Abstract
Purpose
To assess use, screening, and disclosure of perinatal marijuana and other illicit drugs during first obstetric visits.
Design
Observational study that qualitatively assesses provider screening and patient disclosure of substance use.
Setting
Study sites were five urban outpatient prenatal clinics and practices located in Pittsburgh, Pennsylvania.
Participants
Pregnant patients and obstetric providers were recruited as participants.
Methods
We audio recorded patient-provider conversations during first obstetric visits and obtained patient urine samples for drug analyses. Audio recordings were reviewed for provider screening and patient disclosure of illicit drug use. Urine analyses were compared with audio recordings to determine disclosure.
Results
Four hundred and twenty-two pregnant patients provided complete audio recordings and urine samples for analyses. Providers asked about illicit drug use in 81% of the visits. One hundred twenty-three patients (29%) disclosed any current or past illicit drug use; 48 patients (11%) disclosed current use of marijuana while pregnant. One hundred and forty-five samples (34%) tested positive for one or more substances; marijuana was most commonly detected (N = 114, 27%). Of patients who tested positive for any substance, 66 (46%) did not disclose any use; only 36% of patients who tested positive for marijuana disclosed current use.
Conclusion
Although marijuana is illegal in Pennsylvania, a high proportion of pregnant patients used marijuana, with many not disclosing use to their obstetric care providers. (Am J Health Promot 0000; 00[0]:000–000.)
Keywords: Communication, Physician Counseling, Pregnant Women, Marijuana, Prenatal Care, Prevention Research. Manuscript format: research, Research purpose: descriptive, Study design: qualitative, Outcome measure: behavioral, Setting: outpatient obstetrics clinics and offices, Health focus: illicit drug use, Strategy: patient-provider screening communication, Target population age: adult reproductive-age women (18 to 44 years), Target population circumstances: pregnant women and obstetric care providers
PURPOSE
Perinatal illicit drug use puts pregnant women and their fetuses at risk for negative outcomes.1–3 Cocaine use during pregnancy is associated with increased risk of placental abruption4 and low-birth-weight babies.5 Opiate use is associated with increased rates of premature births6 and neonatal abstinence syndrome.7 Recent studies showed marijuana use during pregnancy is associated with increased risk of preterm births, small-for-gestational-age infants, and admissions to the neonatal intensive care unit.8,9 Other studies have noted associations between perinatal marijuana use with cognitive, learning, and behavioral problems in exposed children and adolescents.10–20 Despite such risks, prior studies have estimated that between 2.8% and 7% of pregnant women continue to use illicit drugs during pregnancy.2,21–23
The American College of Obstetricians and Gynecologists and the American Academy of Pediatrics recommend that clinicians caring for pregnant women ask their patients at the initial prenatal visit about illicit drug use.24,25 Research indicates 89% of obstetricians ask about illicit drug use.26 However, these results used self-reported data from questionnaires. Multiple studies have shown that clinicians’ self-report of recommended behaviors is unreliable.27–29
Most studies assessing maternal disclosure of illicit drug use have relied on medical record abstraction.30–35 These studies noted that many pregnant women do not disclose illicit drug use to their health care providers. Among the 11% to 21% of pregnant women who had positive toxicology in studies performing universal urine testing for illicit drugs, between 38.5% and 100% had pregnancy care documentation that indicated no illicit drug use during their pregnancy.30,32,33 However, studies that rely on data from medical records may be limited by reporting bias and incomplete documentation.34,35 In a multisite study in which 8527 newborns had meconium samples tested for metabolites of illicit drugs, 23% of the mothers whose infants tested positive had denied use when asked by social workers or nurses during their postpartum in-hospital recovery period.31 In this study, timing of the assessments may have affected disclosure rates.
No prior studies have examined perinatal illicit drug use screening and disclosure by directly observing patient-provider communication during first obstetric visits. Directly observing the clinical interaction not only allows verification that illicit drug use screening and/or disclosure occurred but also provides the opportunity to examine the communication styles and approaches providers used to assess perinatal illicit drug use. We chose to focus on the first obstetric visit as this tends to be when the most thorough assessment of medical and behavioral risks occurs. Our study objective was to examine audio-recorded first obstetric visits to assess rates of screening, use, and disclosure of marijuana and illicit drugs during pregnancy and to compare patient illicit drug use disclosure to urine drug screening results.
DESIGN
Data for this analysis come from an observational study of audio-recorded first obstetric visits. The study focus was patient-provider communication regarding substance use, including smoking, alcohol, or illicit drug use. The study was observational only; no interventions were tested.
SETTING
We audio recorded first obstetric visits between patients and obstetric care providers in five outpatient obstetrics and gynecology clinics located in Pittsburgh, Pennsylvania. These clinical sites were chosen based on our preliminary medical record assessments, which documented relatively high rates of substance use screening and conversations. In this regard, we were reassured that a good number of substance use screening conversations would be captured in our data collection. We obtained urine samples from patient participants and tested for illicit drugs. The study was approved by the University of Pittsburgh Institutional Review Board (IRB #PR008090530) and the study is ongoing; data included in these analyses were collected from February 2011 through August 2014.
