Abstract
Background
The loss of muscle mass (sarcopenia) and the associated reduced muscle strength are key limiting factors for elderly people's quality of life. Improving muscle performance does not necessarily correlate with increasing muscle mass. In fact, particularly in the elderly, the main explanation for muscle weakness is a reduction of muscle quality rather than a loss of muscle mass, and the main goal to be achieved is to increase muscle strength. The effectiveness of Trimetazidine (TMZ) in preventing muscle functional impairment during ageing was assessed in our laboratory.
Methods
Aged mice received TMZ or vehicle for 12 consecutive days. Muscle function was evaluated at the end of the treatment by a grip test as well as by an inverted screen test at 0, 5, 7 and 12 days of TMZ treatment. After sacrifice, muscles were stored for myofiber cross‐sectional area assessment and myosin heavy chain expression evaluation by western blotting.
Results
Chronic TMZ treatment does not affect the mass of both gastrocnemius and tibialis anterior muscles, while it significantly increases muscle strength. Indeed, both latency to fall and grip force are markedly enhanced in TMZ‐treated versus untreated mice. In addition, TMZ administration results in higher expression of slow myosin heavy chain isoform and increased number of small‐sized myofibers.
Conclusions
We report here some data showing that the modulation of skeletal muscle metabolism by TMZ increases muscle strength in aged mice. Reprogramming metabolism might therefore be a strategy worth to be further investigated in view of improving muscle performance in the elderly.
Keywords: Aging, Exercise pill; Metabolic reprogramming; Neuro‐rehabilitation; Sarcopenia
Introduction
Ageing is an extremely complex biological phenomenon. In general terms, aged individuals lose the capacity to maintain their physiological homeostasis. Many vital organs undergo atrophy or degeneration, in particular, those characterized by post‐mitotic cells such as the skeletal muscle. At the cellular level, ageing is characterized by accumulation of damaging events such as genomic instability and stem cell exhaustion (reviewed by Kroemer).1
The loss of muscle mass (often referred to as sarcopenia) is one of the most relevant changes occurring in ageing. Indeed, sarcopenia and the associated reduced muscle strength are key limiting factors for elderly people's quality of life, resulting in reduced mobility, difficulties in ordinary daily activities, loss of independence and increased risk of fractures. It is estimated that by 2060 the percentage of elderly people (over 65 years) in Europe will have increased by around 80%.2 Because of the growing number of elderly individuals, age‐related diseases and disabilities are rapidly becoming a major health and social problem; indeed, the profound change of family organization (most of the components are out of home during the day) renders external assistance to the elderly unavoidable. It is therefore mandatory to develop strategies aimed at increasing elderly people's self‐sufficiency, making seniors able to perform the activities of daily living, such as rising from a chair, carrying shopping bags and climbing stairs.3, 4 To achieve this goal, increased muscle strength and improved cardio‐respiratory capacity should be obtained.5
Muscle wasting in the elderly is not necessarily associated with body weight loss or reduced body mass index; indeed, increased fat mass (sarcopenic obesity) might hide muscle depletion.6 In contrast, sarcopenia is very often associated with reduced myofiber cross‐sectional area (CSA; atrophy) and muscle fibre loss (hypoplasia),7, 8 which might be induced by the age‐related decrease in the levels of anabolic factors promoting protein synthesis (e.g. Growth Hormone and Insulin Growth Factor 1), by the increased expression of pro‐inflammatory factors mediating protein degradation, by the loss of motoneurons and by apoptosis.9 Basal protein breakdown and synthesis do not change much in ageing,10 whereas it seems that aged adults have a blunted response to anabolic stimuli such as nutrients, insulin and resistance exercise, which likely contributes to the loss of skeletal muscle mass in the elderly.9, 11, 12 Sarcopenia has also been associated with reduced myogenic capacity; muscles in aged individuals display a low reservoir of skeletal muscle stem cells, these having impaired myogenic potential and ability to self‐renew and restore the reservoir.7, 13, 14, 15
As for the mechanisms underlying the pathogenesis of sarcopenia, various hypotheses have been proposed. Ageing is characterized by chronic inflammation, with enhanced production of pro‐inflammatory cytokines such as interleukin‐6 (IL‐6) and tumor necrosis factor α, well‐known mediators of muscle protein catabolism.16, 17 Moreover, neuroendocrine changes and increased mitochondrial production of reactive oxygen species contribute to the neuromuscular junction (NMJ) degeneration and to the muscle denervation occurring in the elderly.18, 19, 20, 21, 22 Finally, muscles of aged individuals frequently show accumulation of adipose and connective tissue within the muscles, respectively resulting in lipodystrophy and fibrosis.13 In addition to these features, also profound metabolic changes occur in myofibers during ageing—above all, reduced mitochondrial mass and function, this likely resulting in low ATP production.21, 23, 24 This would compromise cell functions and reduce contractile force generation, so leading to loss of muscle mass and strength.25 In the elderly, type II (glycolitic) myofibers are more prone to atrophy than type I (oxidative) fibres8, 26, 27, and a switch from type II to type I fibres has often been reported.7, 28, 29, 30, 31 NMJ degeneration has also been associated with mitochondrial dysfunctions.21 Therefore, forcing myofiber metabolism to optimize energy production might be an interesting route to improve muscle function.
