IL‐1β protein, possibly synthesized by the stromal vascular fraction, as suggested by the high total tissue concentration of this cytokine in the depot. In contrast, isolated adipocytes from the mesenteric and epididymal pads showed a clear pro‐inflammatory profile. Ea secretes cytokines already at an early stage, while MEa inflammation was found in advanced cachexia. However, adipocytes from both of these depots contribute consistently to local inflammation at the terminal phase of cachexia and may be relevant to the onset of systemic inflammation. Such differences confirm the heterogeneity of adipocytes obtained from particular depots; while MEa and Ea exhibited a progressive pro‐inflammatory pattern, RPa increased the protein expression of IL‐10. A recent study showed that different adipose pads derive from distinct embryonic origins.40 We may thus suggest that perhaps, the heterogeneous response displayed by the isolated cells from the depots may be related with intrinsic diversity of these cells.
After examining inflammatory cytokine production by cells from the different pads, we investigated the intracellular pathways that could be associated with the modulation of adipocyte inflammation. Firstly, we analysed the classic pathway in chronic inflammatory processes involving NF‐κB.41, 42 The literature provides detailed evidence that the NF‐κB pathway in adipose tissue is modulated in obesity,26, 43, 44 and the same signal route is suggested to be augmented in the adipose tissue of cachectic patients and animals.14, 34 Our results confirm that increased pro‐inflammatory cytokine content in the adipose tissue was accompanied by higher expression of transcription factors key proteins, such as NF‐κB p65. Myd88 protein content was increased both in MEa and Ea and, in both cell populations, associated with increased NF‐κB protein expression. These findings indicate that the adipocyte NF‐κB pathway is modulated during the progression of the syndrome, especially at its end point. In obesity, increased NF‐κB activity has been attributed to infiltrating immune cells, rather than to adipocytes.45, 46, 47 To our best knowledge, this is the first report of adipocyte NF‐κB involvement in WAT inflammation in cachexia.
We report increased IL‐1β protein content in all WAT pads and in two different isolated adipocyte populations. An increase of the cleaved, bioactive IL‐1β form was found to be present only in MEa, while IL‐1β content was high as a consequence of cachexia, in the whole adipose pads studied. IL‐1β is one of the main targets of the inflammasome pathway.31 Therefore, in addition to showing that the NF‐κB pathway is modulated in the adipocytes of WAT during cachexia, the results indicate that cachexia was able to modulate the inflammasome pathway in the cells. In obesity, increased inflammasome activity is related with disease progress. The proteins NLRP 3 and Caspase 1 play an important role in this pathway.32, 48 A study with a knockout model for NLRP 3 and Caspase 1 found the animals to be resistant to obesity after being submitted to a high‐fat diet. These animals also showed improved insulin sensitivity after the high‐fat diet protocol, as compared with controls.49, 50 We believe we are the first to show a modulation of the inflammasome pathways in adipocytes during cachexia.
In summary, our results indicate a heterogeneous response of the different white adipocytes to cachexia and a role in the modulation of adipose tissue inflammation. While MEa and Ea show a clear pro‐inflammatory response during the development of the syndrome, the inflammatory factors we found to be increased in cachexia in the retroperitoneal tissue are probably produced by the stromal vascular fraction. Additionally, we demonstrate, for the first time, that the inflammasome pathway may be involved in adipose inflammation in cancer cachexia. Taken together, the results suggest that the inflammasome pathway could be a target for pharmacological treatment in cachexia.
Funding
FAPESP grants numbers 2010/51078‐1 (M.L.B. Jr.) and 2012/50079‐0 (M.S.) and CAPES (Coordenação de Aperfeiçoamento de Nível Superior).
Conflict of interest
None declared.
Acknowledgements
The authors thank Emilia Ribeiro and Rosana Prisco for the technical assistance. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of FAPESP (Fundação de Amparo á Pesquisa do Estado de São Paulo). The authors of this paper certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia, and Muscle 2010;1:7–8 (von Haehling S., Morley J. E., Coats A. J., and Anker S. D.).