PARTICIPANTS
Patient and provider participants were recruited for enrollment in the study. Patients were eligible if they were pregnant, 18 years of age or older, English speaking, and attending their first obstetric visit. Providers were eligible if they saw patients for first obstetric visits at the participating sites. Each study site served racially diverse populations of women with high proportions reliant on medical assistance (between 50% and 100%). Patient and provider participants gave written informed consent for audio recording the visit. All participants were informed they were participating in a study regarding patient-provider communication and were not initially aware of the focus on substance use. Immediately after the audio-recorded visits, patient participants were debriefed on the study focus and asked to provide a urine sample for testing. Patient participants provided additional written consent for this subsequent portion of the study. In order to minimize the chance that providers would change their behavior knowing the study focus on substance use,36,37 providers were not debriefed on the true study focus until they had participated in recordings with 10 patients or were leaving their position at the study site.
METHOD
A digital voice recorder was placed in each of the patient exam rooms to record the entire first obstetrical visit. Recordings were transcribed verbatim and reviewed for accuracy. Two trained coders independently reviewed transcripts and audio recordings for any provider screening or patient disclosure communication regarding illicit drug use. Provider drug screening communication was coded as present or not. This included any provider-initiated inquiry addressing illicit drug use in general (e.g., “Drugs?” “Any street drugs?” “Do you use recreational drugs?”) or naming of specific substances, which were coded as detailed screening (e.g., “Any marijuana use?” “Any marijuana, cocaine, heroin?”). We also coded screening communication that specifically assessed current use (e.g., “Any drug use right now?” “Any drug use during this pregnancy?”) or past use (e.g., “Any history of drug use?” “Ever use any drugs in your life?”). Inquiries regarding smoking without specific mention of marijuana were determined to represent tobacco screening and thus were not included as provider drug screening communication. Patient drug disclosure during the audio-recorded visit conversation was coded as present or absent. Disclosure was subcategorized as current, past, or unspecified timing of last use. Current use was defined as use within the last 30 days of the patient participant’s audio-recorded visit. Any use greater than 30 days prior to the visit was coded as past. If the patient did not specify when last use occurred, this was coded as last use unspecified.
Urine samples were collected from patient participants using sterile urine specimen cups; lab technicians retrieved the samples within 3 to 5 hours of collection, and samples were tested for 10 different illicit drugs. iCassette Drug Screen 9 Panel Pipette urine toxicology radio assay tests were used to test for marijuana, cocaine, opiates, amphetamines, methamphetamines, PCP, benzodiazepines, barbiturates, and methadone.38 MP Biomedicals Cassette Rapid Drug Tests were used to test for buprenorphine. The tests are FDA approved, use Substance Abuse and Mental Health Services Administration cutoff levels, and have a 97% accuracy rate.39 Table 1 depicts the cutoff and approximate detection time in urine for each substance. We based our definition of current versus past illicit drug use on the longest detection time (15–30 days) noted for marijuana. Drug testing was conducted within 24 hours of collection and the remainder of each patient participant sample was frozen. All urine drug testing was performed by experienced, independent lab technicians, and each result was verified by a second trained technician.
Table 1.
i9 Cassette Drug Test and MP Biomedical Test Card Details
| Drug | Cutoff Level, ng/mL | Approximate Detection Time in Urine, d |
|---|---|---|
| Amphetamine | 1000 | 2–4 |
| Cocaine | 300 | 2–4 |
| Marijuana | 50 | 15–30 |
| Methamphetamine | 1000 | 3–5 |
| Opiates | 2000 | 2–4 |
| Phencyclidine (PCP) | 25 | 7–14 |
| Barbiturates | 300 | 4–7 |
| Benzodiazepines | 300 | 3–7 |
| Methadone | 300 | 3–5 |
| Buprenorphine | 30 | 7 |
All statistical analyses were conducted using Stata (ver. 14; StataCorp LP, College Station, Texas). Current illicit drug use was calculated by combining patient subjects who disclosed current use to their obstetric provider and those whose urine toxicology was positive. Disclosure rates were calculated by determining the proportion of patient subjects who disclosed current illicit drug use to their provider among those whose urine toxicology was positive. Descriptive statistics summarized demographic data, disclosure rates, and urine drug screening results.
RESULTS
Patient Participant Demographics
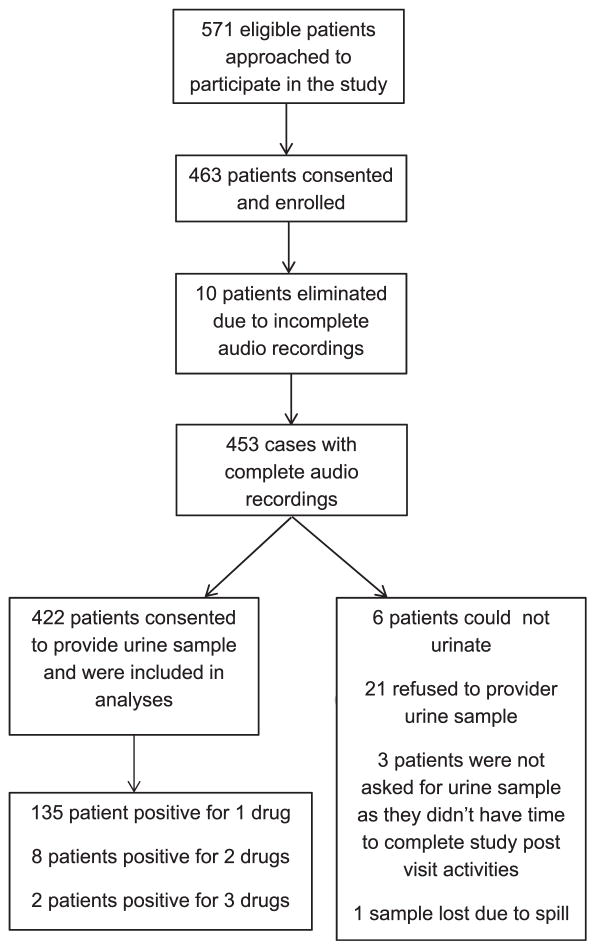
Between February 2011 and August 2014, 571 eligible patients were approached for study participation at the participating sites and 463 patients were enrolled. Of the 463 enrolled, 10 were eliminated because of incomplete audio recording resulting from recorder malfunctions. Of the remaining 453, 422 agreed to provide a urine sample for testing (6 patients could not urinate when asked, 21 refused to provide a sample, 3 patients were not asked as they did not complete the study postquestionnaire because of lack of time, and 1 sample was lost because of spillage). The Figure shows the flow diagram of patient participant eligibility and inclusion. A total of 422 audio-recorded visits and patient participants were used in these analyses. Characteristics of patient participants are shown in Table 2. The majority of our sample was young (mean age 25 years), unmarried, and educated, with slightly more than half identifying their race as African-American. The majority (76%) had an individual income of less than $20,000 per year. Thirty-six percent described themselves as tobacco smokers. For 28%, this was their first pregnancy; 42% had not carried a pregnancy to viability before. The mean gestational age at the time of this first obstetric visit was 12 weeks.