The metabolic modulator trimetazidine (TMZ) optimizes heart metabolism by impinging on myocardial substrate utilization.32, 33 As a result of its action, oxidation is shifted from free fatty acids to glucose, so glycolysis to glucose oxidation coupling is improved (Figure 1).34 Based on these premises, it is conceivable that the metabolic switch triggered by TMZ in the heart might also occur in the skeletal muscle, this likely improving muscle performance. Such a hypothesis is supported by previous observations showing that TMZ also improves exercise capacity in patients with angina.35, 36 Furthermore, recent observations showed for the first time that TMZ directly acts on skeletal muscle cells in culture protecting them from hypotrophy induced by different agents.37 In the current study, we treated elderly mice with TMZ in order to analyse the effect of this metabolic modulator on skeletal muscle force and mass. The results obtained show that TMZ administration is indeed able to improve skeletal muscle strength, without significantly impinging on muscle mass.
Figure 1.
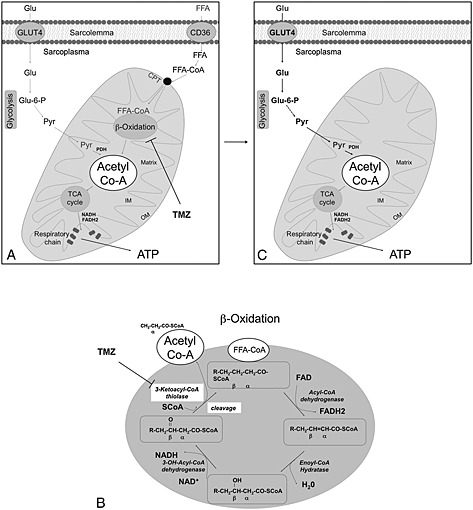
Mechanism of action of trimetazidine (TMZ). (a) Free fatty acids (FFA) are taken up by the cells either via diffusion or via transporters such as CD36. Inside the cytosol, FFA are esterified to fatty acyl‐CoA (FFA‐CoA) which might be catalized by β‐oxidation, this producing acetyl‐CoA. In order to cross, the outer and inner membranes (OM and IM) of mitochondria where FFA‐CoA undergo β‐oxidation, the acyl group of FFA‐CoA must be transferred to carnitine via carnitine palmitoyltransferase1. The acylcarnitine is then shuttled into the mitochondria, where it is converted back to FFA‐CoA by CPT2 (CPT1 and 2 are generically indicated in this figure as CPT). (b) Fatty acid β‐oxidation involves four enzymes (Acyl‐CoA dehydrogenase, Enoyl‐CoA hydratase, 3‐OH Acyl‐CoA dehydrogenase and 3‐Ketotacyl‐CoA thiolase). Upon each cycle of the β‐oxidation, a molecule of acetyl‐CoA and a FFA‐CoA two carbons shorter than the one entering the cycle are produced. The acetyl‐CoA produced enters the TCA cycle. The acetyl‐CoA necessary to feed the TCA cycle derives also from glycolysis, and in particular, from the conversion of pyruvate by the pyruvate dehydrogenase (PDH) complex. The metabolic modulator TMZ inhibits one the four enzymes of the β‐oxidation cycle, the 3‐Ketotacyl‐CoA thiolase (b), this partially inhibiting β‐oxidation (a). (c) As consequence, to obtain acetyl‐CoA needed to feed the TCA cycle and to allow ATP production by respiratory chain, the cells necessarily use more glucose.
Methods
Animals and experimental design
Aged (22 months old) C57BL6/J male mice were used. They were maintained on a regular dark‐light cycle (light from 08:00 to 20:00), with free access to food (Piccioni, Brescia, Italy) and water during the whole experimental period, including the night before sacrifice. Experimental animals were cared for in compliance with the Italian Ministry of Health Guidelines (n° 86609 EEC, permit number 106/2007‐B) and the Policy on Humane Care and Use of Laboratory Animals (NIH 1996). Sample size has been calculated on the basis of previous results showing that 5–6 animals aged 22 months are necessary to detect a significant reduction of gastrocnemius (GSN) and tibialis anterior (TA) mass.38
The animals were divided into two groups (n = 6 each) randomized according to their body weight on the day before the beginning of TMZ administration; one group received TMZ 5 mg/kg intraperitoneal (i.p.) injection twice a day for 12 consecutive days, the other group (Ctrl) received i.p. injections with vehicle only (PBS). Twelve days after the beginning of TMZ treatment, the animals were weighed and anesthetized by isoflurane inhalation. The blood was collected by cardiac puncture from anesthetized animals and monitored for glucose concentration by using the Glucocard G‐sensor strips and apparatus (Menarini Diagnostics). Mice were then sacrificed by cervical dislocation, TA or GSN muscles were rapidly excised, weighed and frozen in liquid N2‐cooled isopentane and finally stored at −80°C.
