Abstract
Purpose
Cancer-related cognitive impairment (CRCI) is a common, fatigue-related symptom that disrupts cancer survivors’ quality of life. Few interventions for CRCI exist. As part of a randomized pilot study targeting cancer-related fatigue, the effects of mindfulness-based stress reduction (MBSR) on survivors’ cognitive outcomes were investigated.
Methods
Breast and colorectal cancer survivors (n=71) with moderate-to-severe fatigue were randomized to MBSR (n=35) or a fatigue education and support (ES; n=36) condition. The Attentional Function Index (AFI) and the Stroop test were used to assess survivors’ cognitive function at baseline (T1), after the 8-week intervention period (T2), and 6 months later (T3) using intent-to-treat analysis. Mediation analyses were performed to explore mechanisms of intervention effects on cognitive functioning.
Results
MBSR participants reported significantly greater improvement on the AFI total score compared to ES participants at T2 (d=0.83, p=0.001) and T3 (d=0.55, p=0.021). MBSR also significantly outperformed ES on most AFI subscales, although both groups improved over time. MBSR produced greater Stroop accuracy rates relative to ES at T2 (r=0.340, p=0.005) and T3 (r=0.280, p=0.030), with improved accuracy over time only for the MBSR group. There were no significant differences in Stroop reaction time between groups. Improvements in mindfulness mediated the effect of group (e.g., MBSR vs. ES) on AFI total score at T2 and T3.
Conclusions
Additional randomized trials with more comprehensive cognitive measures are warranted to definitively assess the efficacy of MBSR for CRCI.
Implications for Cancer Survivors
This pilot study has important implications for all cancer survivors as it is the first published trial to show that MBSR offers robust and durable improvements in CRCI.
Keywords: Cancer, Oncology, Mindfulness, MBSR, Cognition, Attention
Introduction
Cancer-related cognitive impairment (CRCI) is a prevalent and disruptive symptom [1], affecting 15–75 % of patients with breast and colorectal cancer [2–4]. Attention, memory, and executive function often are compromised [5, 6]. Cognitive impairment has been associated with higher levels of depressive symptoms, anxiety, and fatigue [1, 7], which are the most common symptoms reported among cancer survivors post-treatment [7]. Cognitive deficits can persist up to 20 years post-treatment [8]. Without supportive treatment, persistent cognitive impairment can negatively affect cancer survivors’ self-confidence [9], social relationships [9, 10], work ability [9, 11], and quality of life [9, 12].
Despite CRCI’s prevalence, few interventions targeting this symptom have been evaluated [13]. A number of interventional studies have shown promise in reducing the stress associated with CRCI [11]; however, few have directly addressed CRCI itself. An integrative therapeutic intervention that has become increasingly popular and that may hold promise in relieving CRCI is mindfulness-based stress reduction (MBSR) [14]. Through a variety of guided, experiential mindfulness meditation practices, individuals in an MBSR course learn to focus their attention on present-moment experience with an attitude of curiosity, openness, and acceptance. Mindfulness training is thought to improve cognitive functioning through mechanisms of focused attention (e.g., directing and sustaining attention on a selected object, such as one’s breath, while noticing and disengaging from distractions and mind wandering) and open monitoring (e.g., non-reactive meta-cognitive attending to experience, including automatic cognitive and emotional interpretation of stimuli) [15]. Through the attentional control mechanisms of mindfulness [16, 17], cognitive deficits in attention, memory, and executive function among cancer survivors could be alleviated.
Empirical evidence has emerged that suggests that mindfulness may lead to improved cognitive function. A recent systematic review found that mindfulness meditation improved working memory as well as sustained and selective attention in non-cancer populations with no prior meditation experience [18]. Although MBSR has shown efficacy in reducing a range of cancer-related symptoms, such as depression, anxiety, and fatigue, [19–22], studies are lacking regarding its impact on CRCI [23]. In the largest randomized MBSR trial for post-treatment breast cancer survivors to date (n=229), Hoffman and colleagues [19] investigated the effects of MBSR on mood compared to a wait-list control group. They noted significant post-intervention improvement on the confusion subscale of the Profile of Moods States (POMS) favoring the MBSR group. However, the between-group difference was no longer significant at 12 weeks. Another randomized trial comparing MBSR to wait-list control among a heterogeneous sample of cancer outpatients similarly found short-term benefits of MBSR in reducing confusion on the POMS [24]; however, at 6-month follow-up, between-group differences were not found [25]. Despite these promising findings in cancer survivors, as well as direct evidence that MBSR improves cognitive function in healthy samples [18], no randomized trial has examined the effects of MBSR on CRCI.
The purpose of the current study was to examine the impact of an 8-week MSBR program compared to an 8-week fatigue education and support (ES) intervention on subjectively and objectively assessed cognitive function among cancer survivors with fatigue. Potential mechanisms of intervention effects on subjective and objective cognitive functioning also were explored. We hypothesized that MBSR would be superior to ES in improving cognitive outcomes for cancer survivors and that mindfulness would mediate the relationship between intervention and cognitive outcomes. Notably, the results reported here reflect a secondary analysis of a trial testing the efficacy of MBSR on cancer-related fatigue (CRF), and these results have been presented elsewhere [26]. Given that the nature of the relationship between CRF and CRCI is unknown, the findings of this study only apply to post-treatment cancer survivors with fatigue.
Methods
Design
This pilot study was a two-arm randomized clinical trial. Institutional Review Boards at Indiana University and Community Health Network approved study procedures. Written informed consent was obtained from all participants. The study is registered with ClinicalTrials.gov (NCT01919853).
Participants
Over a 5-month period in 2012–2013, consecutive breast (n=60) and colorectal (n=11) cancer survivors (BCS and CRCS, respectively) with CRF were recruited. BCS and CRCS were included because these are two of the most common cancers with a sizeable number of patients experiencing fatigue after treatment with curative intent [27]. Survivors were identified at a midwestern National Cancer Institute–designated cancer center and its affiliated oncology clinics, as well as through a tumor registry and community cancer clinic. Potential participants were informed of the study’s purpose, and interested individuals were assessed for eligibility.
Participants were eligible for the study if they were at least 18 years of age, had a first-time diagnosis of stage 0–III breast or colorectal cancer that had been treated with chemotherapy and/or radiotherapy, and had clinically significant CRF (Fatigue Symptom Inventory severity composite score ≥4 [28]) that had persisted for at least 8 weeks. Excluded were those who had received chemotherapy, radiation therapy, or surgery less than 3 months or more than 5 years prior to enrollment; reported severe depressive symptoms (Patient Health Questionnaire-8 score ≥20 [29]); or reported previous participation in a mindfulness class and/or ongoing meditation practice. Eligibility was not based upon the presence of CRCI, but upon CRF, a symptom often associated with cognitive impairment.