Figure.
Patient Participant Eligibility and Inclusion
Table 2.
Patient and Provider Characteristics*
| Variable | Category | No. (%) |
|---|---|---|
| Patients (N = 422) | ||
| Age, y (mean = 25, SD = 5.191, min/max = 18/45) | <20 | 47 (11.1) |
| 20–29 | 289 (68.5) | |
| 30–39 | 83 (19.7) | |
| 40+ | 3 (0.7) | |
| Ethnicity | African-American | 232 (55.0) |
| White | 138 (32.7) | |
| Other | 46 (10.9) | |
| Asian | 3 (0.7) | |
| Hispanic/Latina | 3 (0.7) | |
| Marital status | Single | 183 (43.4) |
| Living with partner | 172 (40.8) | |
| Married | 52 (12.3) | |
| Separated | 7 (1.7) | |
| Divorced | 7 (1.7) | |
| Widowed | 1 (0.2) | |
| Highest level of education completed | Grade school | 55 (13.0) |
| High school/GED | 169 (40.0) | |
| Associates degree | 41 (9.7) | |
| Some college | 119 (28.2) | |
| Finished college | 32 (7.6) | |
| Graduate school | 6 (1.8) | |
| Yearly income, $ | 0–4999 | 162 (38.4) |
| 5000–9999 | 61 (14.5) | |
| 10,000–14,999 | 58 (13.7) | |
| 15,000–19,999 | 39 (9.2) | |
| 20,000 and higher | 94 (22.3) | |
| Refused | 8 (1.9) | |
| Type of provider who conducted patient visit | First- to fourth-year resident | 280 (66.3) |
| Nurse midwife | 37 (8.8) | |
| Nurse practitioner | 82 (19.4) | |
| Physician assistant | 10 (2.4) | |
| Faculty physician | 13 (3.1) | |
| Gravidity (mean = 2.82, SD = 1.941, min/max = 1/14) | Primagravida | 120 (28.4) |
| Parity (mean = 1.08, SD = 1.339, min/max = 0/8) | Nullipara | 177 (41.9) |
| Average gestational age at new OB appt, wk | Mean = 12.29, SD = 6.900, min/max = 4.2/39.3 | |
| Providers (N = 64) | ||
| Gender | Female | 58 (90.6) |
| Male | 6 (9.4) | |
| Ethnicity | White | 52 (81.3) |
| Black/African-American | 5 (7.8) | |
| Other | 4 (6.3) | |
| Asian | 3 (4.7) | |
| Type of provider (when recruited for study) | First-year resident | 27 (42.2) |
| Second-year resident | 8 (12.5) | |
| Third-year resident | 10 (15.6) | |
| Fourth-year resident | 2 (3.1) | |
| Nurse midwife | 5 (7.8) | |
| Nurse practitioner | 9 (14.1) | |
| Physician assistant | 1 (1.6) | |
| Faculty physician | 2 (3.1) | |
| No. of patients provider saw in the study | Mean = 7.44, SD = 4.223, min/max = 1/21 | |
min/max indicates minimum/maximum; GED, general equivalency diploma; OB, obstetrics; and appt, appointment.
Provider Participant Demographics
Eighty obstetric care providers, including first- to fourth-year residents, nurse midwives, and faculty physicians, consented to study participation. Of the 80, 64 participated in audio-recorded visits included in these analyses. The characteristics of these 64 are shown in Table 2. A majority of the providers were female (91%), white (81%), and obstetrics and gynecology residents (73%). The mean number of audio-recorded encounters across participating providers was 7 (SD =4.223).
Screening
Of the 422 visits for which we obtained both complete audio recordings and urine drug analyses, obstetric care providers asked their pregnant patients about illicit drug use in 81%. One hundred twenty-three patients (29%) disclosed any current or past illicit drug use to their obstetrics provider. Eighty-three (20%) patients disclosed current illicit drug use within the last 30 days to their provider. An additional 34 patients (8%) disclosed only past illicit drug use, specifically describing their last use of any illicit drug as more than 30 days prior to the recorded visit. Six patients (1%) disclosed they had used drugs at some point in their lifetime but did not describe when this use most recently occurred. Of those who disclosed illicit drug use, 48 patients (11%) disclosed current use of marijuana to their provider and another 30 patients (7%) disclosed past use of marijuana.