Skeletal muscle function analysis
The hanging grid test (or inverted grip‐hanging test),39, 40, 41, 42 able to measure muscle force of the four limbs, was performed on a 22 months old C57BL6/J male mice treated or not with TMZ at 0, 5, 7 and 12 days; untrained mice were individually placed at the centre of a wire mesh screen (10 × 14 cm; wire thickness, 2 mm), then the grid was inverted upside‐down with the mouse's head declining first, and latency to fall off was recorded. The screen was held steadily 40–50 cm above a padded surface to protect the mouse from injuring itself. Each day of testing, the latency for the mouse to release the grid, was recorded in three independent trials conducted approximately 15 min apart, and data from all three trials were averaged together. In addition, a forelimb grip strenght test was performed after 12 days of TMZ treatment by using a grip strength metre (Columbus Instruments). Mice held by the tail were gently allowed to grasp a wire grid with the fore paws. Mice were then gently pulled by the tail until they released their grip. The force achieved by the mouse was recorded during three trials and averaged.
Protein isolation and western blotting
Tissue samples from GSN muscles were homogenized and lysed in ice cold Cosper and Leinwand Myosin Extraction Buffer (300 mM NaCl, 0.1 M NaH2PO4, 0.05 M Na2HPO4, 0.01 M Na4P2O7, 1 mM MgCl2, 10 mM EDTA, 1 mM DTT pH 6.5)43 supplemented with a protease inhibitor cocktail (Roche) and a phosphatase inhibitor cocktail (Sigma‐Aldrich). As for myosin heavy chain (MyHC), low ionic strength lysis buffers were reported as not adequate for MyHC analysis because they do not solubilize myosin from thick filaments, resulting in low MyHC levels in the lysate supernatant.44 In order to achieve an accurate extraction of MyHC, we homogenized muscles in a high‐salt buffer (see preceding text).43 A clear supernatant was obtained by centrifugation of lysates at 13 000 g for 20 min at 4°C. Protein concentration in the supernatant was determined by Bradford protein assay (Bio‐Rad). Aliquots of total cell lysates were then separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, and proteins were transferred to nitrocellulose membranes (Hybond‐C Extra; Amersham Biosciences). Membranes were blocked overnight at 4°C with 5% non‐fat milk in T‐TBS (Tris‐Buffered Saline with 0.05% Tween 20) and then probed using the following antibodies directed against: slow MyHC (myosin heavy chain) (M8421), desmin (D1033), fast MyHC (M4276) and α‐tubulin (T5168), all obtained from Sigma‐Aldrich. We also used the monoclonal MF20 antibody (Developmental Studies Hybridoma Bank at the University of Iowa), which recognizes all isoforms of sarcomeric MyHC. The appropriate secondary horseradish peroxidase‐conjugated antibodies from Jackson Immunoresearch were used in blocking solution for 1 h at room temperature. Immunoreactive bands were visualized by SuperSignal West Pico Chemioluminescent substrate kit (Pierce). Equal loading of samples was confirmed by α‐tubulin normalization and quantified by densitometry using the ImageQuant TL software from GE Healthcare Life Sciences.
Immunohistochemistry and cross‐sectional area evaluation
Myofiber CSA measurement was performed on TA. Serial muscle sections (9 µm) were obtained from the mid‐belly region of the TA muscles, which had been embedded in OCT. A CM1900 cryostat (Leica, Wetzlar, Germany) at −20°C was used. Sections were fixed in 4% PFA (paraformaldehyde) and stained with an anti‐laminin (L9393) antibody from Sigma‐Aldrich. The Alexa Fluor 488 anti rabbit IgG (A11008) from Life Technologies was used as secondary antibody. Nuclei were visualized with the DNA dye 40,6‐diamidino‐2‐phenylindole, and the samples were mounted in SlowFade Gold mounting media (Life Technologies). The images were acquired with a Leica TCS SP5 confocal microscope. In the stained muscle sections, automated CSA determination along the laminin‐stained border of each fibre was evaluated by using Image J.45 Because errors in fibre border recognition might occur (i.e. either the fibres might not be recognized or several fibres/non‐fibre regions might be interpreted as a single fibre), a manual correction of myofiber border misinterpretation was performed.
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). Statistical differences between groups were verified by Student's t‐test (2‐tailed). P < 0.05 was considered significant.
