Abstract
Background
Moderate diagnostic accuracy of MRI and initial cerebrospinal fluid (CSF) cytology analysis results in at least 10%–15% false negative diagnoses of leptomeningeal metastases (LM) of solid tumors, thus postponing start of therapy. The aim of this prospective clinical study was to determine the diagnostic value of epithelial cell adhesion molecule (EpCAM)–based flow cytometry versus cytology in CSF for the diagnosis of LM in patients with epithelial tumors.
Methods
Patients with a clinical suspicion of LM but a negative or inconclusive MRI in whom a diagnostic lumbar puncture has to be performed were included. At least 5 mL of CSF for cytology, 5 mL for flow cytometry, 2 mL for cell count and biochemistry, and 8 mL whole blood samples for circulating tumor cells measurements and biochemistry were drawn. Tumor cells in CSF and whole blood were detected by multiparameter flow cytometry using EpCAM antibody.
Results
In total 29 eligible patients were enrolled in the study. Thirteen patients were ultimately diagnosed with LM. The flow cytometry assay showed 100% sensitivity and 100% specificity for diagnosing LM, while sensitivity of CSF cytology was only 61.5%. Cell count or biochemical parameters in CSF were abnormal in 100% of patients with LM.
Conclusions
Our results suggest that the EpCAM-based flow cytometry assay is superior to CSF cytology for the diagnosis of LM in patients with an epithelial tumor, a clinical suspicion of LM, and a nonconclusive MRI. Confirmation of these data is needed in a larger dataset to recommend dual CSF diagnostics for LM.
ClinicalTrials.gov Identifier
Keywords: carcinomatous meningitis, cerebrospinal fluid (CSF), circulating tumor cells (CTC), epithelial cell adhesion molecule (EpCAM), leptomeningeal metastases (LM)
Leptomeningeal metastases (LM), also known as meningeal carcinomatosis or neoplastic meningitis, is a diffuse dissemination of tumor cells into the cerebrospinal fluid (CSF) and leptomeninges.1 Up to 8% of all patients with cancer ultimately develop LM.2 The highest incidence of LM is seen in patients with breast cancer (in particular lobular carcinoma), small cell lung carcinoma (SCLC) and non–small cell lung carcinoma (NSCLC), and melanoma.3 Due to the spread of tumor cells in the CSF, LM is characterized by multifocal symptomatology (cerebral/cerebellum, cranial nerve, and/or spinal nerve dysfunction). Gadolinium-enhanced MRI of the symptomatic sites of the nervous system is the radiological diagnostic method of choice when LM is clinically suspected. In patients with cancer, the diagnosis of LM can be made when clinical signs are compatible with LM and MRI shows subarachnoid tumor nodules or contrast enhancement of the leptomeninges or cranial or spinal nerves. The sensitivity of MRI with gadolinium for LM is 75% and the specificity 77%.4 When MRI does not show equivocal abnormalities, CSF cytology needs to be performed. Malignant cells are found in the first CSF sample in 50%–67% of patients with LM.5–8 The sensitivity rises to 80%–90% after a second CSF sampling.6 The volume of the CSF sample determines partly the sensitivity of CSF cytology. If possible, it is advised to draw 10 mL of CSF and process the CSF as quickly as possible.9 Cell count and clinical chemical analysis of the CSF (leukocyte count, lactate dehydrogenase [LDH], total protein, and glucose concentration) are aberrant in 90% of patients with LM.6,10 However, abnormal cell count and biochemical parameters in the CSF do not prove LM, as these can also be found in other neurological diseases, including infectious or aseptic meningitis.10 Therefore, improved CSF diagnostics for LM are needed to either rule out the diagnosis or expedite treatment without further delay.
Isolation and quantification of circulating tumor cells (CTCs) in whole blood using flow cytometry is a proven valuable prognostic marker in metastatic breast, prostate, and colorectal cancer.11 Concurrent flow cytometry to standard CSF cytology is already being used in confirming the initial diagnosis and monitoring leptomeningeal dissemination from hematologic malignancies.12–15 Flow cytometry allows detection of multiple fluorescent markers simultaneously. In epithelial tumors, a fluorescent antibody against epithelial cell adhesion molecule (EpCAM) is used, as this protein is expressed in various epithelial cancers, such as breast cancer (ductal type more frequently than lobular type), upper digestive and respiratory tract, gastrointestinal cancer, cancers of the genital and urinary tract, neuroendocrine tumors, and some types of soft tissue sarcoma.16 Malignant melanoma and glioblastoma do not express EpCAM. Recently, a few studies have suggested that an EpCAM-based CTC assay could be used for detection of tumor cells in CSF in patients with solid tumors.14–18 Patel et al17 found up to 104 CTCs in 7.5 mL of CSF of 5 patients with LM from breast cancer using a rare cell capture technology with an immunomagnetic platform and anti-EpCAM covered ferroparticles (Veridex) and showed an association between the number of CTCs and response to intrathecal and systemic chemotherapy.
