Abstract
Background
Sarcopenic obesity, age‐related muscle loss, which is compensated by an increase in fat mass, impairs quality of life in elderly people. Although the increase in intramuscular fat is associated with decreased insulin sensitivity and increased metabolic risk factors, the origin of diabetes‐associated intramuscular fat has not been elucidated. Here, we investigated intramuscular fat deposition using a muscle injury model in type 2 diabetic mice.
Methods
Male 8‐week‐old C57BL/6 and 8‐week‐old and 26‐week‐old KKAy underwent intramuscular injection of cardiotoxin (Ctx) (100 μL/10 μM) into the tibialis anterior (TA) muscles. After 2 weeks, the muscles were removed and evaluated.
Results
KKAy exhibited impaired muscle regeneration and ectopic fat deposition. Such impairment was more marked in older KKAy. These changes were also observed in another diabetic mouse model, db/db and diet‐induced obese mice but not in streptozocin‐induced diabetic mice. Deposited fat was platelet‐derived growth factor (PDGF) receptor alpha positive and its cytoskeleton was stained with Masson's trichrome, indicating it to be of fibro‐adipocyte progenitor cell origin. Expression of a myogenic marker, myoD, was lower and that of PDGF receptor alpha and CCAAT/enhancer binding protein (CEBP) alpha was higher in Ctx‐injured TA of KKAy compared with that of C57BL/6. Peroxisome proliferator‐activated receptor γ (PPARγ) was highly expressed in fat‐forming lesions in older KKAy. Treatment with all‐trans retinoic acid prevented the formation of intramuscular fat; however, treatment with GW9662, a PPARγ antagonist, increased the fibrotic change in muscle.
Conclusions
Diabetic mice showed impaired muscle regeneration with fat deposition, suggesting that diabetes may enhance sarcopenic obesity through a mechanism involving anomalous fibro‐adipocyte progenitor cell differentiation.
Keywords: Diabetes mellitus, Muscle regeneration, Ectopic fat deposition, Fibro‐adipocyte progenitor cell, Sarcopenic obesity
Introduction
Sarcopenia is the degenerative loss of skeletal muscle mass, which is related to frailty and the geriatric syndrome1 and impairs quality of life in elderly people.2, 3 On the other hand, sarcopenic obesity (SO) has been defined as a combination of excess weight gain and reduced muscle mass and/or strength.4 SO is also highlighted and reported to worsen cardio‐metabolic outcome.5, 6 Interestingly, SO is modestly associated with increased risk in cardiovascular disease (CVD), while sarcopenia or obesity alone is not sufficient to increase the risk of CVD.7 Moreover, SO is independently associated with and precedes the onset of disability in instrumental activities of daily living, more than in lean sarcopenia or non‐sarcopenic obesity, in the community‐dwelling elderly population.8 Recently, we observed that one‐leg standing time and sway are significantly impaired in subjects with sarcopenic visceral obesity compared with those in subjects with sarcopenia or visceral obesity alone.9 These results indicate that SO is a high risk for impairment of quality of life in elderly people.
Certain conditions have strong potential to coexist with sarcopenia to accelerate the progression of muscle atrophy in elderly people.10 The prevalence of sarcopenia is higher in patients with type 2 diabetes mellitus (T2DM) than in non‐diabetic subjects.11 DM may accelerate the development of age‐associated changes in body composition through a number of mechanisms. T2DM is associated with excessive loss of skeletal muscle and increased trunk fat mass in community‐dwelling older adults.12 Although multiple mechanisms, such as oxidative injury, subclinical inflammation and insulin resistance, have been proposed to be involved in acceleration of sarcopenia in diabetic patients, the detailed mechanism is not yet well understood.
Excessive intramuscular fat (IMF) in skeletal muscle is observed in T2DM patients13 and is associated with decreased insulin sensitivity,14, 15, 16, 17 indicating that an increase in IMF causes a vicious cycle of glucose metabolism. Recently, IMF has been shown to be also associated with a wide range of metabolic risk factors such as dysglycemia, dyslipidemia and hypertension.18 These results indicate that prevention of excessive IMF may ameliorate sarcopenic obesity and contribute to the improvement of the quality of life in T2DM patients. However, the detailed mechanism of diabetes‐induced sarcopenia and the correlation between muscle atrophy and excessive IMF in diabetic patients are enigmas. Here, we investigated the possible mechanism of intramuscular fat deposition using a muscle injury model in diabetic mice, KKAy.
Materials and methods
This study was performed in accordance with the National Institutes of Health guidelines for the use of experimental animals. All animal studies were reviewed and approved by the Animal Studies Committee of Ehime University.
Animals
C57BL6, wild‐type (WT) mice (CLEA, Tokyo, Japan), type 2 diabetes model mice, KKAy (CLEA, Tokyo, Japan) and db/db and diet‐induced obesity (DIO) mice (Charles River Laboratories Inc., Kanagawa, Japan) were used in the following experiments. Some WT mice and DIO mice were fed with high‐cholesterol diet (HCD) (high‐fat diet (HFD)‐60, Oriental Yeast Co., Ltd., Tokyo, Japan). Some WT mice were intraperitoneally injected with streptozocin at 250 mg/kg/day to induce experimental diabetes. A blood glucose level exceeding 300 mg/dL was considered to indicate diabetes. Mice were kept in a room in which lighting was controlled (12 h on and 12 h off) and temperature was kept at 25°C. They were given a standard diet (MF, Oriental Yeast, Tokyo, Japan) and water ad libitum. Some mice were treated with all‐trans retinoic acid (ATRA) intraperitoneally at 1 mg/kg/day with or without GW9662, a peroxisome proliferator‐activated receptor γ (PPARγ) antagonist, in water at 0.0005% (w/w).