Study procedures
Eligible participants were invited to attend one of several group enrollment sessions, which included informed consent, collection of self-reported socio-demographic and medical characteristics, completion of baseline surveys, and randomization in a 1:1 ratio to MBSR or ES. The allocation sequence was generated by coin toss in randomly varied block sizes of 4 or 6 by the principal investigator. The allocation sequence was concealed from participants and research assistants through the use of opaque and sequentially numbered envelopes. Participants were blinded to study hypotheses and to the content of the course to which they were not assigned. Once participants were randomized, they met with a facilitator of their assigned intervention for a brief orientation. During orientation, participants assigned to the ES group were asked to not participate in mindfulness training during the study. The 8-week group interventions began the following week.
Interventions
The MBSR and ES interventions each consisted of 8 weekly 2-h classes led by skilled facilitators following standardized procedures. Each group was limited to approximately ten participants to encourage group cohesion and ensure that each group received equal amounts of facilitator attention and time for group discussion and interaction. MBSR and ES classes were held on the same day and time; however, care was taken to keep the MBSR and ES participants in separate locations to limit contamination. MBSR and ES facilitators contacted participants who missed a class by phone or email, and if the participant was willing and able, highlights of the missed class were covered and class materials were sent. Fidelity to MBSR and ES was ensured through the use of standardized manuals, and sessions were audio-recorded. External reviewers used checklists created for each intervention condition to evaluate 25 % of randomly selected sessions for each intervention condition to ensure adherence to the protocol.
MBSR training included instruction on formal mindfulness meditation practices, including body scan, mindful movement featuring hatha yoga poses, sitting meditation, and lovingkindness meditation, and incorporated informal practices to cultivate present-moment awareness in everyday life. The goal of these practices is to facilitate adaptive, non-reactive, and non-judgmental relating to thoughts, feelings, and bodily sensations by enhancing awareness of the present moment. Classes also included didactic instruction that covered all the MBSR standard curriculum topics, and interactive group discussion of the development and integration of mindfulness-based self-regulatory skills in daily life. MBSR facilitators were a physician and a doctoral-level clinical health psychologist with 9 and 3 years of MBSR teaching experience, respectively. Each had completed the professional training leading to eligibility for MBSR Teacher Certification Review through the Center for Mindfulness at the University of Massachusetts.
Meditation guidance was provided in the weekly classes and through audio recordings outside of class consistent with standard MBSR curriculum [30]. To make the training more feasible for fatigued cancer survivors, classes were 2 h instead of the MBSR standard of 2.5 h due to slight decreases in length of in-session meditation practices. The standard all-day retreat was not included, and formal home meditation practices were 20 min instead of the MBSR standard of 45 min. Participants used logs to track the type and amount of formal meditation practices completed daily.
ES sessions were led by masters-level oncology social workers and included a hybrid of didactic teaching and opportunities to share experiences living with cancer-related symptoms. Class topics, which varied each week, focused on fatigue and challenges faced during survivorship (e.g., lingering and late effects of cancer treatment and impact of fatigue on relationships), strategies for symptom self-management (e.g., sleep hygiene and coping with distress), eating to maximize energy, increasing physical activity, and survivorship care planning. For between-session home practice, participants were provided weekly readings that supplemented course content. Participants were invited to log the amount of time they spent doing reading assignments and other self-care strategies discussed in class on weekly home practice logs. At the end of the study, participants were provided with information on how to access MBSR classes.
Measures
Cognitive function and mindfulness were evaluated at baseline (T1), after the 8-week intervention period (T2), and 6 months post-intervention (T3). The study used both subjective and objective measures of cognitive function, as recommended in a recent systematic review; despite the documented discrepancy between objectively confirmed impairment and perceived impairment, measures of each type yield relevant information about the cognitive functioning of cancer survivors [31]. The data were collected by research assistants blinded to participants’ intervention group. Participants received $25 at each assessment period as an incentive for completing the following measures.
Subjective measure
The Attentional Function Index (AFI) is a 13-item, self-report measure that assesses perceived effectiveness in common activities requiring attention and working memory (e.g., formulating plans, carrying out tasks, making decisions, keeping a train of thought, and attending to what others are saying) [32]. Each item of the AFI was evaluated using a linear analog scale, consisting of a 100-mm horizontal line anchored with opposite phrases from “not at all” (0 mm) to “extremely well or a great deal” (100 mm). Respondents were asked to place a mark through the horizontal line to indicate how well they were functioning at the time. Scores for each item were determined by measuring the distance in millimeters from the lower end of the scale to a participant’s indicated mark along the line. Measurements were made by two research assistants, and discrepancies were discussed until consensus was reached. Total score was calculated as the average of the 13 items. Mean scores also were calculated for each of the AFI’s three subscales: effective action (seven items), attentional lapses (three items), and interpersonal effectiveness (three items). Higher mean scores indicate greater capacity to direct attention. The AFI has well-established reliability and has been validated in cancer populations [32]. For the present study, Cronbach’s alpha for AFI total at T1 was 0.87. Participants completed the AFI on paper at the study site, with the exception of three participants who did not complete their assigned intervention. These participants continued completing the study measures at home, either on paper or online, as part of the intent-to-treat design.
Objective measure
The Stroop color-word test [33] is a test of executive attentional functioning, including selective attention and cognitive flexibility and control [34]. The Stroop test was administered via a laptop computer. Participants were presented with a fixation cross (+) for 500 ms, followed by a color word—BLUE, GREEN, ORANGE, or PURPLE—in either congruent or incongruent script color. After each word presentation, participants were instructed to identify the actual script color by pressing a corresponding key on the laptop keyboard as quickly as possible, while ignoring the habitual process of word reading. Several practice trials were allowed prior to testing to ensure that participants understood the instructions. Participants then completed two randomized blocks of 40 experimental trials, one block consisting of congruent trials and one consisting of incongruent trials. Accuracy (error) rate and average reaction time (RT) for each block were recorded.
The Stroop test is widely used in psychological and medical research because of its flexibility. Multiple parameters (e.g., color, order, speed, and mode of administration) can be varied to tailor the test to the sample of interest. Participants in this study were suffering with CRF, and therefore the Stroop test was modified, using a relatively small number of words and blocks, to minimize its burden. Unfortunately, norms for the Stroop test are rarely available in research contexts precisely because of the test’s flexibility. Yet, the Stroop test has been used extensively for decades across multiple disciplines because, despite the many ways it can be administered, it produces valid and reliable results.
Mindfulness
The 39-item Five Facet Mindfulness Questionnaire (FFMQ) [35] was used to evaluate the tendency to be mindful in daily life. Participants rated the extent to which they were mindful on a five-point Likert scale (1=never or very rarely true; 5=very often or always true), with higher scores representing greater mindfulness. Mean scores were calculated for each of the measure’s five subscales, which are observing, describing, acting with awareness, non-judgment of inner experience, and non-reactivity to inner experience. Validity and internal consistency for the FFMQ are good [35, 36].
Attendance and home practice
Study interventionists tracked attendance of each participant at each session. Participants were asked to track the type and amount of meditation practice (MBSR group) and the number of minutes spent completing reading assignments and using self-care strategies (ES group) between sessions on logs they placed in a sealed envelope each week during the intervention period. These logs were not seen by the study interventionists. At the 6-month follow-up, MBSR participants were asked how many days per week, on average, they had participated in formal mindfulness practice (i.e., body scan, yoga, and sitting meditation) since completing the MBSR class. A similar item asking about continued informal mindfulness practice (doing everyday activities mindfully) was also included.