Neves, R. X. , Rosa‐Neto, J. C. , Yamashita, A. S. , Matos‐Neto, E. M. , Riccardi, D. M. R. , Lira, F. S. , Batista, M. L. Jr , and Seelaender, M. (2016) White adipose tissue cells and the progression of cachexia: inflammatory pathways. Journal of Cachexia, Sarcopenia and Muscle, 7: 193–203. doi: 10.1002/jcsm.12041.
References
- 1. Tisdale MJ, McDevitt TM, Todorov PT, Cariuk P. Catabolic factors in cancer cachexia. In Vivo 1996;10:131–136. [PubMed] [Google Scholar]
- 2. Todorov P, Cariuk P, McDevitt T, Coles B, Fearon K, Tisdale M. Characterization of a cancer cachectic factor. Nature 1996;379:739–742. [DOI] [PubMed] [Google Scholar]
- 3. Tisdale MJ. Cancer cachexia: metabolic alterations and clinical manifestations. Nutrition 1997;13:1–7. [DOI] [PubMed] [Google Scholar]
- 4. Argilés JM, Moore‐Carrasco R, Fuster G, Busquets S, López‐Soriano FJ. Cancer cachexia: the molecular mechanisms. Int J Biochem Cell Biol 2003;35:405–409. [DOI] [PubMed] [Google Scholar]
- 5. Argilés JM, Anker SD, Evans WJ, Morley JE, Fearon KC, Strasser F, Muscaritoli M, Baracos VE. Consensus on cachexia definitions. J Am Med Dir Assoc 2010;11:229–230. [DOI] [PubMed] [Google Scholar]
- 6. Zeyda M, Stulnig TM. Adipose tissue macrophages. Immunol Lett 2007;112:61–67. [DOI] [PubMed] [Google Scholar]
- 7. Inui A. Cancer anorexia–cachexia syndrome: current issues in research and management. CA Cancer J Clin 2002;52:7291. [DOI] [PubMed] [Google Scholar]
- 8. Topkan E, Yavuz AA, Ozyilkan O. Cancer cachexia: pathophysiologic aspects and treatment options. Asian Pac J Cancer Prev 2007;8:445–451. [PubMed] [Google Scholar]
- 9. Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar‐Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, Najand A, Ponikowski P, Rossi Fanelli F, Schambelan M, Schols A, Schuster M, Thomas D, Wolfe R, Anker SD. Cachexia: a new definition. Clin Nutr 2008;27:793–799. [DOI] [PubMed] [Google Scholar]
- 10. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 11. Bing C, Russell S, Becket E, Pope M, Tisdale MJ, Trayhurn P, Jenkins JR. Adipose atrophy in cancer cachexia: morphologic and molecular analysis of adipose tissue in tumour‐bearing mice. Br J Cancer 2006;95:1028–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bing C, Bao Y, Jenkins J, Sanders P, Manieri M, Cinti S, Tisdale MJ, Trayhurn P. Zinc‐alpha2‐glycoprotein, a lipid mobilizing factor, is expressed in adipocytes and is up‐regulated in mice with cancer cachexia. Proc Natl Acad Sci U S A 2004;101:2500–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fouladiun M, Körner U, Bosaeus I, Daneryd P, Hyltander A, Lundholm KG. Body composition and time course changes in regional distribution of fat and lean tissue in unselected cancer patients on palliative care‐‐correlations with food intake, metabolism, exercise capacity, and hormones. Cancer 2005;103:2189–2198. [DOI] [PubMed] [Google Scholar]
- 14. Batista ML Jr, Olivan M, Alcantara PS, Sandoval R, Peres SB, Neves RX, Silverio R, Maximiano LF, Otoch JP, Seelaender M. Adipose tissue‐derived factors as potential biomarkers in cachectic cancer patients. Cytokine 2013;61:532–539. [DOI] [PubMed] [Google Scholar]
- 15. Machado AP, Costa Rosa LF, Seelaender MC. Adipose tissue in Walker 256 tumour‐induced cachexia: possible association between decreased leptin concentration and mononuclear cell infiltration. Cell Tissue Res 2004;318:503–514. [DOI] [PubMed] [Google Scholar]
- 16. DiGirolamo M, Fine JB, Tagra K, Rossmanith R. Qualitative regional differences in adipose tissue growth and cellularity in male Wistar rats fed ad libitum . Am J Physiol 1998;274:R1460–1467. [DOI] [PubMed] [Google Scholar]