Urine Drug Screen Results
Of the 422 patient urine samples provided, 277 were negative for any of the 10 illicit drugs for which we tested and 145 (34%) tested positive for one or more illicit drugs. The most frequent drug for which patients tested positive was marijuana (N =114, 27%), followed by methadone (N = 28, 6%), with a very small number testing positive for benzodiazepines (N = 4, <1%), cocaine (N = 3, <1%), and opioids (N = 3, <1%), and less than .5% for barbiturates, amphetamines, methamphetamine, and buprenorphine. The majority of the subjects with positive urine drug screens tested positive for only one drug (135); eight were positive for two drugs, one positive for three.
Prevalence of Current Illicit Drug Use
One hundred and fifty-seven of the 422 (37%) patients who provided urine samples either had positive urine testing for illicit drugs or disclosed current illicit drug use to their obstetric provider during the recorded visit; thus, 37% of these patient subjects were currently using illicit drugs. One-hundred and twenty-one (29%) tested positive for or admitted current use of marijuana.
Disclosure Rates Among Pregnant Substance Users
Among the 83 patients who disclosed current illicit drug use, the majority (87%) had positive urine drug screens for substances; 11 had negative urine testing. Among the 34 patients who disclosed only past illicit drug use, 14 (41%) had positive urine testing. Among those whose last illicit drug use was undefined, 50% had positive urine testing. Table 3 shows the details of disclosures among participants whose urine testing was positive for drugs. Of the 145 patient participants whose urine samples tested positive for illicit drugs, 66 (46%) did not disclose the use of that drug (either past, current, or undefined) to their obstetric provider during the recorded visit. Among those whose urine tests were positive for marijuana, only 41 (36%) of the patient participants who tested positive for marijuana disclosed current marijuana use to their provider; another 14 (12%) told their provider their marijuana use was in the past.
Table 3.
Disclosure and Results of Urine Drug Screen (UDS)
| No. of Substances Tested Positive | Substance Tested Positive for— Results of UDS | No. (% of 422) | Disclosed Current Substance Use | Disclosed Past Substance Use | No Disclosure of Substance Use |
|---|---|---|---|---|---|
| 1 (n = 135) | Marijuana | 107 (25.4) | 38 | 13 | 56 |
| Methadone | 20 (4.7) | 20 | — | — | |
| Benzodiazepines | 2 (0.5) | — | — | 2 | |
| Opiates | 2 (0.5) | — | — | 2 | |
| Buprenorphine | 2 (0.5) | 2 | — | — | |
| Cocaine | 1 (0.2) | — | 1 | — | |
| Barbiturates | 1 (0.2) | — | — | 1 | |
| 2 (n = 8) | Marijuana + methadone | 4 (0.9) | 2 marijuana 3 methadone |
1 marijuana | 1 marijuana |
| Marijuana + cocaine | 1 (0.2) | Cocaine | — | Marijuana | |
| Methadone + Benzodiazepines | 1 (0.2) | Methadone, benzodiazepines | — | — | |
| Methadone + cocaine | 1 (0.2) | Methadone | — | Cocaine | |
| Benzodiazepines + buprenorphine | 1 (0.2) | Benzodiazepines, buprenorphine | — | — | |
| 3 (n = 2) | Marijuana + methadone + amphetamines | 1 (0.2) | Methadone | — | Marijuana, amphetamines |
| Marijuana + methadone + opiates | 1 (0.2) | Marijuana, methadone | Opiates | — |
Disclosure Communication
In most visits, this screening communication was general and did not specifically assess current or past use; 29% of visits’ drug screening communication named specific drugs; 7% asked about current use; 10%, past. Type of screening communication was also not associated with disclosure; 84% of the 422 cases included were coded for type of illicit drug screening (current, past, detailed). We saw no differences in disclosure rates in visits when providers asked about illicit drug use using general or descriptive questions and no differences when providers asked specifically about current or past use.
CONCLUSION
In the majority of visits, obstetric care providers asked their pregnant patients about illicit drug use. This rate reflects the screening rates reported in other studies.26 Providers at our institution are reminded to screen for a variety of behavioral issues including substance use through visual prompt in the electronic medical chart. This prompt likely contributed to our high screening rates. The prompt, however, is not a field that requests input of detailed information or specifically guides providers on how to frame their substance use questions; obstetric providers individually choose their screening communication styles and approaches.