Statistical analysis
Intent-to-treat analyses were conducted. All participants and their available data were included in the analyses according to participants’ randomly assigned group membership regardless of attendance or engagement in the intervention. Imputation was used to fill in missing data. When computing scale scores, a person-specific and scale-specific mean of non-missing items was substituted for missing items if 33 % or fewer of the scale’s items were missing. Groups were compared on demographic and medical characteristics and symptom reports (i.e., fatigue, depression, anxiety, sleep disturbance, and pain) at T1 using chi-square and Fisher’s exact test for categorical variables and t tests for continuous variables (Table 1). Although there were no statistically significant differences between groups on these variables at p<0.05, we controlled for characteristics thought to be clinically/theoretically relevant [37] in an investigation of CRCI and/or those where the between-group difference was p<0.10. Thus, we used age, education, income, and cancer type as covariates in all multivariable models to minimize potential confounding.
Table 1.
Demographic and medical characteristics
| MBSR n=35 |
ES n=36 |
p value | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years), mean (SD) | 56.9 (9.9) | 56.4 (12.7) | 0.85 |
| Female (%) | 94.3 | 86.1 | 0.43 |
| White (%) | 77.1 | 63.9 | 0.22 |
| Married/partnered (%) | 62.9 | 47.2 | 0.19 |
| College degree (%) | 42.9 | 44.4 | 0.89 |
| Employed (%) | 51.4 | 52.8 | 0.91 |
| Comfortable income (%) | 62.9 | 41.7 | 0.07 |
| Medical characteristics | |||
| Cancer type | 0.35 | ||
| Breast cancer (%) | 51.7 | 48.3 | |
| Colorectal cancer (%) | 36.4 | 63.6 | |
| Cancer stage at diagnosis (%) | 0.75 | ||
| 0 | 128 | 5.3 | |
| I | 41.0 | 36.8 | |
| II | 20.5 | 23.7 | |
| III | 20.5 | 29.0 | |
| Years since cancer treatment completion, mean (SD) | 2.2 (1.4) | 2.5 (1.6) | 0.48 |
| Chemotherapy (%) | 65.7 | 80.6 | 0.16 |
| Radiation (%) | 80.0 | 75.0 | 0.61 |
| Chemo-radiation (%) | 45.7 | 55.6 | 0.41 |
| Current endocrine therapy (%) | 46.0 | 46.0 | 1.00 |
| Co-morbid medical conditions in addition to cancer, mean (SD) | 1.8 (1.5) | 1.7 (1.2) | 0.75 |
| Symptoms and mental health treatment | |||
| Fatigue interference (FSI) | 4.91 (2.17) | 5.06 (1.50) | 0.74 |
| Fatigue severity (FSI) | 5.24 (1.57) | 5.48 (1.30) | 0.49 |
| Depression (PHQ-8) | 11.35 (5.57) | 12.53 (4.90) | 0.35 |
| Anxiety (GAD-7) | 7.46 (5.50) | 8.57 (5.31) | 0.39 |
| Sleep disturbance (ISI) | 15.34 (6.45) | 17.33 (6.27) | 0.19 |
| Pain (PEG) | 3.95 (3.09) | 3.43 (2.80) | 0.45 |
| Mental health treatment—current (%) | 17.1 | 22.2 | 0.59 |
| Mental health treatment—past (%) | 25.7 | 41.7 | 0.16 |
MBSR mindfulness-based stress reduction, ES education/support, SD standard deviation, FSI Fatigue Symptom Inventory (range 0–10), PHQ8 eight-item Patient Health Questionnaire depression scale (range, 0–24), GAD7 seven-item Generalized Anxiety Disorder anxiety scale (range, 0–21), ISI Insomnia Severity Index (range, 0–28), PEG three-item abbreviated version of Brief Pain Inventory (range 0–10).
Analysis of covariance (ANCOVA) was used to test efficacy by comparing MBSR to ES on AFI outcomes at T2 and separately at T3, while adjusting for covariates and baseline scale scores for each variable. With a sample size that was powered as a pilot study, there were limits to the number of covariates that could be added to the model without destabilizing the model. A linear model should have at least ten persons per parameter, where the intercept also counts as a parameter; thus, a sample size of 71 should contain no more than six 1-degree-of-freedom covariates; otherwise, inflated standard errors could occur. The six independent variables in the current model were age, education, income, cancer type, intervention group, and baseline AFI. The symptom scores were not used as covariates because, with the exception of pain, they were significantly correlated with the baseline AFI total score (Table 2). As there were no significant group differences in baseline symptom scores (Table 1), it is unlikely that these scores could confound the intervention effects on AFI at T2 and T3. Effect sizes (Cohen’s d) for each AFI outcome variable were calculated as the standardized mean difference between the MBSR and ES groups at T2 and T3, divided by the pooled baseline standard deviation of the particular outcome variable.
Table 2.
Pearson correlation coefficients (p values) at T1
| Fatigue interference (FSI) |
Fatigue severity (FSI) |
Depression (PHQ-8) |
Anxiety (GAD-7) |
Sleep disturbance (ISI) |
Pain (PEG) |
AFI total | |
|---|---|---|---|---|---|---|---|
| Fatigue interference (FSI) | 1.00 | ||||||
| Fatigue severity (FSI) | 0.61 (<0.0001) |
1.00 | |||||
| Depression (PHQ-8) | 0.67 (<0.0001) |
0.41 (0.000) |
1.00 | ||||
| Anxiety (GAD-7) | 0.45 (<0.0001) |
0.28 (0.019) |
0.71 (<0.0001) |
1.00 | |||
| Sleep disturbances (ISI) | 0.41 (0.000) |
0.31 (0.009) |
0.53 (<0.0001) |
0.35 (0.003) |
1.00 | ||
| Pain (PEG) | 0.35 (0.003) |
0.38 (0.001) |
0.32 (0.007) |
0.26 (0.023) |
0.17 (0.16) |
1.00 | |
| AFI total | −0.40 (0.001) |
−0.36 (0.002) |
−0.54 (<0.0001) |
−0.55 (<0.0001) |
−0.33 (0.005) |
0.05 (0.70) |
1.00 |
FSI Fatigue Symptom Inventory, PHQ-8 eight-item Patient Health Questionnaire depression scale, GAD-7 seven-item Generalized Anxiety Disorder anxiety, ISI Insomnia Severity Index, PEG three-item abbreviated version of Brief Pain Inventory, AFI Attentional Function Index
The paired t test was used to assess within-group improvements on all AFI outcomes for each group at T2 and T3 as compared to T1 scores on each variable. Effect sizes (standardized response mean, SRM) were calculated for each AFI within-group test as the within-group difference between means (T1 to T2; or T1 to T3) divided by the standard deviation (SD) of change scores. Two-sided 95 % confidence intervals were computed for effect sizes.
Because of technology issues and participant dropout, Stroop data could not always be collected from all participants. Stroop group means are based on available data, resulting in different sample sizes across the three time points.