Nayak et al7 demonstrated a sensitivity of 100% and a specificity of 97.2% for the EpCAM-based Cell Search Veridex system in CSF compared with cytology (sensitivity 67%, specificity 100%) in patients with suspected LM. Using the same assay in CSF, Lin et al19 showed a sensitivity of 95% and specificity of 83% for the diagnosis of LM. Subirá et al20 demonstrated an 80% sensitivity of flow cytometry versus cytology (50%) in CSF for LM but a lower specificity (84% vs 100%) using an EpCAM-based flow cytometry assay.
In our laboratory, we have developed an improved EpCAM-based flow cytometry assay that can quantify CTCs with both high and low DNA content, which greatly increases CTC detection sensitivity.21
The primary aim of this prospective study was to evaluate the diagnostic value of conventional cytology and our improved EpCAM-based flow cytometry CTC assay in the CSF of patients with epithelial tumors, a clinical suspicion of LM, but nonconfirmatory MRI. The secondary aims were to analyze CSF cytology using EpCAM immunohistochemistry and compare EpCAM flow cytometry results from CSF with EpCAM flow cytometry from blood in patients with and without confirmation of LM.
Patients and Methods
Patients
The Ethics Committee of the Netherlands Cancer Institute—Antoni van Leeuwenhoek Hospital approved the study.
Patients ≥18 years with a solid tumor and clinical suspicion of LM but a normal or inconclusive MRI who had to undergo a diagnostic lumbar puncture (LP) were asked to participate.
Exclusion criteria were: (i) intracranial or intraspinal tumor with mass effect heralding the risk of herniation during LP and (ii) uncorrected thrombocytopenia or coagulation disorders.
CSF and Blood Analysis
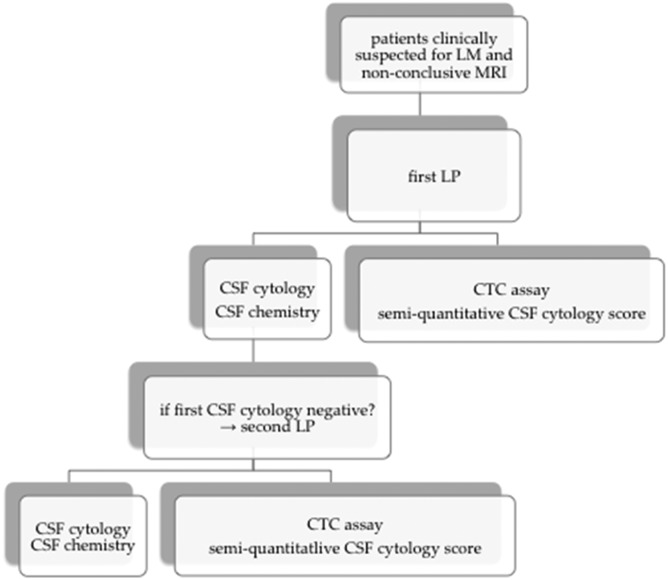
Standard CSF evaluation consisted of CSF pressure measurements, cell counts, and biochemical parameters (leucocyte count, total protein, glucose and LDH concentrations, at least 2 mL CSF), CSF cytology (at least 5 mL of CSF), and tumor cell measurement using an EpCAM-based flow cytometry assay (at least 5 mL of CSF) (Fig. 1). In addition, 8 mL of blood was drawn for cell count and biochemistry for glucose, LDH, and total protein serum concentrations. For the CTC assay, blood was drawn in three 8-mL cell preparation tubes (BD Biosciences). In patients with a high clinical suspicion of LM and a negative CSF cytology at first CSF examination, a second LP could be performed to again collect CSF and blood for cytology, biochemistry, and the CTC assay.
Fig. 1.
Study design.
CSF Cytology
CSF cytology was performed as a 2-step cytocentrifuge method (1600 rpm; 1 min and 10 min, respectively), which deposits cells from a fluid sample directly onto slides (cytospin). Giemsa staining was performed on 2 cytospins.22 The Giemsa stainings of the CSF samples were examined by the pathologist and scored as negative (no tumor cells), positive (tumor cells), or inconclusive (atypical cells). The results of CSF cytology were used in clinical decision making.
