Abstract
Triple negative breast cancer (TNBC), characterized by an abundance of treatment-resistant breast cancer stem cells (CSCs), has a poorer prognosis than other types of breast cancers. Despite its aggressiveness, no effective targeted therapy exists for TNBC. Here, we demonstrate that CQ effectively targets CSCs via autophagy inhibition, mitochondrial structural damage, and impairment of double-stranded DNA break repair. Electron microscopy demonstrates CQ-induced mitochondrial cristae damage, which leads to mitochondrial membrane depolarization with a significant reduction in the activity of cytochrome c oxidase and accumulation of superoxide and double-stranded DNA breaks. CQ effectively diminishes the TNBC cells’ ability to metastasize in vitro and in a TNBC xenograft model. When administered in combination with carboplatin, CQ effectively inhibits carboplatin-induced autophagy. This combination treatment significantly diminishes the expression of DNA repair proteins in CSC subpopulations, resulting in tumor growth reduction in carboplatin-resistant BRCA1 wild-type TNBC orthotopic xenografts. As TNBC’s high treatment failure rate has been attributed to enrichment of CSCs, CQ, an autophagy inhibitor with anti-CSC effects, may be an effective adjunct to current TNBC chemotherapy regimens with carboplatin.
Keywords: breast cancer stem cells, chloroquine, mitochondrial damage, oxidative stress
1. Introduction
Triple negative breast cancer (TNBC), characterized by the absence of estrogen receptors, progesterone receptors, and human epidermal growth factor receptors, is known to be poorly differentiated on histology and is associated with lower rates of survival at each stage compared to other subtypes of breast cancers [1]. Unfortunately, the incidence of TNBC has increased in the last several decades [2]. TNBC occurs more frequently in younger women and grows more rapidly, making early mammographic detection more difficult [1, 3]. Furthermore, TNBC is more likely to have visceral metastasis, particularly to the lungs and brain [2, 3]. Despite its aggressiveness, there are limited targeted therapeutic options for TNBC.
Frequent local relapse and distant metastasis in TNBC may be due to an abundance of CD44+/CD24−/low cancer stem cells (CSCs) [4]. These tumorigenic CSCs are cells that display stem cell-like properties, including self-renewal and tumor initiation [5]. Since current chemotherapeutic agents are most effective at targeting rapidly proliferating cells, these slowly proliferating CSCs are likely responsible for treatment failures [4–6]. Enrichment of CSCs has been detected in residual breast cancer after endocrine-based therapy and chemotherapy, as well as in metastatic lesions [7, 8]. Therefore, developing effective therapies that specifically target treatment-resistant CSCs may prevent local recurrences and distant metastases, improving overall survival and outcomes in TNBC patients.
Previously, we reported that chloroquine (CQ), an autophagy inhibitor, eliminates CSCs via an epigenetic mechanism by altering DNA methylation [9]. However, it is hypothesized that CQ may also affect other pathways, resulting in anti-CSC effects, and thus provides synergy with chemotherapeutic agents. Other reports have indicated that CQ can induce severe oxidative stress in cancer cells via inhibition of autophagy [10–12]. In this study, we show that CQ causes mitochondrial damage, resulting in excessive oxidative DNA damage in TNBC CSCs and subsequent cell death. Furthermore, we investigated the therapeutic potential of CQ in combination with carboplatin, a platinum chemotherapeutic agent. The current standard of care for TNBC patients is anthracycline and/or taxane based chemotherapy [2]. With that therapeutic regimen, the pathological complete response rate in TNBC patients is estimated to be between 25 to 35% [2]. Clinical trials have been completed combining platinum-based agents with standard chemotherapy, which showed even higher rates of pathological complete response [13, 14]. However, better combination treatment is necessary to enhance the efficacy of platinum agents to prevent local relapse and distant metastases by targeting CSCs.
2. Materials and methods
2.1 Materials and Cell cultures
Three TNBC cell lines (Hs578t, MDA-MB-231, and SUM159) were purchased from the American Type Culture Collection (Manassas, VA, USA). All cells were maintained in Dulbecco’s Modified Eagle’s Medium (Invitrogen, Grand Island, NY) with 10% fetal bovine serum (Thermos Scientific Hyclone, Rockford, IL) in a humidified 5% CO2 incubator at 37°C. Chloroquine diphosphate salt (Sigma-Aldrich, St. Louis, MO) and carboplatin (Teva Pharmaceuticals, Sellersville, PA) were dissolved in cell culture-grade water at the concentration of 10 mM. CD marker antibodies (BD Biosciences, San Jose, California), MitoSOX Red Mitochondrial Superoxide Indicator (Thermo Fisher Scientific, Waltham, MA), Cell Proliferation Reagent WST-1 (Clontech, Mountain View, CA) were acquired. Cytochrome C, LC-3B, p62, NQO1, Rad50, PARP and β-actin antibodies were purchased from Cell Signaling Technology (Danvers, MA). Rad51, β-tubulin, and Tom20 antibodies were purchased from Santa Cruz Biotechnology Inc. Dallas, TX. γ-H2AX (EMD Millipore, Billerica, MA) and bcl-2 antibodies (BioLegend, San Diego, CA), were purchased from their respective companies.
2.2 Cancer stem cells assays
Fluorescence-activated cell sorting (FACS) analysis and mammosphere formation efficiency (MSFE) assays were performed, as described previously [9]. Cells stained with antibodies against CD44-APC and CD24-FITC were sorted, as previously described [15]. Isotype control antibodies were used to serve as negative controls. FACS sorting and analysis were performed at the Houston Methodist Research Institute flow cytometry core, using BD FACS Aria II and BD FACS Fortessa, respectively.
2.3 In vivo experiments
The Houston Methodist Hospital Research Institute Animal Care and Use Review Office approved all animal procedures in this study. In the metastatic model experiment, MDA-MB-231 cells expressing GFP tagged Luciferase (MDA-MB-231 G/L) were treated with CQ, and 200,000 of these pre-treated cells in 100 μl PBS were injected intravenously into each immunodeficient SCID-Beige mice. The mice were imaged as previously described [9], using Xenogen IVIS image station (PerkinElmer, Waltham, MA). Two hundred thousands untreated MDA-MB-231 G/L cells were injected into each mouse in the control group. In the orthotopic xenograft model, 1 million SUM159 cells in 100 μl PBS were injected into the left mammary fat pad. When the tumor volume reached approximately 250 mm3, mice were randomly assigned to groups and were treated with carboplatin 24 mg/kg weekly and CQ 30 mg/kg every 3 days for 3 weeks. Tumor volumes were measured twice a week at 3- to 4-day intervals.