Results
Effect of TMZ administration on glycemia, muscle mass and myofiber CSA
A slight reduction of blood glucose concentrations was observed in TMZ‐treated animals (Figure 2a), consistently with previous data showing that this drug enhanced cell glucose uptake.37 However, the difference between TMZ‐treated and untreated aged mice did not reach significance, likely due to the low sample size. Skeletal muscle mass measured in both the TA and the GSN of mice treated with TMZ for 12 days was not different from that of untreated age‐matched controls (Figure 2b). Myofiber CSA was also evaluated in both TMZ‐administered and control mice. Transverse sections of TA muscles were stained with an antibody against laminin, a major component of the basal lamina, and counterstained with 40,6‐diamidino‐2‐phenylindole to detect nuclei. Frequency histograms revealed that, in comparison to untreated controls, the muscle of TMZ‐treated mice displayed a shift towards myofibers with small CSA (Figure 2c and 2d). Consistently, we also found that TMZ treatment triggered an increase of centronucleated fibres as reported in Figure 2c and 2e.
Figure 2.
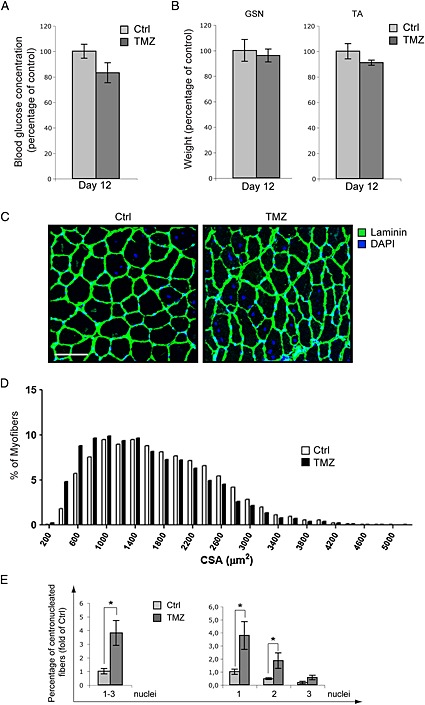
Effect of TMZ administration on glycemia, muscle mass and myofiber CSA. (a) Blood glucose concentration measured before sacrifice in old mice treated with TMZ. Blood was collected by cardiac puncture from anesthetized animals. Data were presented as percentage of untreated mice (Ctrl). (b) Average weight of gastrocnemius (GSN) and tibialis anterior (TA) muscles after 12 days of TMZ treatment, reported as a percentage of the untreated mice weight (Ctrl). (c) Representative images of TA muscle sections stained with antibody to Laminin and 40,6‐diamidino‐2‐phenylindole to detect nuclei are shown. TA sections from 3 untreated (Ctrl) mice and 3 TMZ‐treated mice were stained. Scale bar: 100 µm. (d) Frequency histogram showing the distribution of myofiber CSA measured on transversal sections of TA muscles from TMZ‐treated and untreated (Ctrl) mice. CSA of at least 7000 myofibers from 3 untreated mice and at least 7000 myofibers from 3 TMZ‐treated mice was measured. (e) Histograms show the percentage of centronucleated myofibers with respect to the total number of fibres counted in transversal sections of TA muscles from TMZ‐reated and untreated (Ctrl) mice. On the left panel, centronucleated myofibers with 1, 2 and 3 nuclei per fibre were counted. On the right panel, centronucleated myofibers showing 1 or 2 or 3 nuclei were counted separately. Each value indicates the mean±S.E.M. (reported as fold‐change of Ctrl) of the percentages calculated evaluating a total of at least 7000 myofibers from 3 untreated mice and at least 7000 myofibers from 3 TMZ‐treated mice *P ≤ 0.05 with respect to Ctrl. Scale bar: 10 µm.
Muscle strength increased upon TMZ treatment
To test TMZ effectiveness in preventing the functional impairment associated with sarcopenia, muscle strength was measured by the inverted grip‐hanging test in 22 months old TMZ‐treated C57BL6/J male mice at 0, 5, 7 and 12 days of treatment. As shown in Figure 3, chronic TMZ treatment (that did not exert detectable evidence of toxicity) significantly increased muscle strength. Indeed, TMZ‐treated mice were able to grip the screen almost three times longer than untreated mice. Latency to fall down increased already after 5 days of TMZ treatment (Figure 3a and 3b). This difference was still present after 7 days of treatment, while it ceased to be significant as of day 12, although the tendency to increase was quite clear (Figure 3a and 3b). Of interest, grip force of both untreated and TMZ‐treated mice progressively increased over time (Figure 3a). In untreated mice, however, strength increase was slower than in TMZ‐treated mice (Figure 3a).
Figure 3.