Immunohistochemistry and Semiquantitative Cytology Score in CSF
Two additional cytospins were used for immunohistochemical staining with an antibody against EpCAM. Immunohistochemistry was performed on a BenchMark Ultra autostainer (Ventana Medical Systems).
Briefly, cytospins were washed in reaction buffer (Ventana Medical Systems) and loaded in an autostainer. EpCAM was detected by incubating slides with the antibody clone Ber-EP4/EpCAM (M0804; Dako). After incubation with the primary antibody, an amplification step was used to increase the signal intensity of weak staining of (mouse and rabbit) primary antibodies.
Specific antibody reactions (ie, bound primary antibodies to the specific EpCAM epitopes) were detected using the UltraView Universal DAB Detection Kit (Ventana Medical Systems), and slides were counterstained with hematoxylin.
Tumor cells in these EpCAM-stained cytospins were semiquantitatively scored by the pathologist: a score of 0 for 0 tumor cells/cytospin area, a score of 1 for 1–10 tumor cells/cytospin area, a score of 2 for 11–100 tumor cells/cytospin area, a score of 3 for 101–500 tumor cells/cytospin area, and a score of 4 for >500 tumor cells/cytospin area. The semiquantitative CSF cytology method using EpCAM immunohistochemistry was an exploratory assay only and results were not used in clinical decision making.
Circulating Tumor Cell Flow Cytometry Assay
CSF for the CTC flow cytometry was collected in a conical 50-mL tube. Whole blood was drawn in 3 cell preparation tubes per patient and centrifuged for 25 min at 1500 g at room temperature. The upper phase of the separated plasma from 3 cell preparation tubes was transferred in three 50-mL centrifuge tubes. After fixation with 4% formaldehyde, all 4 samples (1 CSF and 3 plasma) were washed twice with up to 50 mL physiologic saline and centrifuged at 1000 g for 7 minutes at 4°C. The pellets were resuspended in 50% methanol/phosphate buffered saline and stored at −80°C for a maximum of 6 months.
CTC measurements were performed in batches. After defrosting on ice, samples were washed twice with 1 mL of ice-cold beads buffer (phosphate buffered saline with 2 mM EDTA and 0.5% weight/volume bovine serum albumin). After each wash, samples were centrifuged at 1000 g for 4 min at 4°C. Next, supernatant was removed and the pellets were resuspended in the remaining 100 µL of beads buffer. Subsequently, tumor cells were immunomagnetically enriched from the samples using anti-human EpCAM MicroBeads (Miltenyi). Next, tumor cells were fluorescently labeled using anti–EpCAM-phycoerythrin (PE). Hoechst 33258 was used for nuclear staining and anti–CD45-fluorescein isothiocyanate (FITC) for leucocyte labeling. After removal of unreacted antibodies, tumor cells were quantified by fluorescent activated cell sorting (FACS). The details of the EpCAM-based CTC assay were described by Pluim et al.21 With this method, both tumor cells with a normal amount of DNA and tumor cells with less DNA can be quantified.23 The flow cytometric EpCAM assay has a lower limit of quantification of 2 tumor cells per 8 mL of whole blood. Therefore, blood samples with <2 tumor cells/8 mL were considered negative. Results of the flow cytometry assay and cytology in the CSF were both obtained independently. The results of the flow cytometry assay were not used in clinical decision making.
Clinical Follow-up
Clinical signs and symptoms were recorded and neurological examination was done in the week before the LP. Three days after the LP, patients were asked by telephone for symptoms of post-LP headache (with nausea increasing in an upright position). When post-LP headache was diagnosed, follow-up was performed until 28 days after the LP. Both clinical records were studied for the course of neurological symptoms, and subsequent MRIs and/or repeated CSF samples were evaluated to conclude the final diagnosis (definitive LM vs no LM).
Diagnosis of Leptomeningeal Metastases
The diagnosis of LM was considered definitive when at least one of the following conditions was fulfilled:
positive CSF cytology in the initial LP or repeated LP performed within 2 weeks after the initial LP
a follow-up MRI of the brain or spine performed after the diagnostic LP within 2 months following the first MRI, showing unequivocal evidence of LM
progressive neurological symptoms compatible with LM and exclusion of other causes (eg, infectious meningitis, treatment side effects).
Statistics
The total sample size was calculated with nQuery Version 7.024 to target the 0.95 sensitivity of the new test (CTC flow cytometry assay), while allowing the lower limit of the 95% confidence interval to be equal to or higher than the highest reported sensitivity (0.67) of the standard method for LM diagnosis (CSF cytology) in the literature.7 The prevalence of LM among the enrolled patients with an epithelial primary tumor was 0.45. Descriptive demographics were used and sensitivity and specificity of used methods were calculated using SPSS.
