Abstract
Atypical teratoid/rhabdoid tumor (AT/RT) is the most common malignant CNS tumor of children below 6 months of age. The majority of AT/RTs demonstrate genomic alterations in SMARCB1 (INI1, SNF5, BAF47) or, to a lesser extent, SMARCA4 (BRG1) of the SWItch/sucrose nonfermentable chromatin remodeling complex. Recent transcription and methylation profiling studies suggest the existence of molecular subgroups. Thus, at the root of these seemingly enigmatic tumors lies a network of factors related to epigenetic regulation, which is not yet completely understood. While conventional-type chemotherapy may have significant survival benefit for certain patients, it remains to be determined which patients will eventually prove resistant to chemotherapy and thus need novel therapeutic strategies. Elucidation of the molecular consequences of a disturbed epigenome has led to the identification of a series of transduction cascades, which may be targeted for therapy. Among these are the pathways of cyclin D1/cyclin-dependent kinases 4 and 6, Hedgehog/GLI1, Wnt/ß-catenin, enhancer of zeste homolog 2, and aurora kinase A, among others. Compounds specifically targeting these pathways or agents that alter the epigenetic state of the cell are currently being evaluated in preclinical settings and in experimental clinical trials for AT/RT.
Keywords: AT/RT, BAF47, epigenetics, INI1, rhabdoid tumor, SMARCB1, SNF5, SWI/SNF
Tremendous advances in technologies for cancer research have been seen in recent years—among many others, the tools for array hybridization, massive parallel sequencing, gene targeting, cell and tissue engineering, and, foremost, bioinformatics.1,2 The resulting data have altered our view on the mechanisms of cancer as such but have also modified some of the previously purely descriptive, morphology-based classification systems of malignancies toward more molecular and genetic assessments of cancer. Even more importantly, these findings have significant impact on the clinical management of affected patients.3
In 1978 Beckwith and Palmer4 initially described malignant rhabdoid tumors as a separate entity distinct from Wilms tumor on the basis of morphology. Subsequent en detail research recognized atypical teratoid/rhabdoid tumors (AT/RTs) as the CNS counterpart of rhabdoid tumors in the kidney and soft tissues.5 Further analyses have led to the elucidation of mutations in genes for components of the chromatin remodeling complex SWItch/sucrose nonfermentable (SWI/SNF) (foremost SMARCB1, rarely SMARCA4) as the only recurring theme.6,7 Rhabdoid tumors within and outside the CNS represent one end of a spectrum of malignancies characterized by a rather simple genome compared with other cancers, especially of adults, that may exhibit hundreds of mutations in an individual tumor.8 Most recently, profiling the transcript and epigenome has shed new light on AT/RT biology and suggests possible subgroup classification.9
While revisions of neuropathology taxonomy may not immediately impact clinical management, defining molecular mechanisms is a desperately needed key for treatment. The first phase I/II trials employing agents targeting molecular defects specifically designed for rhabdoid tumors are beginning to emerge, and the list of promising compounds is growing at a steady pace. AT/RTs are no longer enigmatic but rather increasingly understood malignancies that are approached in a systematic fashion from diagnosis to therapy.10
The Burden of Atypical Teratoid/Rhabdoid Tumors
Within registries (eg, the Central Brain Tumor Registry of the United States, with 16 044 children registered from 2007–2011), AT/RTs account for ∼40%–50% of all embryonal CNS tumors in the first year of life.11 Age-specific incidence rates decrease thereafter: 8.1 per million below 1 year, 2.2 at 1–4 years, 0.6 at 5–9 years, and close to zero at 10–14 years (http://www.kinderkrebsregister.de/dkkr/veroeffentlichungen/jahresbericht.html). The median age of onset in most series is ∼18 months. All series report a male predominance, with a 1.3 to 1.5 male to female ratio. AT/RT is the most common malignant CNS tumor in children below 1 year of age.11–13
Primary locations may be the CNS, peripheral nerve roots, kidneys, head and neck, paravertebral muscles, liver, mediastinum, retroperitoneum, bladder, pelvis, heart, scrotum, and subcutis.14–16 Rhabdoid tumors may occur synchronously in 2 or more locations, typically due to the patient carrying a germline SMARCB1 alteration.17 In most instances, one location will be the CNS.
Among 116 AT/RTs in the European Rhabdoid Registry (EU-RHAB), 57 (49%) were located within the cerebellum or IVth ventricle; 40 (34%) were located within the hemispheres including the basal ganglia; 5 (4%) each were detected in the mesencephalic and pineal regions, respectively; 2 (1.7%) were found in the spine; and 7 (6%) had extended across anatomic borders so that no clear origin could be determined (M. Frühwald, data from EU-RHAB). Metastases via cerebrospinal fluid are common and may be found in 20%–30% of cases at diagnosis.18
Demonstration of loss of the SWI/SNF-related matrix-associated actin-dependent regulator of chromatin B1 (SMARCB1) protein has tremendously helped in defining this entity and is close to pathognomonic for AT/RT.19 Rare AT/RTs with preserved SMARCB1 are on record, but novel entities such as CRINET (cribriform neuroepithelial tumor) have recently been described that also demonstrate inactivating mutations in SMARCB1.20,21 Previous reports on choroid plexus tumors and CNS primitive neuroectodermal tumors with lost SMARCB1 protein expression may in fact represent AT/RT variants with a less prominent rhabdoid component and more undifferentiated features.22
Patients with multiple primary rhabdoid tumors and those from rare families with more than one affected sibling have a genetic predisposition to a rhabdoid tumor due to an underlying germline copy number alteration or mutation in SMARCB1 or SMARCA4. Mutations of SMARCB1 in the germline have been documented in ∼25%–35% of patients with AT/RT, who are in general younger and exhibit more extensive disease.23,24 Despite the presence of a germline mutation, long-term survival has been recorded in some affected individuals.17,25 Apart from SMARCB1, SMARCA4 may be mutated in the germline.26 The majority of germline mutations appear de novo, and pedigrees with transmission across generations are rare.27,28 It is presumed that gonadal mosaicism accounts for familial cases with incomplete penetrance. Rhabdoid tumors have also been reported following in vitro fertilization, although it remains to be established whether the incidence is significantly increased.29
State of the Art Clinical Management of Atypical Teratoid/Rhabdoid Tumors
Survival rates for patients with AT/RT are generally poor but have improved over recent years (Table 1). This is due to the development of trials specifically designed for this entity and to an improvement in focus on the vulnerability of affected young children.30 A standard of therapy has yet to be defined.
Table 1.
Selection of larger data sets for patients with AT/RT in consistent registries and clinical trials
| Reference Time |
n | Age | M+ | Surgery (GTR) | Chemotherapy | Radiotherapy | Outcome | Comment |
|---|---|---|---|---|---|---|---|---|
| J. Hilden 200413 n.s. |
42 | 12 ≥ 3 y 20 < 3 y |
n = 9 | 20 | COG 99703 n = 8; CCG 9921 n = 6 and individual; n = 16 i.th. therapy; n = 13 HDCT |
n = 9 tumor bed; n = 4 CSI; various doses |
≥3 y: median EFS 16 mo; <2 y: median EFS 7.75 mo 2–3 y: median EFS 10.5 mo |
14 long-term survivors; GTR and older age prognostic |
| T. Tekautz 200512 1984–2003 |
31 | 9 ≥ 3 y 22 < 3 y |
6/31 | 21 | Non-uniform: ≥3 y, n = 7 SJMB96: <3 y, n = 7 BB98 and various others |
<3 y, n = 2 local, n = 1 CSI + boost ≥3 y, n = 7 CSI + boost |
<3 y: 2-y EFS ∼11% ± 6%; 2-y OS ∼17% ± 8% ≥3 y: 2-y EFS ∼78% ± 14%; 2-y OS ∼89% ± 11% |
Age >3 y prognostic; both long-term survivors received radiation |
| S. Chi 200933 2004–2006 |
20 | Median 26 mo 2.4 mo–9.5 y |
6/20 | 10 | IRS III–like | 11 conformal 4 CSI 4 none 1 off study 54 Gy focal, 36 Gy CSI + boost |
2-y PFS 53% ± 13% 2-y OS 70% ± 10% |
Only prospective phase II trial exclusively for AT/RT |
| K. von Hoff 201147 1988–2004 |
56 | Median 1.2 y 0.1–14 y | 26/56 | 18 | HIT medulloblastoma protocols |
n = 15 on primary therapy n = 14 at relapse 10/29 focal 19/29 CSI focal: 44.5–59.4 Gy CSI: 23.4–36.8 Gy |
3-y EFS: 13% ± 5% 3-y OS: 22% ± 6% |
Retrospective analysis of medulloblastoma cohort; age, achievement of CR, and M-disease prognostic |
| C. Dufour 2012116 1998–2008 |
58 | 38 ≤ 2 y | 17/58 10 < 2 y |
27 18 ≤ 2 y |
n = 24 ATRT04; n = 9 baby SFOP; n = 11 HDCT | Radiation in all but baby SFOP n = 16 7 ≤ 2 y |
Median OS 9 mo 1-y EFS: 17% 1-y OS: 41% |
Age <2 y, M+ disease and strong claudin; 6 staining negative prognosticators |
| L. Lafay-Cousin 201231 1995–2007 |
50 | 12 ≥ 3 y 21 = 1–3 y 17 < 1 y |
19/50 | 15 | 22 conventional; 18 HDCT eg, baby brain, IRS III–like, ICE; n = 9 anthracyclines |
21 as part of initial regimen; 6 at relapse |
2-y OS: 36.4 ± 7.7 median survival 9.6 mo in <1 y; 19.1 mo ≥ 3 y |
6/12 survivor no radiation HDCT: 2-y OS: 47.9% ± 12.1% convent.: 2-y OS 27.3% ± 9.5% |
| I. Slavc 201454 1992–2012 |
22 A: 9 B: 13 |
A: 24 mo B: 30 mo median age |
A: 4 B: 3 |
A: 5 B: 5 |
Cohort A: MUV (IRS III–like + HD-MTX + HDCT) cohort B: HIT-SKK, PEI, and others |
A: focal in all B: 3 focal, 4 CSI, 6 none |
Cohort A: 5-y OS 100%; 5-y EFS 88.9% ± 10.5% cohort B: 5-y OS and EFS: 28.8% ± 13.1% |
various i.th. regimens |
| K. Bartelheim submitted 2015 2005–2009 |
12 < 1 y; 11 @1–3 y; 8 ≥ 3 y | 6 | 10 | Rhabdoid 2007 = IRS III–like | <18 mo and in localized disease focal 54 Gy in CSI ≥ 18 mo 24 Gy + boost |
6-y OS: 46% ± 0.10% and 6-y EFS 45% ± 0.09% |
Germline mutation in 29%; age, achievement of CR prognostic |
Abbreviations: M+, metastatic; n.s., not specified; i.th., intrathecal; SFOP, French Society of Pediatric Oncology; HIT-SKK, Therapieprotokoll für Säuglinge und Kleinkinder mit Hirntumoren [Brain Tumor Radiotherapy for Infants and Toddlers with Medulloblastoma]; PEI, percutaneous ethanol injection; COG, Children's Oncology Group; SJMB, St Jude Medulloblastoma trial; ICE, ifosfamide/carboplatin/etoposide; IRS III, intergroup rhabdomypsarcoma study III.
