Abstract
Meningococci spread via respiratory droplets, whereas the closely related gonococci are transmitted sexually. Several outbreaks of invasive meningococcal disease have been reported in Europe and the United States among men who have sex with men (MSM). We recently identified an outbreak of serogroup C meningococcal disease among MSM in Germany and France. In this study, genomic and proteomic techniques were used to analyze the outbreak isolates. In addition, genetically identical urethritis isolates were recovered from France and Germany and included in the analysis. Genome sequencing revealed that the isolates from the outbreak among MSM and from urethritis cases belonged to a clade within clonal complex 11. Proteome analysis showed they expressed nitrite reductase, enabling anaerobic growth as previously described for gonococci. Invasive isolates from MSM, but not urethritis isolates, further expressed functional human factor H binding protein associated with enhanced survival in a newly developed transgenic mouse model expressing human factor H, a complement regulatory protein. In conclusion, our data suggest that urethritis and outbreak isolates followed a joint adaptation route including adaption to the urogenital tract.
Introduction
Neisseria meningitidis causes severe and life threatening invasive infections that manifest mainly as meningitis and/or septicemia. The annual incidence of invasive meningococcal disease (IMD) varies geographically from 0.3 to 3.37 cases per 100, 000 inhabitants in the United States and Europe. The Incidence is the highest among infants. Male to female ratio is close to one [1, 2]. Endogenous and exogenous risk factors for IMD have been described [3]. Meningococci show high genomic plasticity associated with marked genetic diversity. Typing methods are based on DNA sequencing and cluster meningococcal isolates into several genotypes (sequence types, ST and clonal complexes, cc) [4]. Meningococci are transmitted via droplets and colonize the nasopharynx. In contrast, their close relative, the gonococcus (Neisseria gonorrhoeae), is transmitted sexually and infects the urogenital tract, with occasional colonization of the rectal and pharyngeal mucosa. Case reports of meningococcal urethritis [5] suggest that meningococci may share urogenital colonization mechanisms with gonococci. Clusters of IMD among men who have sex with men (MSM) were reported on several occasions [5,6–7]. A recent report of IMD among MSM in Berlin, Germany described the emergence of a particular hyperinvasive genotype, (i.e. serogroup C, PorA type P1.5–1,10–8, FetA type F3-6 and cc11), associated with high case-fatality [6]. A similar observation was also reported in France [8]. Furthermore, all isolates displayed a characteristic point mutation in the fumC gene that is an indicative of the ET-15 clone, [9] a derivative of cc11, which was first observed in Canada in the 1980s and since then spread globally, causing outbreaks in various countries [10]. HIV infection was not linked to this outbreak as most of the cases were negative for HIV [6]. The Berlin cluster occurred simultaneously with IMD cases in MSM in Paris, France, which were caused by the same genotype C:P1.5–1,10–8:F3-6:cc11:ET-15, and with a protracted serogroup C outbreak in MSM in New York City [6, 8, 11]. Efficient meningococcal C vaccination programs were not implemented beforehand in adolescents and young adults in these countries and vaccine coverage was low among these groups [12]. Therefore, circulation of serogroup C isolates in young adults was likely as the genotype C:P1.5–1,10–8:F3-6:cc11:ET-15 is frequent among invasive serogroup C isolates. In addition, the reference laboratories for meningococci in France and Germany over the years had both received meningococci with the identical genotype C:P1.5–1,10–8:F3-6:cc11:ET-15 from cases with urethritis/proctitis. An epidemiological link of these cases to MSM was not explored, although cases of urethritis have been reported among MSM [13]. The analysis of urethritis isolates provides the opportunity to analyze whether similar genetic modifications occurred in urethritis isolates and isolates from invasive disease in MSM, suggesting that transmission networks were at least partially driven by sexual contact. Indeed, an increase in the number of sexual partners and higher risk sexual practices in MSM social networks was suggested to explain an increase in sexually transmitted infections among MSM observed between 2001 and 2003 [14]. Orogenital and anogenital contacts were postulated to explain anogenital meningococcal infections observed in MSM [15]. The available collection of genetically related strains allowed testing this hypothesis through genome sequencing, proteome analysis and animal infection models. We therefore conducted a study to analyze the genetic adaptation associated with the emergence of this outbreak and urethritis.
Methods
(For full description see the S1 Text).