Magnetic resonance imaging
Muscle in the lower limbs of 18‐week‐old WT and KKAy was evaluated with a magnetic resonance imaging (MRI) system, MRmini SA (DS Pharma Biomedical, Osaka, Japan), consisting of a 1.5‐Tesla permanent magnet made of neodymium magnets (Nd‐Fe‐B). After appropriate positioning was confirmed on localizer images, axial and sagittal MR images were obtained using a T1‐weighted multi‐slice sequence. MRI parameters were set with average = 3 and thickness = 1.5 mm.
Muscle injury
Eight‐week‐old (young) and 26‐week‐old (older) mice underwent intramuscular injection of cardiotoxin (Ctx) (100 μL/10 μM) into either the tibialis anterior (TA) or gastrocnemius (GA) muscle as described previously.19 After 2 weeks, the muscles were removed and histological evaluation was performed with haematoxylin and eosin staining following fixation in 4% paraformaldehyde. Some mice were perfused with Indian ink (Kuretake Co. Ltd., Nara, Japan) (Indian ink: phosphate buffer saline (PBS) = 2:1) following injection of PBS with 0.4% NaNO3 for vascular staining.
Generation of chimeric mice
To analyse the functional role of bone marrow cells (BMC) in muscle repair, we generated chimeric mice as described previously, with minor modification.20 Briefly, 8‐week‐old male KKAy was exposed to a total dose of 8 Gy whole‐body irradiation and used as recipients. BMC were isolated from six crushed bones (bilateral tibias, femurs and iliac bones) from 8‐week‐old male mice overexpressing green fluorescent protein (GFP) (green mouse FM131: provided by Dr Masaru Okabe, Osaka University). Bulk BMC (1.0 × 106 cells) diluted in PBS (200 μL) were injected via the tail vein immediately after irradiation. The mice were used for the experiments 6 weeks after transplantation.
Immunohistochemical staining
Formalin‐fixed, paraffin‐embedded sections were prepared from tibialis anterior muscle 2 weeks after Ctx injection. Endogenous peroxidase was blocked by incubation in 3% H2O2 for 15 min, and nonspecific protein binding was blocked by incubation for 10 min in a blocking reagent (Nichirei Bioscience Inc., Tokyo, Japan). Then, the sections were incubated overnight at 4°C with the primary antibody as shown in Supporting information Table S1. Primary antibody binding was visualized with specific secondary fluorescent antibodies as shown in Table S1. Samples were examined with a fluorescence microscope (Keyence BZ‐9000, Osaka, Japan) equipped with a computer‐based imaging system.
Real time reverse transcription polymerase chain reaction
RNA was extracted from tibialis anterior muscles. Real‐time quantitative reverse‐transcription polymerase chain reaction (PCR) was performed with a SYBR Premix Ex Taq (Takara Bio Inc., Japan). PCR primers were as shown in Table S2.
Materials
Reagents not listed earlier were purchased from Sigma–Aldrich Inc. (St. Louis, MO, USA).
Statistical analysis
All values are expressed as mean ± standard deviation in the text and figures. Data were evaluated by ANOVA. If a statistically significant effect was found, post hoc analysis was performed to detect the difference between the groups. Values of P < 0.05 were considered statistically significant.
Results
Characterization of intramuscular fat deposition in KKAy using magnetic resonance imaging
First, we compared the characteristics of skeletal muscle and intramuscular fat between 18‐week‐old C57BL6 (as WT mice) and KKAy using MRI. As shown in Figure 1A, in coronal T1 images of the lower limbs, an increase in subcutaneous and intramuscular fat deposition was observed in KKAy. Moreover, the diameter of the lower limbs tended to be smaller in KKAy compared with WT. Sagittal T1 images suggested the presence of muscle atrophy in the soleus of KKAy (Figure 1B). Histological analysis of the lower limbs of 18‐week‐old KKAy showed slight ectopic fat deposition around blood vessels (Figure 1C).
Figure 1.
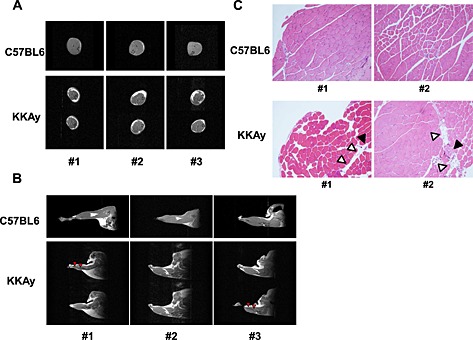
Magnetic resonance imaging of muscles in lower limbs of 18‐week‐old C57BL6 (wild‐type) and KKAy. 1.5 T magnetic resonance imaging T1 images in coronal (A) and sagittal (B) planes were obtained as described in Methods. Representative photos from three mice in each group are shown. Red arrows show muscle atrophy. (C) Haematoxylin‐eosin staining of tibialis anterior muscle of 18‐week‐old WT and KKAy at ×100 magnification. White triangles show ectopic fat deposition. Black triangles show blood vessels.
Muscle repair after cardiotoxin injection
Impairment of muscle repair following injury is one of the factors contributing to the pathogenesis of sarcopenia.21, 22, 23 Thus, we focused on muscle regeneration in the following experiments. Ctx was injected into the TA muscle. The muscle was repaired with satellite cells 2 weeks after Ctx injection, following marked inflammation (data not shown). Muscle regeneration was analysed using muscle samples 2 weeks after Ctx treatment in younger (8‐week‐old) and older (26‐week‐old) WT and KKAy. In younger mice, 2 weeks after Ctx treatment, Ctx‐treated lower limb weight had a tendency to be lower in KKAy, while Ctx‐treated lower limb weight had almost recovered to that of the non‐Ctx‐treated side in WT (Figure 2A). This reduction in weight was marked in older mice (Figure 2B). In older mice, the weight of TA muscle was approximately half in Ctx‐treated KKAy compared with that in other groups (Figure 2B). A marked increase in blood vessel formation was observed in KKAy after Ctx treatment, by examination of mice perfused with Indian ink (Figure S1). Histological analysis demonstrated honeycomb formation in KKAy muscle (Figure 2C). This structural change was markedly enhanced in aged KKAy (Figure 2D and E). These honeycomb structures were stained for perilipin 1, which is a lipid droplet‐associated protein, indicating that they were ectopic fat deposits (Figure 2F). Such change was also observed in another diabetic mouse model, db/db, but not in streptozocin‐induced diabetic mice (Figure 3). Interestingly, WT fed HCD (HFD‐60) and DIO mice fed HFD‐60 showed a reduction of Ctx‐treated lower limb weight compared with WT fed normal chow (Figure 4A). Histological analysis revealed more honeycomb structures in these mice (Figure 4B). These mice exhibited hyperglycemia and higher insulin levels in oral glucose tolerance test (Figure 4C) and insulin resistance in insulin tolerance test (Figure 4D).