Hilden et al13 reported that only 4 of 22 children with less than gross total resection (GTR) remained free of disease (21.5–96 mo from diagnosis). Lafay-Cousin and Hilden and their colleagues reported GTR in 30% (n = 50) and 48% (n = 42), respectively.13,31 A meta-analysis by Athale et al32 and reports by Chi et al33 and Lafay-Cousin and colleagues31 demonstrated improved survival rates for patients with GTR (19 vs 14.6 mo mean survival in complete vs partial resections, with 2-y overall survival [OS] of 60% ± 12.6% vs 21.7% ± 8.5%). Conversely, several cases are on record of long-term survival without radical surgery using aggressive multimodality regimens.33,34
The first successful therapy of AT/RT was reported by Olson et al35 in 3 patients who survived for more than 5 years ( F. Ruymann, personal communication).
The Children's Cancer Group study CCG-9921 identified a 1.5-fold lower risk of ensuing death in infants with progressive disease if they had received radiotherapy (AT/RT n = 28). Five-year event-free survival (EFS) for AT/RT was low at 14% ± 7%.36 Pai Panandiker et al37 reported 2-year progression-free survival (PFS) of 32.2% ± 10% and OS of 53.5% ± 10%, revealing delayed radiation therapy (>1 mo from surgery) as more likely to induce an event in 31 patients. Seventeen patients from Taipei (1990–2003) received craniospinal irradiation (CSI) ranging from 25.5 to 36 Gy or 36 Gy plus a focal boost up to 44 Gy for spinal seeds. Multivariate analysis demonstrated a significant prognostic role of both time from surgery to radiotherapy and time to radiotherapy completion.38 Lafay-Cousin and colleagues31 retrospectively reported 50 patients (1995–2007). Radiation at any time during therapy (adjuvant or salvage) significantly influenced survival (median survival 17.8 mo vs 14 mo; P = .64). In reviewing the Surveillance, Epidemiology, and End Results collection (1973–2008) of 144 patients, Buscariollo and colleagues18 verified a benefit of radiation when used as part of initial therapy. Curiously patients above 4 years of age experienced less benefit than younger patients.18
The significant risk for leukoencephalopathy or even overt radionecrosis in the vulnerable nervous system of infants, who may additionally have been treated with intraventricular methotrexate (MTX), raises the concern as to whether radiotherapy may be either postponed or even replaced by alternative therapeutic means such as high-dose chemotherapy (HDCT).39 In the series of 50 consecutive patients with AT/RT from the Canadian Paediatric Brain Tumor Consortium, a significant number (6/12) of surviving children had never received radiotherapy.31 In a correlation of molecularly defined AT/RT subgroups with clinical variables, Torchia et al9 speculated that a group of patients defined by expression of the Notch signaling pathway gene ASCL1 may have an improved outcome even without radiotherapy. This will have to be validated in future prospective clinical trials.
In another approach to minimize the side effects of radiotherapy, a major focus of research has been on the development of more focal and thus potentially less harmful radiotherapy (eg, proton beam radiotherapy).40–43 The Massachusetts General Hospital's experience enumerates 10 consecutive patients in whom proton therapy succeeded in sparing at-risk organs such as the hypothalamus and cochlea.40 Researchers at MD Anderson treated 31 patients by proton beam. Median PFS and OS were 20.8 and 34.3 months, respectively.41 Five patients developed radiation reactions in the brainstem necessitating use of bevacizumab or steroids. A series from Indianapolis demonstrated radiographic signs of radionecrosis in 3/3 patients with AT/RT.39 In a Swiss study (n = 15), 2-year OS and PFS were 64.6% and 66.0%, respectively. Furthermore, toxicity was encouraging, with no greater than grade 2 acute toxicity and an estimated 2-year toxicity-free survival of 90%. Using the PedsQoL tool, no decrease in quality of life was noted.44
Whether the long-term benefits (eg, avoidance of infertility, hypothyroidism, cardiac toxicity, and pulmonary fibrosis) will outweigh the risks of complications such as radionecrosis deserves investigation ideally in the frame of a controlled clinical trial.
Patients with AT/RT have displayed rather poor median survival when treated in protocols for other CNS tumors affecting the same age groups, such as medulloblastoma and CNS primitive neuroectodermal tumor (15.4 mo vs 156.4 mo; n = 11 and n = 121, respectively).45 Weinblatt and Kochen46 reported success employing focal radiotherapy (41.4 Gy) and rhabdomyosarcoma-based therapy in a single patient. In a follow-up, Olson et al35 published the long-term survivors mentioned above. Tekautz et al12 conveyed the experience comprising 31 patients at St Jude Children's Research Hospital. Eighty-nine percent of children below 3 years and thus the majority (n = 20) succumbed to the disease. In the German HIT-trials (1988–2004), 29 of 56 patients were <1.5 years old. Patients with metastases were typically younger. GTR was achieved in 18 cases. Three-year EFS and OS were 22% ± 6% and 13% ± 5% , respectively. Children who achieved complete remission (CR) following induction chemotherapy had higher OS than patients with less than CR. By multivariate analyses, age at diagnosis was the only independent prognostic factor.47 Currently the only published data from a formal trial specifically designed for AT/RT are from the Dana-Farber Cancer Institute (NCT00084838). Following intensive anthracycline-based induction chemotherapy including intraventricular chemotherapy, early radiotherapy was followed by continuation therapy. The OS and EFS rates at 2 years were 70% ± 10% and 53% ± 13%, respectively. Toxicity of the regimen was pronounced with 1 toxic death and severe adverse events such as transverse myelitis and radiation recall.33,48
As 2 primary rhabdoid tumors may present in a synchronous fashion, especially in patients with germline mutations, it appears judicious to target intra- as well as extracranial rhabdoid tumors (eg, the Third Intergroup Rhabdomyosarcoma Study [IRS III]).17 The EU-RHAB registry has included rhabdoid tumors regardless of anatomic origin since 2005 and demonstrated the feasibility of a multimodal regimen even in the youngest and in those with synchronous tumors.14 Data from the Dana-Farber Cancer Institute and EU-RHAB demonstrate OS and EFS rates of ∼45% (Table 1).
In 1998 Hilden and colleagues49 reported a series of patients who had undergone HDCT as an alternative consolidation. One of these patients remained alive 46 months from diagnosis. The HDCT regimens of Head Start (HS) I and HS II proved toxic, with 8 events of bacterial sepsis in 6 patients in HS I and in all 7 HS II patients. One patient in HS II died of bacterial meningitis. At the time of publication, 3 of 13 patients were alive (all HS II). The 3-year EFS was 43% ± 19%.50 In HS III (2003–2009), 5 intensive induction cycles with high-dose (HD) MTX in 3 cycles plus temozolomide, etoposide, vincristine, and cyclophosphamide in 2 were followed by second-look surgery and HDCT. Among 19 children, only 4 completed induction. Four survivors were noted at 40, 42, 46, and 79 months. Worrisome were 5 toxic deaths and a rather insufficient 3-year EFS of 21% ± 9% and OS of 26% ± 10%.51 Four of 6 patients treated in Toronto between 2003 and 2008 were alive with no evidence of disease after HDCT and a median follow-up of 52 months.34
A phase I trial using tandem HDCT with thiotepa plus BCNU in the first course and thiotepa plus carboplatin in the second included 2 patients with AT/RT. Both were alive more than 7 years post-HDCT.52 Lafay-Cousin et al31 reported 18 patients (45%) who received HDCT. Various induction regimens were utilized, essentially “baby brain protocols,” IRS III, intergroup rhabdomypsarcoma study III, and anthracycline-based approaches. As HDCT 4 patients received HS I consolidation therapy (see above), 7 received 3 sequential cycles of HD carboplatin and thiotepa, and another 7 an MTX-based induction followed by 3 sequential high doses of carboplatin and thiotepa. Patients with HDCT fared significantly better, achieving 2-year OS of 47.9% ± 12.1% (n = 18) compared with 27.3% ± 9.5% (n = 22). In a prospective phase I/II trial, Park et al53 reported a total of 9 patients (<3 y) treated with 6 courses of induction. Six children received tandem HDCT with carboplatin, thiotepa, and etoposide followed by cyclophosphamide and melphalan. All 5 survivors had received tandem HDCT and radiotherapy. Three-year OS was 53.3% ± 17.3%.53
In a series of 10 patients treated from 1997 to 2012 in an approach termed MUV-ATRT from the Medical University of Vienna, 5-year OS and EFS were reported as 100% and 88.9% ± 10.5%, respectively. The regimen consisted of three 9-week courses of doxorubicin, cyclophosphamide, vincristine, ifosfamide, cisplatinum etoposide, and HD-MTX augmented by intrathecal drugs (liposomal cytarabine, etoposide) and completed by HDCT similar to HS II. These data have not been confirmed in a multi-institutional setting but are quite provocative. Other HDCT approaches employing HD-MTX–based induction such as the HS III regimen have been much less successful.54
Investigators from EU-RHAB analyzed 19 patients who had undergone HDCT.55 Before HDCT 6 were in CR, 8 in partial remission, and 2 each had stable disease and progressive disease (in 1 no data). Fourteen patients progressed with a median follow-up time of 16 months. The EFS and OS at 2 years were estimated at 29% ± 11% and 50% ± 12%, respectively. It remains undetermined whether conventional or HDCT offers the greater survival benefit for affected patients. In line with this issue, the Children's Oncology Group since 2008 has been recruiting patients into a clinical trial employing induction chemotherapy with HD-MTX, focal radiotherapy in children as young as 6 months, and 3 cycles of HDCT with thiotepa and carboplatin followed by stem cell rescue (NCT00653068).