2.4 Cell migration and invasion assays
Cultrex 96 Well Cell Migration Assay kit and Cultrex 96 Well BME Cell Invasion Assay kit were purchased from Trevigen, Gaithersburg, MD and were used for cell migration and cell invasion assays, respectively, following the manufacturer’s instructions. 30% FBS medium was used as chemoattractant. Migration was assessed via fluorescence measurement after 6 hours at 485nm excitation and 520 nm emission, using Tecan Infinite microplate reader. For the invasion assay, the chambers were coated with 0.1x BME solution, and invasion was assessed after 24 hours via fluorescence measurement.
2.5 Electron microscopy (EM)
Hs578t cells were prepared as previously described in the literature [9]. Samples stained with uranyl acetate and lead citrate were examined in a JEM 1010 transmission electron microscope (JEOL, USA, Inc., Peabody, MA), and the digital images were obtained using AMT Imaging System (Advanced Microscopy Techniques Corp, Danvers, MA).
2.6 JC-1 Mitochondrial membrane potential measurement
Untreated control cells and CQ-treated cells were incubated with 0.5 μM final concentration JC-1 dye (Cayman Chemical, Ann Arbor, MI) in 37°, 5% CO2 for 20 minutes. Analysis was performed by flow cytometry, using BD FACS Fortessa. Mitochondrial membrane potential was quantified by calculating JC-1 red to green fluorescence.
2.7 Electron transport chain (ETC) complex IV, cytochromc c oxidase activity analysis
SUM159 cells were sonicated and used for an ETC enzyme assay, using Tecan Infinite M200, as previously described [16]. ETC complex IV, cytochrome c oxidase activity measurements were normalized to the total cell protein levels.
2.8 Mitochondrial superoxide measurement
SUM159 cells were sorted into CSCs and non-CSCs using FACS, as above. Superoxide levels were measured by FACS after staining cells with 2.5μM MitoSOX Red indicator for 10 minutes.
2.9 Immunofluorescence staining for γ-H2AX foci detection
Control and CQ-treated SUM159 cells on a cover-slip bottom 13 mm dish (Cell E&G, Houston, TX) were fixed in 4% formalin solution. After blocking with 10% FBS in DMEM, samples were stained with 1:300 monoclonal mouse γ-H2AX antibody at room temperature for 1 hour and washed in PBS with 0.1% Tween-20. Anti-mouse antibodies-Alexa 488 (Invitrogen, Grand Island, NY) was used as the secondary antibody, diluted 1:1000 in blocking solution. Hoechst 33342 was layered on top of the cells, and cells were imaged using a confocal microscope (Olympus FluoView FV1000, Olympus USA, Center Valley, PA). The positive γ-H2AX foci per nucleus were quantified using ImageJ software.
2.10 WST-1 cell viability measurement
10% WST-1 reagent was added to all groups of cells after treatment with CQ and/or carboplatin, and absorbance of WST-1 reagent was measured with a Tecan Infinite microplate reader at 570nm, to determine the mitochondrial metabolic activity levels.
2.11 Trypan blue viability assay
After 48 hour treatment with CQ and/or carboplatin, cells were harvested by trypsinization, stained with 0.4% trypan blue solution (Invitrogen), and counted using a Countess automated cell counter (Invitrogen).
2.12 Western blot
Cells were lysed in a lysis buffer (1.5% Triton X-100 and 10% glycerol in DPBS) with a proteinase and phosphatase inhibitor cocktail (Thermo Scientific Pierce Protein Biology, Rockford, IL). Mitochondria fractionation was carried out using Mitochondria Insolation Kit for Mammalian Cells (Thermo Scientific, Rockford, IL), using manufacturer’s instructions. Then, western blotting experiments were performed with the listed primary antibodies as described previously [17].
2.13 Statistical analysis
Statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC). A two sample t-test (Satterthwaite or pooled, as appropriate) was used to investigate difference in means between two independent groups. One-way analysis of variance (ANOVA) was used to examine differences among multiple groups, with the Tukey-Kramer single-step multiple comparison procedure used for all ad hoc pairwise comparisons between treatment group means. For in vivo experiments, the treatment group differences were assessed using a generalized linear mixed-effects model which included fixed main effects for treatment group, time period, and the interaction of main effects as well as a random subject effect with a heterogeneous first-order autoregressive variance-covariance structure to account for intra-animal correlation over time and the variance heterogeneity observed between treatments. Time point specific treatment differences were investigated by partitioning the interaction by time to obtain the F-tests of the simple effects. The simple effects p-values were adjusted for multiplicity using Hommel’s method. P-values less than 0.05 were considered statistically significant.
3. Results
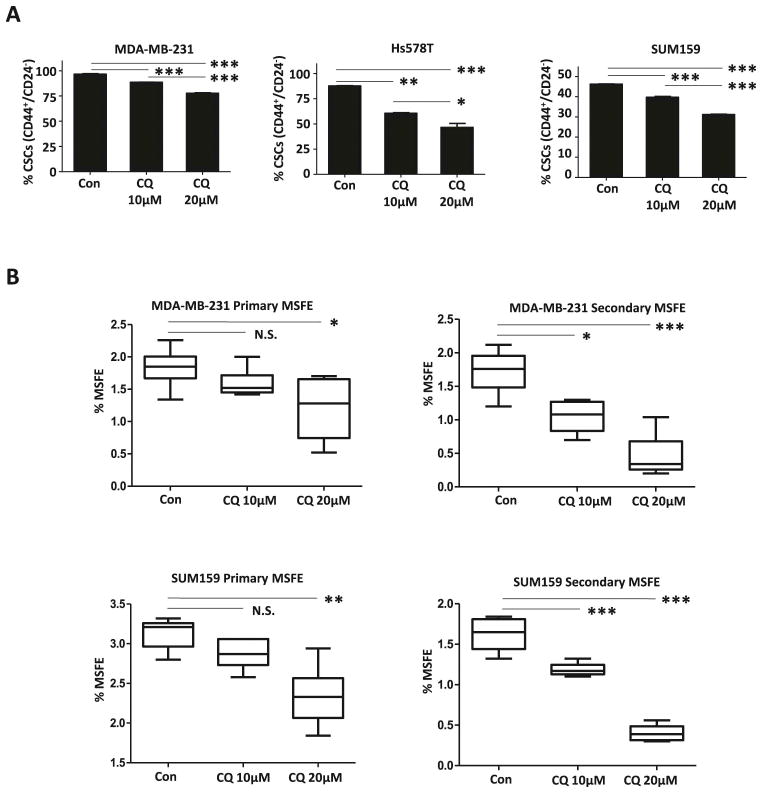
3.1 CQ reduces the population size of CD44+/CD24−/low CSCs and MSFE in TNBC cell lines
Although there was a difference in sensitivities in the MDA-MB-231, HS578t, and SUM159 cell lines, a significant reduction in the number of CD44+/CD24−/low CSCs was observed in all TNBC cells treated with CQ in a dose-dependent manner (Fig. 1A). The Hs578t cell line exhibited the greatest sensitivity CQ, with a reduction in its CSC population from 87.63±0.47% to 60.57±0.99% with 10μM CQ and 46.53±6.79% with 20μM CQ. With 10μM CQ treatment, there was no significant reduction in primary MSFE; however, it reduced secondary MSFE from 1.72±0.31% to 1.05±0.24% in the MDA-MB-231 cell line and from 1.62±0.20% to 1.19±0.08% in the SUM159 cell line (Fig. 1B). When treated with 20μM CQ, there was a significant reduction in both primary and secondary MSFE.