Figure 2.
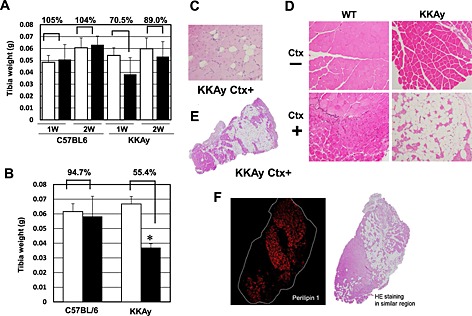
Comparison of tibialis anterior (TA) muscles of C57BL6 (wild‐type (WT)) and KKAy with or without cardiotoxin (Ctx) injection. Muscle weight in WT and KKAy at 8 weeks of age (A) and 26 weeks of age (B). Muscle weight of WT and KKAy with or without Ctx. White bars = no treatment; black bars = Ctx treatment. n = 4 for each. *P = 0.00026 versus Ctx (−). Haematoxylin‐eosin staining of TA muscle in WT and KKAy with or without Ctx at 8 weeks of age (C) and 26 weeks of age (D) at ×100 magnification. (E) Macroimage of TA muscle in KKAy at 26 weeks of age after Ctx injection. (F) Perilipin 1 staining of TA muscle in KKAy at 26 weeks of age after Ctx injection. HE: hematoxylin and eosin.
Figure 3.
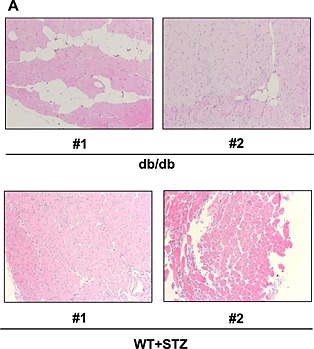
Haematoxylin‐eosin staining of tibialis anterior muscle of db/db mice and streptozocin‐injected mice 2 weeks after cardiotoxin treatment at 8 weeks of age at ×100 magnification. Representative photos of tibialis anterior muscle are shown (#1 and #2 indicate different samples).
Figure 4.
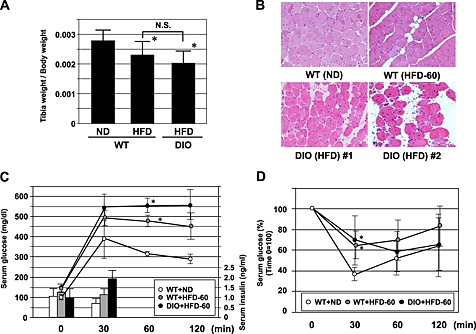
Comparison of tibialis anterior (TA) muscle weight (A), haematoxylin‐eosin staining of TA muscle at ×100 magnification (B) 2 weeks after cardiotoxin treatment of wild‐type (WT) fed with normal chow (WT + normal diet (ND)) and high‐cholesterol diet (high fat diet (HFD)‐60) (WT + HFD‐60) and diet‐induced obesity (DIO) mice fed HFD‐60. (C) Change of glucose and insulin levels in oral glucose tolerance test. (D) Change of glucose levels in insulin tolerance test. *P < 0.05 versus WT + ND.
Origin of cardiotoxin‐induced ectopic fat deposits
We further investigated fat deposition in skeletal muscle of Ctx‐treated KKAy. Abundant blood vessels were observed in fat‐forming lesions in Ctx‐treated KKAy (Figure S2). In this study, fat formation was detected as vimentin‐positive areas, indicating mesenchymal origin. However, vimentin‐positive areas were only observed in fat‐forming lesions (Figure 5A). Masson's trichrome staining demonstrated collagen fibres around the muscle. Interestingly, not only the extracellular matrix around fat‐forming lesions but also the cytoskeleton within lesions seemed to be positively stained by Masson's trichrome (Figure 5B and C).
Figure 5.
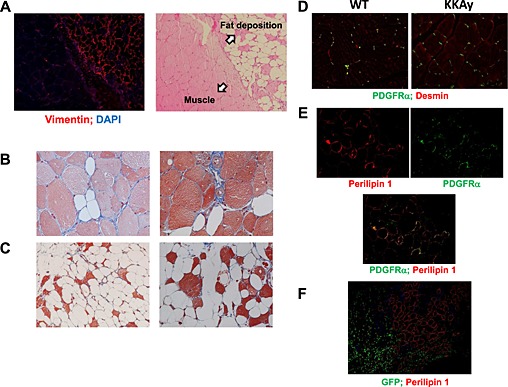
Origin of fat formation after cardiotoxin treatment in KKAy. (A) Immunofluorescent staining with anti‐vimentin antibody at the border between fat formation and muscle of 26‐week‐old mice at ×100 magnification. Masson's trichrome staining of fat formation in KKAy 2 weeks after cardiotoxin treatment of 8‐week‐old (B) and 26‐week‐old (C) mice at ×200 magnification. (D) Immunofluorescent staining with anti‐platelet‐derived growth factor (PDGF) receptor alpha and anti‐desmin antibodies in non‐injured muscle at ×100 magnification. (E) Representative photos of immunofluorescent staining with anti‐perilipin 1 and anti‐PDGF receptor alpha antibodies at ×200 magnification. (F) Representative photos of immunofluorescent staining with anti‐perilipin 1 and anti‐GFP antibodies in green fluorescent protein (GFP)‐chimeric KKAy at ×100 magnification.
