Abstract
Background
It has been reported that 4,4′‐diamino‐diphenyl sulfone (DDS), the longtime treatment of choice for leprosy, prolongs the lifespan and increases mobility in animal models by reducing the levels of reactive oxygen species and inhibiting muscle pyruvate kinase activity. This study aimed to investigate whether sarcopenic status in leprosy survivors was influenced by recent history of DDS medication.
Methods
Forty‐one elderly female leprosy survivors were recruited. The DDS group was defined as survivors who had been taking the drug for the past year or more. Body composition measured by dual energy X‐ray absorptiometry, limb muscle strength, short physical performance battery, and International Physical Activity Questionnaire in Korean were compared.
Results
The DDS group tended to have higher skeletal muscle mass index (24.4 ± 2.7 vs. 22.6 ± 2.2%, P = 0.066) and regional skeletal muscle mass index in non‐dominant leg (8.9 ± 1.0 vs. 7.9 ± 0.9%, P = 0.018) than those of the control group although they had significantly worse leprosy disability than the control group (P = 0.027). The DDS group had greater strength than the control group in non‐dominant shoulder abductor, elbow flexor, hip flexor, and knee extensor (P = 0.005, P = 0.029, P = 0.021, and P = 0.002, respectively). Weekly walking amount was significantly longer (P = 0.020) in the DDS group than the control group. The total lifetime DDS exposure significantly correlated with skeletal muscle mass of the lower extremity in non‐dominant leg (r = 0.379, P = 0.015).
Conclusions
DDS‐taking leprosy survivors had larger skeletal muscle mass and greater muscle strength over non‐taking survivors. There was a dose–response relationship between total lifetime DDS exposure and skeletal muscle mass of lower extremity. These findings might suggest potential anti‐sarcopenic effects of DDS.
Keywords: Sarcopenia, Ageing, Leprosy, Dapsone
Introduction
Although resistance exercise is crucial for sarcopenia prevention,1 poor compliance related with low motivation and physical capacity in elderly people are challenges.2 To overcome these issues, several drugs3, 4 and anabolic hormones5 have been introduced as exercise mimetics. However, hormones have systemic and metabolic adverse effects,6 and newly introduced exercise mimetics await multiple phases of pre‐clinical and clinical trials. At present, it appears more promising to find connections between an existing drug product to sarcopenia—drug repositioning or drug repurposing.7 Because extensive information on the pharmacology and toxicity of repositioned drugs is available, their clinical use may be safer than that of newly developed drugs.8
There was a report that 4,4′‐diamino‐diphenyl sulfone (DDS), a sulfonamide antibiotic and a longtime treatment of choice for leprosy, prolonged lifespan and increased mobility in nematodes.9 Reduction in levels of reactive oxygen species and inhibition of muscular pyruvate kinase activity by DDS were proposed as the mechanisms underlying the increase in longevity in the animal model. These metabolic effects were replicated in mouse muscle tissue. Notably, compared with controls, DDS‐treated aged worms showed significantly faster body movements, which lead us to hypothesize that DDS may have sarcopenia‐preventive effects in humans.
An interesting phenomenon was noted in the authors' country that many leprosy survivors living in specialized colonies continue to take DDS, at varying doses, for a long time after their diseases have been eradicated, probably owing to an irrational fear of disease recurrence. This peculiar situation provides an opportunity to investigate the anti‐sarcopenic effects of DDS in humans. To our knowledge, whether long‐term DDS treatment alters sarcopenic status among leprosy survivors has not been previously investigated, although leprosy survivors are at a greater risk of developing sarcopenia than healthy individuals.10
We aimed to compare skeletal muscle mass and strength in leprosy survivors who had been taking DDS to those who had not. DDS‐taking leprosy survivors were compared with survivors not taking DDS with respect to body composition, muscular strength, functional performance, and activity levels. Dose–response relationships between the total lifetime DDS exposure and muscle mass/strength were also analysed. We hypothesized that DDS‐taking elderly female leprosy survivals would show greater skeletal muscle mass and strength than non‐takers.