It has become evident that children with AT/RT should be treated in trials targeted specifically for this entity, potentially along with rhabdoid tumors in other anatomic locations. Aggressive multidrug regimens containing anthracyclines and alkylating agents may be viewed as standard induction chemotherapy measures, which along with resection (if possible, complete) offer the highest chance at a cure. It remains to be determined whether consolidation by HDCT and/or radiotherapy adds crucial survival benefit and/or improved functional outcome for affected patients.
The roles of intrathecal chemotherapy as a potential replacement for radiotherapy and maintenance therapy as an approach for relapse prevention remain currently unclear.
Advances in Understanding the Molecular Biology of AT/RT
In order to specifically target AT/RT with novel, potentially less toxic compounds, it is imperative to clearly understand the driving molecular mechanisms.
Mutation of the SWI/SNF Chromatin Remodeler Is the Central Event in AT/RT
Chromatin, the term for DNA wrapped around histone supports, plays an important role in gene regulation and is modified through movement of histones along the DNA strand, by control of degree of compaction, as well as by covalent modification of histones.56 Changes of the nucleosome, the most basic unit of chromatin consisting of DNA wrapped around a histone octamer, are mediated by the action of different multiprotein chromatin remodelers such as the SWI/SNF complex. The core component proteins of this complex (SMARCC1, SMARCC2, and SMARCB1) are complemented by a single ATPase (mutually exclusively either SMARCA4 or SMARCA2) and up to 15 known accessory subunits, potentially combining to hundreds of different versions of the complex.57 At least 9 subunit genes of the SWI/SNF complex are recurrently mutated in cancer, spanning malignancies from virtually every tissue type and collectively occurring in up to 20% of all human malignancies.24,58 A study by Kadoch et al58 determined SWI/SNF as the most frequently mutated chromatin remodeler in human cancer.
Murine knockout models of the core component SMARCB1 exhibit embryonic lethality in homozygously deleted animals and a predisposition to aggressive tumors, including those with classic rhabdoid histology, in heterozygotes.59–63 Interestingly, mice with a conditional biallelic inactivation of SMARCB1 employing a construct of the Mx1-Cre transgene rapidly develop mature CD8+ peripheral lymphomas with a short delay (median of 11 wk, compared with 20 wk following inactivation of tumor protein 53); however, no brain tumors have been observed.60 Recently a mouse model was developed with conditional inactivation of SMARCB1 using the Rosa26CreERT2 system (Han et al, submitted). SMARCB1 inactivation at early embryonal stages induced aggressive CNS tumors resembling human AT/RT, with complete penetrance within a short time frame (median 2.5 mo). Notably, tumors form only when SMARCB1 is inactivated before day E9 of development, raising the possibility that the cell of origin might exist only during fetal development. Genetically engineered homozygous knockout mice for SMARCA4 are embryonic lethal, while heterozygous animals do not show rhabdoid tumors but develop gynecologic and pulmonary neoplasms.64,65 In an attempt to specify the cell of origin for AT/RT, Moreno et al66 deleted SMARCB1 and SMARCA4 in cerebellar granule neurons of transgenic mice. While these animals demonstrated a severely hypomorphic cerebellum and neurologic deficits, they did not develop tumors, arguing against a cerebellar granule cell origin for AT/RT.66
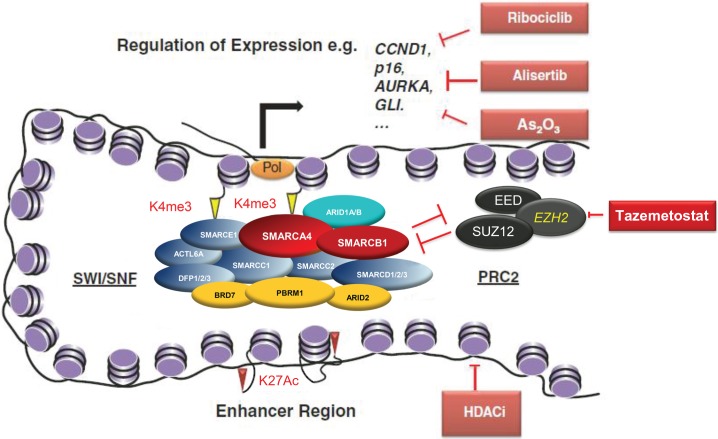
Recent functional analyses demonstrated complex contributions of SWI/SNF to chromatin structure and gene regulation. Tolstorukov and colleagues67 explored the interactions of SWI/SNF and chromatin at transcription start sites (Fig. 1). They revealed a landscaping function of SWI/SNF at promoter regions, a role that may also function at enhancers and superenhancers to modulate transcription.67,68 Previous work by Kassabov and others had indicated roles for SWI/SNF in the directional unwrapping of DNA from nucleosomes as well as sliding in cis and nucleosome transfer.69 Employing protein screening and short interfering RNA profiling techniques, it was established that positioning of the SWI/SNF complex at nucleosomes is mediated by interactions with Argonaute2 (AGO2), a protein known to participate in heterochromatin assembly, and a new class of sRNAs. These swiRNAs apparently determine nucleosome occupancy at transcription start sites by recruiting SWI/SNF complexes and AGO2.70 Furthermore, SWI/SNF complexes appear to fulfill central functions in the assembly of nuclear bodies such as paraspeckles, subnuclear structures involved in stress responses. Subunits of SWI/SNF interact with long noncoding RNAs, and inactivation of SWI/SNF components resulted in paraspeckle disintegration.71
Fig. 1.
Epigenetic roles of SMARCB1 in promoter activation, occupancy, and enhancer modification, including potential therapeutic targeting. The SWI/SNF complex modulates gene transcription including by (i) regulation of nucleosome positioning at promoters and consequently transcription factor binding and accessibility to the transcription machinery (Pol), (ii) antagonism of the Polycomb repressive complex 2 (PRC2), thus opposing the repressive H3k27Me3 mark written by PRC2 and favoring an active H3k4Me3 modified promoter, and (iii) localization at enhancers where the complex may contribute to transcription regulation, although the mechanism remains poorly understood. The SWI/SNF complex is bound at most active genes and the transcription of many genes are affected by SMARCB1 loss. Among others these include CCND1, GLI1, and AURKA, which may be therapeutically targeted by ribociclib, arsenic trioxide, and alisertib, respectively. The chromatin antagonizing effects of EZH2 may be therapeutically targeted by tazemetostat.
AT/RTs Display a Lack of Genomic Alterations but Comprise Molecularly Defined Subgroups
Tumors in children on average display fewer mutations than those in adults. In a multiplatform approach employing single nucleotide polymorphism–based oligonucleotide arrays, multiplex ligation-dependent probe amplification, fluorescence in situ hybridization, and sequence analysis, biallelic alterations of SMARCB1 located in chromosome 22q11.2 were detected in 50 of 51 rhabdoid tumors. SMARCB1 inactivation was due to a variety of mechanisms, such as deletions, mutations, and loss of heterozygosity, thus making it the single most important recurrent potentially driving mutation in rhabdoid tumors.72 Experiments using a molecular inversion probe single nucleotide polymorphism assay, whole-exome sequencing, and OncoMap (a mass spectrometric method for allele detection) demonstrated the absence of recurrent genomic alterations other than in SMARCB1 in a combined 76 AT/RTs.6,7,73
As mutations of genes other than SMARCB1/SMARCA4 are exceedingly rare in AT/RT, scanning approaches have been employed to detect further genomic, epigenomic, and transcriptional changes. In a modifier screen of a Drosophila model for SMARCB1-negative AT/RT, Jeibmann and colleagues74 identified changes in members of the Notch and Hippo signaling pathways, with roles in neural development and cell-cell interactions.
Birks et al75 had analyzed the microarray expression profiles of 18 AT/RTs and identified 4 molecularly defined major subgroups. Survival differed significantly among the different groups. Significant upregulation of genes of the bone morphogenic protein signaling cascade (ie, BMP4) was detected in the group with the lowest survival.
More recently, by employing DNA methylation or gene expression analyses, 3 distinct subgroups of AT/RT were described by Johan and colleagues. Whole-genome DNA and RNA sequencing found no other recurrent mutations explaining the differences among subgroups; however, whole-genome bisulfite sequencing furthermore confirmed differences in methylation patterns among the subgroups (Johan et al, submitted).
Utilizing gene expression and high-resolution copy number analyses, Torchia and colleagues9 identified 2–3 distinct AT/RT subgroups correlating not only with anatomic location within the CNS, but also with clinical features. Expression of the gene ASCL1 involved in the Notch activation cascade was more common in supratentorial AT/RT and associated with improved 5-year OS rates (34% vs 9% for achaete-scute homolog 1 [ASCL1] positive vs negative tumors).
Whether the molecular heterogeneity of AT/RT may also explain the differences seen in response to treatments needs to be validated in clinically annotated and equally treated AT/RT cohorts.
Innovative Treatment in AT/RT: Compound-Driven Approaches
A first description of resistance to cytostatic agents followed by a confirmatory report demonstrated doxorubicin and actinomycin-D as the only compounds with a sizable response.76,77 Employing in vitro proliferation assays, Lunenburger et al. demonstrated in vitro activity of vinorelbine, sorafenib, rapamycin, and the herbal compound curcumin. Another group utilized antisense oligonucleotides against insulin-like growth factor 1 receptor to establish chemosensitization against cytostatics such as cisplatin and doxorubicin.78
As opposed to the above-mentioned single system approaches, systematic preclinical testing programs such as the PPTP (Pediatric Preclinical Testing Program) have detected responses to conventional and targeted drugs in vitro and in vivo in a series of compounds including multiple tyrosine kinase inhibitors such as sorafenib and mammalian target of rapamycin inhibitors as well as oncolytic viruses and others (Tables 2 and 3). Testing combinations of tyrosine kinase inhibitors with topoisomerase 1 inhibitors, synergistic growth inhibition was observed in 2 cell lines.79 As primary AT/RTs (18/23) expressed high levels of the stem cell factors LIN28A/B, Weingart and colleagues80 evaluated signaling pathways such as the mitogen-activated protein kinase cascade. They presented excellent in vitro responses of AT/RT cell lines to the selective mitogen/extracellular signal-regulated kinase (MEK) inhibitor selumetinib. MEK inhibitors that potentially penetrate the blood–brain barrier are in phase I trials for children (NCT02124772).