Skeletal muscle contains several components of mesenchymal stem‐progenitor cells. Fibro‐adipocyte progenitor (FAP) cells have potential to differentiate into fibroblasts and adipocytes but not into myocytes.24, 25, 26 FAPs are platelet‐derived growth factor (PDGF) receptor‐alpha positive and reside around blood vessels in non‐injured muscle.27 Our results showed that PDGF receptor‐alpha‐positive cells existed in the interstitial space between the myofibres of non‐injured muscle in WT and KKAy, with no difference in the number between them (Figure 5D). In Ctx‐injured KKAy, perilipin 1‐positive cells were also PDGF receptor‐alpha positive, indicating that they were of FAP origin (Figure 5E).
To exclude the possibility that this fat formation was of haematopoietic cell origin, we generated GFP‐chimeric mice with bone marrow transplantation in KKAy irradiated with a half‐lethal dose of X‐ray. As shown in Figure 5F, there was no correlation between GFP‐positive cells and perilipin 1‐positive cells, suggesting that fat formation was not derived from bone marrow cells.
Muscle repair genes in diabetic mice
In younger mice, reverse transcription‐PCR analysis showed that an expression of a myogenic marker, myoD, was lower than that of a mesenchymal marker, PDGFRα, and an adipogenic marker, CCAAT/enhancer binding protein‐alpha(CEBPα), was higher in Ctx‐injured muscle of KKAy compared with that of WT (Figure 6), indicating that the muscle repair response was impaired in KKAy.
Figure 6.
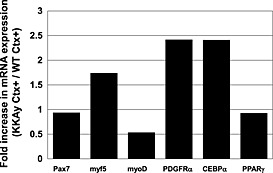
Reverse transcription polymerase chain reaction analysis of various factors involved in muscle repair: adipogenic markers, chondrogenic markers and mesenchymal markers in cardiotoxin (Ctx)‐injured tibialis anterior muscle of C57BL/6 and KKAy. Values are shown as mRNA expression ratio of Ctx‐treated KKAy to Ctx‐treated wild‐tpye (WT). (PPARγ), peroxisome proliferator‐activated receptor γ.
Correlation between fibro‐adipocyte progenitors and myotubes
Recently, interaction between FAPs and muscle fibres has been reported.25, 26, 28 After muscle damage, FAPs are induced to proliferate, but not differentiate, and produce muscle repair factors such as interleukin 6. However, when damage leads to muscle degeneration, FAPs differentiate into adipocytes or fibroblasts. Our results also showed that proliferated mesenchymal cells were present between fat‐forming lesions (Figure 7A). Fat deposition seemed to spread from the region of proliferated mesenchymal cells. Vimentin‐positive fat deposition gradually increased away from the border. Interestingly, PPARγ was highly expressed in fat‐forming lesions in aged mice. As shown in Figure 7B and C, there were two types of cells in fat‐forming lesions, vimentin‐positive but desmin‐negative cells and vimentin‐negative but desmin‐positive cells; the latter are thought to be myogenic cells. PPARγ was highly expressed in both cell types.
Figure 7.
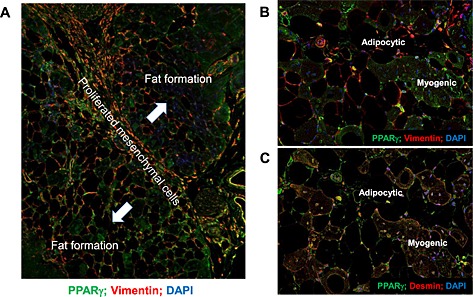
(A) Immunofluorescent staining with anti‐vimentin and anti‐peroxisome proliferator‐activated receptor gamma (PPARγ) antibodies in border between fat formation and satellite cells in 26‐week‐old KKAy after cardiotoxin treatment. Blue line shows the border. High magnification (×400) view of immunofluorescent staining with anti‐vimentin and anti‐PPARγ antibodies (B) and anti‐desmin and anti‐PPARγ antibodies (C) in the border between fat formation and satellite cells in 26‐week‐old KKAy after cardiotoxin treatment. DAPI, 4',6‐diamidino‐2‐phenylindole.
Effect of retinoic acid on ectopic fat deposition
Retinoic acid is known to be a potent inhibitor of adipogenesis, with transcriptional modulation during the early stage of differentiation.29, 30 Therefore, we evaluated the effect of ATRA and PPARγ on such ectopic fat deposition in skeletal muscle using younger mice. Administration of ATRA significantly prevented fat formation in the lower limbs after Ctx treatment (Figure 8A and B). However, co‐administration of GW9662, a PPARγ antagonist, with ATRA significantly enhanced the white area in macroscopic images of TA muscle (Figure 8A). Histological analysis demonstrated that co‐administration of GW9662 with ATRA markedly increased fibrosis in the TA muscle of KKAy after Ctx treatment (Figure 8B).
Figure 8.
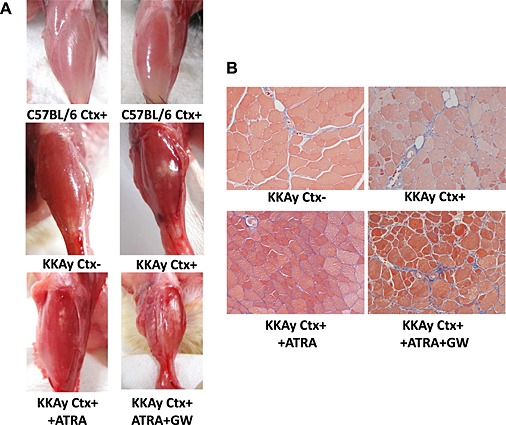
Effect of all‐trans‐retinoic‐acid (ATRA) and GW9662 on cardiotoxin (Ctx)‐induced muscle injury. (A) Representative photos of tibialis anterior muscle with each treatment. (B) Masson's trichrome staining of tibialis anterior muscle of 18‐week‐old KKAy at ×100 magnification. ATRA and GW9662 were administered as described in Methods.