Figure 1.
(A) There is a significant reduction in the CD44+/CD24−/low CSC population of 3 TNBC cell lines following CQ treatment, in a dose-dependent manner. (B) CQ reduced primary and secondary MSFE in a dose-dependent manner. One-way ANOVA was used for statistical analysis. This figure represents the mean difference in CSC population with standard deviation of three technical repeats and the mean difference in MSFE with max and min of six replicates. These effects were confirmed in independently repeated experiments. N.S. indicates not significant. * indicates p<0.05. ** indicates p<0.001. *** indicates p<0.0001).
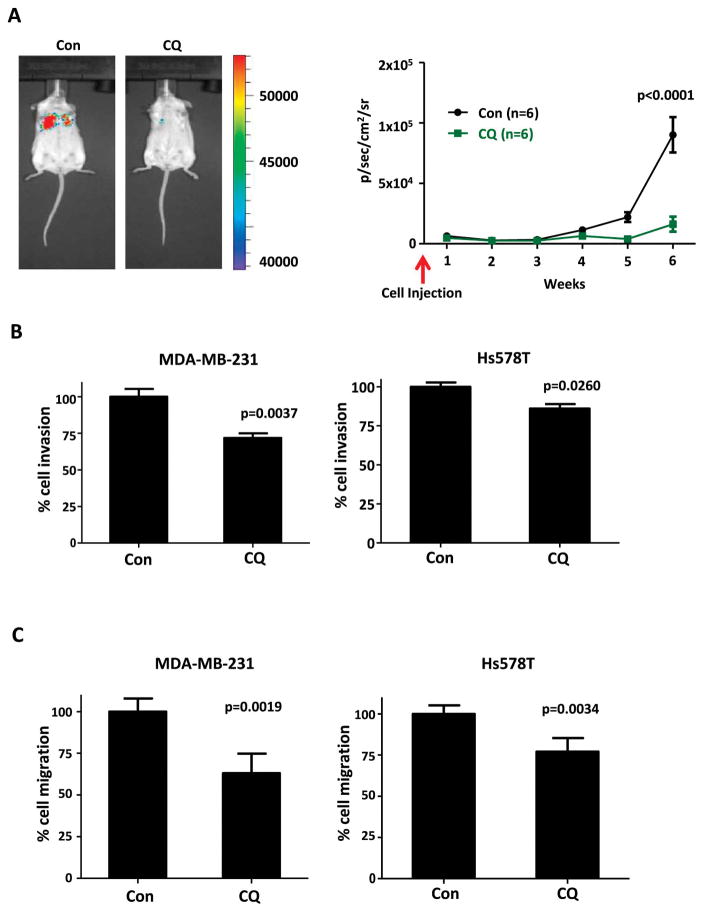
3.2 By targeting CSCs, CQ reduces the metastatic potential of TNBC
With evidence that CQ effectively targets CSCs, we investigated the metastatic potential of MDA-MB-231 G/L treated with CQ. In vivo, CQ effectively eliminated the intravenously injected MDA-MB-231 G/L cells’ ability to initiate distant metastatic tumor formation in lungs (Fig. 2A). Distant metastasis is a problem often seen in TNBC patients even after an initial successful response to treatment. CQ’s ability to suppress the invasiveness of the TNBC cells was further confirmed via an in vitro Boyden chamber with 8μm pores. As exhibited in Fig. 2B, CQ successfully hindered the invasion of MDA-MB-231 cells by 28.15± 8.65% (p=0.0037) and HS578t cells by 13.97±4.96% (p=0.0260). Furthermore, CQ impaired the migratory capacity of MDA-MB-231 cells and HS578t cells by 36.94±9.93% (p=0.0019) and 22.99±6.93% (p=0.0034), respectively, when compared to the untreated control group (Fig. 2C).
Figure 2.
(A) Pre-treatment with CQ inhibited the metastatic potential of MDA-MB-231 G/L cells injected intravenously (n=6 per group). Total photon counts were converted to log10.. As Satterthwaite’s method demonstrated significant interaction effect (p<0.0001), the differences in the total photon counts between the control group and CQ-treated group were investigated by the F-tests. F-tests with p-values adjusted for multiplicity using Hommel’s method indicated significantly reduced lung metastatic burden in CQ-treated group at week 4 (p=0.0096), week 5 (p=0.0002), and week 6 (p<0.0001). (B) CQ treated MDA-MB-231 and HS578t cells had decreased ability to invade in vitro. (C) CQ treated MDA-MB-231 and HS578t cells had decreased migratory capacity in vitro. (C and D) Data shown is mean normalized to untreated control group with standard deviation obtained from three independent experiments. Pooled two independent sample t-test was used for statistical analysis.