Methods
Participants
A cross‐sectional study was performed at a local leprosy rehabilitation hospital in Yeosu, South Korea, between March 2012 and September 2013. At the time of the study, 82 female leprosy survivors resided in two leprosy villages (Dosung and Yeochun) affiliated with the hospital. Among them, 46 were elderly (aged 65 years or older) and ambulatory with mild or no physical impairment of the limbs. Survivors who had systemic or structural disorders that affected mobility or body composition, amputation proximal to the level of the wrist or ankle joint as a leprosy sequela, a history of central nervous system disease such as cerebrovascular accident or spinal cord injury, or a surgical history that could disturb the analysis of body composition (e.g. joint arthroplasty) were excluded. After five patients were excluded due to a history of arthroplasty (n = 3), cerebrovascular accident (n = 1), and poor cooperation (n = 1), 41 survivors were recruited (Figure 1). This study was approved by the institutional review board of our hospital (IRB No. H‐1109‐078‐378). Written informed consents were obtained from all female leprosy survivors.
Figure 1.
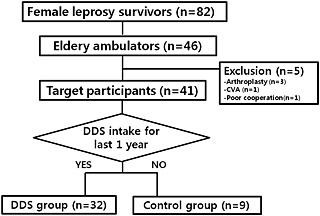
Flow diagram of participant recruitment. DDS, 4,4′‐diamino‐diphenyl sulfone.
Medical history
The medical history of the participants was obtained by one‐on‐one interviews to assess whether they had been taking DDS (dapsone) for the past year or longer, when they were diagnosed with leprosy, when they started DDS medication at a therapeutic dosage (100 mg/day), when they switched to a self‐selected, sub‐therapeutic dosage, and what dosage they were taking.
The DDS group was defined as survivors who were still taking the drug in the year prior to the study while the control group had not continued to take the drug. One year was selected as the cut‐off point of a long‐term medication period, which could be easily recalled by the elderly. There were 32 in the recent drug exposure group (DDS group) and only nine in the group without recent exposure (control group). The total DDS treatment period and the periods of therapeutic and sub‐therapeutic dosage were determined to estimate the total lifetime DDS exposure as follows: [period of therapeutic dosage (years) × 1 (tablet/day) × 365 days/year] + [period of sub‐therapeutic dosage (years) × self‐selected dosage per month (tablet/month) × 12 months/year].
The severity of disability of each participant was determined based on the World Health Organization leprosy disability grading system (WHO‐DG),11, 12 which was evaluated at the beginning of one's leprosy history from each participant's medical record.
Measurements
Baseline characteristics
Basic anthropometric data including height and weight were measured using standard methods. Body mass index (BMI) was calculated as weight divided by the square of one's height (kg/m2). Obesity was defined as a BMI over 25 kg/m2.13 Waist circumference (cm) was checked at the midpoint between the lower margin of the last palpable rib and the top of the iliac crest at the end of a normal expiration with the arms relaxed at the sides.
Body composition
Dual energy X‐ray absorptiometry (DISCOVERY‐W fan‐beam densitometer; Hologic Inc., Bedford, MA) was used to analyse body composition including lean body and fat masses. Appendicular skeletal muscle mass was calculated by obtaining the sum of the lean mass in bilateral upper and lower extremities.14 Skeletal muscle mass index (SMI, %) was calculated from appendicular muscle mass divided by total body weight.15 Regional SMI was defined as the skeletal muscle mass of each extremity normalized by total body weight (%). The cut‐off value from the fourth Korea National Health and Nutrition Examination Survey (2009) in Korean women (SMI = 23.2%) was accepted for low appendicular muscle mass.16 The participant was classified as having sarcopenic obesity if the conditions for both obesity and low appendicular muscle mass were met.17
Muscular strength
Muscular strength was assessed using a handheld dynamometer (PowerTrack II; JTECH Medical, Salt Lake City, UT).18, 19 Maximal voluntary contractions (MVC) were assessed for bilateral shoulder abduction, hip flexion, elbow flexion, and knee extension, in that order. One of the authors (S.G.C.) checked isometric MVCs of all the participants based on the ‘make and break’ technique.20 Four bilateral muscle groups were tested per trial. Three trials were conducted with 5 min of rest between trials. The highest peak value among three trials was determined as MVC for each functional muscle group.21 All MVCs were normalized to total body weight (MVC/TBW).