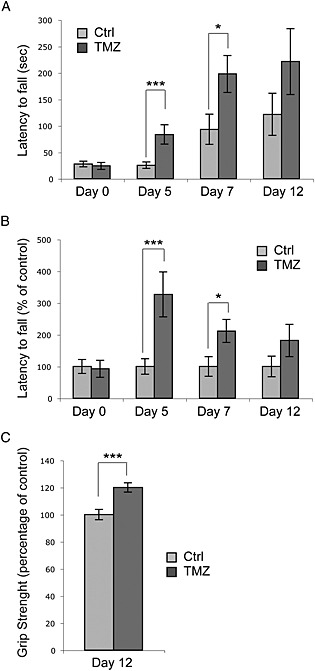
Trimetazidine (TMZ) increases grip strength in aged mice. (a) An inverted grip‐hanging test was performed on 22 months old C57BL6/J male mice treated or not with TMZ. Mice were injected with TMZ for consecutive 12 days. Latency to fall off was recorded at 0, 5, 7 and 12 days of TMZ treatment. The latency to fall from the screen is significantly higher in TMZ‐treated aged mice (TMZ) compared with PBS‐injected aged mice (Ctrl). (b) The same data as (a) are reported as a percentage of control. n = 6 animals were tested for each treatment (Ctrl and TMZ). Each day of testing, for each mouse, three independent trials were conducted 15 min apart. Data are reported as the mean±S.D. (c) Fore grip strength was measured after 12 days of TMZ treatment by using a grip strenght metre. The force achieved by the mouse was recorded for three trials and averaged. *P ≤ 0.05, ***P ≤ 0.005 with respect to Ctrl by student t‐test.
Grip strength has also been measured at 12 days of TMZ‐treatment with a commercial digital grip strength metre (Figure 2c; 20% increase in TMZ‐treated vs untreated mice, P < 0.005), supporting the results obtained by the inverted screen test. These data were allowed to conclude that TMZ administration resulted in a significant increase of muscle strength in aged mice, likely because of its action as a metabolic modulator.
Slow MyHC isoform over‐expression upon TMZ treatment
Muscle atrophy might be associated with a fibre type shift, which also occurred during ageing. On this line, the expression levels of MyHC isoforms were evaluated by western blotting (Figure 4a). The results showed that total levels of the structural proteins MyHC, fast MyHC and desmin did not significantly change upon TMZ treatment, whereas the levels of MyHC slow isoform increased (Figure 4a).
Figure 4.
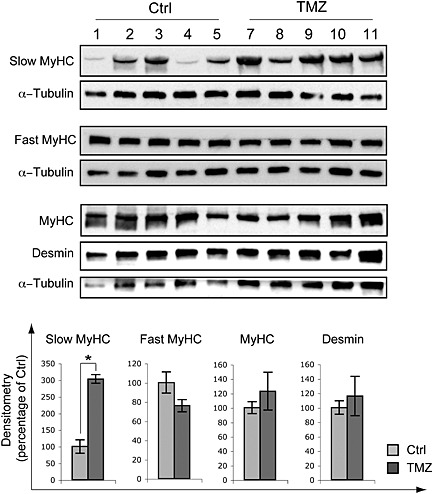
Trimetazidine (TMZ) triggers slow MyHC isoform over‐expression. Gastrocnemius extracts from untreated (Ctrl) and TMZ‐treated mice were assayed for slow MyHC, fast MyHC, total MyHC and desmin protein levels. Protein levels of representative 5 out of 6 untreated mice (1–5) and 5 out of 6 TMZ‐treated mice (7–;11) are shown. α‐Tubulin was used as loading control. Density of immunoreactive bands was calculated using the ImageQuant TL software from GE Healthcare Life normalized for α‐tubulin. Each value indicates the mean± S.E.M. (reported as percentage of Ctrl) of the densitometric analysis on three independent immunoblots. *P ≤ 0.05 with respect to Ctrl.
Discussion
The purpose of this study was to investigate the effect of TMZ treatment on skeletal muscle mass and strength in aged mice. We revealed that chronic TMZ treatment in ageing significantly increases muscle strength. Our experiments show that grip force of untreated and TMZ‐treated mice progressively increases over time. However, in untreated mice, strength increase is slower than in TMZ‐treated mice. Such a progressive increase likely depends on an adaptation to exercise; in this regard, TMZ appears to facilitate force improvement, somehow mimicking and anticipating exercise effects.
It is noteworthy that, contrarily to force, there was no corresponding increase of muscle mass and myofiber CSA in aged animals upon TMZ treatment (Figure 2a, 2b and 2c). This is inconsistent with the finding that the drug counteracts hypotrophy in C2C12 myotube cultures induced by TNF‐α and serum starvation.37 However, it is in line with several studies reporting that improved muscle force does not necessarily correlate with muscle hypertrophy; this is particularly evident upon endurance exercise. In addition, strength decrease in the elderly does not parallel muscle depletion, being about four‐fold greater.9 Indeed, muscle weakness in the elderly mainly derives from impaired muscle quality rather than from loss of muscle mass; this has been associated with decreased fibre specific tension and with other factors such as the loss of motor units, NMJ degeneration, reduction of excitation‐contraction coupling and decreased transmission of lateral force.9, 46, 47 Taking these observations into consideration, it is conceivable that strategies able to increase muscle force in the elderly are more relevant than those aimed at obtaining a mere restoration of muscle mass.