Our rates of illicit drug and perinatal marijuana use are higher than those found in other studies and previously reported in the literature. Population-based studies of pregnant women in California and Florida using urine drug testing found 5.2% and 14.8% positive for perinatal illicit drug use, respectively.40,41 Analyses of the 2011 National Survey on Drug Use and Health (NSDUH) data showed only 5% of pregnant women reporting any illicit drug use during pregnancy.42 Like other studies, we noted that marijuana was the most commonly used drug during pregnancy.50–57 Our perinatal marijuana rates were higher than the 4.6% noted in the NSDUH 2009 report or the 6% found in a recent study that included 1632 women from four cities across the United States.43,44
Characteristics of our study population, such as the high proportion of African-American and lower–socioeconomic-status women, likely contribute to our higher rates of perinatal illicit drug and marijuana use. Earlier demographic studies indicated higher rates of perinatal illicit drug use among African-American patients and patients of lower socioeconomic status.45–47 Other studies noted higher rates (5%–10%) of illicit drug use among pregnant women receiving medical assistance compared to those with private insurance (1%–1.9%).46,47
Our study’s focus on patient-provider communication and our study design combining analyses of audio-recorded clinical visits with urine testing using two separate consenting processes may have also contributed to our higher detection rates. Studies relying on self-report or medical record data are susceptible to reporting bias; others focusing on biochemical testing may suffer potential selection bias. Another study using a similar two-phased consenting process with subjects first participating in a computerized survey of risk behaviors and then consenting to providing biologic samples for testing found perinatal illicit drug use rates similar to ours (29%).48
Our study corroborates other studies that indicate a large proportion of pregnant women who use illicit drugs do not disclose this use to their obstetric provider.30–33 Our study also did not show that any specific screening communication approach was associated with disclosure. Explanations for this lack of difference may be the rather limited range of screening questions used or the small proportion of visits in which providers asked about illicit drugs by name rather than asking more generally. Potentially, patients may be influenced by the providers’ overall interaction style or communication elements used when discussing other topics. Style or type of screening communication may also be less influential to disclosure than women’s perceived concerns or risks related to telling an obstetric provider about her illicit drug use. Other studies have shown that pregnant women who are using illicit drugs worry about stigma and judgment that inhibits their willingness to disclose or seek treatment. They described feeling embarrassed and guilty about their illicit drug use, and fearing imprisonment, prosecution, or losing custody of their child/ children.49,50 In this regard, policies, practices, or laws related to perinatal illicit drug use that women perceive as punitive may have a greater impact on disclosure rates.
There are some limitations to our study. The characteristics of our patient population may limit generalizability of our study findings. Our patients were recruited from urban prenatal clinics in Pittsburgh, Pennsylvania, that primarily served low-income women on medical assistance. In this regard, our findings may not be representative of other patient populations, clinical settings, or geographic locations. Even among a substance-using population, the proportions and types of illicit drugs use likely vary across states and regions. Our sites were also chosen specifically because high substance use screening rates documented our review of their medical records. In this regard, then, the screening rates may not reflect usual screening rates in other obstetric clinics or practices.
Additionally, although providers and patients consented to audio recording their visit, this may have altered their behavior regarding asking and disclosing illicit substances while being recorded. However, all participants were aware that the study data’s confidentiality is protected by a National Institutes of Health Certificate of Confidentiality. Furthermore, when patient participants were asked immediately after the visit whether the audio recording affected their behavior, 98% reported it did not affect honesty in their responses to the provider’s questions during their clinic visit. Additionally, none of the 34 provider participants who were interviewed when they completed the study revealed that they knew the focus of the study was on substance use.
The audio recordings used in this study also only captured discussions in the first obstetric visit. As a result, we are unable to provide information regarding illicit drug screening and disclosure communication that may occur in subsequent patient-provider interactions. In this manner, our data do not account for the influence of rapport or relationship building between patients and providers over the course of multiple pregnancy visits. The majority of our patients also presented for the first obstetric visit early in their pregnancy; we are unable to then explore differences in illicit drug use when women are further along in their pregnancy.
Another limitation is that marijuana can appear in urine analysis for a period of time after cessation and thus the findings in our urine samples may not be limited to current use. This certainly can explain the 12% of women with urine testing positive for marijuana who had described their marijuana use as in the past. However, 52% of those testing positive for marijuana denied any (current or past) marijuana use. Additionally, a recent review of marijuana elimination times notes that prolonged periods of continued marijuana positivity (e.g., 30 days or more) were exceptional findings and based on studies that either used highly sensitive but less specific testing or did not specify type of testing performed. This review found that at the 50 ng/mL cutoff concentration for detection of marijuana metabolites in urine, there would be low likelihood of even chronic users having a positive test more than 10 days from the last use.51
Finally, only conversations between obstetrics providers and patients were obtained. Discussions with other clinical personnel such as nurses, medical assistants, and social workers were not included; it is possible that perinatal drug screening and disclosure may have occurred in these other conversations.
Despite these limitations, our study suggests that the rates of perinatal marijuana use may be higher than previous estimates and that although obstetric providers are asking patients about illicit drug use, the majority of patients are not disclosing this use. According to the 2006 NSDUH report, drug and/or alcohol interventions address the needs of only 10.8% of those meeting criteria for alcohol and/ or illicit drug dependence or abuse; among the other 89% who had not accessed services, only 4.5% recognized a need for treatment.22 Given that a large proportion of substance users are likely unknown to providers because of lack of disclosure, it is imperative to explore more accurate methods of detecting illicit drug use in pregnant patients. This then raises questions regarding what methods are most effective for increasing disclosure, and thus detection. Other approaches to perinatal illicit drug use detection include a variety of screening tools and approaches, all with varied effectiveness.48,52,53 Although there may be a temptation to argue for more widespread or even universal biochemical drug testing of pregnant patients, such policies, in turn, could generate some ethical concerns and controversies, particularly if these practices target only certain populations of pregnant women. There may also be other unintended consequences such as impaired patient-provider trust54 or delayed or reduced use of prenatal care.
Further research is needed to understand what inhibits or contributes to a patient’s willingness to disclose perinatal illicit drug use. We are furthering our analyses to explore patient attitudes and beliefs regarding perinatal illicit drug and marijuana use and their thoughts and concerns regarding disclosing such use to their obstetric providers. Additionally, more study is needed to better understand how obstetrics providers counsel patients using illicit drugs, whether this counseling differs based on the type of drug (e.g., cocaine versus marijuana), and how provider responses affect pregnant women who use these substances. Future research is also needed regarding effective strategies to prevent perinatal illicit drug use.