Physical performance and activity levels
Physical performance and activity levels were measured using functional examination and questionnaires. Functional examination was conducted using the short physical performance battery (SPPB) derived from three objective physical function tests, that is, the time taken to cover 4 m at a comfortable walking speed, time taken to move as quickly as possible to standing from sitting in a chair five times without stopping, and ability to maintain balance for 10 s in three different foot positions at progressively more challenging levels.22 A score from 0 to 4 was assigned to performance on each task, with higher scores indicating better lower body function. The questionnaire to assess physical activity level was a short version of International Physical Activity Questionnaire in Korean (K‐IPAQ).23, 24
Statistical analysis
Welch's t‐tests were used for comparison of means of anthropometric data, SMI, regional SMI, whole‐body fat mass, and MVC for each functional muscle group. The unequal variances t‐tests were applied because the two groups had unequal sample sizes.25 The same test was employed to compare the components of the K‐IPAQ and SPPB between the two groups. The χ2 test was used to compare the prevalence of low appendicular muscle mass, obesity, and sarcopenic obesity between the two groups. Pearson's correlation coefficient was used for analysing the relationship between total lifetime DDS exposure and sarcopenic variables. The linear by linear association test was also used to compare WHO‐DG between the two groups. PASW Statistics 18 (SPSS Inc., Chicago, IL, USA) was used for all analyses. P‐values < 0.05 were considered statistically significant.
Results
Subjects characteristics
Thirty‐two had been taking DDS for the past 1 year and more (DDS group) and nine had not (control group). Intergroup differences in age and period since leprosy diagnosis were not significant. However, the total DDS treatment period and the period of therapeutic dose were significantly longer (53.5 ± 6.7 vs. 31.9 ± 18.0 years, P = 0.007; 28.1 ± 22.1 vs. 12.7 ± 14.2 years, P = 0.027, respectively), and total lifetime DDS exposure of the DDS group was significantly larger (11 551 ± 6109 vs. 4496 ± 4535 tablets, P = 0.001) in the DDS group. However, the control group had significantly milder disability (eight with WHO‐DG 0 and one with WHO‐DG 1) than the DDS group (P = 0.027) (Table 1).
Table 1.
Age and medical history of the DDS and control groups
| DDS (n = 32) | Control (n = 9) | P‐valuea | ||
|---|---|---|---|---|
| Age (years) | 74.3 ± 7.8 | 72.7 ± 6.2 | 0.519 | |
| Period since leprosy diagnosis (years) | 57.0 ± 8.6 | 55.8 ± 6.1 | 0.635 | |
| Total DDS‐taking period (years) | 53.5 ± 6.7 | 31.9 ± 18.0 | 0.007 | |
| Period of Therapeutic dosage (years) | 28.1 ± 22.1 | 12.7 ± 14.2 | 0.027 | |
| Total lifetime DDS exposure (tablets) | 11 551 ± 6 109 | 4 496 ± 4 535 | 0.001 | |
| WHO disability grade | 0 | 16 | 9 | 0.027b |
| 1 | 2 | 1 | ||
| 2 | 7 | 0 | ||
| 3 | 6 | 0 | ||
Data are presented as mean ± standard deviation.
DDS, 4,4′‐diamino‐diphenyl sulfone; WHO, World Health Organization.
Welch's t‐test for group differences and
linear by linear association for WHO disability grade comparison.
Comparisons of body compositional characteristics
The DDS group showed a lower BMI (24.9 ± 2.8 vs. 27.2 ± 3.7 kg/m2, P = 0.107) and higher SMI (24.4 ± 2.7 vs. 22.6 ± 2.2%, P = 0.066) than the control group. The DDS group had lower prevalences of low appendicular muscle mass (34 vs. 67%, P = 0.082), and sarcopenic obesity (16 vs. 56%, P = 0.014) than those of the control group (Table 2). Regional SMIs were higher in the DDS group than in the control group for all four limbs. The differences were statistically significant in both dominant and non‐dominant legs (9.11 ± 1.08 vs. 8.41 ± 0.77%, P = 0.044 and 8.85 ± 0.85 vs. 7.92 ± 0.89%, P = 0.018, respectively) (Figure 2).
Table 2.