Discussion
In the present study, we demonstrated that T2DM mice, KKAy, showed ectopic fat deposition in the lower limb evaluated by MRI. Moreover, in a Ctx‐treated muscle injury model, KKAy exhibited excessive IMF deposition. IMF seemed to be of FAP origin, and anomalous FAP differentiation was suggested to be involved in IMF deposition. Such change was also observed in another diabetic mouse model, db/db and diet‐induced insulin‐resistant mice, but not in streptozocin‐induced diabetic mice, indicating that insulin resistance may be involved in such anomalous FAP differentiation. These results suggest that sarcopenic obesity with loss of skeletal muscle mass and excessive IMF accumulation enhances insulin resistance and further enhances IMF deposition with abnormal FAP differentiation and results in further loss of muscle. This vicious cycle is considered to be one of the possible mechanisms of sarcopenic obesity. A thematic presentation of our working hypothesis is shown in Figure 9.
Figure 9.
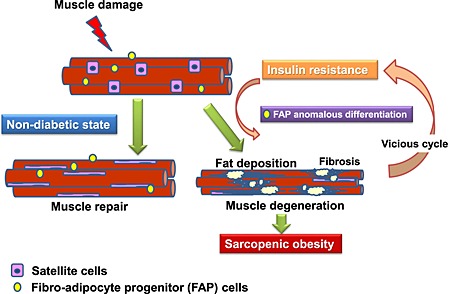
Thematic presentation of diabetes‐induced sarcopenic obesity involving anomalous fibro adipocyte progenitor (FAP) differentiation.
The muscle progenitor cell population, particularly the muscle satellite cell population, is adversely affected by the diabetic environment. D'Souza's review indicated that satellite cell function in T2DM depends on T2DM disease severity, and long‐term exposure to T2DM may promote detrimental epigenetic changes in satellite cells.31 Akhmedov et al. also examined obesity‐induced and diabetes‐induced muscle regeneration including impairment of the potential for satellite cell‐mediated repair.32 However, the detailed mechanism is still under investigation. Nguyen et al. reported impairment of muscle regeneration in ob/ob and db/db mice.33 They focused on angiogenesis, cell proliferation and myoblast accumulation associated with impaired macrophage accumulation. Skeletal muscle macrophages, which induce chronic tissue inflammation, play a fundamental role in inflammation, repair and pathogen clearance and contribute to insulin resistance.34, 35 Recent reports on the interaction between FAPs and muscle degeneration involving muscle repair factors such as IL‐625, 26, 28 may explain the increase in IMF deposition because of anomalous FAP differentiation into adipocytes or fibroblasts via such diabetes‐induced impairment of muscle repair. IMF accumulation in obese patients is positively correlated with insulin resistance and reduced muscle performance.36, 37 Lipid overload causes impaired skeletal muscle function because of a reduction of muscle mass and ultrastructural damage38; therefore, lipotoxic species induced via IMF deposition also interfere with insulin signalling and muscle repair. Moreover, muscle regenerative capacity declines with ageing.39 These reports and our findings suggest a vicious cycle of reduced satellite cell function related to ageing, inflammation, insulin resistance and IMF in patients with sarcopenic obesity. Because the present study did not compare macrophage filtration in injured skeletal muscle between diabetic and non‐diabetic mice, we should investigate the interaction between inflammatory cells and satellite cell function as a key determinant of diabetes‐induced muscle degeneration in future experiments.
Fibro‐adipocyte progenitor cells have been highlighted as a key determinant in the pathogenesis of muscular diseases, including Duchenne muscular dystrophy. Dong et al. also showed that glucocorticoids stimulate FAPs to differentiate into adipocytes in injured muscle and that IL‐4 inhibited their differentiation process.40 Moreover, Cordani et al. suggested a preventive effect of nitric oxide on FAP differentiation into adipocytes via increased expression of miR‐27b, leading to downregulation of PPARγ expression.41 Very recently, Saccone et al. reported that the dystrophic muscle environment causes FAPs to adopt a chromatin state that imparts these cells with myogenic potential.42 Interestingly, such myogenic potential of FAPs is limited to cells derived from muscle in young mdx mice. Moreover, they demonstrated that treatment of muscle with a histone deacetylase inhibitor blocked adipogenesis and driving muscle differentiation. These findings support that ageing, cytokines, nuclear receptor signalling and epigenetic changes may synergistically induce FAP differentiation. In the present study, we used half‐a‐year‐old mice to study sarcopenia. Mice at this age are young and not close to the sarcopenic threshold; therefore, we should perform similar experiments, especially therapeutic analysis, using more aged mice to confirm age‐related sarcopenia as a geriatric phenomenon.
A recent report demonstrated that a PPARγ‐activating microenvironment, such as treatment with fatty acids, caused fibroblasts to differentiate into adipocytes.43 In contrast, myogenic cells did not undergo adipogenesis. Our results demonstrated that aged KKAy exhibited higher expression of PPARγ in injured muscle, while the expression of PPARγ did not change in young KKAy (Figures 4 and 5). Therefore, high PPARγ expression may markedly induce differentiation of FAPs into adipocytes in aged KKAy. Wu et al. reported that visceral adipose tissue of old C57BL mice showed significantly higher mRNA expression of proinflammatory cytokines and lower expression of anti‐inflammatory factors such as PPARγ than those in young mice.44 Moreover, relative PPARγ expression is increased in omental fat in obesity,45 and a highly significant negative correlation between adipocyte PPARγ expression and BMI was observed.46 These reports suggest that relative PPARγ expression is increased in aged obese diabetic mice. This PPARγ‐activating microenvironmental change may enhance adipocyte differentiation into FAP cells.