MyHC is the core of the contractile apparatus, while desmin forms a scaffold around the Z‐disc of the sarcomere, connecting it to the cell cytoskeleton and maintaining the structural integrity of myofibers. At variance with the expression levels of the structural proteins MyHC and desmin, which do not seem to vary upon TMZ administration in aged mice, the slow MyHC isoform is definitively up‐regulated by TMZ‐treatment. Interestingly, endurance exercise has been associated with marked modifications of myofiber contractile properties due to a fibre type shift towards the slow‐twitch contractile apparatus.48, 49 Exercise triggers both a metabolic and structural remodelling in the skeletal muscle in order to reduce muscle fatigue; indeed, the shift towards long‐twitch and slow MyHC isoforms contributes to improve the energetic efficiency.50 The increased expression of slow MyHC induced by TMZ might suggest that the drug could trigger some effects similar to those induced by exercise, thus acting like other pharmacological compounds defined as ‘exercise mimetics’, although this point still needs to be demonstrated. Notably, also the exercise mimetics GW1516 and AICAR are metabolic remodelling agents that recapitulate some exercise effects aimed at achieving the best metabolic energetic efficiency. Similarly, TMZ modulates cell metabolism through a mechanism, which might be beneficial during exercise where there is a high oxygen expenditure. In this regard, the choice of glucose as a substrate triggered by TMZ induces a more efficient utilization of the oxygen available; this could increase skeletal muscle metabolism efficiency and contractile performance, as already observed for cardiac muscle function under transitory hypoxia.34
Finally, our experiments demonstrate that, in aged mice, TMZ triggers a shift towards myofiber characterized by a low CSA. Notably, it has been shown that the CSA of type I (slow) myofibers is smaller than that of type II (fast) fibres, and this strongly correlates with our finding that TMZ‐treatment enhances the expression of the slow MyHC isoform. In addition, we found that TMZ enhances the number of centronucleated fibres (Figure 2c and 2e), which strongly suggests an increase of newly formed myofibers in vivo. This might contribute to explaining the increased number of small‐sized myofibers we found upon TMZ treatment of aged mice. Further studies are in progress to better investigate this point.
The data here reported suggest that the positive effects of TMZ on muscle force might be directly exerted acting on myofibers and might derive from metabolic modulations. However, we cannot exclude that such effects could be secondary to effects on other organs—such as improved cardiac function (often impaired in the elderly)—and which might result in increased nutrient and oxygen availability to the skeletal muscle. On the whole, because TMZ appears to clearly improve muscle function in aged animals, this drug appears appealing for a possible reappraisal for the increase of muscle force in the elderly, and further experiments would be welcome and helpful in order to explore the molecular mechanism underlying such effects.
Ethical standards statement
Experimental animals were cared for in compliance with the Italian Ministry of Health Guidelines (n° 86609 EEC, permit number 106/2007‐B) and the Policy on Human Care and Use of Laboratory Animals (NIH 1996). The experimental protocol was approved by the Bioethical Committee of the University of Turin and by the Italian Ministry of Health, Italy, and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Conflict of interest
Elisabetta Ferraro, Fabrizio Pin, Stefania Gorini, Laura Pontecorvo, Alberto Ferri, Vincenzo Mollace, Paola Costelli and Giuseppe Rosano declare that they have no conflict of interest.
Acknowledgements
This work was supported by the Italian Ministry of Health (Ricerca Finalizzata‐2010‐2318508 to Elisabetta Ferraro), University of Turin (ex‐60% funds) and Associazione Italiana per la Ricerca sul Cancro (AIRC‐PC:IG9153). We wish to thank MW Bennett for the valuable editorial work. The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle (von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7–8).
Ferraro, E. , Pin, F. , Gorini, S. , Pontecorvo, L. , Ferri, A. , Mollace, V. , Costelli, P. , and Rosano, G. (2016) Improvement of skeletal muscle performance in ageing by the metabolic modulator Trimetazidine. Journal of Cachexia, Sarcopenia and Muscle, 7: 449–457. doi: 10.1002/jcsm.12097.