Comparisons of anthropometric data and sarcopenic indices between the DDS and control groups
| DDS (n = 32) | Control (n = 9) | P‐valuea | |
|---|---|---|---|
| Height (cm) | 147.7 ± 6.0 | 147.7 ± 8.4 | 1.000 |
| Weight (kg) | 54.5 ± 8.6 | 59.7 ± 11.5 | 0.232 |
| BMI (kg/m2) | 24.9 ± 2.8 | 27.2 ± 3.7 | 0.107 |
| Waist circumference (cm) | 82.9 ± 7.8 | 86.5 ± 7.1 | 0.237 |
| SMI (%) | 24.4 ± 2.7 | 22.6 ± 2.2 | 0.066 |
| Whole‐body fat mass (kg) | 19.1 ± 5.0 | 22.5 ± 8.1 | 0.293 |
| Low appendicular muscle mass (%) | 11/32 (34%) | 6/9 (67%) | 0.082b |
| Obesity (%) | 14/32 (44%) | 6/9 (67%) | 0.224b |
| Sarcopenic obesity (%) | 5/32 (16%) | 5/9 (56%) | 0.014b |
Data are presented as mean ± standard deviation.
BMI, body mass index; SMI, skeletal muscle mass index; DDS, 4,4′‐diamino‐diphenyl sulfone.
Welch's t‐test and
χ2 test for group differences.
Figure 2.
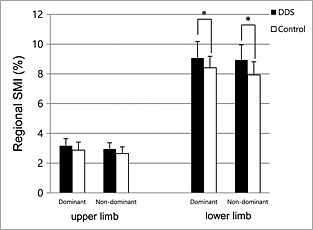
Comparisons of regional skeletal muscle mass index (SMI) of each limb between the 4,4′‐diamino‐diphenyl sulfone (DDS) and control groups. *p < 0.05 by Welch's t‐test.
Comparisons of muscular strength, physical performance, and activity levels
In all the tested muscle groups, the DDS group had larger MVC/TBWs than the control group. Shoulder abductors, elbow flexors, hip flexor, and knee extensors of the non‐dominant side of the DDS survivors were significantly stronger than those of controls (2.14 ± 0.68 vs. 1.49 ± 0.48 N/ kg, P = 0.005; 2.85 ± 0.80 vs. 2.20 ± 0.69 N/kg, P = 0.029; 2.47 ± 0.76 vs. 1.96 ± 0.46 N/kg, P = 0.021; and 3.91 ± 1.12 vs. 2.71 ± 0.81 N/kg, P = 0.005, respectively) (Figure 3).
Figure 3.
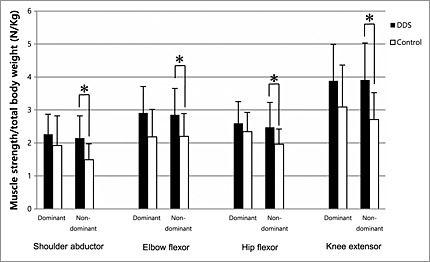
Comparisons of maximal voluntary contraction of dominant and non‐dominant shoulder abductor, elbow flexor, hip flexor, and knee extensor between the 4,4′‐diamino‐diphenyl sulfone (DDS) and control groups. Muscular strength was normalized by total body weight. *p < 0.05 by Welch's t‐test.
Three components of the SPPB of the DDS group were higher than those of the control group, and total scores of SPPB also showed an insignificant difference between the DDS and control groups (9.3 ± 2.6 vs. 7.6 ± 3.2, P = 0.155) (Table 3). With regard to activity levels assessed using K‐IPAQ, the DDS group demonstrated longer daily walking duration (P = 0.005) and larger weekly walking amounts calculated from the duration and frequency of walking (P = 0.020) (Table 4).
Table 3.
Comparisons of physical performance by short physical performance battery between the DDS and control groups
| DDS (n = 32) | Control (n = 9) | P‐valuea | |
|---|---|---|---|
| Balance | 2.8 ± 1.2 | 2.0 ± 1.8 | 0.216 |
| Walking speed | 3.3 ± 0.9 | 2.9 ± 0.8 | 0.218 |
| Chair stand | 3.2 ± 1.2 | 2.7 ± 1.3 | 0.308 |
| Total score | 9.3 ± 2.6 | 7.6 ± 3.2 | 0.155 |
Data are presented as mean ± standard deviation.
DDS, 4,4′‐diamino‐diphenyl sulfone.
Welch's t‐test for group differences.
Table 4.