To maintain the quality of skeletal muscle, prevention of muscle fibrosis is also important as well as intramuscular fat deposition. Satellite cells from aged mice tend to convert from a myogenic to a fibrogenic lineage associated with Wnt signalling pathway.47 Very recently, Fry et al. reported that the loss of satellite cells may contribute to age‐related muscle fibrosis using a genetic mouse model that allows for the specific, inducible depletion of satellite cells in adult skeletal muscle.48 Moreover, Krause et al. demonstrated the impairment of satellite cell infiltration in diabetic skeletal muscle.49 Therefore, fibrotic change may be involved in muscle quality of diabetic skeletal muscle. On the other hand, increased collagen content is observed in insulin‐resistant skeletal muscles of humans.50 Inoue et al. reported that lack of thrombospondin 1 state protects mice from HFD‐induced muscle fibrosis and insulin resistance.51 Moreover, extracellular matrix remodelling is an important factor for determining muscle insulin resistance in the presence of HFD.52 Although we have not assessed insulin resistance in KKAy mice with or without fibrosis in skeletal muscle, these reports suggest that muscle fibrosis may also enhance insulin resistance. Therefore, impaired muscle quality with an increase in not only fat but also fibrosis of skeletal muscle aft1er injury may cause vicious cycle of insulin resistance. Further investigation of muscle fibrosis involving satellite cells is necessary in the future.
Treatment with retinoic acid (RA) has partly prevented fat deposition; however, co‐administration with a PPARγ antagonist has enhanced fibrosis. RA is reported to inhibit differentiation of 3T3‐F442A cells into adipocytes53 and upregulate preadipocyte genes such as cellular RA binding protein type II and nuclear RA receptors to block adipogenesis and suppress diet‐induced obesity.54 Recently, it was reported that combined treatment with three ligands, PPARs, ATRA and the retinoic X receptor, 9‐cis, prevented liver fibrosis in rat primary hepatic stellate cells,55 indicating that cooperation of several factors including PPARs, RA and nuclear receptors regulates FAP cell differentiation after muscle injury.
Some of the limitations of this study are as follows. The results were mainly obtained from immunohistological analysis and lacked quantitative analysis. It is necessary to identify concrete mechanisms to address the connection between intramuscular lipid and muscle degeneration. In the present study, we used obese diabetic mice. It is not clear which intramuscular fat deposition is induced by obesity or diabetes. To clarify the relation, we could use animal models of obesity such as diet‐induced obesity or ob/ob mice in the future. Moreover, in a previous study, 16‐week‐old KKAy exhibited an increase in creatinine level, which suggests early onset of kidney failure.56 Therefore, it cannot be excluded that muscle atrophy is associated with nephropathy. Furthermore, it is not known how ‘acute muscle injury’ is involved in sarcopenic obesity as a chronic disease. Our findings should be investigated from a clinical perspective in patients with diabetes.
In conclusion, the mice with T2DM showed significantly increased IMF deposition, possibly due to anomalous cell differentiation. To prevent sarcopenic obesity, prevention of IMF deposition with consideration of muscle degeneration should be one of the critical targets and its mechanism should be investigated, focusing on anomalous FAP differentiation.
Funding
JSPS KAKENHI Grant Numbers 25293310 (M.H.), 25462220 (M.M.). Research grants from pharmaceutical companies: Astellas Pharma Inc., Bayer Yakuhin, Ltd., Daiichi‐Sankyo Pharmaceutical Co. Ltd., Nippon Boehringer Ingelheim Co. Ltd., Novartis Pharma K. K., Shionogi & Co., Ltd. and Takeda Pharmaceutical Co. Ltd.
Conflict of interest
Masatsugu Horiuchi has received research grants from pharmaceutical companies: Astellas Pharma Inc., Bayer Yakuhin, Ltd., Daiichi‐Sankyo Pharmaceutical Co. Ltd., Nippon Boehringer Ingelheim Co. Ltd., Novartis Pharma K. K., Shionogi & Co., Ltd. and Takeda Pharmaceutical Co. Ltd. All other authors have declared no conflict of interest.
Supporting information
Table S1. List of antibodies.
Table S2. List of primer sequences used for RT‐PCR analysis.
Figure S1. Comparison of tibialis anterior (TA) muscles of C57BL6 (WT) and KKAy with or without Ctx injection. Representative photos of TA muscle with or without Indian ink.
Figure S2. Fat formation and blood vessels. Hematoxylin‐eosin staining (upper panel) and immunofluorescent staining with anti‐CD31 and anti‐perilipin antibodies (lower panel) of tibialis anterior muscles in 26‐week‐old KKAy after Ctx treatment.
Supporting info item
Supporting info item
Acknowledgements
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle (von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7‐8.)
We thank Dr Masaru Okabe from the Department of Experimental Genome Research, Osaka University for generously providing the Green mouse, FM131. We also thank Takeshi Kiyoi from the the Integrated Center for Sciences, Ehime University for providing experimental technological assistance.
Mogi, M. , Kohara, K. , Nakaoka, H. , Kan‐no, H. , Tsukuda, K. , Wang, X‐L. , Chisaka, T. , Bai, H‐Y. , Shan, B‐S. , Kukida, M. , Iwanami, J. , Miki, T. , and Horiuchi, M. (2016) Diabetic mice exhibited a peculiar alteration in body composition with exaggerated ectopic fat deposition after muscle injury due to anomalous cell differentiation. Journal of Cachexia, Sarcopenia and Muscle, 7: 213–224. doi: 10.1002/jcsm.12044.