Results
Patient Characteristics
In total 31 patients with solid tumors from epithelial origin, a clinical suspicion of LM, and a nonconfirmatory MRI were enrolled. All patients were undergoing an initial LP for diagnosis of LM. Two patients were not eligible as LPs were performed because of the location of the tumor (near the ventricles), but there were no clinical symptoms of LM. Distribution of the primary tumor types is presented in Table 1. In total, 13 patients were ultimately diagnosed with definitive LM (45%). In 16 patients with clinical suspicion of LM at study inclusion, the diagnosis of LM was not confirmed eventually. They received varying diagnoses, such as skull base metastases, cervical myelopathy, radiation-induced opticopathy, hepatic encephalopathy, anterior cutaneous nerve entrapment syndrome, vestibular neuritis, cerebral ischemia, and infectious meningitis.
Table 1.
Tumor type distribution in included patients
| Tumor Type | Number of Patients |
|---|---|
| Breast cancer | n = 13 (45%) |
| NSCLC | n = 6 (21%) |
| SCLC | n = 2 (7%) |
| Gastrointestinal | n = 3 (10%) |
| Ovarian cancer | n = 1 (3%) |
| Other tumor types | n = 4 (14%) |
| Total | n = 29 |
Abbreviations: SCLC, small cell lung cancer; NSCLC, non-small cell lung cancer.
Gastrointestinal tumors: 2 neuroendocrine colorectal tumors and 1 gastrointestinal stromal tumor. Other tumor types: 1 nasopharyngeal carcinoma, 1 urothelial cell carcinoma, 1 renal cell cancer, and 1 parotid gland carcinoma.
Nine of 13 patients (69%) with a definitive diagnosis of LM were female. Primary tumors were breast cancer (n = 7, 54%: 3 lobular and 4 ductal), NSCLC (n = 4, 31%), colon carcinoma (n = 1), and urothelial cell cancer (n = 1) (Table 2). Eleven of these patients (85%) had systemic metastases. Four patients had concomitant brain metastases (31%). Neurological symptoms and signs in patients with definitive LM were classified as cerebral/cerebellar symptoms, including headache, vomiting, nausea, encephalopathy, dysphasia, dysarthria, ataxia (n = 11, 78%); cranial nerve symptoms, including decreased or double vision, facial paresis, tinnitus (n = 7, 50%); and spinal symptoms, including sensibility disorders and/or paresis of arms and legs, bladder, and/or bowel dysfunction (n = 3, 21%).
Table 2.
Characteristics of patients with definitive LM
| Patients with Definitive LM | 13/29 (45%) |
|---|---|
| Age | |
| Median (range) | 55 (20–82) y |
| Gender | |
| Male | 4 (31%) |
| Female | 9 (69%) |
| WHO | |
| 0 | 2 (15%) |
| 1 | 3 (23%) |
| 2 | 6 (46%) |
| 3 | 2 (15%) |
| Primary tumor type | |
| Breast carcinoma | 7 (54%) |
| NSCLC | 4 (31%) |
| Other* | 2 (15%) |
| Metastases (any) | 12 (92%) |
| Lymph nodes | 7 (54%) |
| Bone | 7 (54%) |
| Lung | 4 (31%) |
| Brain | 5 (38%) |
Abbreviation: WHO, World Health Organization performance status.
*Other, other primary tumor types: urothelial cell carcinoma and colon cancer.
CSF Pressure, Leukocyte Count, and Biochemical Parameters
CSF pressure was increased (>20 cm H2O) in 6 of 13 patients (46%) with definitive LM. In 100% of patients with LM, either leukocyte count or biochemical parameters in CSF were abnormal. Increased total protein CSF levels (>0.45 g/L) were present in 85% of patients with LM (median 0.72 g/L, range 0.27–4.51 g/L). Leucocyte counts were increased (>3/mm3) in 61% (median 4/mm3, range 1–100/mm3), LDH levels were increased (>40 U/L) in 54% (median 48 U/L, range 18–1287 U/L), and glucose CSF/serum ratio was decreased (<0.66) in 85% of patients with LM. Two of 29 eligible patients (7%) had post-LP headache.