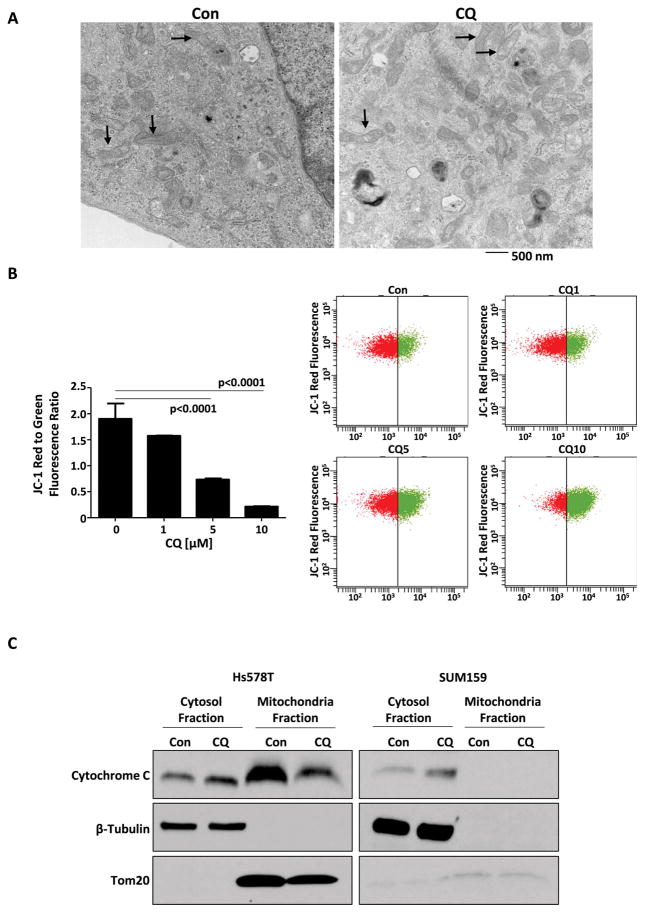
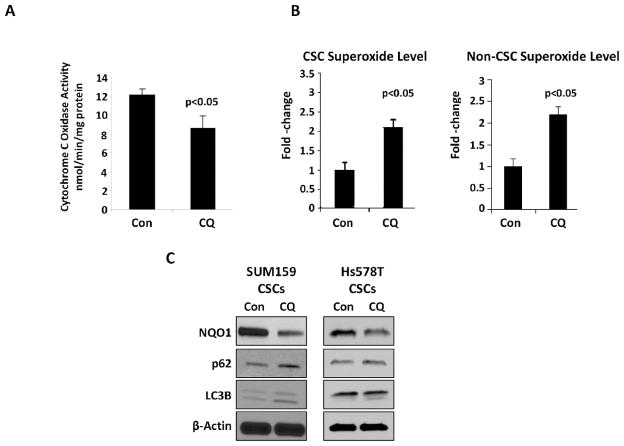
3.3 CQ induces mitochondrial structural damage, resulting in impaired activity of cytochrome c oxidase and increased cellular oxidative stress
When compared to non-stem cell population, CSCs have been shown to have different mitochondrial metabolic states, with increased number of mitochondria and greater ATP content, suggesting higher oxygen consumption rates [18]. In addition, CSCs’ quiescent state has been associated with autophagy [18]. Therefore, we investigated CQ’s effect on mitochondrial activity. CQ’s effects on the ultramicroscopic features of TNBC cells’ mitochondria were evident on electron microscopy. In untreated cells, mitochondrial architecture remained intact. In cells treated with CQ, disruption of mitochondrial cristae resulted in vacuolization within mitochondria (Fig. 3A). This mitochondrial injury resulted in mitochondrial membrane depolarization, in a dose dependent manner, as measured by JC-1 red to green fluorescence ratio (Fig. 3B) and release of cytochrome C from mitochondria into cytosol (Fig. 3C). Furthermore, CQ treatment led to a reduction in the activity of cytochrome c oxidase, the terminal enzyme of the mitochondrial ETC (Fig. 4A). Cytochrome c oxidase is responsible for the transfer of electrons from cytochrome c to oxygen, and its inhibition is known to result in increased levels of superoxide [19, 20]. Therefore, MitoSOX Red indicator, a highly selective detector of mitochondrial superoxide, was used to quantify superoxide levels. TNBC cells treated with CQ for 48 hours had a marked increase in mitochondrial superoxide level, when compared to the untreated control group (Fig. 4B), in both CSC and non-CSC populations. Oxidative stress from increased superoxide level in CSCs was closely associated with inhibition of autophagy by CQ and a decrease in NAD(P)H:quinone oxidoreductase 1 (NQO1), a superoxide scavenging protein (Fig. 4C). High level of NQO1 is known to correlate with aggressiveness of breast cancer with worse prognoses in patients [21].
Figure 3.
(A) EM stained with uranyl acetate and lead citrate shows intact mitochondrial structures in an untreated Hs578t TNBC cell. There is visible vacuolization of cristae, indicating structural mitochondrial damage in Hs578t TNBC cell treated with CQ. Arrow indicates mitochondria. (B) CQ treatment led to a dose-dependent depolarization of mitochondrial membrane, as indicated by reduction in JC-1 red fluorescence to JC-1 green fluorescence ratio. (C) Cytochrome c levels in the cytosolic and mitochondrial fractions of HS578t and SUM159 were analyzed by Western blot analysis. β-Tubulin and Tom20 were used as cytoplasm and mitochondria internal control, respectively.
Figure 4.
(A) With CQ treatment, there is a decrease in the activity of mitochondrial ETC complex IV, cytochrome c oxidase in SUM159 cells. (B) Increased superoxide in both CSC and non-CSC populations with CQ treatment in SUM159 cells correlated with impaired mitochondrial function. (C) Western blotting was performed from lysates of SUM159 and HS578t CSCs after CQ treatment. Up-regulation of LC3B-II and p62 confirmed autophagy inhibition by CQ. CQ treatment reduced the expression of superoxide scavenging protein, NQO1. β-actin was used as loading control.
3.4 CQ results in accumulation of γ-H2AX, a marker of double-stranded DNA damage
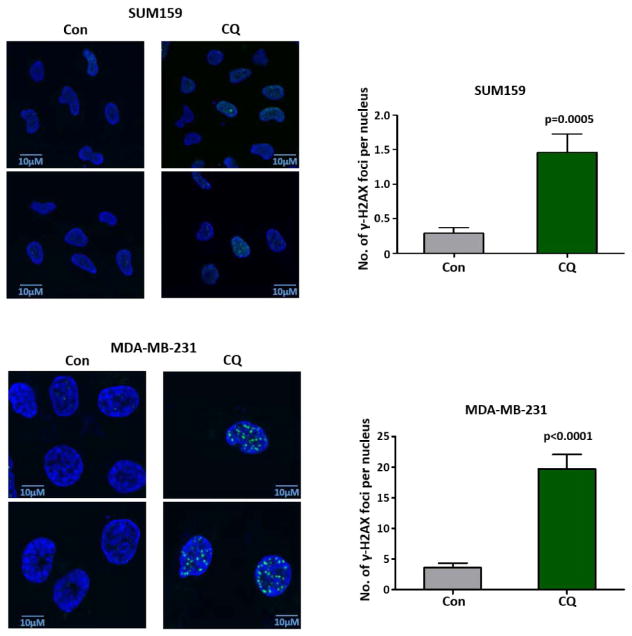
CQ is a well-known DNA intercalating drug that can unwind the DNA double helix, altering the conformation of DNA [22, 23]. Therefore, we hypothesized that with its oxidative stress-inducing property, CQ can induce DNA damage. We detected γ-H2AX by immunofluorescence in CQ treated cells. Because one of the most well recognized processes of DNA-damage responses is the phosphorylation of the SQ motif in H2AX histone, γ-H2AX is an excellent marker of double-stranded DNA damage [24, 25]. The number of γ-H2AX foci per nucleus was significantly higher in SUM159 and MDA-MB-231 cells treated with CQ, when compared with untreated cells (Fig. 5).