Table 2.
Phase I/II trials recruiting patients with AT/RT
| Phase | Title | Status | IND | Target/Comment |
|---|---|---|---|---|
| I | Simvastatin with Topotecan and Cyclophosphamide in Relapsed and/or Refractory Pediatric Solid and CNS Tumors (Aflac ST1402) | Recruiting | Simvastatin | HMG CoA reductase |
| I | p28 in Treating Younger Patients with Recurrent or Progressive Central Nervous System Tumors | Recruiting | p28 | Azurin-derived cell-penetrating peptide p28 |
| I/II | Molecular-Guided Therapy for Childhood Cancer | Recruiting | n.a. | Guided therapy |
| I/II | Crizotinib in Treating Young Patients with Relapsed or Refractory Solid Tumors or Anaplastic Large Cell Lymphoma | Recruiting | Crizotinib | ALK-, MET-, ROS1-tyrosine kinases |
| II | Phase 2 Study of Alisertib Therapy for Rhabdoid Tumors | Recruiting | Alisertib | Aurora kinase A |
| II | Iodine I 131 Monoclonal Antibody 3F8 in Treating Patients with Central Nervous System Cancer or Leptomeningeal Cancer | Recruiting | 3F8-I131 | Gd2 |
| III | Risk-Adapted Therapy for Young Children with Embryonal Brain Tumors, Choroid Plexus Carcinoma, High Grade Glioma or Ependymoma | Recruiting | n.a. | Feasibility study |
| I | Study of Safety and Efficacy in Patients with Malignant Rhabdoid Tumors (MRT) and Neuroblastoma | Active, not recruiting | Ribociclib | Cyclin D1/CDK4, 6 |
| I | Aflac ST0901 CHOANOME Sirolimus in Solid Tumors | Active, not recruiting | Sirolimus | mTOR |
| I | AZD2171 in Treating Young Patients with Recurrent, Progressive, or Refractory Primary CNS Tumors | Active, not recruiting | Cediranib | VEGFR 1–3 |
| I/II | Methotrexate Infusion into the Fourth Ventricle in Children with Malignant Fourth Ventricular Brain Tumors: A Pilot Study | Active, not recruiting | Methotrexate | DHFR inhibition |
| I/II | Dasatinib, Ifosfamide, Carboplatin, and Etoposide in Treating Young Patients with Metastatic or Recurrent Malignant Solid Tumors | Active, not recruiting | Dasatinib | BCR/ABL and SRC-TK |
| I | ABT-888 and Temozolomide in Treating Young Patients with Recurrent or Refractory CNS Tumors | Completed | Veliparib | PARP inhibitor; DNA repair inhibition |
| I | Vorinostat with or without Isotretinoin in Treating Young Patients with Recurrent or Refractory Solid Tumors, Lymphoma, or Leukemia | Completed | Vorinostat | HDAC |
| I | Vorinostat and Temozolomide in Treating Young Patients with Relapsed or Refractory Primary Brain Tumors or Spinal Cord Tumors | Completed | Vorinostat | HDAC |
| I | Lenalidomide in Treating Young Patients with Recurrent, Progressive, or Refractory CNS Tumors | Completed | Lenalidomide | Immune modulation |
| I | Talabostat Combined with Temozolomide or Carboplatin in Treating Young Patients with Relapsed or Refractory Brain Tumors or Other Solid Tumors | Completed | Talabostat | Inhibitor of dipeptidyl peptidase IV |
| I | SCH 66336 in Treating Children with Recurrent or Progressive Brain Tumors | Completed | Lonafarnib | Farnesyl transferase |
| I | Temozolomide, Vincristine, and Irinotecan in Treating Young Patients with Refractory Solid Tumors | Completed | Irinotecan | Conventional chemotherapy |
| II | Oxaliplatin in Treating Children with Recurrent or Refractory Medulloblastoma, Supratentorial Primitive Neuroectodermal Tumor, or Atypical Teratoid Rhabdoid Tumor | Completed | Oxaliplatin | Cytostatic |
| I | Gamma-Secretase Inhibitor RO4929097 in Treating Young Patients with Relapsed or Refractory Solid Tumors, CNS Tumors, Lymphoma, or T-Cell Leukemia | Terminated | RO4929097 | γ-Secretase |
| I | MK0752 in Treating Young Patients with Recurrent or Refractory CNS Cancer | Terminated | MK0752 | γ-Secretase inhibitor, which reduces Aβ40 production |
| Trials involving combinations of conventional chemotherapy (incl. HDCT) | ||||
| III | Combination Chemotherapy, Radiation Therapy, and an Autologous Peripheral Blood Stem Cell Transplant in Treating Young Patients with Atypical Teratoid/Rhabdoid Tumor of the Central Nervous System | Ongoing, not recruiting | NCT00653068 | |
| III | Treatment of Patients with Newly Diagnosed Medulloblastoma, Supratentorial Primitive Neuroectodermal Tumor, or Atypical Teratoid Rhabdoid Tumor | Ongoing, not recruiting | NCT00085202 | |
| I | Chemotherapy Plus Peripheral Stem Cell Transplantation in Treating Infants with Malignant Brain or Spinal Cord Tumors | Completed | NCT00003141 | |
| I | Chemotherapy and Stem Cell Transplantation in Treating Children with Central Nervous System Cancer | Completed | NCT00053118 | |
| III | Combination Chemotherapy Followed by Second-Look Surgery and Radiation Therapy in Treating Children with Nonmetastatic Medulloblastoma or Primitive Neuroectodermal Tumor | Completed | NCT00006461 | |
| III | Combination Chemotherapy with or without Etoposide Followed by an Autologous Stem Cell Transplant in Treating Young Patients with Previously Untreated Malignant Brain Tumors | Unknown | Head Start III | NCT00392886 |
| III | Intrathecal and Systemic Chemotherapy Combined with Radiation Therapy in Treating Young Patients with Newly Diagnosed Central Nervous System Atypical Teratoid/Rhabdoid Tumors | Unknown |
NCT00084838 Chi et al, JCO 2009 |
|
Abbreviations: IND, investigational new drug; HMG CoA, 3-hydroxy-3-methylglutaryl coenzyme A; ALK, anaplastic lymphoma kinase; VEGFR, vascular endothelial growth factor receptor; DHFR, dihydrofolate reductase; PARP, poly(ADP-ribose) polymerase; DNA, desoxyribonucleic acid; n.a., depending on the identified target; BCR/ABL and SRC-TK, BCR/ABL and SRC tyrosine kinases.
Table 3.
Preclinical and clinical aspects of selected targeted compounds for rhabdoid tumor therapy
| Compound | Target | Xenograft Response | Date | Ref. | Translational Aspects |
|---|---|---|---|---|---|
| Irofulven | Alkylating agent | 3/6 CR; 3/6 PR | 2002 | PPTP | Derivative of mushroom toxin illudin; mechanism not clear |
| Gefitinib | EGFR family | 2/2 CR | 2004 | 113 | No clinical trials in rhabdoid tumors |
| Dasatinib | BCR/ABL-, SRC-TK | No effect (1 cell line sensitive) | 2007 | PPTP | In clinical trials for rhabdoid tumors |
| Rapamycin | mTORC1 inhibition | 1/1 PR | 2007 | PPTP | In clinical trials for rhabdoid tumors |
| ABT-751 | Tubulin binding agent | 1/2 SD, 1/2 PD | 2008 | PPTP | 7% OR in phase I trial on neuroblastoma |
| Sunitinib | Multi-TK inhibitor | 1/3 CR | 2008 | PPTP | Response in an adult with rhabdoid renal cell carcinoma |
| Flavopiridol | CDK4,6 and cyclin D1 | 1/1 OR | 2008 | 114 | Phase I with related compound ribociclib completed |
| Ispinepib | Kinesin spindle protein inhibitor | 1/2 CR | 2009 | PPTP | Phase I in children completed; no rhabdoid patients included |
| Seneca Valley Virus | Oncolytic virus | 1/3 OR | 2010 | PPTP | Phase I in adults completed, phase II on lung cancer active |
| MLN8237 | Aurora kinase A | 1/3 CR; 1/3 SD | 2010 | 92 | Phase I/II; compound: alisertib |
| GSK923295A | CENP-E inhibition | 2/3 CR | 2011 | PPTP | Phase I in adults completed; no trials for children |
| AZD8055 | mTORC1 and 2 inhibition | 1/2 PR | 2012 | PPTP | Phase I in adults completed; no trials for children |
| Genz-644282 | Topoisomerase 1 | 1/1 PD | 2012 | PPTP | Phase I in adults completed; no trials for children |
| Lapatinib | EGFR | 1/1 CR | 2013 | 81 | Phase II in children with ependymoma completed |
| EPZ-6438 | EZH2 | 1/1 CR | 2013 | 104 | Phase I in adults recruiting |
| SAHA | HDAC | n.a. | 2013 | 107 | In phase I trials in children (combination therapy) |
| NVP-BG398 | FGFR | 1/1 OR | 2013 | 115 | No clinical trials yet; |
| As2O3 (ATO) | GLI1 | 1/1 CR | 2014 | 96 | multiple phase I/II trials in children; no clinical trials in rhabdoid tumors |
| JQ1 | BRD4 | n.a.a | 2014 | 108 | Bromodomain inhibitors in phase I trials in adults |
Abbreviations: PR, partial response; SD, stable disease; PD, progressive disease; CENP-E, centrosome-associated protein E; EGFR, epidermal growth factor receptor; mTORC, mammalian target of rapamycin complex; TK, tyrosine kinase; FGFR, fibroblast growth factor receptor; BRD4, bromodomain containing protein 4; OR, objective response; BCR/ABL-, SRC-TK, BCR/ABL- and SRC tyrosine kinases.
PPTP: results may be viewed at http://gccri.uthscsa.edu/pptp/.
aCompound tested in HH-driven medulloblastomas.
Major drawbacks of the aforementioned analyses are the inherent inefficiencies of testing drugs in cell lines (eg, culture dependency, selection) and dependent xenografts. Not surprisingly, a whole array of compounds prompt responses in the cell line KT16 in vitro and in vivo, while very few affect the AT/RT-derived BT29 (http://gccri.uthscsa.edu/pptp/).