Comparisons of the functional activity by a short version of International Physical Activity Questionnaire in Korean between the DDS and control group
| DDS (n = 9, 28%) | Control (n = 2, 22%) | P‐valuea | ||
|---|---|---|---|---|
| Moderate activity | Frequency (day/week) | 1.4 ± 2.5 | 1.1 ± 2.4 | 0.753 |
| Duration (minute/day) | 22.6 ± 49.9 | 40.0 ± 79.4 | 0.547 | |
| Amount (minute/week) | 107.6 ± 233.8 | 200.0 ± 435.8 | 0.555 |
| DDS (n = 24, 77%) | Control (n = 6, 67%) | P‐valuea | ||
|---|---|---|---|---|
| Walking | Frequency (day/week) | 4.4 ± 3.1 | 4.0 ± 3.3 | 0.764 |
| Duration (minute/day) | 43.8 ± 46.4 | 14.4 ± 15.1 | 0.005 | |
| Amount (minute/week) | 254.5 ± 323.3 | 94.4 ± 99.6 | 0.020 | |
Data are presented as mean ± standard deviation.
The subject numbers denoted by ‘n’ are the number of subjects who gave positive responses to each question.
DDS, 4,4′‐diamino‐diphenyl sulfone.
Welch's t‐test for group differences.
Correlations with total lifetime DDS exposure
The total lifetime DDS exposure significantly correlated with regional SMI of non‐dominant leg (r = 0.379, P = 0.015). SMI and regional SMI of dominant leg also showed positive correlations with DDS dosage (r = 0.254, P = 0.109, and r = 0.280, P = 0.076, respectively) (Table 5 and Figure 4).
Table 5.
Correlations between total lifetime 4,4′‐diamino‐diphenyl sulfone exposure and body composition and muscle strength
| Pearson's correlation coefficient (r) | P‐valuea | |
|---|---|---|
| BMI | −0.159 | 0.321 |
| SMI | 0.254 | 0.109 |
| Regional SMI | ||
| Dominant arm | −0.035 | 0.827 |
| Non‐dominant arm | 0.006 | 0.969 |
| Dominant leg | 0.280 | 0.076 |
| Non‐dominant leg | 0.379 | 0.015 |
| Muscular strength | ||
| Dominant shoulder abductor | 0.040 | 0.811 |
| Dominant elbow flexor | 0.155 | 0.347 |
| Dominant hip flexor | 0.090 | 0.587 |
| Dominant knee extensor | 0.128 | 0.439 |
| Non‐dominant shoulder abductor | 0.174 | 0.282 |
| Non‐dominant elbow flexor | 0.139 | 0.392 |
| Non‐dominant hip flexor | 0.170 | 0.293 |
| Non‐dominant knee extensor | 0.204 | 0.206 |
BMI, body mass index; SMI, skeletal muscle mass index.
Pearson's correlation with total lifetime 4,4′‐diamino‐diphenyl sulfone exposure.
Figure 4.
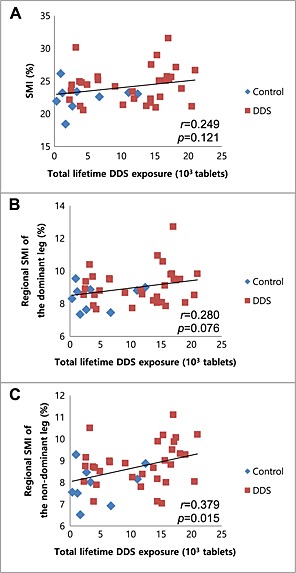
Correlations between total lifetime 4,4′‐diamino‐diphenyl sulfone (DDS) exposure and sarcopenic indices: (A) skeletal muscle mass index (SMI), (B) regional SMI of the dominant leg, and (C) regional SMI of the non‐dominant leg.
Discussion
Body composition, muscular strength, physical performance, and activity level were examined in all the community‐ambulating elderly female leprosy survivors with mild or no physical impairment living in a leper colony comprising of two villages. Survivors who had been taking DDS had larger regional SMI in both legs, lower prevalence of sarcopenic obesity, greater muscular strength of non‐dominant shoulder abductors, elbow flexors, hip flexor, and knee extensors, and higher walking scores in K‐IPAQ than survivors not on the medication. Most importantly, there was a significant dose–response relationship between total lifetime DDS exposure and regional SMI of the lower extremity. To our knowledge, this is the first report to reveal different sarcopenic status in humans associated with DDS medication.