Ethical statement
Invasive meningococcal isolates were sent to the National Reference Centres for meningococci in France and Germany as part of national laboratory surveillance systems for invasive meningococcal disease. Animal work in this study was carried out at the Institut Pasteur in strict accordance with the European Union Directive 2010/63/EU (and its revision 86/609/EEC) on the protection of animals used for scientific purposes. The laboratory at the Institut Pasteur has the administrative authorization for animal experimentation (Permit Number 75–1554) and the protocol was approved by the Institut Pasteur Review Board that is part of the Regional Committee of Ethics of Animal Experiments of Paris Region (Permit Number: 99–174). All the invasive procedures were performed under anesthesia with sodium pentobarbital (Sanofi Sante. Animale, Libourne, France) and all possible efforts were made to minimize animal suffering by limiting the experiment to 24 hours after infection and by inspecting the conditions of animal three times during the experiment at 2, 6 and 10 hours after infection.
The animals were euthanatized by injection of high dose of chemical anesthetics (pentobarbital) which was performed before blood sampling.
Bacterial strains and typing
Invasive N. meningitidis isolates were sent to the National Reference Centres for meningococci as part of national laboratory-based surveillance systems for invasive meningococcal disease. Isolates linked to the MSM community were included as well as the available urethritis and proctitis isolates showing the same genotype. The latter isolates are not usually part of the surveillance of meningococcal disease (S1 Table). The serogroup was determined by slide agglutination. Genotyping (multilocus sequence typing [MLST] and antigen typing [porA, fhbp and fetA]) were performed as previously described [16–21]. Sequence types (STs), clonal complexes (cc), and antigen types were determined through the meningococcal typing website (http://neisseria.org/nm/typing/).
Whole genome sequencing, assembly and cgMLST for phylogenomic analysis
Genomic DNA was extracted and whole-genome sequencing was then performed using Illumina HiSeq 2000 sequencer or by Illumina MiSeq sequencer. After sequencing, the reads were quality-trimmed and then assembled using the CLC Genomics Workbench software version 6.0 (CLC bio, Aarhus, Denmark). The resulting assembly files were exported as ACE files and imported into SeqSphere+ software version 2.3 (Ridom GmbH, Münster, Germany). The assembly contigs together with epidemiologic meta-data were also deposited to the Neisseria BIGSdb website [22].
A core genome multi-locus sequence typing (cgMLST) target set was determined using all finished N. meningitidis genomes available in GenBank as of February 2014 (n = 14) [23]. The genome of strain FAM18 (NC_008767) was used as a reference. Complete sequence for each gene was analyzed in comparison to the FAM18 reference. The combination of all alleles in each strain formed an allelic profile. The exported aligned sequences were then imported into MEGA6 and a neighbor-joining tree was generated with default parameters and the bootstrap option (with 1.000 replications) turned on [24]. All classical typing results achieved by Sanger sequencing were confirmed by WGS data analysis [25].
Determination of AniA nitrite reductase activity and growth under anaerobic conditions in the presence of nitrite
Nitrite reductase activity was monitored by nitrite consumption according to published protocols [26] with slight modifications. For anaerobic growth, meningococcal cultures on sheep blood agar (bioMérieux) were grown aerobically overnight at 37°C and 5% CO2. Meningococci that are capable of nitrite respiration grow in a characteristic halo around the nitrite disk, while meningococci that are incapable of nitrite respiration do not grow.
Construction, characterization and experimental meningococcal infection of transgenic mice expressing the human factor H
Transgenic mice expressing the human factor H (a regulatory protein of the complement system, S2 Table for primers) were constructed and backcrossed to BLAB/c background. Flow cytometry was performed as previously described [27] with a FACSCalibur flow cytometer (BD Biosciences, France) to monitor the activation of complement at the bacterial surface by detecting the deposition of C3b on bacteria. Detection of fHbp by Western blotting using anti-fHbp antibodies was performed as previously described [27]. For colony blot analysis, colonies from GCB plates were transferred to nitrocellulose membrane and revealed using anti-fHbp antibodies [27].
Proteomic analysis
This approach was used for detecting proteins that are differentially expressed between meningococcal isolates from MSM and those from adolescents of a cluster of cases from non-MSM subjects in Schwerte, Germany, 2003 [28].