CSF Cytology Versus CTCs in Solid Tumors from Epithelial Origin
Twenty-nine CSF samples were obtained from 29 eligible patients with epithelial primary tumors with a clinical suspicion of LM. In 8 of 13 patients with definitive LM, both cytology and the flow cytometry assay in CSF were positive (61.5%, median 316.5 CTCs/mL, range 160–4503 CTCs/mL). Five of 13 patients (38.5%) showed a discrepancy between CSF cytology and the flow cytometry assay. In these patients CSF cytology was negative, but CTC measurements showed ≥2 CTCs/mL (median 17.2 CTCs/mL, range 3–41.8 CTCs/mL). Patients having no LM (n = 16) showed 0–0.2 CTCs/mL (0 or 1 CTC per 5 mL CSF). Therefore, CSF with ≥2 CTCs per 5 mL was considered positive.
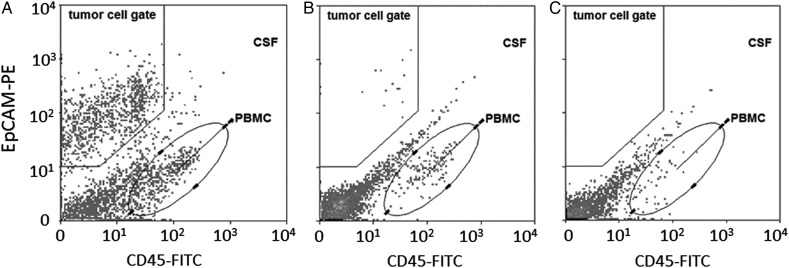
Sensitivity of CSF cytology for the diagnosis of LM was 61.5% (95% CI, 32%–86%), with a specificity of 100% (95% CI, 79%–100%). Sensitivity and specificity of the CTC assay were both 100% (sensitivity: 95% CI, 75%–100%; specificity: 95% CI, 79%–100%) (Table 3). See also Fig. 2 for flow cytometry plots in CSF of individual patients.
Table 3.
Sensitivity and specificity of CSF cytology, CTC flow cytometry assay, and semiquantitative CSF cytology score (%) for diagnosing LM in EpCAM-positive tumors
| Sensitivity, % | Specificity, % | PPV, % | NPV, % | |
|---|---|---|---|---|
| CSF cytology | 61.5 | 100 | 100 | 76 |
| Semiquantitative CSF cytology score | 75 | 100 | 100 | 84 |
| CTC assay | 100 | 100 | 100 | 100 |
Abbreviations: PPV, positive predictive value; NPV, negative predictive value.
Fig. 2.
Examples of EpCAM-based flow cytometry plots in CSF in individual patients. (A) NSCLC patient with LM with EpCAM-positive CTCs (162 CTCs/mL); CSF cytology was positive (not shown). (B) Breast cancer patient with LM with EpCAM-positive CTCs (3 CTCs/mL). CSF cytology was negative (not shown). (C) Breast cancer patient without LM. No EpCAM-positive CTCs in CSF. CSF cytology was also negative (not shown).
Semiquantitative CSF Cytology Based on Immunohistochemistry—EpCAM Staining of CSF
Immunohistochemistry for EpCAM of CSF cytospins was positive in 9 of 12 available cytospins of patients with definitive LM and available EpCAM-stained cytospins. In 8 of these patients, both CSF cytology and the flow cytometry assay were positive. In one patient with a positive EpCAM staining of CSF (semiquantitative cytology score 2), CSF cytology was negative, but the flow cytometry assay was positive.
The semiquantitative CSF cytology method had 75% sensitivity (95% CI, 43%–94%) and 100% specificity (95% CI, 79%–100%) and performed slightly better than standard CSF cytology, but less than the flow cytometry assay.
Immunohistochemistry EpCAM Staining of Primary Tumors
In less than half of included patients, the primary tumor histology samples were collected and stained for EpCAM. In 7 of 13 patients with LM, EpCAM staining of the primary tumor was performed. Three primary tumors were strongly positive for EpCAM (NSCLC, colon and ductal breast cancer) and 4 were weakly positive (ductal breast cancer [n = 3], rectal cancer [n = 1]). In 8 of the 16 patients with no LM, EpCAM staining of the primary tumors was done. Four primary tumors were weakly positive for EpCAM and 4 showed positivity to some degree (EpCAM staining in some areas of the tumor).
CTC Flow Cytometry Assay in Blood
Nine of 13 patients with LM had ≥2 CTCs/8 mL in their blood samples (median 89, range 10–2554 CTCs/8 mL). All 9 patients had active systemic metastases. In one patient, blood samples for CTC measurements were not obtained. Four of 16 patients with no LM showed ≥2 CTCs/8 mL blood (median 27, range 2–102 CTCs/8 mL). All 4 patients had systemic metastases.