Figure 5.
SUM159 and MDA-MB-231 cells were stained against γH2AX after 48 hour treatment with 10 μM CQ. Negative control (not shown) was stained with secondary antibody alone without primary antibody against γH2AX. All images were taken using an oil-immersion 100x objective lens with 2.5 optical zoom and the same light exposure. γH2AX foci was counted using ImageJ. Based on a two-sided Fisher’s exact test, the difference in the distribution of foci per nucleus is significantly different between control and CQ-treated groups.
3.5 CQ inhibits tumor growth in vivo in combination with carboplatin in BRCA wild-type TNBC
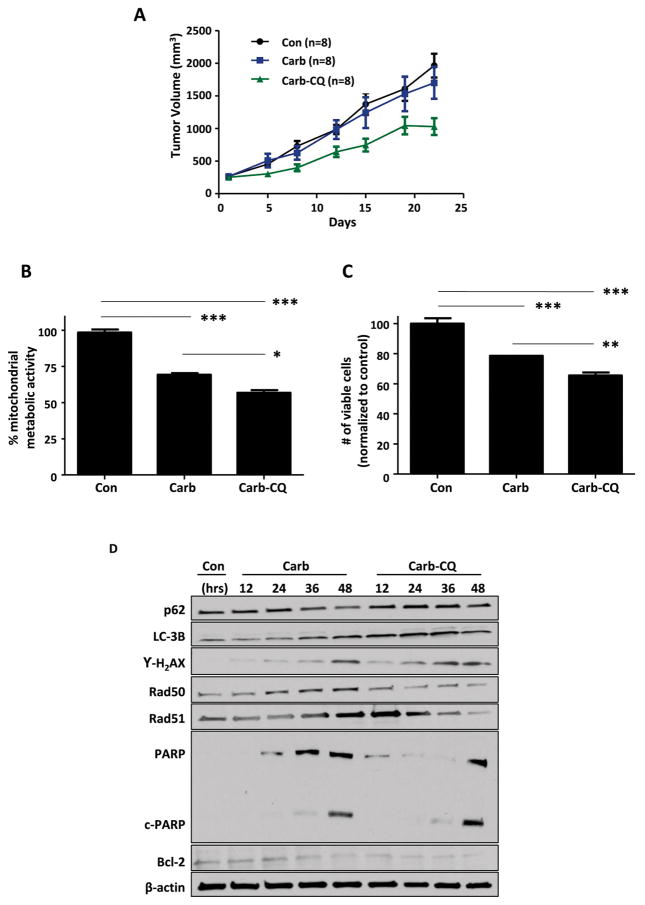
As CQ specifically targets CSCs and has minimal effects on overall tumor growth in vivo, when used as a single agent in BRCA1 wild-type SUM159 orthotopic xenografts (Supplementary Figure), we investigated its effect on tumor growth in combination with carboplatin. In BRCA-mutated TNBC patients, addition of carboplatin to conventional neoadjuvant chemotherapy has been shown to significantly increase pathological complete response rate [26]. However, in SUM159 orthotopic xenografts with intact BRCA-associated DNA repair mechanisms, tumor growth rate in the carboplatin-treated group was essentially identical to that of the untreated control group (Fig. 6A). However, with addition of CQ, there was a significant reduction in tumor growth rate. The effect of combination treatment with carboplatin and CQ was associated with decreased mitochondrial metabolic activity (Fig. 6B) and with decreased number of viable cells (Fig. 6C). Furthermore, increasing levels of LC3B-II and p62 demonstrated that CQ can successfully inhibit autophagy induced by carboplatin (Fig. 6D). A time-dependent increase in cell death by addition of CQ closely correlated with increased DNA damage leading to apoptosis, as evident by accumulation of γ-H2AX as well as an increased level of cleaved poly ADP ribose polymerase (PARP). Additionally, when treated with carboplatin alone, TNBC cells had the ability to repair double-stranded DNA breaks, as seen by increasing Rad50 and Rad51 expression in a time-dependent manner. However, the combination treatment with CQ effectively decreased these proteins involved in double-stranded DNA damage response via homologous recombination [13], indicating sustained DNA damage within TNBC cells, ultimately leading to increased cell death. Furthermore, anti-apoptotic protein Bcl-2 was dramatically reduced at an early time point by addition of CQ to treatment regimen. All together, these data suggest that addition of CQ to carboplatin increases TNBC cell death by impairing repair mechanisms for of oxidative DNA damage induced by mitochondrial dysfunction.
Figure 6.
(A) CQ and carboplatin combination treatment reduced the orthotopic SUM159 tumor growth (n=8 in each group). As Satterthwaite’s method demonstrated significant interaction effect (p=0.0172), the differences in the tumor volume between the carboplatin group and carboplatin-CQ combination group were investigated by the F-tests. F-tests with p-values adjusted for multiplicity using Hommel’s method indicated significantly reduced tumor volume in combination group on day 12 (p=0.0371), on day 15 (p=0.0271), and day 22 (p=0.0137). (B) Mitochondrial metabolic activity determined by MTT assay was decreased further by addition of CQ to carboplatin. (C) The number of viable cells after 48 hour treatment was significant reduced by addition of CQ to carboplatin. One-way ANOVA was used for statistical analysis. Data in this figure are represented as mean percent untreated with standard deviation of quadruplicate (B) and triplicate (C) experiments. *** indicates p<0.0001. ** indicates p<0.01. * indicates p<0.05. (D) Results of western blot for p62, LC-3B, γ-H2AX, Rad50, Rad51, PARP, and Bcl-2 after time-dependent treatment with 10 μM carboplatin and 10μM CQ are shown. β-actin was used as loading control.