The proteomes of three meningococcal strains (DE12845, DE12939, DE12957) from MSM from Berlin and three meningococcal strains (DE9273, DE9301, DE9425) from adolescents of the cluster in Schwerte, Germany, 2003 [28] were compared by high-sensitivity mass spectrometry as described recently [29]. The protocol included an internal control generated by metabolic labeling of neisserial proteins with 15N. After protein quantification, equal amounts of 14N samples and the 15N labeled reference were combined and subjected to GeLCMS-analyses as previously described in detail in [30].
Results
Genome sequencing analysis of meningococcal isolates
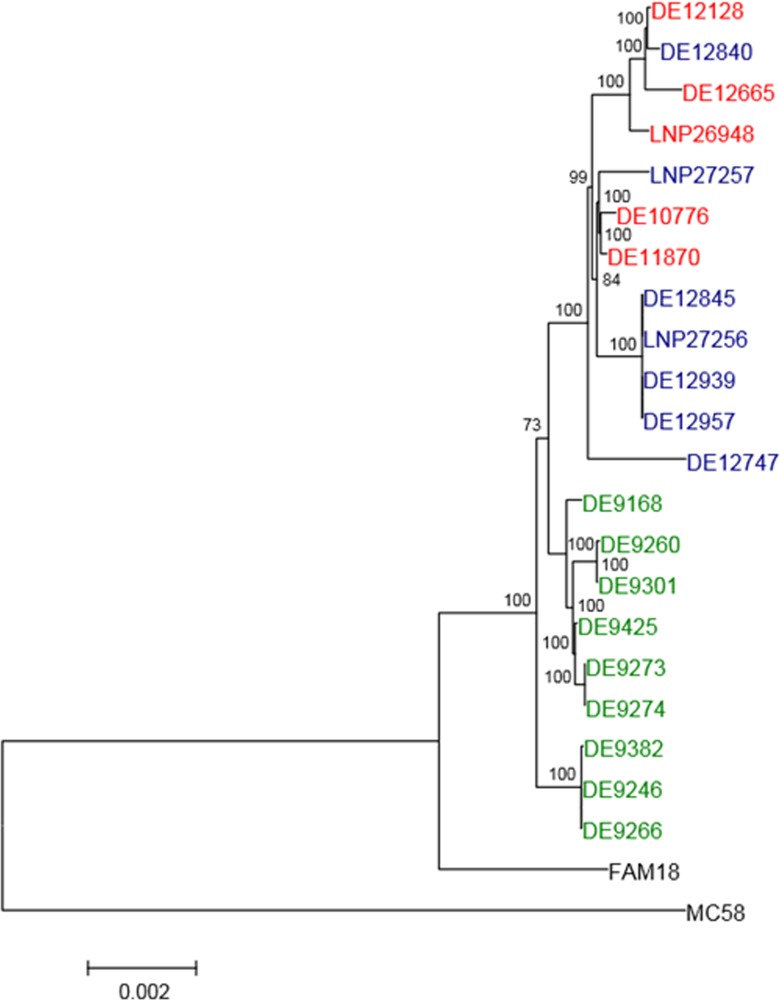
We first performed a deep genetic analysis on the MSM isolates. The origins of the isolates and their corresponding clinical presentation and typing are shown in the S1 Table. Genome sequencing of several invasive isolates from the outbreak among MSM in 2012–2013 and isolates from cases of urethritis/proctitis isolated in 2006–2012 was performed. We analyzed the phylogenetic relationships between these isolates and other C:P1.5–1,10–8:F3-6:cc11/ET-15 isolates from children and adolescents, which were identified in Germany in 2003 in four Federal States. The isolates were from sporadic disease and from two clusters (European Nucleotide Archive (ENA) study accession number PRJEB7500) [28]. A core genome multi-locus sequence typing (cgMLST as designated in the SeqSphere+ software used here) approach was chosen for genome comparison [31, 32]. Fig 1 shows that the invasive isolates from MSM clustered with urethritis and proctitis strains in a separate branch distinct from other cc11/ET-15 isolates. This finding proved the clonal relationship of the outbreak isolates as well as their similarity to urethritis isolates collected in the two countries.
Fig 1. Whole genome sequence based phylogenetic tree.
The neighbor-joining tree was calculated from 1,056 concatenated and multiple aligned core genome genes shared by all studied isolates. Numbers at the branches indicate the percentage of bootstrap support (1,000 replications). Labels in blue represent IMD isolates in MSM; in red urethritis and proctitis isolates; in green adolescent IMD isolates; and in black FAM18 cc11 and MC58 outgroup [42, 43]. A distance scale-bar is shown at the bottom left.