References
- 1. Kinney JM. Nutritional frailty, sarcopenia and falls in the elderly. Curr Opin Clin Nutr Metab Care 2004;7:15–20. [DOI] [PubMed] [Google Scholar]
- 2. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc 2011;12:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roubenoff R. Sarcopenic obesity: the confluence of two epidemics. Obes Res 2004;12:887–888. [DOI] [PubMed] [Google Scholar]
- 5. Kohara K, Ochi M, Tabara Y, Nagai T, Igase M, Miki T. Arterial stiffness in sarcopenic visceral obesity in the elderly: J‐SHIPP study. Int J Cardiol 2012;158:146–148. [DOI] [PubMed] [Google Scholar]
- 6. Dominguez LJ, Barbagallo M. The cardiometabolic syndrome and sarcopenic obesity in older persons. J Cardiometab Syndr 2007;2:183–189. [DOI] [PubMed] [Google Scholar]
- 7. Stephen WC, Janssen I. Sarcopenic‐obesity and cardiovascular disease risk in the elderly. J Nutr Health Aging 2009;13:460–466. [DOI] [PubMed] [Google Scholar]
- 8. Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res 2004;12:1995–2004. [DOI] [PubMed] [Google Scholar]
- 9. Ochi M, Tabara Y, Kido T, Uetani E, Ochi N, Igase M, et al Quadriceps sarcopenia and visceral obesity are risk factors for postural instability in the middle‐aged to elderly population. Geriatr Gerontol Int 2010;10:233–243. [DOI] [PubMed] [Google Scholar]
- 10. Buford TW, Anton SD, Judge AR, Marzetti E, Wohlgemuth SE, Carter CS, et al Models of accelerated sarcopenia: critical pieces for solving the puzzle of age‐related muscle atrophy. Ageing Res Rev 2010;9:369–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim TN, Park MS, Yang SJ, Yoo HJ, Kang HJ, Song W, et al Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: the Korean Sarcopenic Obesity Study (KSOS). Diabetes Care 2010;33:1497–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park SW, Goodpaster BH, Lee JS, Kuller LH, Boudreau R, de Rekeneire N, et al Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care 2009;32:1993–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Loon LJ, Goodpaster BH. Increased intramuscular lipid storage in the insulin‐resistant and endurance‐trained state. Pflugers Arch 2006;451:606–616. [DOI] [PubMed] [Google Scholar]
- 14. Komiya H, Mori Y, Yokose T, Kurokawa N, Horie N, Tajima N. Effect of intramuscular fat difference on glucose and insulin reaction in oral glucose tolerance test. J Atheroscler Thromb 2006;13:136–142. [DOI] [PubMed] [Google Scholar]
- 15. Pigeon E, Couillard E, Tremblay A, Bouchard C, Weisnagel SJ, Joanisse DR. Mid‐thigh subcutaneous adipose tissue and glucose tolerance in the Quebec family study. Obes Facts 2008;1:310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ingram KH, Lara‐Castro C, Gower BA, Makowsky R, Allison DB, Newcomer BR, et al Intramyocellular lipid and insulin resistance: differential relationships in European and African Americans. Obesity (Silver Spring) 2011;19:1469–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ingram KH, Hill H, Moellering DR, Hill BG, Lara‐Castro C, Newcomer B, et al Skeletal muscle lipid peroxidation and insulin resistance in humans. J Clin Endocrinol Metab 2012;97:E1182–E1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Therkelsen KE, Pedley A, Speliotes EK, Massaro JM, Murabito J, Hoffmann U, et al Intramuscular fat and associations with metabolic risk factors in the Framingham Heart Study. Arterioscler Thromb Vasc Biol 2013;33:863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garry DJ, Yang Q, Bassel‐Duby R, Williams RS. Persistent expression of MNF identifies myogenic stem cells in postnatal muscles. Dev Biol 1997;188:280–294. [DOI] [PubMed] [Google Scholar]
- 20. Iwanami J, Mogi M, Tsukuda K, Min LJ, Sakata A, Jing F, et al Effect of angiotensin II type 2 receptor deletion in hematopoietic cells on brain ischemia‐reperfusion injury. Hypertension 2011;58:404–409. [DOI] [PubMed] [Google Scholar]
- 21. Carlson ME, Suetta C, Conboy MJ, Aagaard P, Mackey A, Kjaer M, et al Molecular aging and rejuvenation of human muscle stem cells. EMBO Mol Med 2009;1:381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch‐mediated restoration of regenerative potential to aged muscle. Science 2003;302:1575–1577. [DOI] [PubMed] [Google Scholar]
- 23. Lee CE, McArdle A, Griffiths RD. The role of hormones, cytokines and heat shock proteins during age‐related muscle loss. Clin Nutr 2007;26:524–534. [DOI] [PubMed] [Google Scholar]
- 24. Giordani L, Puri PL. Epigenetic control of skeletal muscle regeneration: integrating genetic determinants and environmental changes. FEBS J 2013;280:4014–4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol 2010;12:143–152. [DOI] [PubMed] [Google Scholar]
- 26. Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, et al Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 2010;12:153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pretheeban T, Lemos DR, Paylor B, Zhang RH, Rossi FM. Role of stem/progenitor cells in reparative disorders. Fibrogenesis Tissue Repair 2012;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rodeheffer MS. Tipping the scale: muscle versus fat. Nat Cell Biol 2010;12:102–104. [DOI] [PubMed] [Google Scholar]
- 29. Xue JC, Schwarz EJ, Chawla A, Lazar MA. Distinct stages in adipogenesis revealed by retinoid inhibition of differentiation after induction of PPARgamma. Mol Cell Biol 1996;16:1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marchildon F, St‐Louis C, Akter R, Roodman V, Wiper‐Bergeron NL. Transcription factor Smad3 is required for the inhibition of adipogenesis by retinoic acid. J Biol Chem 2010;285:13274–13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. D'Souza DM, Al‐Sajee D, Hawke TJ. Diabetic myopathy: impact of diabetes mellitus on skeletal muscle progenitor cells. Front Physiol 2013;4:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Akhmedov D, Berdeaux R. The effects of obesity on skeletal muscle regeneration. Front Physiol 2013;4:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nguyen MH, Cheng M, Koh TJ. Impaired muscle regeneration in ob/ob and db/db mice. Scientific World Journal 2011;11:1525–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pillon NJ, Bilan PJ, Fink LN, Klip A. Cross‐talk between skeletal muscle and immune cells: muscle‐derived mediators and metabolic implications. Am J Physiol Endocrinol Metab 2013;304:E453–E465. [DOI] [PubMed] [Google Scholar]