3.6 Effects of CQ is robust in CSC subpopulation
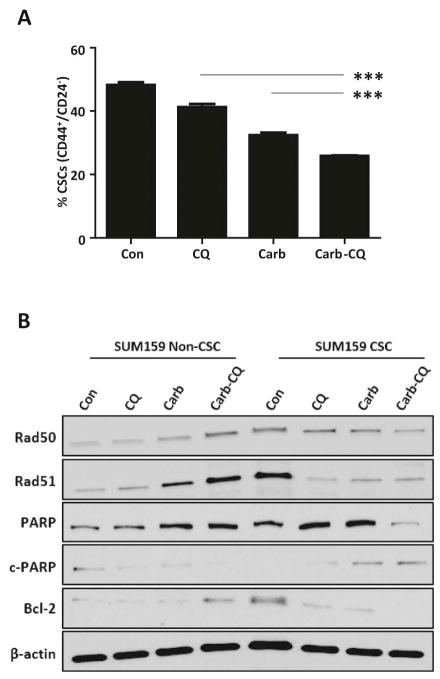
In clinical samples of breast cancer patients, conventional chemotherapeutic agents, docetaxel or doxorubicin and cyclophosphamide, have been shown to increase the percentage of tumorigenic CD44+/CD24−/low CSC subpopulations [27]. Interestingly, in Fig. 7A, carboplatin efficiently decreased the CSC population in the SUM159 cell line from 48.30±0.82% to 32.40±0.80%. This reduction by carboplatin is even greater than the effect of CQ, which decreased the CSC population to 41.27±0.99%. However, with these two agents together, the CSC population was further reduced by an additional 15.37±0.7 % (p<0.0001) when compared to CQ treatment alone and an additional 6.50±0.57% (p<0.0001) when compared to carboplatin treatment alone. Furthermore, this combination effect was calculated using a two-way ANOVA, which included an interaction term. The combination effect demonstrated an insignificant interaction term (p=0.56), indicating an additive combination effect of carboplatin and CQ, rather than a synergistic effect.
Figure 7.
(A) There is a significant additive reduction in the CD44+/CD24−/low CSC population following CQ and carboplatin combination treatment (p<0.0001). This data represents the mean difference in CSC population with standard deviation of three technical repeats. (B) Results of western blot for DNA repair proteins and anti-apoptotic protein are shown and compared between non-CSC and CSC subpopulations after 48 hour treatment with 10 μM carboplatin and/or 10μM CQ. β-actin was used as loading control.
In Fig. 7B, we show that the combination treatment effects in TNBC are much more robust in CSC subpopulations when compared to non-CSC subpopulations. Untreated CSCs inherently have greater capacity to repair DNA damage, as evident by significantly higher expressions of Rad50 and Rad51 in CSCs when compared to those in non-CSCs. In non-CSCs, combination treatment with carboplatin and CQ significantly increased the expressions of Rad50 and Rad51, demonstrating a robust attempt at double-stranded DNA damage repair as more oxidative stress was induced by the combination treatment. However, in CSCs, combination treatment effectively eliminated the ability to repair oxidative double-stranded DNA damage, as indicated by the reduction in Rad50 and Rad51 expressions. Therefore, we can presume that the impairment of double-stranded DNA repair by carboplatin and CQ together may be unique to CSC subpopulations. In addition, increased expression of cleaved PARP (c-PARP), indicating greater DNA-damage dependent apoptotic cell death by combination treatment, was only evident in CSCs. PARP is one of the key players in DNA repair to maintain cells’ viability during periods of oxidative cellular stress. It is also one of the main cleavage targets of caspase-3, serving as a reliable marker of DNA fragmentation, the final stage of apoptotic cell death pathway [28]. The level of intact PARP is well sustained in non-CSCs with negligible level of cleaved PARP, despite accumulating oxidative stress by the combination therapy. However, in CSCs, there is a dramatic reduction in intact PARP with accumulation of cleaved PARP upon combination treatment. Furthermore, there is a significantly higher baseline level of anti-apoptotic protein Bcl-2 in CSCs, compared to non-CSCs. Combination treatment induced expression of Bcl-2 in non-CSCs, but eliminated its expression in CSCs. In summary, CSCs express higher level of DNA repair proteins and anti-apoptotic proteins than their non-CSC counterparts at baseline, likely contributing to their chemoresistance. However, as a combination therapy, CQ and carboplatin together can successfully overcome these mechanisms and eliminate CSCs.
4. Discussion
Previously, our group has demonstrated that CQ targets CSCs in TNBC via inhibition of autophagy and epigenetic regulation by DNA demethylation [9]. This current study suggests that CQ also causes mitochondrial dysfunction, resulting in oxidative DNA damage, impairment of effective DNA repair, and apoptotic cell death of TNBC CSCs. There is growing evidence that while cancer cells have elevated levels of ROS, CSCs are able to maintain relatively low ROS levels, in part due to augmented free radical scavenging systems [29–31]. Post-chemotherapy breast cancer cells were found to have enrichment of CSCs with less ROS and more antioxidant proteins, compared to pretreatment tumors [32]. Thus, depletion of ROS scavengers can lead to sensitization to currently available cancer therapy, specifically by targeting chemotherapy-resistant CSCs [31]. In our study, treatment with CQ not only decreased CSC subpopulations, but also reduced superoxide scavenging protein NQO1.
Here, we showed a dramatic reduction in tumor growth in vivo when CQ was administered in combination with carboplatin. Carboplatin is a platinum analog that impedes DNA transcription and replication, eventually resulting in single- or double-stranded DNA breaks [26]. As BRCA1 and BRCA2 genes encode proteins essential for homologous recombination, the cell’s most efficient DNA repair pathway, it is hypothesized that cancers harboring BRCA mutations are particularly susceptible to platinum analogs, such as carboplatin [26, 33]. Interestingly, many of breast cancer patients with BRCA1 mutations have tumors that are triple-negative and particularly aggressive basal-like subtype [26, 34, 35]. Despite limited efficacy as a single agent in heavily treated metastatic breast cancers [35], carboplatin has shown to be an excellent addition to treatment regimen in TNBC, conferring increasing rates of pathological complete response and survival benefits to patients [13, 14, 36, 37]. Unfortunately, treatment failure is eventually seen in a substantial number of TNBC patients due to chemoresistance over time. Chemoresistance to platinum drugs has been attributed to several mechanisms, including alterations in apoptotic signaling pathways preventing cancer cells from undergoing cell death and new genomic changes that restore the ability to repair DNA damage [33, 38]. In our study, with carboplatin alone, robust DNA damage repair was triggered in BRCA1 intact TNBC cells. However, CQ effectively diminished this response, especially in CSC subpopulations. Therefore, we propose that addition of CQ to carboplatin may be superior to carboplatin alone in TNBC patients, preventing local relapse and distant metastasis due to CSCs, problems often seen in TNBC patients even after initial pathological complete response.