- 17. Hausman DB, Fine JB, Tagra K, Fleming SS, Martin RJ, DiGirolamo M. Regional fat pad growth and cellularity in obese zucker rats: modulation by caloric restriction. Obes Res 2003;11:674–682. [DOI] [PubMed] [Google Scholar]
- 18. Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo . Cell 2008;135:240–249. [DOI] [PubMed] [Google Scholar]
- 19. Tran KV, Gealekman O, Frontini A, Zingaretti MC, Morroni M, Giordano A, Smorlesi A, Perugini J, De Matteis R, Sbarbati A, Corvera S, Cinti S. The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab 2012;15:222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Batista ML Jr, Neves RX, Peres SB, Yamashita AS, Shida CS, Farmer SR, Seelaender M. Heterogeneous time‐dependent response of adipose tissue during the development of cancer cachexia. J Endocrinol 2012;215:363–373. [DOI] [PubMed] [Google Scholar]
- 21. Bertevello PS, Seelaender MC. Heterogeneous response of adipose tissue to cancer cachexia. Braz J Med Biol Res 2001;34:1161–1167. [DOI] [PubMed] [Google Scholar]
- 22. Esser N, L'homme L, De Roover A, Kohnen L, Scheen AJ, Moutschen M, Piette J, Legrand‐Poels S, Paquot N. Obesity phenotype is related to NLRP3 inflammasome activity and immunological profile of visceral adipose tissue. Diabetologia 2013;56:2487–2497. [DOI] [PubMed] [Google Scholar]
- 23. Seelaender MC, Nascimento CM, Curi R, Williams JF. Studies on the lipid metabolism of Walker 256 tumour‐bearing rats during the development of cancer cachexia. Biochem Mol Biol Int 1996;39:1037–1047. [DOI] [PubMed] [Google Scholar]
- 24. Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. NF‐kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF‐1alpha. Nature 2008;453:807–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 2008;9:367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yin J, Peng Y, Wu J, Wang Y, Yao L. Toll‐like receptor 2/4 links to free fatty acid‐induced inflammation and β‐cell dysfunction. J Leukoc Biol 2014;95:47–52. [DOI] [PubMed] [Google Scholar]
- 27. Latz E. The inflammasomes: mechanisms of activation and function. Curr Opin Immunol 2010;22:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stienstra R, Tack CJ, Kanneganti TD, Joosten LA, Netea MG. The inflammasome puts obesity in the danger zone. Cell Metab 2012;15:10–18. [DOI] [PubMed] [Google Scholar]
- 29. Lappas M. Activation of inflammasomes in adipose tissue of women with gestational diabetes. Mol Cell Endocrinol 2014;382:74–83. [DOI] [PubMed] [Google Scholar]
- 30. Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol 2013;13:397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schroder K, Tschopp J. The inflammasomes. Cell 2010;140:821–832. [DOI] [PubMed] [Google Scholar]
- 32. Benetti E, Chiazza F, Patel NS, Collino M. The NLRP3 inflammasome as a novel player of the intercellular crosstalk in metabolic disorders. Mediators Inflamm 2013;2013:678627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Seelaender MC, Curi R, Colquhoun A, Williams JF, Zammitt VA. Carnitine palmitoyltransferase II activity is decreased in liver mitochondria of cachectic rats bearing the Walker 256 carcinosarcoma: effect of indomethacin treatment. Biochem Mol Biol Int 1998;44:185–193. [DOI] [PubMed] [Google Scholar]
- 34. Donatto FF, Neves RX, Rosa FO, Camargo RG, Ribeiro H, Matos‐Neto EM, Seelaender M. Resistance exercise modulates lipid plasma profile and cytokine content in the adipose tissue of tumour‐bearing rats. Cytokine 2013;61:426–432. [DOI] [PubMed] [Google Scholar]
- 35. Lira FS, Rosa JC, Zanchi NE, Yamashita AS, Lopes RD, Lopes AC, Batista ML Jr, Seelaender M. Regulation of inflammation in the adipose tissue in cancer cachexia: effect of exercise. Cell Biochem Funct 2009;27:71–75. [DOI] [PubMed] [Google Scholar]