Quantity and quality of skeletal muscle
Although the statistical significance was not high, the SMI of the DDS group tended to be higher than that of the control group (24.4 ± 2.7 vs. 22.6 ± 2.2%, P = 0.066). It is of note that the DDS group had more disabilities than the control group according to the disability status in leprosy as shown in Table 1, which could have caused survivors receiving DDS to lead more sedentary lives and be more vulnerable to having low appendicular muscle mass. Further, the findings of lower prevalence of low appendicular muscle mass and sarcopenic obesity in the DDS group are suggestive of potential anti‐sarcopenic effects of DDS.
The quantitative superiority of skeletal muscles in the DDS group was reinforced by comparing muscle quality. The non‐dominant shoulder abductor, elbow flexor, hip flexor, and knee extensor were significantly stronger in the DDS group. This finding is especially interesting because there are several supporting reports26, 27, 28 that loss of muscle power, called dynapenia,26 correlates more closely with physical function and risk for disability than reduction of skeletal muscle mass.29, 30 Considering that sarcopenia is regarded as a secondary outcome of dynapenia,26, 27 it is remarkable that DDS may have preventive effects on both sarcopenia and dynapenia. Stronger muscular strength in the DDS‐taking survivors was also reflected in functional performance scores and levels of physical activity.
Dose–response relationship of DDS in anti‐sarcopenia
To reveal the anti‐sarcopenic effects of the drug, correlations between the total lifetime DDS exposure and sarcopenic variables were obtained. Regional SMI of non‐dominant leg showed a significant correlation with total DDS exposure and that the correlation of low extremity muscle mass with the walking function31 corroborates the results of comparative analyses demonstrating higher quantity (regional SMI) and better quality (muscular strength) in lower extremities. Considering that the current study was not a controlled clinical trial and that the number of participants was not large enough to overcome several hidden confounders, a positive correlation between DDS exposure and regional SMI supports the hypothesis that DDS has anti‐sarcopenic effects.
Target of DDS in skeletal muscle
Even though the target of DDS in eukaryotic cells is not fully understood, pyruvate kinase (PK) is the best candidate. It has been suggested that DDS might inhibit the biochemical activity of PK and the pyk‐1 gene, the PK‐encoding gene, which are mainly expressed in skeletal muscles in animals.9 They also proposed that the inhibition of PK activity might lead to down‐regulation of the entire tricarboxylic acid cycle as a compensatory mechanism. This suggested mechanism is also supported by the finding that PK deficiency in humans results in hemolytic anaemia,32 the most common side effect of DDS.
In contrast, some investigators showed that the serum level of pyruvate in leprosy patients who had been taking DDS was higher than that of the control group.33 If DDS is inhibiting PK activity, the pyruvate level should have been down‐regulated. Another conflicting result comes from the study of pyk‐1 deletion mutant (ok1754) worms.9 The ok1754 worms treated with DDS lived longer than untreated pyk‐1 mutant animals, but just as long as normal worms treated with DDS. This indicates that there must be additional DDS targets for extending the lifespan. Further investigations for identifying the interaction between DDS and PK are needed.
Limitations of this study
There are several limitations of this study. First, the number of participants was relatively small to obtain the representativeness of total leprosy population. However, considering that all of the community‐ambulating female elderly survivors in the colony were recruited, the results of this study would be reflective of general characteristics in those leprosy regions. In addition, unequal sample sizes between the DDS and control groups (32 vs. 9) could also limit the statistical power. To address the issue, Welch's t‐test, an adaptation of Student's t‐test for unequal variances and unequal sample sizes, was utilized.25 Second, health behaviours such as taking medicines regularly or walking frequently might have been confounding factors. Because the DDS group had more disabilities than the control group at the onset of the disease, they might have been more concerned about health‐related issues, which could have raised their tendency to take DDS and to increase their physical activities, resulting in higher muscle mass and better muscle quality. Third, the authors defined sarcopenia based only on muscle mass reduction in contrast to recent consensus recommendations34, 35 suggesting inclusion of hand grip strength and gait speed. While we checked all participants' gait speeds as one of the tasks of SPPB, hand grip strength was unreliable to measure because some of the subjects had minor hand deformities as sequelae of leprosy. Therefore, we applied the classical definition of sarcopenia. Instead of hand grip strength, we tested muscle strength of major joints (shoulder, elbow, hip, and knee) and analysed them quantitatively. Lastly, with the genuine limitation of a cross‐sectional study, it is hard to establish a strong causal relationship between DDS and sarcopenia at the present stage. Therefore, further randomized controlled trials are needed.