In keeping with prior work [38, 39], reaction times from the Stroop task were trimmed and transformed. At each time point, reaction times exceeding 2.5 SDs were recoded as the mean latency plus 2.5 SDs for that time point. Because RT scores tend to be positively skewed, scores were then log-transformed at each time point. Stroop interference scores for RT data, which reflect the extent to which participants experience difficulty inhibiting prepotent responses, an indicator of executive attentional function [34], were computed by subtracting congruent (i.e., control) trial means from incongruent trial means for each participant. This commonly used approach controls for base rate RT [38, 40], which is a particular concern when using fatigued participants. A similar approach was used for Stroop accuracy (i.e., error) rates, which were computed by subtracting the congruent (i.e., control) trial accuracy rate from the incongruent trial accuracy rate for each participant. Accuracy rate data from the Stroop test were compared between groups at T2 and T3 using the two-sided Wilcoxon rank sum test and within groups across time points using the two-sided Wilcoxon signed rank test because the Stroop variable was highly skewed even after transformation. Effect size, r, was computed by dividing the z obtained from the Wilcoxon rank tests by the square root of the total sample size.
Mediational analysis
To provide insight into how MBSR benefitted participants’ cognitive function, we examined whether changes on each of the five subscales of the FFMQ mediated the effect of group (i.e., MBSR vs. ES) on AFI total scores and on Stroop accuracy rates at T2 and T3. For each mediator, indirect effects and the 95 % confidence interval were computed for each of 10,000 bootstrapped samples [41]. We examined each potential mediator separately because the FFMQ subscales are considered to be independent from a theoretical perspective, an assumption borne out by the low correlations we found among the changes on each subscale at each time point (all r<.46).
Results
Enrollment and attrition
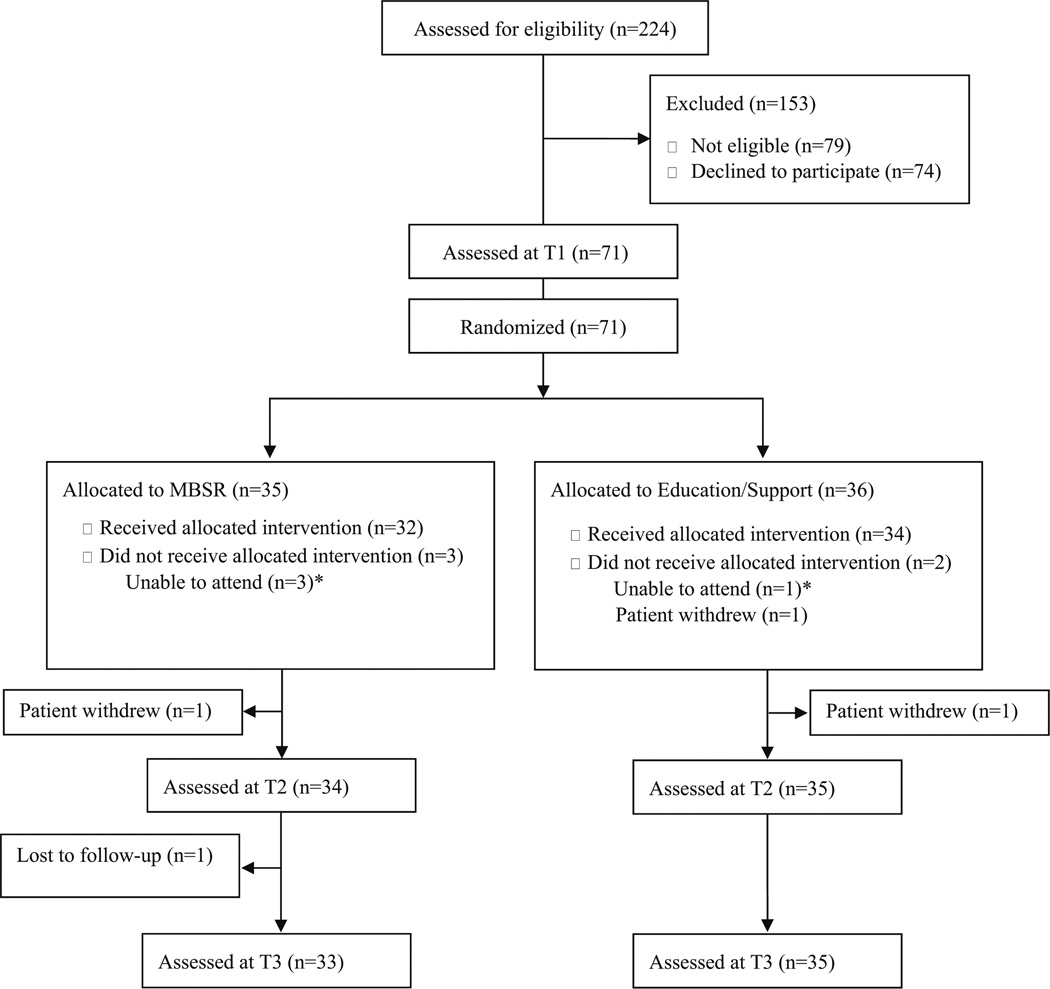
Of the 224 consecutive survivors screened for eligibility, 153 were excluded (see Fig. 1). Of the eligible survivors, 49.0 % (n=71) were enrolled and randomized. Retention rates for both groups were high at T2 (MBSR=97.1 %; ES=97.2 %) and at T3 (MBSR=94.3 %; ES=97.2 %). Two individuals dropped out between T1 and T2 due to lack of interest (one from each group), and one individual completed the MBSR intervention and T2 assessment but was lost to follow-up at T3. Notably, four participants (MBSR=3; ES=1) were unable to attend any of their respective intervention sessions, yet they continued to complete all study measures in keeping with intent-to-treat analysis.
Fig. 1.
CONSORT diagram.
*Participants did not receive intervention; however, they completed all surveys as part of intent-to-treat analysis
Baseline participant characteristics
As reported in Table 1, the sample was predominantly white (70.4 %) and female (90.1 %), and a little more than half did not have a college degree (56.3 %). About half were employed (52.1 %), were married/partnered (54.9 %), and reported having a “comfortable” income (52.1 %). Average time since completion of cancer treatment was approximately 2.4 years. At the time of enrollment, 46 % of breast cancer survivors were on endocrine therapy.
Attendance and home practice
Attendance was similar between groups, with MBSR participants attending 5.8 sessions (SD=2.1) compared to 6.3 sessions (SD=1.9) for ES participants [t(68)=0.96, p=0.30]. Although the difference was not statistically significant, MBSR participants reported more minutes per week on average completing meditation practice assignments at home [117.6 (SD=85.9)] compared to ES participants’ time completing reading assignments or using self-care strategies discussed in class [92.5 (SD=92.1); t(69)=1.19, p=0.24]. Notably, at the 6-month follow-up, 75.8 % of MBSR participants reported continued formal mindfulness practice, such as body scan, sitting meditation, and yoga; however, the majority of these (63.6 %) reported formal practice only once or twice per week. Most MBSR participants (84.8 %) reported continued informal mindfulness practice at the 6-month follow-up.