Cell Viability and DNA Quantity of CTCs in CSF Versus Blood
Cell viability and DNA quantity of CTCs in the CSF were measured and compared with simultaneously withdrawn blood samples. The relative amount of DNA-positive CTCs in CSF was 2.67-fold higher than in blood (66.1% ± 19.1% DNA-positive CTCs in CSF vs 24.7% ± 20.8% in blood, P = .0001), confirming a higher cell viability of CTCs in CSF.
The EpCAM mean fluorescent intensity of the DNA-positive CTCs in CSF was 3.23-fold higher than in blood (average blood EpCAM mean fluorescent intensity was 111.6 ± 59.8 vs 360.7 ± 149.1 in CSF, P = .0002), which may indicate an important role for EpCAM expression in the process of metastasis and survival of CTCs in the CSF compartment.
Discussion
This study shows a 100% sensitivity and specificity for the EpCAM-based CTC flow cytometry assay for the diagnosis of LM in patients with epithelial primary tumors, a clinical suspicion of LM but a nonconfirmatory MRI. The sensitivity of this assay in CSF is higher than for conventional cytology (61.5%) with an equal specificity.
Using this dual diagnostic method (ie, standard CSF cytology with morphological cell assessment by the pathologist and flow cytometric CTC assay), patients with a clinical suspicion of LM and a nonconfirmatory MRI scan can be diagnosed for LM in a timely manner with a very low chance of false negative results. This will diminish the diagnostic uncertainty of LM and prevent multiple lumbar punctures. Moreover, treatment for LM can be initiated without further delay to prevent progressive neurological symptoms and provide substantial benefit in quality of life.25
The EpCAM-based flow cytometry assay in CSF brings higher sensitivity for the diagnosis of LM, especially when the CTC count in the CSF is below 50 cells/mL CSF and cytology becomes negative.
Four earlier studies on CTC number measurements in the CSF of patients with LM have been performed by Nayak et al,7 Subirá et al,8,20 and Lin et al (Table 4).19 They included 51, 78, 94, and 62 patients, respectively, with suspected LM. All patients had EpCAM-positive primary tumors. In the studies by Nayak et al and Lin et al, EpCAM-positive CTCs in the CSF were found in 95%–100% of patients against 76%–80% in the 2 studies by Subirá et al, which overlap in patient's inclusion. Specificity of the CSF assays ranged from 83% to 97%.7,8,19,20 In all 4 studies the diagnosis of LM was clinically suspected, and MRI, CSF cytology, and CTC assay were performed synchronously. This implies that patients with unequivocal radiological evidence of LM were also included in these studies.
Table 4.
Results from studies in literature using EpCAM-based CTC assays in CSF in patients with (suspected) LM compared with our study
| Assay Used | Technology | Positive Aspects | Negative Aspects | Patients | Sensitivity | Specificity | Reference |
|---|---|---|---|---|---|---|---|
| Flow cytometry assay developed by NKI-AVL | MACS with EpCAM MicroBeads, CTC detection by EpCAM-PE; CD45-FITC and Hoechst33258 | Automated tumor cell recognition, common clinical chemistry equipment (FACS), also quantifies CTC with low DNA content | Noncommercial, no visual verification of CTC | 29 Pts with solid tumors and suspected LM | 100% CI, 75%–100% |
100% CI, 79%–100% |
Current study |
| CellSearch system (Veridex) | MACS with EpCAM ferrofluid, CTC detection by CK, CD45 and DAPI staining | Commercial, FDA approved, visual verification of CTC by imaging | Human observer needed, only CTC with normal DNA content quantified, CSF needs to be spiked in blood, expensive ($450–$600/sample) | 5 Pts with breast cancer and LM | Not applicable | Not applicable | Patel et al, 201117 |
| CellSearch system (Veridex) | MACS with EpCAM ferrofluid, CTC detection by CK, CD45 and DAPI staining | Commercial, FDA approved, visual verification of CTC by imaging | Human observer needed, only CTCs with normal DNA content quantified, CSF needs to be spiked in blood, expensive ($450–600/sample) | 51 Pts with solid tumors and suspected LM | 100% CI, 78%–100% |
97% CI, 85%–100% |
Nayak et al, 20137 |
| CellSearch system (Veridex) | MACS with EpCAM ferrofluid, CTC detection by CK, CD45 and DAPI staining | Commercial, FDA approved, visual verification of CTC by imaging | Human observer needed, only CTCs with normal DNA content quantified, CSF needs to be spiked in blood, expensive ($450–600/sample) | 62 Pts with solid tumors and suspected LM | 95% | 83% | Lin et al, 201519 |
| Flow cytometry assay | CTC detection by 2-clone anti-EpCAM Mab-PE and FITC, CK, DRAQ5 nuclear staining | Use of common laboratory equipment (FACS), also inflammatory cells are quantified | Noncommercial, no visual verification of CTCs | 78 Pts with solid tumors and suspected LM | 76% | 96% | Subirá et al, 20128 |
| Flow cytometry assay | CTC detection by 2-clone anti-EpCAM Mab-PE and FITC, DRAQ5 nuclear staining, no CK | Use of common laboratory equipment (FACS), also inflammatory cells are quantified | Noncommercial, no visual verification of CTCs | 94 Pts with solid tumors and suspected LM | 80% | 84% | Subirá et al, 201520 |
Abbreviations: MACS, magnetic antibody cell sorting; CK, cytokeratin; DAPI, 4′,6-diamidino-2-phenylindole; pts, patients.