On a broader scale, researchers from POETIC (the Pediatric Oncology Experimental Therapeutics Investigators’ Consortium) profiled 129 small-molecule inhibitors in 3 AT/RT-derived cell lines. While these authors presented only data on the efficacy of the compound lapatinib, a dual epidermal growth factor receptor/Her-3 neu inhibitor, they present a vast amount of information on compounds yet to be tested (see Table 1).81
As an asset to cells grown in culture and/or xenografts, patient-derived orthotopic tumor models have been employed. Girard et al82 recently provided efficacy data of the drug cabazitaxel in patient-derived orthotopic tumor models of embryonal CNS tumors including AT/RT.
Innovative Treatment in AT/RT: Mechanism-Driven Approaches
Only very recently have phase I/II trials specifically aimed at AT/RT been introduced, in part due to the elucidation of involved signaling pathways (Table 2).
Changes in the function of the SWI/SNF complex may affect a whole array of signal transduction cascades (eg, cyclin-dependent kinases [CDKs] 4 and 6/cyclin D1/retinoblastoma, Sonic Hedgehog [SHH], Polycomb complexes) with significance for the origin and/or spread of tumors.24,83,84 Proteomic, bioinformatic, and functional epigenetic analyses indicate that up to one third of all promoters of genes may be bound by SWI/SNF complexes.58,64,67
Targeting Cell Cycle Regulators in Rhabdoid Tumors
Evaluating a cohort of infant brain tumors, Fujisawa et al85 detected overexpression of cyclin D1 in AT/RT. Experimental reintroduction of SMARCB1 into cell lines resulted in G0/G1 cell cycle arrest, repression of cyclin D1, induction of p16INK4A, and hypophosphorylation of retinoblastoma, thus offering a growth advantage via release of the oncogene E2F.86,87 Crossing mice negative for cyclin D1–/– with those heterozygous for SMARCB1–/+, the occurence of rhabdoid tumors was abolished.63 Not surprisingly pan-CDK inhibitors such as flavopiridol combined with tamoxifen affected cyclin D1 and inhibited rhabdoid tumor cell growth.88,89 However, in a murine xenograft model, animals bearing tumors with high levels of cyclin D1 demonstrated resistance to flavopiridol.89 Recently a clinical trial, the results of which are pending, employed the CDK4/6 inhibitor ribociclib in patients with rhabdoid tumors, neuroblastomas, and CDK4-amplified malignancies (Table 2). It is to be anticipated that such a compound may be used in a combinatorial trial with conventional chemotherapy.
Aurora Kinase A Inhibitors for AT/RT Treatment
Tyrosine kinases are expressed in cell lines and in primary rhabdoid tumor samples. Imatinib reduced rhabdoid cell growth in vitro.90 Genome-wide analyses demonstrated that the serine/threonine kinase aurora A is highly expressed in rhabdoid tumors. Reexpression of SMARCB1 downregulates aurora A by repression of promoter activity. Targeting aurora A in rhabdoid tumor cell lines by short interfering (si)RNA or small-molecule inhibitors induced cell death in vitro.91 Rhabdoid tumor xenograft models demonstrated intermediate to strong responses to the inhibitor MLN8237.92 Aurora A inhibition enhanced radiation sensitivity in rhabdoid tumor cell lines, making this compound attractive for combined therapy.93
The aurora kinase inhibitor MLN8237 is currently in clinical trials in phase I/II for different tumor entities in adults and children (Table 2). Employing single agent MLN8237, also known now as alisertib, has produced noteworthy responses. Four patients affected by relapsed or progressive AT/RT received 80 mg/m2 alisertib by mouth. All 4 displayed disease stabilization and/or regression of tumors and 2 are alive 1 and 2 years, respectively, on therapy.94 A trial combining alisertib with conventional therapy in newly diagnosed patients with rhabdoid tumors is currently recruiting patients (NCT02114229).
Hedgehog/GLI Inhibition as a Window of Therapeutic Opportunity
Employing chromatin immunoprecipitation assays, Jagani et al95 determined that SMARCB1 and GLI1 were enriched at regions upstream of the transcriptional start sites of both promoters. They furthermore demonstrated that SMARCB1 directly interacts with GLI1 on a protein level and that loss of SMARCB1 increased expression of GLI1 up to 10-fold in cell lines.95 Furthermore GLI1 was upregulated in primary AT/RT compared with control samples. As expected, inhibitors of Patched or Smoothened (eg, LDE225) do not show any inhibiting effect on rhabdoid tumor cell growth, as activation of the SHH pathway mediated by GLI1 in these tumors is downstream of the receptors Patched and Smoothened.95 Using cytotoxicity assays, xenograft models, and siRNA targeting, Kerl et al96 demonstrated that the GLI1 inhibitor arsenic trioxide (As2O3) may significantly inhibit the growth of Hedgehog-driven rhabdoid cells in vitro and in vivo.96 Linking the HH/GLI pathway with epigenetic changes, Tang et al97 demonstrated excellent control of HH-driven tumors by employing the bromodomain and extraterminal inhibitor JQ1.97 It appears that bromodomains recognize lysine residues in the N-terminal tails of histone H3. Bromodomains containing complexes often localize with the promoter of proproliferative genes such as the oncogene Myc. It has been demonstrated that SMARCA4 antagonizes Myc activity.98
Wnt/ß-Catenin Signal Alterations in AT/RT
Similar to canonical Wnt signaling, the SWI/SNF complex has repeatedly been implicated in lineage specification, proliferation, and differentiation. By the use of conditional mouse and cell culture models, Mora-Blanco et al99 demonstrated that inactivation of SMARCB1 leads to aberrant body patterning due to overexpression of the Wnt/ß-catenin pathway. Employing the bioinformatics tool of Gene Set Enrichment Analysis (GSEA), these authors exposed an elevation of Wnt-target genes in SMARCB1-deficient rhabdoid tumors. Upon reexpression of SMARCB1, ß-catenin target genes such as AXIN2, APC, ßTRCP, LEF1, and HDAC4 were inhibited.
Analogous to the dependencies of the Hedgehog/GLI pathway, classic upstream inhibition of the ß-catenin pathway did not result in a repression of colony formation or proliferation, suggesting activation of the pathway independently of the canonical Wnt pathway, and more likely on a chromatin and transcriptional level. These experiments underline a tissue- and context-associated regulation of the Wnt pathway by the SWI/SNF complex.
Employing transcriptome sequencing analyses, significant upregulation of WNT5B was revealed. SiRNAs against the gene induced a decrease in expression of the genes for the receptors FRIZZLED 1 and RYK. It furthermore influenced cell viability significantly.100
Therapeutic Approaches Targeting the Histone Trimethylase Enhancer of Zeste Homolog 2
It has been demonstrated that SWI/SNF and the Polycomb complex PRC2 possess largely antagonistic properties.57,101,102 Expression experiments demonstrated upregulation of the histone trimethylase EZH2 in SMARCB1-deficient rhabdoid tumors. On further experimentation it was shown that SMARCB1 loss led to an added upregulation of EZH2, widespread trimethylation of histone H3K27, and repression of p16INK4. GSEA comparing primary rhabdoid tumors and normal brain indicated a set of H3K27 modified target genes defined from embryonic stem cells which were negatively enriched in rhabdoid tumors.101
Consistent with these findings, Unland et al103 employed the antagonist of enhancer of zeste homolog 2 (EZH2), DZNep (3-Deazaneplanocin A), a nonspecific S-adenosylhomocysteine hydrolase analogue, alone and combined with drugs such as doxorubicin and etoposide or epigenetically active compounds like the methylation inhibitor 5-Aza-CdR or the histone deacetylase (HDAC) inhibitor suberoylanilide hydroxamic acid (SAHA). Proliferation assays in rhabdoid cell lines demonstrated that DZNep synergistically and significantly enhanced the antiproliferative activity of etoposide, 5-Aza-CdR, and SAHA. Pretreatment with DZNep significantly increased the effects of 5-Aza-CdR and SAHA on apoptosis, cell cycle progression, and clonogenicity. Microarray analyses following sequential treatment with DZNep and 5-Aza-CdR or SAHA revealed changes in global gene expression affecting apoptosis, neuronal development, and metabolic processes. Even closer to the clinical situation were experiments using the EZH2 inhibitor EPZ-6438 (tazemetostat) in vitro and in vivo. Suppression of H3K27 trimethylation (H3K27Me3) induced selective cell killing of human lymphoma cells bearing point mutations in the EZH2 catalytic domain.104 In a murine xenograft model, therapy of EZH2-mutant xenografts with EPZ-6438 caused dose-dependent tumor growth inhibition, including complete and sustained tumor regression with a corresponding reduction in H3K27Me3 levels in tumors and normal tissues. Mice that received the drug for 28 days stayed tumor free for up to 63 days after the compound was stopped. Treatment of mice carrying rhabdoid xenografts with an EZH2 inhibitor was also shown to be beneficial.104 Furthermore it was demonstrated that EZH2 inactivation increased the radiosensitivity of rhabdoid tumor cells.105 A preliminary report of a phase I trial result revealed a complete response in the first rhabdoid tumor patient enrolled. Consequently a clinical trial employing EPZ-6438 in children with rhabdoid tumors is in the planning stages.106
Histone Alterations as Targets for Experimental Therapy
Histone modifications are also affected by SWI/SNF complexes.56,57 Alterations in enzymes influencing the epigenome often cause global effects on chromatin. Acetylation of histones facilitates accession of transcription factors to chromatin.57 The acetylation status of histones is controlled by 2 different groups of enzymes: histone acetyltransferases and HDACs. Targeting histone acetylation presents an attractive tool for AT/RT, as HDAC1 and HDAC2 are overexpressed in primary tumors and cell lines. To evaluate whether inhibitors of HDACs might influence the proliferation of rhabdoid tumor cell lines, Kerl et al107 employed proliferation assays, apoptosis detection, cell cycle analysis, and RNA expression. They detected synergistic actions of the HDAC inhibitor SAHA with fenretinide, tamoxifen, and doxorubicin.107 Targeting HDAC induced cell cycle arrest and apoptosis. GSEA disclosed deregulation of gene sets derived from MYCC, retinoblastoma 1, and the stem cell clusters in treated cells. It appears conceivable that some of the novel HDAC inhibitors might be assets in the treatment of affected patients. While SAHA has successfully been evaluated as a radiosensitizer in a xenograft model,108 other compounds such as panobinostat and resminostat offer potentially favorable pharmacokinetic and pharmacodynamic properties suitable especially for small children.109,110
Chromatin-directed therapy approaches
While at least 9 different subunits of the SWI/SNF complex are recurrently mutated in cancer, emerging data raise the possibility that these mutations do not inactivate the complex but rather result in a dysfunctional residual complex. In the case of cancer cell lines carrying mutations in either the ARID1A or SMARCA4 subunits, it has been shown that these mutations result in related SWI/SNF subunits becoming preferentially essential, and therefore potentially a therapeutic vulnerability. The residual SWI/SNF complex has also been shown to be essential for the growth of SMARCB1 mutant rhabdoid tumors. Whether the residual complex may constitute a therapeutic target remains thus far speculative.57,111,112
Conclusion
It has been recognized that rhabdoid tumors represent a model disease for malignancies not purely based on genomic mutational events but also on epigenetic alterations. Investigations of signaling pathways altered in AT/RT have yielded a whole array of compounds with potential therapeutic activity.81 Recently phase I/II trials have made use of this knowledge and are beginning to enroll affected patients. Major obstacles for rapid success are the time-intensive evaluation process for novel targeted drugs, the rarity of the disease, and the highly vulnerable population of very young children affected by an aggressive malignancy which, once proven resistant to conventional therapy, may grow at a pace too quickly for enrollment into a clinical trial. Additionally it remains to be determined which patients respond to conventional-type chemotherapy and which exhibit primary chemotherapy resistance.