AFI
Between-group effects
As shown in Table 3, the mean scores of the AFI total as well as the Effective Action and Attentional Lapses subscales were significantly and substantially higher in the MBSR group than in the ES group at the end of the intervention (T2; p≤0.004). Importantly, the improved MBSR scores remained stable, and the differences between groups continued to be significant 6 months later at T3 (p<0.027). Effect sizes accompanying the significant differences were moderate to large, ranging from 0.55 to 0.90. Scores on the AFI Interpersonal Effectiveness subscale, although favoring the MBSR group, were not significantly different between groups at any time point. Notably, the MBSR group’s mean scores on the AFI total rose to approximately 65 at T2 and T3, indicating that participants rose from low to moderate cognitive functioning based on the instrument’s reported threshold scores. In addition, AFI Attentional Lapses and Interpersonal Effectiveness rose to just above a score of 65, which was the mean AFI score reported by Cimprich and colleagues in a large validation sample of breast cancer survivors [32]. In contrast, the ES group’s mean scores remained below 65 at every time point on all AFI outcomes.
Table 3.
Between-group analysis for attentional function index total score and subscales
| Dependent variables | MBSR n=35a |
ES n=36b |
Diff | SE diff | p value | Pooled SD | Effect size (d) | 95 % CI effect size |
|---|---|---|---|---|---|---|---|---|
| Baseline (T1) | ||||||||
| AFI total (α=0.87) | 48.00 | 45.83 | 1.73 | 3.84 | 0.65 | 15.59 | 0.11 | −0.37, 0.59 |
| Effective action (α=0.83) | 44.88 | 43.05 | 1.86 | 4.23 | 0.66 | 17.17 | 0.11 | −0.37, 0.59 |
| Attentional lapses (α=0.64) | 51.71 | 47.27 | 3.61 | 4.65 | 0.44 | 18.96 | 0.19 | −0.29, 0.67 |
| Interpersonal effectiveness (α=0.78) | 51.56 | 51.17 | −0.72 | 5.73 | 0.90 | 22.86 | −0.03 | −0.52, 0.46 |
| Post-intervention (T2) | ||||||||
| AFI total (α=0.88) | 64.64 | 52.28 | 12.57 | 3.54 | 0.001 | 15.15 | 0.83 | 0.37, 1.29 |
| Effective action (α=0.86) | 63.16 | 50.09 | 12.84 | 4.28 | 0.004 | 17.78 | 0.72 | 0.25, 1.19 |
| Attentional lapses (α=0.80) | 67.30 | 50.20 | 17.99 | 4.76 | <0.001 | 20.07 | 0.90 | 0.43, 1.36 |
| Interpersonal effectiveness (α=0.78) | 65.54 | 59.50 | 6.99 | 4.26 | 0.106 | 21.02 | 0.33 | −0.06, 0.73 |
| 6-month follow-up (T3) | ||||||||
| AFI total (α=0.90) | 64.83 | 55.21 | 8.90 | 3.75 | 0.021 | 16.16 | 0.55 | 0.10, 1.01 |
| Effective action (α=0.88) | 62.74 | 52.53 | 10.45 | 4.61 | 0.027 | 18.84 | 0.55 | 0.08, 1.03 |
| Attentional lapses (α=0.80) | 68.13 | 55.68 | 11.84 | 4.82 | 0.017 | 19.98 | 0.59 | 0.12, 1.07 |
| Interpersonal effectiveness (α=0.75) | 66.37 | 61.24 | 3.47 | 4.40 | 0.433 | 19.44 | 0.18 | −0.26, 0.62 |
Sample size for MBSR group was 35 at baseline, 34 post-intervention, and 33 at 6 months post-intervention
Sample size for education/support was 36 at baseline, 35 post-intervention, and 35 at 6 months post-intervention
MBSR mindfulness-based stress reduction, ES education/support, AFI Attentional Function Index (range 0–100), SE standard error, SD standard deviation, ES Cohen’s d effect size, CI confidence interval, α Cronbach’s alpha
Within-group effects
Both groups experienced statistically significant improvements in cognitive function over time, with effect sizes ranging from 0.40 to 0.96; however, MBSR participants tended to experience larger gains (Table 4). Specifically, the MBSR group exhibited significant improvements at T2 on all AFI subscales, and these improvements were durable, with mean scores remaining stable from T2 to T3. In contrast, by T2, the ES group had improved on only the AFI total and Interpersonal Effectiveness subscale, although gains on the Effective Action subscale approached significance. However, the ES group showed significant improvements on all subscales by T3. Unlike the MBSR group, the ES group continued to show some improvement in cognitive function at T3, although these gains were not sufficient to raise ES participants to the same levels experienced by MBSR participants.
Table 4.
Within-group analysis for attentional function index total score and subscales
| MBSR n=35a |
ES n=36b |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | (SD) | p value | SRM | (95 % CI) | Mean | (SD) | p value | SRM | (95 % CI) | |
| AFI total | ||||||||||
| T1 | 48.00 | 17.09 | 45.83 | 13.99 | ||||||
| T2 | 64.64 | 15.39 | <0.0001 | 0.93 | (0.58,1.28) | 52.28 | 14.90 | 0.0227 | 0.40 | (0.06, 0.75) |
| T3 | 64.83 | 14.48 | <0.0001 | 0.87 | (0.51, 1.22) | 55.21 | 17.59 | 0.0015 | 0.58 | (0.24, 0.93) |
| Effective action | ||||||||||
| T1 | 44.88 | 18.70 | 43.05 | 15.54 | ||||||
| T2 | 63.16 | 17.57 | <0.0001 | 0.96 | (0.61, 1.31) | 50.09 | 17.98 | 0.0578 | 0.33 | (−0.01, 0.68) |
| T3 | 62.74 | 17.42 | <0.0001 | 0.82 | (0.46, 1.17) | 52.53 | 20.09 | 0.0171 | 0.42 | (0.08, 0.77) |
| Attentional lapses | ||||||||||
| T1 | 51.71 | 19.61 | 47.27 | 18.30 | ||||||
| T2 | 67.30 | 19.09 | 0.0004 | 0.68 | (0.33, 1.03) | 50.20 | 20.98 | 0.3762 | 0.15 | (−0.19, 0.50) |
| T3 | 68.13 | 17.77 | 0.0018 | 0.59 | (0.24, 0.95) | 55.68 | 21.85 | 0.0067 | 0.49 | (0.14, 0.83) |
| Interpersonal effectiveness | ||||||||||
| T1 | 51.56 | 25.70 | 51.17 | 19.71 | ||||||
| T2 | 65.54 | 22.87 | 0.0011 | 0.62 | (0.27, 0.96) | 59.50 | 19.05 | 0.0153 | 0.43 | (0.09, 0.78) |
| T3 | 66.37 | 18.44 | 0.0025 | 0.57 | (0.22, 0.93) | 61.24 | 20.34 | 0.0051 | 0.51 | (0.16, 0.85) |
Sample size for MBSR group was 35 at baseline, 34 post-intervention, and 33 at 6 months post-intervention
Sample size for education/support was 36 at baseline, 35 post-intervention, and 35 at 6 months post-intervention
MBSR mindfulness-based stress reduction, ES education/support, AFI Attentional Function Index (range 0–100), SD standard deviation, SRM standardized response mean, CI confidence interval, T1 baseline, T2 immediately post intervention, T3 6-months post-intervention
Stroop test
Between-group effects
No differences emerged between MBSR and ES participants at T2 or T3 (all p>0.64) on Stroop interference scores. Table 5 shows the between-group effects on the Stroop test for accuracy rates. Compared to the ES group, the MBSR group made fewer errors on the incongruent trials relative to errors on the congruent trials at T2 and T3. The groups did not differ at T1, suggesting that the differences at T2 and T3 were due to the effects of MBSR.