References
- 1. Lopez‐Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 2013;153:1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Forte R, Pesce C, Leite JC, De Vito G, Gibney ER, Tomporowski PD, et al. Executive function moderates the role of muscular fitness in determining functional mobility in older adults. Aging Clin Exp Res 2013;25:291–298. [DOI] [PubMed] [Google Scholar]
- 3. Rao SS. Prevention of falls in older patients. Am Fam Physician 2005;72:81–88. [PubMed] [Google Scholar]
- 4. Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol 2004;159:413–421. [DOI] [PubMed] [Google Scholar]
- 5. von Haehling S, Morley JE, Anker SD. From muscle wasting to sarcopenia and myopenia: update 2012. J Cachexia Sarcopenia Muscle 2012;3:213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci 2000;904:437–448. [DOI] [PubMed] [Google Scholar]
- 7. Snijders T, Verdijk LB, van Loon LJ. The impact of sarcopenia and exercise training on skeletal muscle satellite cells. Ageing Res Rev 2009;8:328–338. [DOI] [PubMed] [Google Scholar]
- 8. Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15‐ to 83‐year‐old men. J Neurol Sci 1988;84:275–294. [DOI] [PubMed] [Google Scholar]
- 9. Narici MV, Maffulli N. Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull 2010;95:139–159. [DOI] [PubMed] [Google Scholar]
- 10. Volpi E, Sheffield‐Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA 2001;286:1206–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fry CS, Rasmussen BB. Skeletal muscle protein balance and metabolism in the elderly. Curr Aging Sci 2011;4:260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporosis Int 2010;21:543–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carlson ME, Suetta C, Conboy MJ, Aagaard P, Mackey A, Kjaer M, et al. Molecular aging and rejuvenation of human muscle stem cells. EMBO Mol Med 2009;1:381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kadi F, Charifi N, Denis C, Lexell J. Satellite cells and myonuclei in young and elderly women and men. Muscle Nerve 2004;29:120–127. [DOI] [PubMed] [Google Scholar]
- 15. Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch‐mediated restoration of regenerative potential to aged muscle. Science 2003;302:1575–1577. [DOI] [PubMed] [Google Scholar]
- 16. Roubenoff R. Physical activity, inflammation, and muscle loss. Nutr Rev 2007;65:S208–S212. [DOI] [PubMed] [Google Scholar]
- 17. Neels JG, Olefsky JM. Inflamed fat: what starts the fire? J Clin Invest 2006;116:33–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jang YC, Lustgarten MS, Liu Y, Muller FL, Bhattacharya A, Liang H, et al. Increased superoxide in vivo accelerates age‐associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J 2010;24:1376–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Delbono O. Neural control of aging skeletal muscle. Aging Cell 2003;2:21–29. [DOI] [PubMed] [Google Scholar]
- 20. Fulle S, Protasi F, Di Tano G, Pietrangelo T, Beltramin A, Boncompagni S, et al. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp Gerontol 2004;39:17–24. [DOI] [PubMed] [Google Scholar]
- 21. Ibebunjo C, Chick JM, Kendall T, Eash JK, Li C, Zhang Y, et al. Genomic and proteomic profiling reveals reduced mitochondrial function and disruption of the neuromuscular junction driving rat sarcopenia. Mol Cell Biol 2013;33:194–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Deschenes MR, Roby MA, Eason MK, Harris MB. Remodeling of the neuromuscular junction precedes sarcopenia related alterations in myofibers. Exp Gerontol 2010;45:389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Joseph AM, Adhihetty PJ, Buford TW, Wohlgemuth SE, Lees HA, Nguyen LM, et al. The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high‐ and low‐functioning elderly individuals. Aging Cell 2012;11:801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peterson CM, Johannsen DL, Ravussin E. Skeletal muscle mitochondria and aging: a review. J Aging Res 2012;2012:194821. doi:10.1155/2012/194821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hiona A, Leeuwenburgh C. The role of mitochondrial DNA mutations in aging and sarcopenia: implications for the mitochondrial vicious cycle theory of aging. Exp Gerontol 2008;43:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, et al. Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J Gerontol 1992;47:B71–B76. [DOI] [PubMed] [Google Scholar]
- 27. Andersen JL. Muscle fibre type adaptation in the elderly human muscle. Scand J Med Sci Sports 2003;13:40–47. [DOI] [PubMed] [Google Scholar]
- 28. Holloszy JO, Chen M, Cartee GD, Young JC. Skeletal muscle atrophy in old rats: differential changes in the three fiber types. Mech Ageing Dev 1991;60:199–213. [DOI] [PubMed] [Google Scholar]