As information about potential subgroups and potential drug targets evolves, international collaborations are highly desirable. Recruiting enough patients in a stratified fashion according to novel biomarkers will benefit from international consortia and the use of new statistical approaches in order to test new compounds in a rapid succession.
Despite these challenging obstacles, the pace of new developments and the newly awakened international interest in pediatric cancer—as reflected, for instance, by international funding initiatives—raise high hopes for a steady improvement in outcome for these severely affected patients.
Funding
M.C.F. is supported by funds from the Deutsche Kinderkrebsstiftung, the Gesellschaft für Kinderkrebsforschung, the parents organization Horizonte Weseke, as well as the parent's organization Lichtblicke to the European Registry for Rhabdoid Tumors.
J.A.B. acknowledges the support of a grant from the National Institutes of Health (R0146274).
F.B. acknowledges INSERM U830, which is supported by the Ligue Nationale contre le Cancer; the rhabdoid team of the Curie Institute SiRIC, supported by the Société Française des Cancers de l'Enfant (SFCE), the Federation Enfants et Santé, and the associations Abigael, Marabout de Ficelles, and Hubert Gouin-Enfance et Cancer.
C.W.M.R. was supported by National Institutes of Health grants R01CA172152 and R01CA113794 and received additional support from the Cure AT/RT Now Fund, the Avalanna Fund, the Garrett B. Smith Foundation, Miles for Mary, the Claudia Adams Barr Foundation, and Alex's Lemonade Stand.
S.N.C. was supported in part by a grant from the Cookies for Kids' Cancer Foundation and the Cure AT/RT Now Fund.
Conflict of interest statement. None declared.
References
- 1.Keung AJ, Joung JK, Khalil AS et al. Chromatin regulation at the frontier of synthetic biology. Nat Rev Genet. 2015;16(3):159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garraway LA, Lander ES. Lessons from the cancer genome. Cell. 2013;153(1):17–37. [DOI] [PubMed] [Google Scholar]
- 3.Janeway KA, Place AE, Kieran MW et al. Future of clinical genomics in pediatric oncology. J Clin Oncol. 2013;31(15):1893–1903. [DOI] [PubMed] [Google Scholar]
- 4.Beckwith JB, Palmer NF. Histopathology and prognosis of Wilms tumors: results from the First National Wilms’ Tumor Study. Cancer. 1978;41(5):1937–1948. [DOI] [PubMed] [Google Scholar]
- 5.Rorke LB, Packer RJ, Biegel JA. Central nervous system atypical teratoid/rhabdoid tumors of infancy and childhood: definition of an entity. J Neurosurg. 1996;85(1):56–65. [DOI] [PubMed] [Google Scholar]
- 6.Lee RS, Stewart C, Carter SL et al. A remarkably simple genome underlies highly malignant pediatric rhabdoid cancers. J Clin Invest. 2012;122(8):2983–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hasselblatt M, Isken S, Linge A et al. High-resolution genomic analysis suggests the absence of recurrent genomic alterations other than SMARCB1 aberrations in atypical teratoid/rhabdoid tumors. Genes Chromosomes Cancer. 2013;52(2):185–190. [DOI] [PubMed] [Google Scholar]
- 8.Vogelstein B, Papadopoulos N, Velculescu VE et al. Cancer genome landscapes. Science. 2013;339(6127):1546–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torchia J, Picard D, Lafay-Cousin L et al. Molecular subgroups of atypical teratoid rhabdoid tumours in children: an integrated genomic and clinicopathological analysis. Lancet Oncol. 2015;165:569–582. [DOI] [PubMed] [Google Scholar]
- 10.Kerl K, Holsten T, Fruhwald MC. Rhabdoid tumors: clinical approaches and molecular targets for innovative therapy. Pediatr Hematol Oncol. 2013;30(7):587–604. [DOI] [PubMed] [Google Scholar]
- 11.Ostrom QT, de Blank PM, Kruchko C et al. Alex's Lemonade Stand Foundation infant and childhood primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2015;16(Suppl 10):x1–x36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tekautz TM, Fuller CE, Blaney S et al. Atypical teratoid/rhabdoid tumors (ATRT): improved survival in children 3 years of age and older with radiation therapy and high-dose alkylator-based chemotherapy. J Clin Oncol. 2005;23(7):1491–1499. [DOI] [PubMed] [Google Scholar]
- 13.Hilden JM, Meerbaum S, Burger P et al. Central nervous system atypical teratoid/rhabdoid tumor: results of therapy in children enrolled in a registry. J Clin Oncol. 2004;22(14):2877–2884. [DOI] [PubMed] [Google Scholar]
- 14.Seeringer A, Bartelheim K, Kerl K et al. Feasibility of intensive multimodal therapy in infants affected by rhabdoid tumors—experience of the EU-RHAB registry. Klin Padiatr. 2014;226(3):143–148. [DOI] [PubMed] [Google Scholar]
- 15.Bourdeaut F, Freneaux P, Thuille B et al. Extra-renal non-cerebral rhabdoid tumours. Pediatr Blood Cancer. 2008;51(3):363–368. [DOI] [PubMed] [Google Scholar]
- 16.Rizzo D, Freneaux P, Brisse H et al. SMARCB1 deficiency in tumors from the peripheral nervous system: a link between schwannomas and rhabdoid tumors? Am J Surg Pathol. 2012;36(7):964–972. [DOI] [PubMed] [Google Scholar]
- 17.Seeringer A, Reinhard H, Hasselblatt M et al. Synchronous congenital malignant rhabdoid tumor of the orbit and atypical teratoid/rhabdoid tumor—feasibility and efficacy of multimodal therapy in a long-term survivor. Cancer Genet. 2014;207(9):429–433. [DOI] [PubMed] [Google Scholar]
- 18.Buscariollo DL, Park HS, Roberts KB et al. Survival outcomes in atypical teratoid rhabdoid tumor for patients undergoing radiotherapy in a Surveillance, Epidemiology, and End Results analysis. Cancer. 2012;118(17):4212–4219. [DOI] [PubMed] [Google Scholar]
- 19.Judkins AR, Mauger J, Ht A et al. Immunohistochemical analysis of hSNF5/INI1 in pediatric CNS neoplasms. Am J Surg Pathol. 2004;28(5):644–650. [DOI] [PubMed] [Google Scholar]
- 20.Hasselblatt M, Oyen F, Gesk S et al. Cribriform neuroepithelial tumor (CRINET): a nonrhabdoid ventricular tumor with INI1 loss and relatively favorable prognosis. J Neuropathol Exp Neurol. 2009;68(12):1249–1255. [DOI] [PubMed] [Google Scholar]
- 21.Schneppenheim R, Fruhwald MC, Gesk S et al. Germline nonsense mutation and somatic inactivation of SMARCA4/BRG1 in a family with rhabdoid tumor predisposition syndrome. Am J Hum Genet. 2010;86(2):279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schittenhelm J, Nagel C, Meyermann R et al. Atypical teratoid/rhabdoid tumors may show morphological and immunohistochemical features seen in choroid plexus tumors. Neuropathology. 2011;31(5):461–467. [DOI] [PubMed] [Google Scholar]
- 23.Kordes U, Gesk S, Fruhwald MC et al. Clinical and molecular features in patients with atypical teratoid rhabdoid tumor or malignant rhabdoid tumor. Genes Chromosomes Cancer. 2010;49(2):176–181. [DOI] [PubMed] [Google Scholar]
- 24.Biegel JA, Busse TM, Weissman BE. SWI/SNF chromatin remodeling complexes and cancer. Am J Med Genet C Semin Med Genet. 2014;166C(3):350–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kordes U, Bartelheim K, Modena P et al. Favorable outcome of patients affected by rhabdoid tumors due to rhabdoid tumor predisposition syndrome (RTPS). Pediatr Blood Cancer. 2014;61(5):919–921. [DOI] [PubMed] [Google Scholar]
- 26.Hasselblatt M, Nagel I, Oyen F et al. SMARCA4-mutated atypical teratoid/rhabdoid tumors are associated with inherited germline alterations and poor prognosis. Acta Neuropathol. 2014;128(3):453–456. [DOI] [PubMed] [Google Scholar]
- 27.Ammerlaan AC, Ararou A, Houben MP et al. Long-term survival and transmission of INI1-mutation via nonpenetrant males in a family with rhabdoid tumour predisposition syndrome. Br J Cancer. 2008;98(2):474–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fruhwald MC, Hasselblatt M, Wirth S et al. Non-linkage of familial rhabdoid tumors to SMARCB1 implies a second locus for the rhabdoid tumor predisposition syndrome. Pediatr Blood Cancer. 2006;47(3):273–278. [DOI] [PubMed] [Google Scholar]
- 29.Cecen E, Gunes D, Uysal KM et al. Atypical teratoid/rhabdoid tumor in an infant conceived by in vitro fertilization. Childs Nerv Syst. 2010;26(2):263–266. [DOI] [PubMed] [Google Scholar]
- 30.Ginn KF, Gajjar A. Atypical teratoid rhabdoid tumor: current therapy and future directions. Front Oncol. 2012;2:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lafay-Cousin L, Hawkins C, Carret AS et al. Central nervous system atypical teratoid rhabdoid tumours: the Canadian Paediatric Brain Tumour Consortium experience. Eur J Cancer. 2012;48(3):353–359. [DOI] [PubMed] [Google Scholar]
- 32.Athale UH, Duckworth J, Odame I et al. Childhood atypical teratoid rhabdoid tumor of the central nervous system: a meta-analysis of observational studies. J Pediatr Hematol Oncol. 2009;31(9):651–663. [DOI] [PubMed] [Google Scholar]
- 33.Chi SN, Zimmerman MA, Yao X et al. Intensive multimodality treatment for children with newly diagnosed CNS atypical teratoid rhabdoid tumor. J Clin Oncol. 2009;27(3):385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finkelstein-Shechter T, Gassas A, Mabbott D et al. Atypical teratoid or rhabdoid tumors: improved outcome with high-dose chemotherapy. J Pediatr Hematol Oncol. 2010;32(5):e182–e186. [DOI] [PubMed] [Google Scholar]
- 35.Olson TA, Bayar E, Kosnik E et al. Successful treatment of disseminated central nervous system malignant rhabdoid tumor. J Pediatr Hematol Oncol. 1995;17(1):71–75. [DOI] [PubMed] [Google Scholar]