Table 5.
Within-group and between-group effects on stroop accuracy rates
| Within-group effectsa |
Between-group effectsb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MBSR n=35c |
ES n=36d |
|||||||||
| Stroop accuracy | mean | (SD) | p value | r | Mean | (SD) | p value | r | p value | r |
| T1 | −0.10 | 0.25 | −0.08 | 0.19 | 0.990 | 0.002 | ||||
| T2 | −0.02 | 0.05 | 0.171 | 0.25 | −0.11 | 0.24 | 0.914 | 0.020 | 0.005 | 0.340 |
| T3 | −0.01 | 0.03 | 0.034 | 0.39 | −0.07 | 0.19 | 0.986 | 0.003 | 0.030 | 0.280 |
Wilcoxon signed rank test used for within-group effects
Wilcoxon rank sum test used for between-group tests
Sample size for MBSR group was 33 at baseline, 32 post-intervention, and 30 at 6 months post-intervention
Sample size for education/support was 36 at baseline, 33 post-intervention, and 30 at 6 months post-intervention
MBSR mindfulness-based stress reduction, ES education/support, SD standard deviation, r effect size for Wilcoxon tests, T1 baseline, T2 immediately post-intervention, T3 6-months post-intervention
Within-group effects
Examining the within-group effects revealed that the error rates of the ES group did not differ over time (all p>0.90). Although the MBSR group did not differ between T1 and T2 (p=0.17), MBSR participants had a lower error rate at T3 (MT3=−0.01, SDT3=0.03) relative to T1 (MT1=−0.10, SDT1=0.25; z=−2.12, n=29, p=0.034, r=0.39), indicating improvement over time (Table 5).
Mediational analysis
For AFI total score at T2, changes on FFMQ acting with awareness subscale were identified as a significant mediator. The indirect effect was 1.9544 and the 95 % confidence interval ranged from .1997 to 4.8845 indicating that relative to ES, MBSR exerted its salutary effects in part by increasing participants’ ability to act with awareness. At T3, the same analytic approach revealed three significant mediators: changes on FFMQ acting with awareness (indirect effect=4.1528; 95 % confidence interval (CI) [1.1309, 9.0138]), observing (indirect effect=2.0945, 95 % CI [0.2046, 5.7429]), and non-reactivity to internal experience subscales (indirect effect=3.1655, 95 % CI [0.5692,7.8012]). Over the long run, MBSR seems to have exerted at least some of its positive effects via improving each of these mindfulness-related abilities. When the same analyses were conducted using Stroop accuracy instead of AFI total as the outcome variable, no significant mediators were identified.
Discussion
To our knowledge, this is the first randomized clinical trial to test the effects of MBSR compared to an active control group that included both subjective and objective measures of cognitive impairment in cancer survivors. These exploratory findings suggest that MBSR may offer an efficacious and durable means to relieve CRCI, one of the most pervasive and disruptive sequelae of cancer. In terms of the subjective outcomes assessed by the AFI, MBSR participants experienced significant improvement over time on the AFI total score, as well as the Effective Action and Attentional Lapses subscales. These significantly exceeded improvements experienced by the ES group, often to a substantial degree as indicated by large to moderate effect sizes at T2 and T3. Notably, the MBSR group’s improvements were durable, lasting at least 6 months after the intervention. A similar pattern emerged with respect to the objective outcomes assessed by the Stroop test. Although their reaction time did not improve relative to ES participants, MBSR participants made significantly fewer errors at both T2 and T3, effects that were small to moderate in magnitude, and their accuracy rate increased over time, whereas the accuracy rate of ES participants did not improve. The general convergence across both types of cognitive measures supports the potential of MBSR as an intervention to relieve CRCI.
The primary aim of the parent study from which these data were drawn was to assess the effects of MBSR compared to ES on fatigue, with improved cognitive outcomes as a secondary aim. Notably, mean AFI total scores for participants in our trial were <50 for both groups at baseline, suggesting low cognitive functioning [42]. The MBSR group experienced significant improvements on cognitive outcomes at T2 and T3, and these were more robust than the effects on fatigue interference, which tended to favor the MBSR group only at T2 (d=−0.46, p=0.073). These results are different than those reported in a recent systematic review of the effects of interventions on fatigue outcomes among cancer patients; decreases in mental fatigue, a construct related to CRCI, occurred less frequently and less robustly than reductions in other dimensions of fatigue [43]. Notably, none of the included studies in the review involved mindfulness-based interventions.
To date, studies examining the effects of MBSR on cognitive outcomes among post-treatment cancer survivors have yielded mixed results. Three randomized trials of MBSR in cancer have included a patient-reported cognitive outcome; however, all of these trials used a wait-list control design. Whereas two of the trials reported significant effects favoring MBSR on confusion [19, 24], one reported non-significant effects on trouble remembering [44]. In contrast, the current study compared MBSR to an active control on attentional and executive functions and found significant and long-term effects on both measures. Although further research is needed, it is possible that MBSR has differential effects on different aspects of cognitive functioning. Although we found no significant mediators to explain the effects of mindfulness on an objective measure of executive functioning in our trial, mindfully acting with awareness, observing, and approaching internal experiences with non-reactivity mediated the effects of MBSR on improvement in patient-reported cognitive functioning. These results are consistent with research suggesting that focused attention (e.g., observing) and open monitoring (e.g., non-reactivity) are mechanisms of mindfulness. Cancer survivors with a mindfulness practice may be able to cope with common and poorly addressed symptoms (e.g., fatigue and cognitive impairment) and other survivorship challenges (e.g., fear of recurrence) with greater ease.