SO WHAT? Implications for Health Promotion Practitioners and Researchers.
What is already known on this topic?
Marijuana is the most commonly used recreational drug in the United States, with perinatal use rates ranging between 4.6% and 11.9%.
What does this article add?
Our results raise speculation that estimates of perinatal illicit drug use based on surveys or medical record reviews may underestimate the rates of actual use. The actual rate of current perinatal illicit drug use was 1.9 times the rate based on patient disclosure (37% versus 20%). This highlights the limitations of perinatal illicit drug use studies that rely solely on self-report or medical record data.
What are the implications for health promotion practice or research?
Legal implications for disclosing and detecting perinatal illicit drug use likely complicate these conversations. Various new state laws, such as the Tennessee law passed in 2014 that allows a woman to be charged with assault if her child suffers any negative consequences related to illegal opioid use during pregnancy, could have the unintended effect of reinforcing women’s desire to hide this problem from their obstetric providers and thus reduce use of treatment and support services. Additionally, further study is needed to examine the implications of recent state laws legalizing recreational marijuana on screening and disclosure behaviors for this drug. Our study was conducted in Pennsylvania, a state in which marijuana use is illegal. Screening and disclosure behaviors regarding perinatal marijuana may differ in regions where the use of marijuana is legally sanctioned, such as in Colorado, Washington, Oregon, and Alaska.
Finally, our findings suggest that current methods obstetrics providers use to assess perinatal illicit drug use needs improvement. Future studies are needed to identify assessment approaches more conducive to encouraging open dialogue on perinatal illicit drug use, motivating reduced use, and ultimately ensuring optimal health outcomes for mothers and children.
Acknowledgments
Funding was provided by the National Institute of Drug Abuse (NIDA), a component of the National Institutes of Health (R01 DA026410, PI: J.C.C) and, in part, from a grant from the Pennsylvania Department of Health. The project described was also supported by the National Institutes of Health through Grant Number UL1TR000005. The study sponsors had no role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication. The content does not necessarily represent the official views of NIDA, the National Institutes of Health, or the Pennsylvania Department of Health. No financial disclosures or conflicts of interest were reported by the authors of this paper.
References
- 1.Chasnoff I. Chemical dependency and pregnancy. Clin Perinatol. 1991;18:1–191. [PubMed] [Google Scholar]
- 2.Ebrahim S, Gfroerer J. Pregnancy-related substance use in the United States during 1996–1998. Obstet Gynecol. 2003;101:374–379. doi: 10.1016/s0029-7844(02)02588-7. [DOI] [PubMed] [Google Scholar]
- 3.Sherwood R, Keating J, Kavvadia V, et al. Substance misuse in early pregnancy and relationship to fetal outcomes. Neonatology. 1999;158:488–492. doi: 10.1007/s004310051126. [DOI] [PubMed] [Google Scholar]
- 4.Oyelese Y, Ananth C. Placental abruption. Obstet Gynecol. 2006;108:1005–1016. doi: 10.1097/01.AOG.0000239439.04364.9a. [DOI] [PubMed] [Google Scholar]
- 5.Bada H, Das A, Bauer C, et al. Low birth weight and preterm births: etologic fraction attributable to prenatal drug exposure. J Perinatol. 2005;25:631–637. doi: 10.1038/sj.jp.7211378. [DOI] [PubMed] [Google Scholar]
- 6.Bennett A. Perinatal substance abuse and the drug-exposed neonate. Adv Nurse Pract. 1999;7:32–36. [PubMed] [Google Scholar]
- 7.Elliott M, Cunliffe P, Demianczuk N, Robertson C. Frequency of newborn behaviours associated with neonatal abstinence syndrome: a hospital based study. J Obstet Gynaecol Can. 2004;26:25–34. doi: 10.1016/s1701-2163(16)30693-4. [DOI] [PubMed] [Google Scholar]
- 8.Dekker G, Lee S, North R, et al. Risk factors for preterm birth in an International prospective cohort of nulliparous women. PLoS One. 2012;7:e39154. doi: 10.1371/journal.pone.0039154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayatbakhsh M, Flenady V, Gibbons K, et al. Birth outcomes associated with cannabis use before and during pregnancy. Pediatr Res. 2012;71:215–219. doi: 10.1038/pr.2011.25. [DOI] [PubMed] [Google Scholar]
- 10.Fried P, Watkinson B, Gray R. A follow-up study of attentional behavior in 6-year old children exposed prenatally to marijuana, cigarettes, and alcohol. Neurotoxicol Teratol. 1992;14:299–311. doi: 10.1016/0892-0362(92)90036-a. [DOI] [PubMed] [Google Scholar]
- 11.Richardson G, Ryan C, Willford J, et al. Prenatal alcohol and marijuana exposure: effects on neuropsychological outcomes at 10 years. Neurotoxicol Teratol. 2002;24:309–320. doi: 10.1016/s0892-0362(02)00193-9. [DOI] [PubMed] [Google Scholar]
- 12.Day N, Richardson G, Goldschmidt L, et al. Effect on prenatal marijuana exposure on the cognitive development of offspring at age three. Neurotoxicol Teratol. 1994;16:169–175. doi: 10.1016/0892-0362(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 13.Goldschmidt L, Day N, Richardson G. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol. 2000;22:325–336. doi: 10.1016/s0892-0362(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 14.Richardson G, Day N, Goldschmidt L. Prenatal alcohol, marijuana, and tobacco use: infant mental and motor development. Neurotoxicol Teratol. 1995;17:479–487. doi: 10.1016/0892-0362(95)00006-d. [DOI] [PubMed] [Google Scholar]