Conclusions
This study shows that DDS‐taking leprosy survivors have a significantly lower prevalence of sarcopenic obesity and increased skeletal muscle mass, muscular strength, and walking activity than leprosy survivors not taking DDS. There was a dose–response relationship between total lifetime DDS exposure and skeletal muscle mass of the lower extremities. These findings may suggest one possible hypothesis on the potential sarcopenia‐preventive effects of DDS. Albeit by a cross‐sectional study, this is the first clinical investigation to implicate that sarcopenia can be influenced by a repositioned drug. Further controlled clinical trials are required to confirm the precise effects of DDS.
Conflict of interest
S.Y.L., W.K., H.‐W.P., S.C.P., I.K.K., and S.G.C. declare that they have no conflict of interest.
Acknowledgements
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle (von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7–8). This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean Government (MEST) (No. 2011–0030135).
Lee, S. Y. , Kim, W. , Park, H. ‐W. , Park, S. C. , Kim, I. K. , and Chung, S. G. (2016) Anti‐sarcopenic effects of diamino‐diphenyl sulfone observed in elderly female leprosy survivors: a cross‐sectional study. Journal of Cachexia, Sarcopenia and Muscle, 7: 322–329. doi: 10.1002/jcsm.12074.
References
- 1. Fiatarone MA, O'Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N. Engl. J. Med. 1994;330:1769–1775. [DOI] [PubMed] [Google Scholar]
- 2. Foley A, Hillier S, Barnard R. Effectiveness of once‐weekly gym‐based exercise programmes for older adults post discharge from day rehabilitation: A randomised controlled trial. Br. J. Sports Med. 2011;45:978–986. [DOI] [PubMed] [Google Scholar]
- 3. Richter EA, Kiens B, Wojtaszewski JF. Can exercise mimetics substitute for exercise? Cell Metab. 2008;8:96–98. [DOI] [PubMed] [Google Scholar]
- 4. Matsakas A, Narkar VA. Endurance exercise mimetics in skeletal muscle. Curr. Sports Med. Rep. 2010;9:227–232. [DOI] [PubMed] [Google Scholar]
- 5. Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, et al. Adverse events associated with testosterone administration. N. Engl. J. Med. 2010;363:109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pierorazio PM, Ferrucci L, Kettermann A, Longo DL, Metter EJ, Carter HB. Serum testosterone is associated with aggressive prostate cancer in older men: Results from the Baltimore Longitudinal Study of Aging. BJU Int. 2010;105:824–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sayer AA, Robinson SM, Patel HP, Shavlakadze T, Cooper C, Grounds MD. New horizons in the pathogenesis, diagnosis and management of sarcopenia. Age Ageing 2013;42:145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sardana D, Zhu C, Zhang M, Gudivada RC, Yang L, Jegga AG. Drug repositioning for orphan diseases. Brief. Bioinform. 2011;12:346–356. [DOI] [PubMed] [Google Scholar]
- 9. Cho SC, Park MC, Keam B, Choi JM, Cho Y, Hyun S, et al. DDS, 4,4′‐diaminodiphenylsulfone, extends organismic lifespan. Proc. Natl. Acad. Sci. U. S. A. 2010;107:19326–19331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim W, Park HW, Hwang BK, Bae SO, Kim IK, Chung SG. Comparison of sarcopenic status between elderly leprosy survivors and general population. Arch. Gerontol. Geriatr. 2014;58:134–139. [DOI] [PubMed] [Google Scholar]
- 11. Brandsma W, Larsen M, Richard C, Ebenezer M. Inter‐rater reliability of WHO ‘disability’ grading. Lepr. Rev. 2004;75:131–134. [PubMed] [Google Scholar]
- 12. Nienhuis WA, van Brakel WH, Butlin CR, van der Werf TS. Measuring impairment caused by leprosy: Inter‐tester reliability of the WHO disability grading system. Lepr. Rev. 2004;75:221–232. [PubMed] [Google Scholar]
- 13. Chiu M, Austin PC, Manuel DG, Shah BR, Tu JV. Deriving ethnic‐specific BMI cutoff points for assessing diabetes risk. Diabetes Care 2011;34:1741–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998;147:755–763. [DOI] [PubMed] [Google Scholar]