In comparison, we enrolled 29 eligible patients with tumors with epithelial origin and a clinical suspicion of LM but a nonconfirmatory MRI. This cohort represents a patient group with true diagnostic uncertainty in clinical practice. Despite an anticipated lower average number of CTC/mL of CSF in this patient group, we achieved a sensitivity of the CTC flow cytometry assay that is in line with the results of Nayak et al7 and Lin et al19 obtained with the Cell Search Veridex method and higher than obtained with noncommercial flow cytometry assays in the studies by Subirá et al.8,20 The specificity of our CSF flow cytometry assay is comparable to the study by Nayak et al7 (97%) and Subirá et al8 (96%), but higher than in the other study by Subirá et al20 (84%) and Lin et al19 (83%).
The higher sensitivity of our flow cytometry CSF assay for tumors with epithelial origin is most likely a result of the additional ability to quantify CTCs with low DNA quantity. The Cell Search Veridex assay used by Nayak et al7 and Lin et al19 can only quantify CTCs with a high DNA content and is dependent on a human observer for the final identification of CTCs. In contrast, our noncommercial method is fully objective, using automated CTC recognition. Moreover, our assay can be easily performed with relatively simple and cheap laboratory techniques and equipment. Also possible is cost-effective, centralized analysis as a service for multicenter research, since our fixation method allows batch analysis of samples up to 6 months after collection due to immediate sample fixation.7,9,18
The background of the CTC assay for CSF was ≤0.2 CTCs/mL in patients with no LM in our study, which is similar to the background signal found in blood (≤0.25 CTCs/mL).21
Adding EpCAM staining of CSF cytospins (semiquantitative tumor cell measurements) to standard CSF cytology showed a slight but nonsignificant improvement in diagnosing LM. Although the sensitivity of CSF cytology for LM (61.5%) is comparable to the sensitivity found in earlier studies,5–8 we cannot exclude that sampling of 5 mL instead of 10 mL of CSF for cytology may have affected its sensitivity.
In conclusion, our EpCAM-based flow cytometry assay has a 100% sensitivity and 100% specificity for the diagnosis of LM in patients with primary epithelial tumors and a clinical suspicion but a nonconfirmatory diagnosis of LM on MRI. These results should be confirmed in a large independent and representative cohort to recommend the use of the flow cytometry assay next to cytology in CSF of patients clinically suspected for LM. Furthermore, more research needs to be performed about whether tumor cell measurements by flow cytometry in CSF can be used for LM treatment monitoring.
Funding
This work was financed by the Netherlands Cancer Institute—Antoni van Leeuwenhoek Hospital, Amsterdam, the Netherlands.
There is no external funding of this research to disclose.
Conflict of interest statement. No authors have any conflicts of interest to disclose.
Acknowledgments
We would like to acknowledge NKI-AVL Core Facility Molecular Pathology & Biobanking (CFMPB) for supplying NKI-AVL Biobank material and/or laboratory support.