- 15.Fried P, Watkinson B. Differential effects on facets of attention in adolescents prenatally exposed to cigarettes and marijuana. Neurotoxicol Teratol. 2001;23:421–430. doi: 10.1016/s0892-0362(01)00160-x. [DOI] [PubMed] [Google Scholar]
- 16.Fried P, Watkinson B. 36- and 48-month neurobehavioral follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol. J Dev Behav Pediatr. 1990;11:49–58. [PubMed] [Google Scholar]
- 17.Fried P, Watkinson B. Visuoperceptual functioning differs in 9-to 12-year olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 2000;22:11–20. doi: 10.1016/s0892-0362(99)00046-x. [DOI] [PubMed] [Google Scholar]
- 18.Fried P, Watkinson B, Gray R. Differential effects on cognitive functioning in 9- to 12-year olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 1998;20:293–306. doi: 10.1016/s0892-0362(97)00091-3. [DOI] [PubMed] [Google Scholar]
- 19.Smith A, Fried P, Hogan M, Cameron I. Effects of prenatal marijuana on response inhibition: an fMRI study in young adults. Neurotoxicol Teratol. 2004;26:533–542. doi: 10.1016/j.ntt.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Smith A, Fried P, Hogan M, Cameron I. Effects of prenatal marijuana on visuospatial working memory: an fMRI study in young adults. Neurotoxicol Teratol. 2006;28:286–295. doi: 10.1016/j.ntt.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Greenfield S, Manwani S, Nargiso J. Epidemiology of substance use disorders in women. Obstet Gynecol Clin North Am. 2003;30:412–446. doi: 10.1016/s0889-8545(03)00072-x. [DOI] [PubMed] [Google Scholar]
- 22.Substance Abuse and Mental Heatlh Services Administration (SAMHSA) Results from the 2006 National Survey on Drug use and Health: National Findings. Rockville, Md: Substance Abuse and Mental Health Services Administration, Office of Applied Studies; 2007. [Google Scholar]
- 23.Substance Abuse and Mental Heatlh Services Administration (SAMHSA) [Accessed July 26, 2013];Substance use during pregnancy varies by race and ethnicity. Available at: http://www.samhsa.gov/data/spotlight/Spot062PregnantRaceEthnicity2012.pdf.
- 24.American Academy of Pediatrics, American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. Washington, DC: American Academy of Pediatrics, American College of Obstetricians and Gynecologists; 2007. [Google Scholar]
- 25.American College of Obstetricians and Gynecologists. [Accessed January 22, 2009];All patients should be asked about alcohol and drug abuse. Available at: http://www.acog.org/from_home/publications/press_releases/nr12-12-08.cfm.
- 26.Floyd R, Belodoff B, Sidhu J, et al. A survey of obstetrician-gynecologists on their patients’ use of tobacco and other drugs during pregnancy. Prenat Neonatal Med. 2001;6:201–207. [Google Scholar]
- 27.Bastani R, Glenn BA, Maxwell AE, et al. Validation of self-reported colorectal cancer (CRC) screening in a study of ethnically diverse first-degree relatives of CRC cases. Cancer Epidemiol Biomarkers Prev. 2008;17:791–798. doi: 10.1158/1055-9965.EPI-07-2625. [DOI] [PubMed] [Google Scholar]
- 28.Davis DA, Mazmanian PE, Fordis M, et al. Accuracy of physician self-assessment compared with observed measures of competence: a systematic review. JAMA. 2006;296:1094–1102. doi: 10.1001/jama.296.9.1094. [DOI] [PubMed] [Google Scholar]
- 29.Montano DE, Phillips WR. Cancer screening by primary care physicians: a comparison of rates obtained from physician self-report, patient survey, and chart audit. Am J Public Health. 1995;85:795–800. doi: 10.2105/ajph.85.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gillogley K, Evans A, Hansen R, et al. The perinatal impact of cocaine, amphetamine, and opiate use detected by universal intrapartum screening. Am J Obstet Gynecol. 1990;163(5 pt 1):1535–1542. doi: 10.1016/0002-9378(90)90621-d. [DOI] [PubMed] [Google Scholar]
- 31.Lester B, ElSohly M, Wright L, et al. The Maternal Lifestyle Study: drug use by meconium toxicology and maternal self-report. Pediatrics. 2001;107:309–317. doi: 10.1542/peds.107.2.309. [DOI] [PubMed] [Google Scholar]
- 32.Christmas J, Knisely J, Dawson K, et al. Comparison of questionnaire screening and urine toxicology for detection of pregnancy complicated by substance use. Obstet Gynecol. 1992;80:750–754. [PubMed] [Google Scholar]
- 33.Sanaullah F, Gillian M, Lavin T. Screening of substance misuse during early pregnancy in Blyth: an anonymous unlinked study. J Obstet Gynaecol. 2006;26:187–190. doi: 10.1080/01443610500508121. [DOI] [PubMed] [Google Scholar]
- 34.Peabody JW, Luck J, Glassman P, et al. Comparison of vignettes, standardized patients, and chart abstraction: a prospective validation study of 3 methods for measuring quality. JAMA. 2000;283:1715–1722. doi: 10.1001/jama.283.13.1715. [DOI] [PubMed] [Google Scholar]
- 35.Stange KC, Zyzanski SJ, Smith TF, et al. How valid are medical records and patient questionnaires for physician profiling and health services research? A comparison with direct observation of patient visits. Med Care. 1998;36:851–867. doi: 10.1097/00005650-199806000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Gillespie R. A History of the Hawthorne Effect. Cambridge, Mass: Cambridge University Press; 1991. [Google Scholar]
- 37.Roethlisberger F, Dickson W. Management and the Worker. Cambridge, Mass: Harvard University Press; 1939. [Google Scholar]
- 38.Rapid Detect Inc. [Accessed June 4, 2013];iCassette drug test—9 panel pipette. Available at: http://www.rapiddetect.com/iCassette-Drug-Test-9-Panel-Pipette-p/i-doa-1195.htm.