Several limitations of the current study should be noted. Because this pilot investigation was designed to assess the impact of MBSR on fatigue and related outcomes, the findings of this secondary analysis on cognitive outcomes should be viewed as exploratory and warranting further study. Participants were enrolled on the basis of fatigue, not CRCI. Mental fatigue often co-occurs with physical fatigue, and in keeping with prior research, a large majority of participants reported cognitive impairment, as indicated by low AFI scores at baseline. In fact, the mean AFI total score of the current sample at baseline was approximately 1 standard deviation lower than the mean AFI total score reported by Cimprich et al. [32] in a validation sample of women newly diagnosed with early-stage breast cancer. Notably, the intervention effect on self-reported cognitive improvement could have been mediated by a variety of factors, including improved fatigue or other behavioral confounds. Further research is needed to determine if MBSR has similar benefits for fatigued and non-fatigued survivors with cancer-related cognitive impairment. Another concern is that the current sample was composed of participants with only two types of cancer, breast and colorectal, which limits generalizability, as does the approximately 50 % participation rate. However, generalizability concerns are somewhat offset by the fact that the sample was diverse in other ways: About 30 % of participants were African-American or Hispanic, about half did not have a college degree, and about half reported having an income that was less than comfortable. Additionally, only two cognitive measures were used to avoid overtaxing fatigued cancer survivors. Although these measures included both subjective and objective assessments, a greater variety of methods would have provided better insight into the impact of MBSR on cognitive function. Finally, because we analyzed T2 and T3 as separate time points in an ANCOVA model, due to T2 and T3 being unique occasions, inflated family-wise type I error is a limitation. Because this was a pilot study with a modest sample size (n=71) comparing non-invasive interventions with few side effects, we placed slightly greater importance on reducing type II error over controlling family-wise type I error and higher priority on using a simpler model that could handle small sample sizes rather than a more complex statistical model, such as a mixed linear repeated measures model.
Clinical implications
Although CRCI is a potentially debilitating symptom for cancer survivors, few intervention trials have been conducted to address this symptom [13]. The newly released Survivorship Guidelines from the National Comprehensive Cancer Network (NCCN) recommend stress reduction and optimized management of other symptoms, including fatigue, pain, depression, and sleep disturbance, as general strategies to address CRCI [45]; however, no further information is provided. Findings from this study suggest that MBSR, with further testing, could be an efficacious and cost-effective option to address CRCI among cancer survivors with fatigue. MBSR participants reported high engagement in the intervention, as evidenced by high rates of self-reported home practice of mindfulness during the intervention, and the majority of participants still practiced mindfulness through the 6-month follow-up. Moreover, retention rates in the trial exceeded 95 %, suggesting that MBSR is feasible in clinical settings.
Conclusion
These exploratory findings suggest that MBSR may have positive effects on cognitive outcomes in cancer survivors with fatigue, and further investigation is warranted. Although MBSR has long been shown to improve well-being [19] and symptoms, such as fatigue [19–21, 26], depression, and anxiety [19–22, 46], few studies have examined its role with CRCI. This study provides initial evidence regarding MBSR’s acceptability and preliminary efficacy in addressing CRCI for cancer survivors.
Acknowledgments
The authors thank the participants for their active engagement in this clinical trial and encourage their continued health and wellness.
Compliance with ethical standards
Funding Research reported in this publication was supported by the Walther Cancer Foundation (0106–01), Indiana Clinical and Translational Sciences Institute (Grant # TR000163 and # TR000006), and the National Cancer Institute of the National Institutes of Health under Award Number K05CA175048. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Conflict of interest The authors have no conflict of interest to report.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study
References
- 1.Bender CM, Thelen BD. Cancer and cognitive changes: the complexity of the problem. Semin Oncol Nurs. 2013;29(4):232–237. doi: 10.1016/j.soncn.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Vardy J, Rourke S, Tannock IF. Evaluation of cognitive function associated with chemotherapy: a review of published studies and recommendations for future research. J Clin Oncol. 2007;25(17):2455–2463. doi: 10.1200/JCO.2006.08.1604. [DOI] [PubMed] [Google Scholar]
- 3.Wefel JS, Schagen SB. Chemotherapy-related cognitive dysfunction. Curr Neurol Neurosci Rep. 2012;12(3):267–275. doi: 10.1007/s11910-012-0264-9. [DOI] [PubMed] [Google Scholar]
- 4.Cruzado JA, Lopez-Santiago S, Martinez-Marin V, Jose-Moreno G, Custodio AB, Feliu J. Longitudinal study of cognitive dysfunctions induced by adjuvant chemotherapy in colon cancer patients. Support Care Cancer. 2014;22(7):1815–1823. doi: 10.1007/s00520-014-2147-x. [DOI] [PubMed] [Google Scholar]
- 5.Hodgson KD, Hutchinson AD, Wilson CJ, Nettelbeck T. A meta-analysis of the effects of chemotherapy oncognition in patients with cancer. Cancer Treat Rev. 2013;39(3):297–304. doi: 10.1016/j.ctrv.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Lindner OC, Phillips B, McCabe MG, et al. A meta-analysis of cognitive impairment following adult cancer chemotherapy. Neuropsychology. 2014;28(5):726–740. doi: 10.1037/neu0000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bender CM, Ergyn FS, Rosenzweig MQ, Cohen SM, Sereika SM. Symptom clusters in breast cancer across 3 phases of the disease. Cancer Nurs. 2005;28(3):219–225. doi: 10.1097/00002820-200505000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Koppelmans V, Breteler MM, Boogerd W, Seynaeve C, Gundy C, Schagen SB. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J Clin Oncol. 2012;30(10):1080–1086. doi: 10.1200/JCO.2011.37.0189. [DOI] [PubMed] [Google Scholar]
- 9.Von Ah D, Habermann B, Carpenter J, Schneider B. Impact of perceived cognitive impairment in breast cancer survivors. Eur J Oncol Nurs. 2013;17(2):236–241. doi: 10.1016/j.ejon.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Myers JS. Cancer- and chemotherapy-related cognitive changes: the patient experience. Semin Oncol Nurs. 2013;29(4):300–307. doi: 10.1016/j.soncn.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Feuerstein M, Hansen JA, Calvio LC, Johnson L, Ronquillo JG. Work productivity in brain tumor survivors. J Occup Environ Med. 2007;49(7):803–811. doi: 10.1097/JOM.0b013e318095a458. [DOI] [PubMed] [Google Scholar]
- 12.Von Ah D, Russell KM, Storniolo AM, Carpenter JS. Cognitive dysfunction and its relationship to quality of life in breast cancer survivors. Oncol Nurs Forum. 2009;36(3):326–336. doi: 10.1188/09.ONF.326-334. [DOI] [PubMed] [Google Scholar]
- 13.Von Ah D, Jansen CE, Allen DH. Evidence-based interventions for cancer- and treatment-related cognitive impairment: putting evidence into practice. Clin J Oncol Nurs. 2014;18(Suppl):17–25. doi: 10.1188/14.CJON.S3.17-25. [DOI] [PubMed] [Google Scholar]