- 36.Geyer JR, Sposto R, Jennings M et al. Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: a report from the Children's Cancer Group. J Clin Oncol. 2005;23(30):7621–7631. [DOI] [PubMed] [Google Scholar]
- 37.Pai Panandiker AS, Merchant TE, Beltran C et al. Sequencing of local therapy affects the pattern of treatment failure and survival in children with atypical teratoid rhabdoid tumors of the central nervous system. Int J Radiat Oncol Biol Phys. 2012;82(5):1756–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen YW, Wong TT, Ho DM et al. Impact of radiotherapy for pediatric CNS atypical teratoid/rhabdoid tumor (single institute experience). Int J Radiat Oncol Biol Phys. 2006;64(4):1038–1043. [DOI] [PubMed] [Google Scholar]
- 39.Kralik SF, Ho CY, Finke W et al. Radiation necrosis in pediatric patients with brain tumors treated with proton radiotherapy. AJNR Am J Neuroradiol. 2015;36(8):1572–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Amorim Bernstein K, Sethi R, Trofimov A et al. Early clinical outcomes using proton radiation for children with central nervous system atypical teratoid rhabdoid tumors. Int J Radiat Oncol Biol Phys. 2013;86(1):114–120. [DOI] [PubMed] [Google Scholar]
- 41.McGovern SL, Okcu MF, Munsell MF et al. Outcomes and acute toxicities of proton therapy for pediatric atypical teratoid/rhabdoid tumor of the central nervous system. Int J Radiat Oncol Biol Phys. 2014;90(5):1143–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suneja G, Poorvu PD, Hill-Kayser C et al. Acute toxicity of proton beam radiation for pediatric central nervous system malignancies. Pediatr Blood Cancer. 2013;60(9):1431–1436. [DOI] [PubMed] [Google Scholar]
- 43.Yock TI, Bhat S, Szymonifka J et al. Quality of life outcomes in proton and photon treated pediatric brain tumor survivors. Radiother Oncol. 2014;113(1):89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weber DC, Ares C, Malyapa R et al. Tumor control and QoL outcomes of very young children with atypical teratoid/rhabdoid tumor treated with focal only chemo-radiation therapy using pencil beam scanning proton therapy. J Neurooncol. 2015;121(2):389–397. [DOI] [PubMed] [Google Scholar]
- 45.Ho DM, Hsu CY, Wong TT et al. Atypical teratoid/rhabdoid tumor of the central nervous system: a comparative study with primitive neuroectodermal tumor/medulloblastoma. Acta Neuropathol. 2000;99(5):482–488. [DOI] [PubMed] [Google Scholar]
- 46.Weinblatt M, Kochen J. Rhabdoid tumor of the central nervous system. Med Pediatr Oncol. 1992;20(3):258. [DOI] [PubMed] [Google Scholar]
- 47.von Hoff K, Hinkes B, Dannenmann-Stern E et al. Frequency, risk-factors and survival of children with atypical teratoid rhabdoid tumors (AT/RT) of the CNS diagnosed between 1988 and 2004, and registered to the German HIT database. Pediatric Blood Cancer. 2011;57(6):978–985. [DOI] [PubMed] [Google Scholar]
- 48.Zimmerman MA, Goumnerova LC, Proctor M et al. Continuous remission of newly diagnosed and relapsed central nervous system atypical teratoid/rhabdoid tumor. J Neurooncol. 2005;72(1):77–84. [DOI] [PubMed] [Google Scholar]
- 49.Hilden JM, Watterson J, Longee DC et al. Central nervous system atypical teratoid tumor/rhabdoid tumor: response to intensive therapy and review of the literature. J Neurooncol. 1998;40(3):265–275. [DOI] [PubMed] [Google Scholar]
- 50.Gardner SL, Asgharzadeh S, Green A et al. Intensive induction chemotherapy followed by high dose chemotherapy with autologous hematopoietic progenitor cell rescue in young children newly diagnosed with central nervous system atypical teratoid rhabdoid tumors. Pediatr Blood Cancer. 2008;51(2):235–240. [DOI] [PubMed] [Google Scholar]
- 51.Zaky W, Dhall G, Ji L et al. Intensive induction chemotherapy followed by myeloablative chemotherapy with autologous hematopoietic progenitor cell rescue for young children newly-diagnosed with central nervous system atypical teratoid/rhabdoid tumors: the Head Start III experience. Pediatr Blood Cancer. 2014;61(1):95–101. [DOI] [PubMed] [Google Scholar]
- 52.Gilman AL, Jacobsen C, Bunin N et al. Phase I study of tandem high-dose chemotherapy with autologous peripheral blood stem cell rescue for children with recurrent brain tumors: a Pediatric Blood and MarrowTransplant Consortium study. Pediatr Blood Cancer. 2011;57(3):506–513. [DOI] [PubMed] [Google Scholar]
- 53.Park ES, Sung KW, Baek HJ et al. Tandem high-dose chemotherapy and autologous stem cell transplantation in young children with atypical teratoid/rhabdoid tumor of the central nervous system. Korean Med Sci. 2012;27(2):135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slavc I, Chocholous M, Leiss U et al. Atypical teratoid rhabdoid tumor: improved long-term survival with an intensive multimodal therapy and delayed radiotherapy. The Medical University of Vienna Experience 1992–2012. Cancer Med. 2014;3(1):91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Benesch M, Bartelheim K, Fleischhack G et al. High-dose chemotherapy (HDCT) with auto-SCT in children with atypical teratoid/rhabdoid tumors (AT/RT): a report from the European Rhabdoid Registry (EU-RHAB). Bone Marrow Transplant. 2014;49(3):370–375. [DOI] [PubMed] [Google Scholar]
- 56.Voss TC, Hager GL. Dynamic regulation of transcriptional states by chromatin and transcription factors. Nat Rev Genet. 2014;15(2):69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Helming KC, Wang X, Roberts CW. Vulnerabilities of mutant SWI/SNF complexes in cancer. Cancer Cell. 2014;26(3):309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kadoch C, Hargreaves DC, Hodges C et al. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nature Genet. 2013;45(6):592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roberts CW, Galusha SA, McMenamin ME et al. Haploinsufficiency of Snf5 (integrase interactor 1) predisposes to malignant rhabdoid tumors in mice. Proc Natl Acad Sci U S A. 2000;97(25):13796–13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roberts CW, Leroux MM, Fleming MD et al. Highly penetrant, rapid tumorigenesis through conditional inversion of the tumor suppressor gene Snf5. Cancer Cell. 2002;2(5):415–425. [DOI] [PubMed] [Google Scholar]
- 61.Klochendler-Yeivin A, Fiette L, Barra J et al. The murine SNF5/INI1 chromatin remodeling factor is essential for embryonic development and tumor suppression. EMBO Rep. 2000;1(6):500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guidi CJ, Sands AT, Zambrowicz BP et al. Disruption of Ini1 leads to peri-implantation lethality and tumorigenesis in mice. Mol Cell Biol. 2001;21(10):3598–3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsikitis M, Zhang Z, Edelman W et al. Genetic ablation of cyclin D1 abrogates genesis of rhabdoid tumors resulting from Ini1 loss. Proc Natl Acad Sci U S A. 2005;102(34):12129–12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bultman S, Gebuhr T, Yee D et al. A Brg1 null mutation in the mouse reveals functional differences among mammalian SWI/SNF complexes. Mol Cell. 2000;6(6):1287–1295. [DOI] [PubMed] [Google Scholar]
- 65.Bultman SJ, Herschkowitz JI, Godfrey V et al. Characterization of mammary tumors from Brg1 heterozygous mice. Oncogene. 2008;27(4):460–468. [DOI] [PubMed] [Google Scholar]
- 66.Moreno N, Schmidt C, Ahlfeld J et al. Loss of Smarc proteins impairs cerebellar development. J Neurosci. 2014;34(40):13486–13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tolstorukov MY, Sansam CG, Lu P et al. Swi/Snf chromatin remodeling/tumor suppressor complex establishes nucleosome occupancy at target promoters. Proc Natl Acad Sci U S A. 2013;110(25):10165–10170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shi J, Whyte WA, Zepeda-Mendoza CJ et al. Role of SWI/SNF in acute leukemia maintenance and enhancer-mediated Myc regulation. Genes Dev. 2013;27(24):2648–2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kassabov SR, Zhang B, Persinger J et al. SWI/SNF unwraps, slides, and rewraps the nucleosome. Mol Cell. 2003;11(2):391–403. [DOI] [PubMed] [Google Scholar]
- 70.Carissimi C, Laudadio I, Cipolletta E et al. ARGONAUTE2 cooperates with SWI/SNF complex to determine nucleosome occupancy at human transcription start sites. Nucleic Acids Res. 2015;43(3):1498–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kawaguchi T, Tanigawa A, Naganuma T et al. SWI/SNF chromatin-remodeling complexes function in noncoding RNA-dependent assembly of nuclear bodies. Proc Natl Acad Sci U S A. 2015;112(14):4304–4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jackson EM, Sievert AJ, Gai X et al. Genomic analysis using high-density single nucleotide polymorphism-based oligonucleotide arrays and multiplex ligation-dependent probe amplification provides a comprehensive analysis of INI1/SMARCB1 in malignant rhabdoid tumors. Clin Cancer Res. 2009;15(6):1923–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kieran MW, Roberts CW, Chi SN et al. Absence of oncogenic canonical pathway mutations in aggressive pediatric rhabdoid tumors. Pediatr Blood Cancer. 2012;59(7):1155–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jeibmann A, Eikmeier K, Linge A et al. Identification of genes involved in the biology of atypical teratoid/rhabdoid tumours using Drosophila melanogaster. Nat Commun. 2014;5:4005. [DOI] [PubMed] [Google Scholar]