- 15. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002;50:889–896. [DOI] [PubMed] [Google Scholar]
- 16. Hwang B, Lim JY, Lee J, Choi NK, Ahn YO, Park BJ. Prevalence rate and associated factors of sarcopenic obesity in Korean elderly population. J. Korean Med. Sci. 2012;27:748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prado CM, Wells JC, Smith SR, Stephan BC, Siervo M. Sarcopenic obesity: A critical appraisal of the current evidence. Clin. Nutr. 2012;31:583–601. [DOI] [PubMed] [Google Scholar]
- 18. Harlaar J, Roebroeck ME, Lankhorst GJ. Computer‐assisted hand‐held dynamometer: Low‐cost instrument for muscle function assessment in rehabilitation medicine. Med. Biol. Eng. Comput. 1996;34:329–335. [DOI] [PubMed] [Google Scholar]
- 19. Roy JS, MacDermid JC, Orton B, Tran T, Faber KJ, Drosdowech D, et al. The concurrent validity of a hand‐held versus a stationary dynamometer in testing isometric shoulder strength. J. Hand Ther. 2009;22:320–326. [DOI] [PubMed] [Google Scholar]
- 20. Burns SP, Spanier DE. Break‐technique handheld dynamometry: Relation between angular velocity and strength measurements. Arch. Phys. Med. Rehabil. 2005;86:1420–1426. [DOI] [PubMed] [Google Scholar]
- 21. Shin S, Valentine RJ, Evans EM, Sosnoff JJ. Lower extremity muscle quality and gait variability in older adults. Age Ageing 2012;41:595–599. [DOI] [PubMed] [Google Scholar]
- 22. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower‐extremity function in persons over the age of 70 years as a predictor of subsequent disability. N. Engl. J. Med. 1995;332:556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hagstromer M, Oja P, Sjostrom M. The International Physical Activity Questionnaire (IPAQ): A study of concurrent and construct validity. Public Health Nutr. 2006;9:755–762. [DOI] [PubMed] [Google Scholar]
- 24. Chun MY. Validity and reliability of Korean version of International Physical Activity Questionnaire short form in the elderly. Korean J Fam Med. 2012;33:144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ruxton G. The unequal variance t‐test is an underused alternative to Student's t‐test and the Mann–Whitney U test. Behavioral Ecology 2006;17:688–690. [Google Scholar]
- 26. Clark BC, Manini TM. Sarcopenia ≠ dynapenia. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63:829–834. [DOI] [PubMed] [Google Scholar]
- 27. Manini TM, Clark BC. Dynapenia and aging: An update. J. Gerontol. A Biol. Sci. Med. Sci. 2012;67:28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61:72–77. [DOI] [PubMed] [Google Scholar]
- 29. Doherty TJ. Invited review: Aging and sarcopenia. J. J. Appl. Physiol. (1985). 2003;95:1717–1727. [DOI] [PubMed] [Google Scholar]
- 30. Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB. Sarcopenia: Etiology, clinical consequences, intervention, and assessment. Osteoporos. Int. 2010;21:543–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim SH, Kim TH, Hwang HJ. The relationship of physical activity (PA) and walking with sarcopenia in Korean males aged 60 years and older using the Fourth Korean National Health and Nutrition Examination Survey (KNHANES IV‐2, 3), 2008–2009. Arch. Gerontol. Geriatr. 2013;56:472–477. [DOI] [PubMed] [Google Scholar]
- 32. Zanella A, Fermo E, Bianchi P, Valentini G. Red cell pyruvate kinase deficiency: Molecular and clinical aspects. Br. J. Haematol. 2005;130:11–25. [DOI] [PubMed] [Google Scholar]
- 33. Sinha SN, Gupta SC, Bajaj AK, Srivastava NP, Mehrotra TN. Effect of dapsone on blood lactic and pyruvic acids in leprosy. Int. J. Lepr. Other Mycobact. Dis. 1982;50:468–470. [PubMed] [Google Scholar]
- 34. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014;15:95–101. [DOI] [PubMed] [Google Scholar]
- 35. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]