- 35. Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med 2012;18:363–374. [DOI] [PubMed] [Google Scholar]
- 36. Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr 2000;71:885–892. [DOI] [PubMed] [Google Scholar]
- 37. Hilton TN, Tuttle LJ, Bohnert KL, Mueller MJ, Sinacore DR. Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: association with performance and function. Phys Ther 2008;88:1336–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tamilarasan KP, Temmel H, Das SK, Al Zoughbi W, Schauer S, Vesely P, et al Skeletal muscle damage and impaired regeneration due to LPL‐mediated lipotoxicity. Cell Death Dis 2012;3:e354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jang YC, Sinha M, Cerletti M, Dall'Osso C, Wagers AJ. Skeletal muscle stem cells: effects of aging and metabolism on muscle regenerative function. Cold Spring Harb Symp Quant Biol 2011;76:101–111. [DOI] [PubMed] [Google Scholar]
- 40. Dong Y, Silva KA, Zhang L. Glucocorticoids increase adipocytes in muscle by affecting IL‐4 regulated FAP activity. FASEB J 2014, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cordani N, Pisa V, Pozzi L, Sciorati C, Clementi E. Nitric oxide controls fat deposition in dystrophic skeletal muscle by regulating fibro‐adipogenic precursor differentiation. Stem Cells 2014;32:874–885. [DOI] [PubMed] [Google Scholar]
- 42. Saccone V, Consalvi S, Giordani L, Mozzetta C, Barozzi I, Sandona M, et al HDAC‐regulated myomiRs control BAF60 variant exchange and direct the functional phenotype of fibro‐adipogenic progenitors in dystrophic muscles. Genes Dev 2014;28:841–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Agley CC, Rowlerson AM, Velloso CP, Lazarus NR, Harridge SD. Human skeletal muscle fibroblasts, but not myogenic cells, readily undergo adipogenic differentiation. J Cell Sci 2013;126:5610–5625. [DOI] [PubMed] [Google Scholar]
- 44. Wu D, Ren Z, Pae M, Guo W, Cui X, Merrill A, et al Aging up‐regulates expression of inflammatory mediators in mouse adipose tissue. J Immunol 2007;179:4829–4839. [DOI] [PubMed] [Google Scholar]
- 45. Lefebvre AM, Laville M, Vega N, Riou JP, van Gaal L, Auwerx J, et al Depot‐specific differences in adipose tissue gene expression in lean and obese subjects. Diabetes 1998;47:98–103. [DOI] [PubMed] [Google Scholar]
- 46. Montague CT, Prins JB, Sanders L, Zhang J, Sewter CP, Digby J, et al Depot‐related gene expression in human subcutaneous and omental adipocytes. Diabetes 1998;47:1384–1391. [DOI] [PubMed] [Google Scholar]
- 47. Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, et al Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 2007;317:807–810. [DOI] [PubMed] [Google Scholar]
- 48. Fry CS, Lee JD, Mula J, Kirby TJ, Jackson JR, Liu F, et al Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat Med 2015;21:76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Krause MP, Al‐Sajee D, D'Souza DM, Rebalka IA, Moradi J, Riddell MC, et al Impaired macrophage and satellite cell infiltration occurs in a muscle‐specific fashion following injury in diabetic skeletal muscle. PLoS One 2013;8:e70971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Berria R, Wang L, Richardson DK, Finlayson J, Belfort R, Pratipanawatr T, et al Increased collagen content in insulin‐resistant skeletal muscle. Am J Physiol Endocrinol Metab 2006;290:E560–E565. [DOI] [PubMed] [Google Scholar]
- 51. Inoue M, Jiang Y, Barnes RH 2nd, Tokunaga M, Martinez‐Santibañez G, Geletka L, et al Thrombospondin 1 mediates high‐fat diet‐induced muscle fibrosis and insulin resistance in male mice. Endocrinology 2013;154:4548–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. KangL1 , Mayes WH, James FD, Bracy DP, Wasserman DH. Matrix metalloproteinase 9 opposes diet‐induced muscle insulin resistance in mice. Diabetologia 2014;57:603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kuri‐Harcuch W Differentiation of 3 T3‐F442A cells into adipocytes is inhibited by retinoic acid. Differentiation 1982;23:164–169. [DOI] [PubMed] [Google Scholar]
- 54. Berry DC, DeSantis D, Soltanian H, Croniger CM, Noy N. Retinoic acid upregulates preadipocyte genes to block adipogenesis and suppress diet‐induced obesity. Diabetes 2012;61:1112–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sharvit E, Abramovitch S, Reif S, Bruck R. Amplified inhibition of stellate cell activation pathways by PPAR‐gamma, RAR and RXR agonists. PLoS One 2013;8:e76541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ishizawa K, Izawa‐Ishizawa Y, Yamano N, Urushihara M, Sakurada T, Imanishi M, et al Nitrosonifedipine ameliorates the progression of type 2 diabetic nephropathy by exerting antioxidative effects. PLoS One 2014;9:e86335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of antibodies.
Table S2. List of primer sequences used for RT‐PCR analysis.
Figure S1. Comparison of tibialis anterior (TA) muscles of C57BL6 (WT) and KKAy with or without Ctx injection. Representative photos of TA muscle with or without Indian ink.
Figure S2. Fat formation and blood vessels. Hematoxylin‐eosin staining (upper panel) and immunofluorescent staining with anti‐CD31 and anti‐perilipin antibodies (lower panel) of tibialis anterior muscles in 26‐week‐old KKAy after Ctx treatment.
Supporting info item
Supporting info item