- 75.Birks DK, Donson AM, Patel PR et al. High expression of BMP pathway genes distinguishes a subset of atypical teratoid/rhabdoid tumors associated with shorter survival. Neuro Oncol. 2011;13(12):1296–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rosson GB, Vincent TS, Oswald BW et al. Drug resistance in malignant rhabdoid tumor cell lines. Cancer Chemother Pharmacol. 2002;49(2):142–148. [DOI] [PubMed] [Google Scholar]
- 77.Lunenburger H, Lanvers-Kaminsky C, Lechtape B et al. Systematic analysis of the antiproliferative effects of novel and standard anticancer agents in rhabdoid tumor cell lines. Anticancer Drugs. 2010;21(5):514–522. [DOI] [PubMed] [Google Scholar]
- 78.D'Cunja J, Shalaby T, Rivera P et al. Antisense treatment of IGF-IR induces apoptosis and enhances chemosensitivity in central nervous system atypical teratoid/rhabdoid tumours cells. Eur J Cancer. 2007;43(10):1581–1589. [DOI] [PubMed] [Google Scholar]
- 79.Jayanthan A, Bernoux D, Bose P et al. Multi-tyrosine kinase inhibitors in preclinical studies for pediatric CNS AT/RT: evidence for synergy with topoisomerase-I inhibition. Cancer Cell Int. 2011;11(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weingart MF, Roth JJ, Hutt-Cabezas M et al. Disrupting LIN28 in atypical teratoid rhabdoid tumors reveals the importance of the mitogen activated protein kinase pathway as a therapeutic target. Oncotarget. 2015;6(5):3165–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singh A, Lun X, Jayanthan A et al. Profiling pathway-specific novel therapeutics in preclinical assessment for central nervous system atypical teratoid rhabdoid tumors (CNS ATRT): favorable activity of targeting EGFR-ErbB2 signaling with lapatinib. Mol Oncol. 2013;7(3):497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Girard E, Ditzler S, Lee D et al. Efficacy of cabazitaxel in mouse models of pediatric brain tumors. Neuro Oncol. 2015;17(1):107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alarcon-Vargas D, Zhang Z, Agarwal B et al. Targeting cyclin D1, a downstream effector of INI1/hSNF5, in rhabdoid tumors. Oncogene. 2006;25(5):722–734. [DOI] [PubMed] [Google Scholar]
- 84.Kim KH, Roberts CW. Mechanisms by which SMARCB1 loss drives rhabdoid tumor growth. Cancer Genet. 2014;207(9):365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fujisawa H, Misaki K, Takabatake Y et al. Cyclin D1 is overexpressed in atypical teratoid/rhabdoid tumor with hSNF5/INI1 gene inactivation. J Neurooncol. 2005;73(2):117–124. [DOI] [PubMed] [Google Scholar]
- 86.Zhang ZK, Davies KP, Allen J et al. Cell cycle arrest and repression of cyclin D1 transcription by INI1/hSNF5. Mol Cell Biol. 2002;22(16):5975–5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Betz BL, Strobeck MW, Reisman DN et al. Re-expression of hSNF5/INI1/BAF47 in pediatric tumor cells leads to G1 arrest associated with induction of p16ink4a and activation of RB. Oncogene. 2002;21(34):5193–5203. [DOI] [PubMed] [Google Scholar]
- 88.Smith ME, Cimica V, Chinni S et al. Rhabdoid tumor growth is inhibited by flavopiridol. Clin Cancer Res. 2008;14(2):523–532. [DOI] [PubMed] [Google Scholar]
- 89.Smith ME, Cimica V, Chinni S et al. Therapeutically targeting cyclin D1 in primary tumors arising from loss of Ini1. Proc Natl Acad Sci U S A. 2011;108(1):319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koos B, Jeibmann A, Lunenburger H et al. The tyrosine kinase c-Abl promotes proliferation and is expressed in atypical teratoid and malignant rhabdoid tumors. Cancer. 2010;116(21):5075–5081. [DOI] [PubMed] [Google Scholar]
- 91.Lee S, Cimica V, Ramachandra N et al. Aurora A is a repressed effector target of the chromatin remodeling protein INI1/hSNF5 required for rhabdoid tumor cell survival. Cancer Res. 2011;71(9):3225–3235. [DOI] [PubMed] [Google Scholar]
- 92.Maris JM, Morton CL, Gorlick R et al. Initial testing of the aurora kinase A inhibitor MLN8237 by the Pediatric Preclinical Testing Program (PPTP). Pediatr Blood Cancer. 2010;55(1):26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Venkataraman S, Alimova I, Tello T et al. Targeting aurora kinase A enhances radiation sensitivity of atypical teratoid rhabdoid tumor cells. J Neurooncol. 2012;107(3):517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wetmore C, Boyett J, Li S et al. Alisertib is active as single agent in recurrent atypical teratoid rhabdoid tumors in 4 children. Neuro Oncol. 2015;176:882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jagani Z, Mora-Blanco EL, Sansam CG et al. Loss of the tumor suppressor Snf5 leads to aberrant activation of the Hedgehog-Gli pathway. Nature Med. 2010;16(12):1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kerl K, Moreno N, Holsten T et al. Arsenic trioxide inhibits tumor cell growth in malignant rhabdoid tumors in vitro and in vivo by targeting overexpressed Gli1. Int J Cancer. 2014;135(4):989–995. [DOI] [PubMed] [Google Scholar]
- 97.Tang Y, Gholamin S, Schubert S et al. Epigenetic targeting of Hedgehog pathway transcriptional output through BET bromodomain inhibition. Nature Med. 2014;20(7):732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Romero OA, Setien F, John S et al. The tumour suppressor and chromatin-remodelling factor BRG1 antagonizes Myc activity and promotes cell differentiation in human cancer. EMBO Mol Med. 2012;4(7):603–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mora-Blanco EL, Mishina Y, Tillman EJ et al. Activation of beta-catenin/TCF targets following loss of the tumor suppressor SNF5. Oncogene. 2014;33(7):933–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chakravadhanula M, Hampton CN, Chodavadia P et al. Wnt pathway in atypical teratoid rhabdoid tumors. Neuro Oncol. 2015;17(4):526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wilson BG, Wang X, Shen X et al. Epigenetic antagonism between polycomb and SWI/SNF complexes during oncogenic transformation. Cancer Cell. 2010;18(4):316–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kia SK, Gorski MM, Giannakopoulos S et al. SWI/SNF mediates polycomb eviction and epigenetic reprogramming of the INK4b-ARF-INK4a locus. Mol Cell Biol. 2008;28(10):3457–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Unland R, Borchardt C, Clemens D et al. Analysis of the antiproliferative effects of 3-deazaneoplanocin A in combination with standard anticancer agents in rhabdoid tumor cell lines. Anticancer Drugs. 2015;26(3):301–311. [DOI] [PubMed] [Google Scholar]
- 104.Knutson SK, Kawano S, Minoshima Y et al. Selective inhibition of EZH2 by EPZ-6438 leads to potent antitumor activity in EZH2-mutant non-Hodgkin lymphoma. Mol Cancer Ther. 2014;13(4):842–854. [DOI] [PubMed] [Google Scholar]
- 105.Alimova I, Birks DK, Harris PS et al. Inhibition of EZH2 suppresses self-renewal and induces radiation sensitivity in atypical rhabdoid teratoid tumor cells. Neuro Oncol. 2013;15(2):149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ribrag V, Soria J-C, Reyderman L et al. Phase 1 first-in-human study of the enhancer of zest-homolog 2 (EZH2) histone methyltransferase inhibitor E7438. Annals Oncol. 2015;26(Suppl 2):ii10–ii11. [Google Scholar]
- 107.Kerl K, Ries D, Unland R et al. The histone deacetylase inhibitor SAHA acts in synergism with fenretinide and doxorubicin to control growth of rhabdoid tumor cells. BMC Cancer. 2013;13:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Thiemann M, Oertel S, Ehemann V et al. In vivo efficacy of the histone deacetylase inhibitor suberoylanilide hydroxamic acid in combination with radiotherapy in a malignant rhabdoid tumor mouse model. Radiat Oncol. 2012;7:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sharma S, Witteveen PO, Lolkema MP et al. A phase I, open-label, multicenter study to evaluate the pharmacokinetics and safety of oral panobinostat in patients with advanced solid tumors and varying degrees of renal function. Cancer Chemother Pharmacol. 2015;75(1):87–95. [DOI] [PubMed] [Google Scholar]
- 110.Kitazono S, Fujiwara Y, Nakamichi S et al. A phase I study of resminostat in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol. 2015;75(6):1155–1161. [DOI] [PubMed] [Google Scholar]
- 111.Hohmann AF, Vakoc CR. A rationale to target the SWI/SNF complex for cancer therapy. Trends Genet. 2014;30(8):356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hoffman GR, Rahal R, Buxton F et al. Functional epigenetics approach identifies BRM/SMARCA2 as a critical synthetic lethal target in BRG1-deficient cancers. Proc Natl Acad Sci U S A. 2014;111(8):3128–3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kuwahara Y, Hosoi H, Osone S et al. Antitumor activity of gefitinib in malignant rhabdoid tumor cells in vitro and in vivo. Clin Cancer Res. 2004;10(17):5940–5948. [DOI] [PubMed] [Google Scholar]
- 114.Cimica V, Smith ME, Zhang Z et al. Potent inhibition of rhabdoid tumor cells by combination of flavopiridol and 4OH-tamoxifen. BMC Cancer. 2010;10:634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wohrle S, Weiss A, Ito M et al. Fibroblast growth factor receptors as novel therapeutic targets in SNF5-deleted malignant rhabdoid tumors. PLoS One. 2013;8(10):e77652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dufour C, Beaugrand A, Le Deley MC et al. Clinicopathologic prognostic factors in childhood atypical teratoid and rhabdoid tumor of the central nervous system: a multicenter study. Cancer. 2012;11815:3812–3821. [DOI] [PubMed] [Google Scholar]