- 39.Rapid Detect Inc. [Accessed June 4, 2013];Frequently asked questions. Available at: http://www.rapiddetect.com/frequently-asked-questions-s/437.htm.
- 40.Chasnoff I, Landress H, Barrett M. The prevalence of illicit drug or alcohol use during pregnancy and discrepancies in mandatory reporting in Pinellas County, Florida. N Engl J Med. 1990;322:1202–1206. doi: 10.1056/NEJM199004263221706. [DOI] [PubMed] [Google Scholar]
- 41.Vega W, Kolody B, Hwang J, Noble A. Prevalence and magnitude of perinatal substance exposures in California. N Engl J Med. 1995;329:850–854. doi: 10.1056/NEJM199309163291207. [DOI] [PubMed] [Google Scholar]
- 42.Substance Abuse and Mental Health Services Administration (SAMHSA) Results from the 2011 National Survey on Drug Use and Health: summary of national findings. Substance Abuse and Mental Health Services Administration; [Accessed May 1, 2015]. Available at: http://www.samhsa.gov/data/nsduh/2k11results/nsduhresults2011.htm#2.6. [Google Scholar]
- 43.Havens J, Simmons L, Shannon L, Hansen W. Factors associated with substance use during pregnancy: results from a national sample. Drug Alcohol Depend. 2009;99:89–95. doi: 10.1016/j.drugalcdep.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 44.Arria A, Derauf C, LaGasse L, et al. Methamphetamine and other substance use during pregnancy: preliminary estimates from the Infant Development, Environment, and Lifestyle (IDEAL) Study. Matern Child Health J. 2006;10:293–302. doi: 10.1007/s10995-005-0052-0. [DOI] [PubMed] [Google Scholar]
- 45.Hans S. Demographic and psychosocial characteristics of substance-abusing pregnant women. Clin Perinatol. 1999;26:55–74. [PubMed] [Google Scholar]
- 46.Noble A, Vega W, Kolody B, et al. Prenatal substance abuse in California: findings from the Perinatal Substance Exposure Study. J Psychoactive Drugs. 1997;29:43–53. doi: 10.1080/02791072.1997.10400169. [DOI] [PubMed] [Google Scholar]
- 47.Yawn B, Thompson L, Lupo V, et al. Prenatal drug use in Minneapolis-St Paul, Minn. A 4-year trend. Arch Fam Med. 1994;3:520–527. doi: 10.1001/archfami.3.6.520. [DOI] [PubMed] [Google Scholar]
- 48.Ondersma S, Svikis D, Lebreton J, et al. Development and preliminary validation of an indirect screener for drug use in the perinatal period. Addiction. 2012;107:2099–2106. doi: 10.1111/j.1360-0443.2012.03982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gehshan S. Missed opportunities for intervening in the lives of pregnant women addicted to alcohol or other drugs. J Am Med Womens Assoc. 1995;50:160–163. [PubMed] [Google Scholar]
- 50.Minkoff H, Paltrow L. Melissa Rowland and the rights of pregnant women. Obstet Gynecol. 2004;104:1234–1236. doi: 10.1097/01.AOG.0000146289.65429.48. [DOI] [PubMed] [Google Scholar]
- 51.Cary P. Drug Court Practitioner Fact Sheet. 2 Vol. 4. Alexandria, Va: National Drug Court Institute; 2006. The Marijuana Detection Window: Determining the Length of Time Cannabinoids Will Remain Detectable in Urine Following Smoking. A Critical Review of Relevant Research and Cannabinoid Detection Guidance for Drug Courts. [Google Scholar]
- 52.Yudko E, Lozhkina O, Fouts A. A comprehensive review of the psychometric properties of the Drug Abuse Screening Test. J Subst Abuse Treat. 2007;32:189–198. doi: 10.1016/j.jsat.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 53.Grekin E, Svikis D, Lam P, et al. Drug use during pregnancy: validating the Drug Abuse Screening Test against psychological measures. Psychol Addict Behav. 2010;24:719–723. doi: 10.1037/a0021741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roberts S, Nuru-Jeter A. Women’s perspectives on screening for alcohol and drug use in prenatal care. Womens Health Issues. 2010;20:193–200. doi: 10.1016/j.whi.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Westfall RE, Janssen PA, Lucas P, Capler R. Survey of medicinal cannabis use among childbearing women: patterns of its use in pregnancy and retroactive self-assessment of its efficacy against “morning sickness”. Complement Ther Clin Pract. 2006;12:27–33. doi: 10.1016/j.ctcp.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 56.Kuczkowski KM. The effects of drug abuse on pregnancy. Curr Opin Obstet Gynecol. 2007;19:578–585. doi: 10.1097/GCO.0b013e3282f1bf17. [DOI] [PubMed] [Google Scholar]