References
- 1.Chamberlain M, Soffietti R, Raizer J et al. Leptomeningeal metastasis: a Response Assessment in Neuro-Oncology critical review of endpoints and response criteria of published randomized clinical trials. Neuro Oncol. 2014;169:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drappatz J, Batchelor TT. Leptomeningeal neoplasms. Curr Treat Options Neurol. 2007;9(4):283–293. [DOI] [PubMed] [Google Scholar]
- 3.Le Rhun E, Taillibert S, Chamberlain M. Carcinomatous meningitis: leptomeningeal metastases in solid tumors. Surg Neurol Int. 2013;4(Suppl 4):S265–S288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Straathof CS, de Bruin HG, Dippel DW et al. The diagnostic accuracy of magnetic resonance imaging and cerebrospinal fluid cytology in leptomeningeal metastasis. J Neurol. 1999;246(9):810–814. [DOI] [PubMed] [Google Scholar]
- 5.Van Oostenbrugge RJ, Twijnstra A. Presenting features and value of diagnostic procedures in leptomeningeal metastases. Neurology. 1999;53(2):382–385. [DOI] [PubMed] [Google Scholar]
- 6.Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer. 1982;49(4):759–772. [DOI] [PubMed] [Google Scholar]
- 7.Nayak L, Fleisher M, Gonzalez-Espinoza R et al. Rare cell capture technology for the diagnosis of leptomeningeal metastasis in solid tumors. Neurology. 2013;80(17):1598–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Subirá D, Serrano C, Castanón S et al. Role of flow cytometry immunophenotyping in the diagnosis of leptomeningeal carcinomatosis. Neuro Oncol. 2012;14(1):43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glantz MJ, Cole BF, Glantz LK et al. Cerebrospinal fluid cytology in patients with cancer: minimizing false-negative results. Cancer. 1998;82(4):733–739. [DOI] [PubMed] [Google Scholar]
- 10.Brandsma D, Voest EE, De Jager W et al. CSF protein profiling using Multiplex Immuno-assay: a potential new diagnostic tool for leptomeningeal metastases. J Neurol. 2006;253(9):1177–1184. [DOI] [PubMed] [Google Scholar]
- 11.Balic M, Williams A, Lin H et al. Circulating tumor cells: from bench to bedside. Annu Rev Med. 2012;64:31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cesana C, Klersy C, Scarpati B et al. Flow cytometry and cytomorphology evaluation of hematologic malignancy in cerebrospinal fluids: comparison with retrospective clinical outcome. Ann Hematol. 2011;90(7):827–835. [DOI] [PubMed] [Google Scholar]
- 13.Bromberg JEC, Breems DA, Kraan J et al. CSF flow cytometry greatly improves diagnostic accuracy in CNS hematologic malignancies. Neurology. 2007;68(20):1674–1679. [DOI] [PubMed] [Google Scholar]
- 14.Subirá D, Castañón S, Román A et al. Flow cytometry and the study of central nervous disease in patients with acute leukaemia. Br J Haematol. 2001;112(2):381–384. [DOI] [PubMed] [Google Scholar]
- 15.Wilson WH, Bromberg JEC, Stetler-Stevenson M et al. Detection and outcome of occult leptomeningeal disease in diffuse large B-cell lymphoma and Burkitt lymphoma. Haematologica. 2014;99(7):1228–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Went PT, Lugli A, Meier S et al. Frequent EpCam protein expression in human carcinomas. Hum Pathol. 2004;35(1):122–128. [DOI] [PubMed] [Google Scholar]
- 17.Patel AS, Allen JE, Dicker DT et al. Identification and enumeration of circulating tumor cells in the cerebrospinal fluid of breast cancer patients with central nervous system metastases. Oncotarget. 2011;2(10):752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Rhun E, Massin F, Tu Q et al. Development of a new method for identification and quantification in cerebrospinal fluid of malignant cells from breast carcinoma leptomeningeal metastasis. BMC Clin Pathol. 2012;12:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin X, Fleisher M, Omuro AMP, Shagabayeva L, Pentsova E. Prospective validation of cerebrospinal fluid (CSF) circulating tumor cells (CTC) to diagnose leptomeningeal metastasis (LM) from epithelial tumors. In: ASCO Annual Meeting 2015. Available at: http://meetinglibrary.asco.org/content/153257-156. [Google Scholar]
- 20.Subirá D, Simó M, Illán J et al. Diagnostic and prognostic significance of flow cytometry immunophenotyping in patients with leptomeningeal carcinomatosis. Clin Exp Metastasis. 2015;32(4):383–391. [DOI] [PubMed] [Google Scholar]
- 21.Pluim D, Devriese LA, Beijnen JH, Schellens JHM. Validation of a multiparameter flow cytometry method for the determination of phosphorylated extracellular-signal-regulated kinase and DNA in circulating tumor cells. Cytometry A. 2012;81(8):664–671. [DOI] [PubMed] [Google Scholar]
- 22.Gray W, McKee GT. Diagnostic Cytopathology. 2nd ed. London: Churchill Livingstone; 2003. [Google Scholar]
- 23.Hayes DF, Smerage JB. Circulating tumor cells. Prog Mol Biol Transl Sci. 2010;95:95–112. [DOI] [PubMed] [Google Scholar]
- 24.Elashoff JD. nQuery Advisor Version 7.0 Users's Guide. Los Angeles, CA: Statistical Solutions Ltd; 2007. [Google Scholar]
- 25.Chamberlain MC. Neoplastic meningitis. J Clin Oncol. 2005;23(15):3605–3613. [DOI] [PubMed] [Google Scholar]