- 36. Lira FS, Yamashita AS, Rosa JC, Tavares FL, Caperuto E, Carnevali LC Jr, Pimentel GD, Santos RV, Batista ML Jr, Laviano A, Rossi‐Fanelli F, Seelaender M. Hypothalamic inflammation is reversed by endurance training in anorectic–cachectic rats. Nutr Metab (Lond) 2011;8:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lira FS, Carnevali LC Jr, Zanchi NE, Santos RV, Lavoie JM, Seelaender M. Exercise intensity modulation of hepatic lipid metabolism. J Nutr Metab 2012;2012:809576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lira FS, Yamashita AS, Rosa JC, Koyama CH, Caperuto EC, Batista ML, Seelaender MC. Exercise training decreases adipose tissue inflammation in cachectic rats. Horm Metab Res 2012;44:91–98. [DOI] [PubMed] [Google Scholar]
- 39. Pond CM. Physiological specialisation of adipose tissue. Prog Lipid Res 1999;38:225–248. [DOI] [PubMed] [Google Scholar]
- 40. Chau YY, Bandiera R, Serrels A, Martínez‐Estrada OM, Qing W, Lee M, Slight J, Thornburn A, Berry R, McHaffie S, Stimson RH, Walker BR, Chapuli RM, Schedl A, Hastie N. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol 2014;16:367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tamai M, Shimada T, Hiramatsu N, Hayakawa K, Okamura M, Tagawa Y, Takahashi S, Nakajima S, Yao J, Kitamura M. Selective deletion of adipocytes, but not preadipocytes, by TNF‐alpha through C/EBP‐ and PPARgamma‐mediated suppression of NF‐kappaB. Lab Invest 2010;90:1385–1395. [DOI] [PubMed] [Google Scholar]
- 42. Kim S, Joe Y, Jeong SO, Zheng M, Back SH, Park SW, Ryter SW, Chung HT. Endoplasmic reticulum stress is sufficient for the induction of IL‐1β production via activation of the NF‐κB and inflammasome pathways Innate Immun; 2013; 799–815. [DOI] [PubMed] [Google Scholar]
- 43. Nov, O , Shapiro, H , Ovadia, H , Tarnovscki, T , Dvir, I , Shemesh, E , Kovsan, J , Shelef, I , Carmi, Y , Voronov, E , Apte, RN , Lewis, E , Haim, Y , Konrad, D , Bashan, N , Rudich, A . Interleukin‐1beta regulates fat‐liver crosstalk in obesity by auto‐paracrine modulation of adipose tissue inflammation and expandability. PLoS One 2013;8:e53626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yin J, Lee JH, Zhang J, Gao Z, Polotsky VY, Ye J. Regulation of hepatocyte growth factor expression by NF‐κB and PPARγ in adipose tissue. Am J Physiol Endocrinol Metab 2014;306:E929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee YH, Thacker RI, Hall BE, Kong R, Granneman JG. Exploring the activated adipogenic niche: interactions of macrophages and adipocyte progenitors. Cell Cycle 2014;13:184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee BC, Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity‐induced insulin resistance. Biochim Biophys Acta 2014;1842:446–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rahman SM, Janssen RC, Choudhury M, Baquero KC, Aikens RM, de la Houssaye BA, Friedman JE. CCAAT/enhancer‐binding protein β (C/EBPβ) expression regulates dietary‐induced inflammation in macrophages and adipose tissue in mice. J Biol Chem 2012;287:34349–34360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reynolds CM, McGillicuddy FC, Harford KA, Finucane OM, Mills KH, Roche HM. Dietary saturated fatty acids prime the NLRP3 inflammasome via TLR4 in dendritic cells‐implications for diet‐induced insulin resistance. Mol Nutr Food Res 2012;56:1212–1222. [DOI] [PubMed] [Google Scholar]
- 49. Stienstra R, van Diepen JA, Tack CJ, Zaki MH, van de Veerdonk FL, Perera D, Neale GA, Hooiveld GJ, Hijmans A, Vroegrijk I, van den Berg S, Romijn J, Rensen PC, Joosten LA, Netea MG, Kanneganti TD. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci U S A 2011;108:15324–15329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity‐induced inflammation and insulin resistance. Nat Med 2011;17:179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]