In summary, our study demonstrates that CQ causes sustained oxidative DNA damage in CSCs, which may contribute to its anti-CSC property. TNBC CSCs, with an inherently high capacity for DNA damage repair, were particularly sensitive to the effects of combination therapy with carboplatin and CQ. Currently, CQ is being investigated in multiple clinical trials to treat various types of malignancy. At our institution, a phase II clinical trial is underway to determine the efficacy and safety of CQ in combination with taxane chemotherapy agents in advanced breast cancer patients who have previously failed anthracycline chemotherapy. Because TNBC’s high treatment failure rate has been attributed to enrichment of CSCs as a key element of TNBC relapse and metastasis, the autophagy inhibitor CQ, with its anti-CSC effects, warrants further clinical evaluation as a targeted therapy, especially in combination therapy with agents such as carboplatin, for the comprehensive treatment of TNBC.
Supplementary Material
Highlights.
Chloroquine (CQ) eliminates cancer stem cell (CSC) subpopulations in TNBC.
CQ causes structural mitochondrial damage, resulting in oxidative DNA breaks.
CQ hinders the homologous recombination necessary for DNA break repair in CSCs.
CQ sensitizes platinum chemotherapy-resistant BRCA1 wild-type TNBC to carboplatin.
Acknowledgments
This work was supported by NIH grants RO1 CA138197, U54 CA149196, Golfers against Cancer, Breast Cancer Research Foundation, Causes for a Cure, Team Tiara, Emily W. Herrman Cancer Research Laboratory, Komen for Cure KG 08169c, R21CA179720, and R21CA173150.
Abbreviations
- TNBC
Triple negative breast cancer
- CSCs
Cancer stem cells
- CQ
Chloroquine
- FACS
Fluorescence-activated cell sorting
- MSFE
Mammosphere formation efficiency
- EM
Electron microscopy
- ETC
Electron transport chain
- NQO1
NAD (P) H: quinone oxidoreductase 1
- ROS
Reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Diana H. Liang, Email: jyhwang@houstonmethodst.org.
Dong Soon Choi, Email: DChoi@houstonmethodist.org.
Joe E. Ensor, Email: jeensor@houstonmethodist.org.
Benny A. Kaipparettu, Email: kaippare@bcm.edu.
Barbara L. Bass, Email: BBass@houstonmethodist.org.
Jenny C. Chang, Email: jcchang@houstonmethodist.org.
References
- 1.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 2.Valsecchi ME, Kimmey G, Bir A, Silbermins D. Role of Carboplatin in the Treatment of Triple Negative Early- Stage Breast Cancer. Rev Recent Clin Trials. 2015;10:101–110. doi: 10.2174/1574887110666150624101343. [DOI] [PubMed] [Google Scholar]
- 3.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 4.Idowu MO, Kmieciak M, Dumur C, Burton RS, Grimes MM, Powers CN, Manjili MH. CD44(+)/CD24(−/low) cancer stem/progenitor cells are more abundant in triple-negative invasive breast carcinoma phenotype and are associated with poor outcome. Hum Pathol. 2012;43:364–373. doi: 10.1016/j.humpath.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dave B, Mittal V, Tan NM, Chang JC. Epithelial-mesenchymal transition, cancer stem cells and treatment resistance. Breast Cancer Res. 2012;14:202. doi: 10.1186/bcr2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A, Herschkowitz JI, Fan C, Zhang X, He X, Pavlick A, Gutierrez MC, Renshaw L, Larionov AA, Faratian D, Hilsenbeck SG, Perou CM, Lewis MT, Rosen JM, Chang JC. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A. 2009;106:13820–13825. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu H, Patel MR, Prescher JA, Patsialou A, Qian D, Lin J, Wen S, Chang YF, Bachmann MH, Shimono Y, Dalerba P, Adorno M, Lobo N, Bueno J, Dirbas FM, Goswami S, Somlo G, Condeelis J, Contag CH, Gambhir SS, Clarke MF. Cancer stem cells from human breast tumors are involved in spontaneous metastases in orthotopic mouse models. Proc Natl Acad Sci U S A. 2010;107:18115–18120. doi: 10.1073/pnas.1006732107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi DS, Blanco E, Kim YS, Rodriguez AA, Zhao H, Huang TH, Chen CL, Jin G, Landis MD, Burey LA, Qian W, Granados SM, Dave B, Wong HH, Ferrari M, Wong ST, Chang JC. Chloroquine eliminates cancer stem cells through deregulation of Jak2 and DNMT1. Stem Cells. 2014;32:2309–2323. doi: 10.1002/stem.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Q, Zhang Y, Zhang P, Chao Z, Xia F, Jiang C, Zhang X, Jiang Z, Liu H. Hexokinase II inhibitor, 3-BrPA induced autophagy by stimulating ROS formation in human breast cancer cells. Genes Cancer. 2014;5:100–112. doi: 10.18632/genesandcancer.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas S, Sharma N, Golden EB, Cho H, Agarwal P, Gaffney KJ, Petasis NA, Chen TC, Hofman FM, Louie SG, Schonthal AH. Preferential killing of triple-negative breast cancer cells in vitro and in vivo when pharmacological aggravators of endoplasmic reticulum stress are combined with autophagy inhibitors. Cancer Lett. 2012;325:63–71. doi: 10.1016/j.canlet.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 12.Harhaji-Trajkovic L, Arsikin K, Kravic-Stevovic T, Petricevic S, Tovilovic G, Pantovic A, Zogovic N, Ristic B, Janjetovic K, Bumbasirevic V, Trajkovic V. Chloroquine-mediated lysosomal dysfunction enhances the anticancer effect of nutrient deprivation. Pharm Res. 2012;29:2249–2263. doi: 10.1007/s11095-012-0753-1. [DOI] [PubMed] [Google Scholar]
- 13.von Minckwitz G, Schneeweiss A, Loibl S, Salat C, Denkert C, Rezai M, Blohmer JU, Jackisch C, Paepke S, Gerber B, Zahm DM, Kummel S, Eidtmann H, Klare P, Huober J, Costa S, Tesch H, Hanusch C, Hilfrich J, Khandan F, Fasching PA, Sinn BV, Engels K, Mehta K, Nekljudova V, Untch M. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15:747–756. doi: 10.1016/S1470-2045(14)70160-3. [DOI] [PubMed] [Google Scholar]