- 14.Cullen M. Mindfulness-based interventions: an emerging phenomenon. Mindfulness. 2011;2(3):186–193. [Google Scholar]
- 15.Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends Cogn Sci. 2008;12(4):163–169. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carmody J. Evolving conceptions of mindfulness in clinical settings. J Cogn Psychother. 2009;23(3):270–280. [Google Scholar]
- 17.Gu J, Strauss C, Bond R, Cavanagh K. How do mindfulness-based cognitive therapy and mindfulness-based stress reduction improve mental health and wellbeing? a systematic review and meta-analysis of mediation studies. Clin Psychol Rev. 2015;37:1–12. doi: 10.1016/j.cpr.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Chiesa A, Calati R, Serretti A. Does mindfulness training improve cognitive abilities? a systematic review of neuropsychological findings. Clin Psychol Rev. 2011;31(3):449–464. doi: 10.1016/j.cpr.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman CJ, Ersser SJ, Hopkinson JB, Nicholls PG, Harrington JE, Thomas PW. Effectiveness of mindfulness-based stress reduction in mood, breast- and endocrine-related quality of life, and well-being in stage 0 to III breast cancer: a randomized, controlled trial. J Clin Oncol. 2012;30(12):1335–1342. doi: 10.1200/JCO.2010.34.0331. [DOI] [PubMed] [Google Scholar]
- 20.Lengacher CA, Johnson-Mallard V, Post-White J, et al. Randomized controlled trial of mindfulness-based stress reduction (MBSR) for survivors of breast cancer. Psychooncology. 2009;18(12):1261–1272. doi: 10.1002/pon.1529. [DOI] [PubMed] [Google Scholar]
- 21.Johns SA, Brown LF, Beck-Coon K, Monahan PO, Tong Y, Kroenke K. Randomized controlled pilot study of mindfulness-based stress reduction for persistently fatigued cancer survivors. Psychooncology. 2014 doi: 10.1002/pon.3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zainal NZ, Booth S, Huppert FA. The efficacy of mindfulness-based stress reduction on mental health of breast cancer patients: a meta-analysis. Psychooncology. 2013;22(7):1457–1465. doi: 10.1002/pon.3171. [DOI] [PubMed] [Google Scholar]
- 23.Von Ah D, Storey S, Jansen C, Allen D. Coping strategies and interventions for cognitive changes associated with cancer and cancer therapy. Semin Oncol Nurs. 2013;29(4):288–299. doi: 10.1016/j.soncn.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Speca M, Carlson LE, Goodey E, Angen M. A randomized, waitlist controlled clinical trial: the effect of a mindfulness meditation-based stress reduction program on mood and symptoms of stress in cancer outpatients. Psychosom Med. 2000;62(5):613–622. doi: 10.1097/00006842-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Carlson LE, Ursuliak Z, Goodey E, Angen M, Speca M. The effects of a mindfulness meditation-based stress reduction program on mood and symptoms of stress in cancer outpatients: 6-month follow-up. Support Care Cancer. 2001;9(2):112–123. doi: 10.1007/s005200000206. [DOI] [PubMed] [Google Scholar]
- 26.Johns SA, Talib TL, Brown LF, et al. Transforming Cancer Survivorship Through Research and Best Practice. Cincinnati, OH: 2015. Randomized controlled trial of mindfulness-based stress reduction compared to education/support for persistently fatigued breast and colorectal cancer survivors. [Google Scholar]
- 27.Harrington CB, Hansen JA, Moskowitz M, Todd BL, Feuerstein M. It’s not over when it’s over: long-term symptoms in cancer survivors-a systematic review. Int J Psychiatry Med. 2010;40(2):163–181. doi: 10.2190/PM.40.2.c. [DOI] [PubMed] [Google Scholar]
- 28.Donovan KA, Jacobsen PB, Small BJ, Munster PN, Andrykowski MA. Identifying clinically meaningful fatigue with the fatigue symptom inventory. J Pain Symptom Manag. 2008;36(5):480–487. doi: 10.1016/j.jpainsymman.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kroenke K, Spitzer RL, Williams JBW. The patient health questionnaire-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santorelli S, Kabat-Zinn J. Mindfulness-based stress reduction professional education and training resource manual: MBSR standards of practice, curriculum, and supporting materials. Worcester: Center for Mindfulness in Medicine, Health Care, and Society, University of Massachusetts Medical School; 2011. [Google Scholar]
- 31.Hutchinson AD, Hosking JR, Kichenadasse G, Mattiske JK, Wilson C. Objective and subjective cognitive impairment following chemotherapy for cancer: a systematic review. Cancer Treat Rev. 2012;38(7):926–934. doi: 10.1016/j.ctrv.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Cimprich B, Visovatti M, Ronis DL. The attentional function index-a self-report cognitive measure. Psychooncology. 2011;20(2):194–202. doi: 10.1002/pon.1729. [DOI] [PubMed] [Google Scholar]
- 33.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–622. [Google Scholar]
- 34.MacLeod CM. The stroop task in cognitive research. In: Wenzel A, Rubin DC, editors. Cognitive methods and their application to clinical research. Washington, D. C: Merican Psychological Association; 2005. pp. 17–40. [Google Scholar]
- 35.Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13(1):27–45. doi: 10.1177/1073191105283504. [DOI] [PubMed] [Google Scholar]
- 36.Baer RA, Smith GT, Lykins E, et al. Construct validity of the five facet mindfulness questionnaire in meditating and nonmeditating samples. Assessment. 2008;15(3):329–342. doi: 10.1177/1073191107313003. [DOI] [PubMed] [Google Scholar]
- 37.Senn S. Testing for baseline balance in clinical trials. Stat Med. 1994;13(17):1715–1726. doi: 10.1002/sim.4780131703. [DOI] [PubMed] [Google Scholar]
- 38.Johnson SE, Richeson JA, Finkel EJ. Middle class and marginal? socioeconomic status, stigma, and self-regulation at an elite university. J Pers Soc Psychol. 2011;100(5):838–852. doi: 10.1037/a0021956. [DOI] [PubMed] [Google Scholar]
- 39.Richeson JA, Trawalter S. Why do interracial interactions impair executive function? a resource depletion account. J Pers Soc Psychol. 2005;88(6):934–947. doi: 10.1037/0022-3514.88.6.934. [DOI] [PubMed] [Google Scholar]
- 40.Josefsson T, Borberg A. Mediators and non-mediators on sustained and executive attentional performance. c. 2011;14(3):291–309. [Google Scholar]
- 41.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40(3):879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 42.Cimprich B, So H, Ronis DL, Trask C. Pre-treatment factors related to cognitive functioning in women newly diagnosed with breast cancer. Psychooncology. 2005;14(1):70–78. doi: 10.1002/pon.821. [DOI] [PubMed] [Google Scholar]
- 43.de Raaf PJ, de Klerk C, van der Rijt CCD. Elucidating the behavior of physical fatigue and mental fatigue in cancer patients: a review of the literature. Psycho-Oncology. 2013;22(9):1919–1929. doi: 10.1002/pon.3225. [DOI] [PubMed] [Google Scholar]
- 44.Lengacher C, Reich R, Post-White J, et al. Mindfulness based stress reduction in post-treatment breast cancer patients: an examination of symptoms and symptom clusters. J Behav Med. 2012;35(1):86–94. doi: 10.1007/s10865-011-9346-4. [DOI] [PubMed] [Google Scholar]
- 45.Denlinger CS. [Accessed 20 March 2015];National Comprehensive National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Survivorship, version 1.2015. 2015 http://www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf.
- 46.Henderson VP, Massion AO, Clemow L, Hurley TG, Druker S, Hebert JR. A randomized controlled trial of mindfulness-based stress reduction for women with early-stage breast cancer receiving radiotherapy. Integr Cancer Ther. 2013;12(5):404–413. doi: 10.1177/1534735412473640. [DOI] [PMC free article] [PubMed] [Google Scholar]