- 29. Larsson L, Biral D, Campione M, Schiaffino S. An age‐related type IIB to IIX myosin heavy chain switching in rat skeletal muscle. Acta Physiol Scand 1993;147:227–234. [DOI] [PubMed] [Google Scholar]
- 30. Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci 1995;50:11–16. [DOI] [PubMed] [Google Scholar]
- 31. Ciciliot S, Rossi AC, Dyar KA, Blaauw B, Schiaffino S. Muscle type and fiber type specificity in muscle wasting. Int J Biochem Cell Biol 2013;45:2191–2199. [DOI] [PubMed] [Google Scholar]
- 32. Kantor PF, Lucien A, Kozak R, Lopaschuk GD. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long‐chain 3‐ketoacyl coenzyme A thiolase. Circ Res 2000;86:580–588. [DOI] [PubMed] [Google Scholar]
- 33. Chaitman BR. Efficacy and safety of a metabolic modulator drug in chronic stable angina: review of evidence from clinical trials. J Cardiovasc Pharmacol Ther 2004;9:S47–S64. [DOI] [PubMed] [Google Scholar]
- 34. Lopaschuk GD, Barr R, Thomas PD, Dyck JR. Beneficial effects of trimetazidine in ex vivo working ischemic hearts are due to a stimulation of glucose oxidation secondary to inhibition of long‐chain 3‐ketoacyl coenzyme a thiolase. Circ Res 2003;93:e33–e37. [DOI] [PubMed] [Google Scholar]
- 35. Vitale C, Marazzi G, Pelliccia F, Volterrani M, Cerquetani E, Spoletini I, et al. Trimetazidine improves exercise performance in patients with peripheral arterial disease. Pharmacol Res 2011;63:278–283. [DOI] [PubMed] [Google Scholar]
- 36. Monti LD, Setola E, Fragasso G, Camisasca RP, Lucotti P, Galluccio E, et al. Metabolic and endothelial effects of trimetazidine on forearm skeletal muscle in patients with type 2 diabetes and ischemic cardiomyopathy. Am J Physiol Endocrinol Metab 2006;290:E54–E59. [DOI] [PubMed] [Google Scholar]
- 37. Ferraro E, Giammarioli AM, Caldarola S, Lista P, Feraco A, Tinari A, et al. The metabolic modulator trimetazidine triggers autophagy and counteracts stress‐induced atrophy in skeletal muscle myotubes. FEBS J 2013;280:5094–5108. [DOI] [PubMed] [Google Scholar]
- 38. Penna F, Bonetto A, Muscaritoli M, Costamagna D, Minero VG, Bonelli G, et al. Muscle atrophy in experimental cancer cachexia: is the IGF‐1 signaling pathway involved? Int J Cancer 2010;127:1706–1717. [DOI] [PubMed] [Google Scholar]
- 39. Apolloni S, Amadio S, Montilli C, Volonte C, D'Ambrosi N. Ablation of P2X7 receptor exacerbates gliosis and motoneuron death in the SOD1‐G93A mouse model of amyotrophic lateral sclerosis. Hum Mol Genet 2013;22:4102–4116. [DOI] [PubMed] [Google Scholar]
- 40. Altamirano F, Valladares D, Henriquez‐Olguin C, Casas M, Lopez JR, Allen PD, et al. Nifedipine treatment reduces resting calcium concentration, oxidative and apoptotic gene expression, and improves muscle function in dystrophic mdx mice. PLoS One 2013;8e81222. doi:10.1371/journal.pone.0081222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kaja S, van de Ven RC, van Dijk JG, Verschuuren JJ, Arahata K, Frants RR, et al. Severely impaired neuromuscular synaptic transmission causes muscle weakness in the Cacna1a‐mutant mouse rolling Nagoya. Eur J Neurosci 2007;25:2009–2020. [DOI] [PubMed] [Google Scholar]
- 42. Deacon RM. Measuring the strength of mice. J Visual Exp: JoVE 2013;76. doi:10.3791/2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cosper PF, Leinwand LA. Myosin heavy chain is not selectively decreased in murine cancer cachexia. Int J Cancer 2012;130:2722–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Toth MJ, Miller MS, Callahan DM, Sweeny AP, Nunez I, Grunberg SM, et al. Molecular mechanisms underlying skeletal muscle weakness in human cancer: reduced myosin‐actin cross‐bridge formation and kinetics. J Appl Physiol (1985) 2013;114:858–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012;9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Campbell MJ, McComas AJ, Petito F. Physiological changes in ageing muscles. J Neurol Neurosurg Psychiatry 1973;36:174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang C, Gao Y. Effects of aging on the lateral transmission of force in rat skeletal muscle. J Biomech 2014;47:944–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wilson JM, Loenneke JP, Jo E, Wilson GJ, Zourdos MC, Kim JS. The effects of endurance, strength, and power training on muscle fiber type shifting. Journal Strength Condition Res / National Strength Condition Assoc 2012;26:1724–1729. [DOI] [PubMed] [Google Scholar]
- 49. Gundersen K. Excitation‐transcription coupling in skeletal muscle: the molecular pathways of exercise. Biol Rev Camb Philos Soc 2011;86:564–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ferraro E, Giammarioli AM, Chiandotto S, Spoletini I, Rosano G. Exercise‐induced skeletal muscle remodeling and metabolic adaptation: redox signaling and role of autophagy. Antioxid Redox Signal 2014;21:154–176. [DOI] [PMC free article] [PubMed] [Google Scholar]