- 14.Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM, Kuzma CS, Pluard TJ, Somlo G, Port ER, Golshan M, Bellon JR, Collyar D, Hahn OM, Carey LA, Hudis CA, Winer EP. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance) J Clin Oncol. 2015;33:13–21. doi: 10.1200/JCO.2014.57.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schott AF, Landis MD, Dontu G, Griffith KA, Layman RM, Krop I, Paskett LA, Wong H, Dobrolecki LE, Lewis MT, Froehlich AM, Paranilam J, Hayes DF, Wicha MS, Chang JC. Preclinical and clinical studies of gamma secretase inhibitors with docetaxel on human breast tumors. Clin Cancer Res. 2013;19:1512–1524. doi: 10.1158/1078-0432.CCR-11-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma Y, Bai RK, Trieu R, Wong LJ. Mitochondrial dysfunction in human breast cancer cells and their transmitochondrial cybrids. Biochim Biophys Acta. 2010;1797:29–37. doi: 10.1016/j.bbabio.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JH, Choi DS, Lee OH, Oh SH, Lippman SM, Lee HY. Antiangiogenic antitumor activities of IGFBP-3 are mediated by IGF-independent suppression of Erk1/2 activation and Egr-1-mediated transcriptional events. Blood. 2011;118:2622–2631. doi: 10.1182/blood-2010-08-299784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vlashi E, Lagadec C, Vergnes L, Reue K, Frohnen P, Chan M, Alhiyari Y, Dratver MB, Pajonk F. Metabolic differences in breast cancer stem cells and differentiated progeny. Breast cancer research and treatment. 2014;146:525–534. doi: 10.1007/s10549-014-3051-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu S, Zhou F, Wei Y, Chen WR, Chen Q, Xing D. Cancer phototherapy via selective photoinactivation of respiratory chain oxidase to trigger a fatal superoxide anion burst. Antioxid Redox Signal. 2014;20:733–746. doi: 10.1089/ars.2013.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quoilin C, Mouithys-Mickalad A, Lecart S, Fontaine-Aupart MP, Hoebeke M. Evidence of oxidative stress and mitochondrial respiratory chain dysfunction in an in vitro model of sepsis-induced kidney injury. Biochim Biophys Acta. 2014;1837:1790–1800. doi: 10.1016/j.bbabio.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Zhang Y, Wu Q, Cui X, Lin Z, Liu S, Chen L. Clinical implications of high NQO1 expression in breast cancers. J Exp Clin Cancer Res. 2014;33:14. doi: 10.1186/1756-9966-33-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krajewski WA. Alterations in the internucleosomal DNA helical twist in chromatin of human erythroleukemia cells in vivo influences the chromatin higher-order folding. FEBS letters. 1995;361:149–152. doi: 10.1016/0014-5793(95)00144-x. [DOI] [PubMed] [Google Scholar]
- 23.Mahut M, Leitner M, Ebner A, Lammerhofer M, Hinterdorfer P, Lindner W. Time-resolved chloroquine-induced relaxation of supercoiled plasmid DNA. Analytical and bioanalytical chemistry. 2012;402:373–380. doi: 10.1007/s00216-011-5213-y. [DOI] [PubMed] [Google Scholar]
- 24.Foster ER, Downs JA. Histone H2A phosphorylation in DNA double-strand break repair. FEBS J. 2005;272:3231–3240. doi: 10.1111/j.1742-4658.2005.04741.x. [DOI] [PubMed] [Google Scholar]
- 25.Nikitaki Z, Hellweg CE, Georgakilas AG, Ravanat JL. Stress-induced DNA damage biomarkers: applications and limitations. Front Chem. 2015;3:35. doi: 10.3389/fchem.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sikov WM. Assessing the role of platinum agents in aggressive breast cancers. Curr Oncol Rep. 2015;17:3. doi: 10.1007/s11912-014-0428-7. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC, Wong H, Rosen J, Chang JC. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. Journal of the National Cancer Institute. 2008;100:672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 28.Decker P, Muller S. Modulating poly (ADP-ribose) polymerase activity: potential for the prevention and therapy of pathogenic situations involving DNA damage and oxidative stress. Current pharmaceutical biotechnology. 2002;3:275–283. doi: 10.2174/1389201023378265. [DOI] [PubMed] [Google Scholar]
- 29.Dando I, Cordani M, Dalla Pozza E, Biondani G, Donadelli M, Palmieri M. Antioxidant Mechanisms and ROS-Related MicroRNAs in Cancer Stem Cells. Oxid Med Cell Longev. 2015;2015:425708. doi: 10.1155/2015/425708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi X, Zhang Y, Zheng J, Pan J. Reactive oxygen species in cancer stem cells. Antioxid Redox Signal. 2012;16:1215–1228. doi: 10.1089/ars.2012.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, Joshua B, Kaplan MJ, Wapnir I, Dirbas FM, Somlo G, Garberoglio C, Paz B, Shen J, Lau SK, Quake SR, Brown JM, Weissman IL, Clarke MF. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Achuthan S, Santhoshkumar TR, Prabhakar J, Nair SA, Pillai MR. Drug-induced senescence generates chemoresistant stemlike cells with low reactive oxygen species. J Biol Chem. 2011;286:37813–37829. doi: 10.1074/jbc.M110.200675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto KN, Hirota K, Takeda S, Haeno H. Evolution of pre-existing versus acquired resistance to platinum drugs and PARP inhibitors in BRCA-associated cancers. PLoS One. 2014;9:e105724. doi: 10.1371/journal.pone.0105724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 35.Palma G, Frasci G, Chirico A, Esposito E, Siani C, Saturnino C, Arra C, Ciliberto G, Giordano A, D’Aiuto M. Triple negative breast cancer: looking for the missing link between biology and treatments. Oncotarget. 2015;6:26560–26574. doi: 10.18632/oncotarget.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villarreal-Garza C, Khalaf D, Bouganim N, Clemons M, Pena-Curiel O, Baez-Revueltas B, Kiss A, Kassam F, Enright K, Verma S, Pritchard K, Myers J, Dent R. Platinum-based chemotherapy in triple-negative advanced breast cancer. Breast Cancer Res Treat. 2014;146:567–572. doi: 10.1007/s10549-014-3033-4. [DOI] [PubMed] [Google Scholar]
- 37.Chen XS, Yuan Y, Garfield DH, Wu JY, Huang O, Shen KW. Both carboplatin and bevacizumab improve pathological complete remission rate in neoadjuvant treatment of triple negative breast cancer: a meta-analysis. PLoS One. 2014;9:e108405. doi: 10.1371/journal.pone.0108405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, Castedo M, Kroemer G. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31:1869–1883. doi: 10.1038/onc.2011.384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.