Proteomic analysis of meningococcal isolates
We next used recently published proteomic technology to screen for differentially expressed proteins [29]. Three invasive cc11/ET-15 isolates each from MSM in Berlin and from one of the adolescent clusters unrelated to the MSM community were compared [28]. The mass spectrometry based proteomics data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository [33]. The dataset ID is PXD001498 http://proteomecentral.proteomexchange.org/cgi/GetDataset?ID=PXD000181.
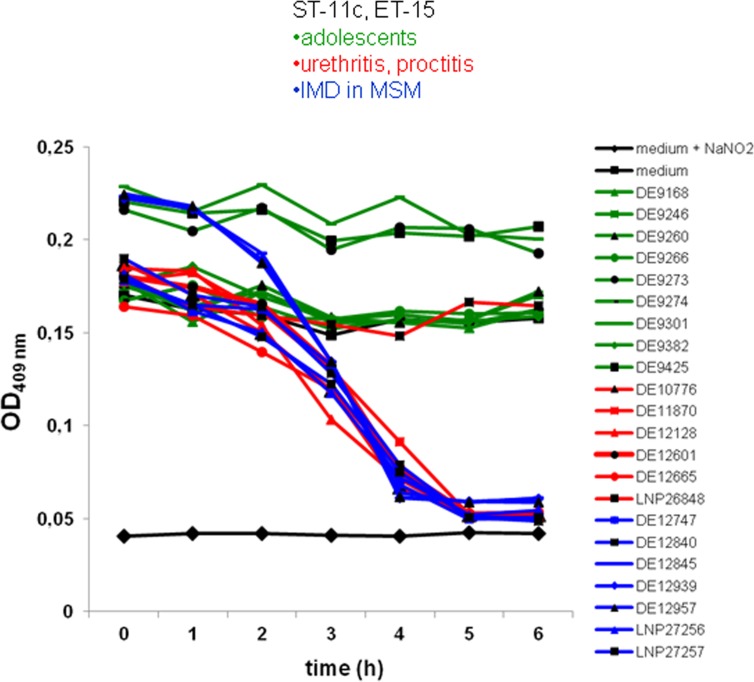
Among the differentially expressed proteins, copper containing nitrite reductase AniA was studied further, as it was only expressed in MSM isolates, but not in isolates from adolescents. AniA is located in the outer membrane of Neisseria, where it is expressed under low-oxic conditions. This permits anaerobic respiration under conditions that gonococci may encounter in the urethra [34]. In meningococcal, but not in gonococcal strains, the aniA gene frequently harbors a point mutation in a homopolymeric polyA tract resulting in the expression of a non-functional truncated protein. This suggests that in contrast to gonococci, meningococci do not necessarily require AniA expression for survival [34,35–36]. The aniA gene was in-frame in all isolates from IMD in MSM and in all but one urethritis/proctitis isolate. In contrast, aniA was out-of-frame in classical cc11/ET-15 strains isolated from IMD cases unrelated to the MSM community (S1 Table). Measurement of the nitrite reductase activity of the meningococcal isolates confirmed the aniA genotype and the results of proteome analysis. All isolates that harbored in-frame aniA (MSM/urethritis isolates) were able to reduce nitrite, whereas no activity was detected in the out-of-frame urethritis isolate or in isolates unrelated to the MSM outbreak (Fig 2). In addition, we tested the ability of meningococcal isolates to grow under anaerobic conditions on agar plates in the presence of disks soaked with nitrite. All six tested MSM/urethritis isolates grew under anaerobic conditions except for the one isolate that harbored an out-of-frame aniA gene. Isolates from cases of IMD unrelated to the MSM community were also unable to grow under these conditions (S1 Table).
Fig 2. AniA nitrite reductase activity as measured by sodium nitrite consumption.
cc11/ET-15 isolates from MSM (blue), urethritis (red) and adolescents (green) were investigated. The black curve corresponds to the control (culture medium without NaNO2).
The expression of AniA in meningococcal isolates from MSM and urethritis cases may therefore reflect a selection of isolates adapted to anaerobic growth resembling the phenotype of gonococci, which are sexually transmitted. Expression of AniA is thought to support gonococci in their survival under anaerobic and acidic pH in the urethra [35].
Impact of the expression of factor H binding protein
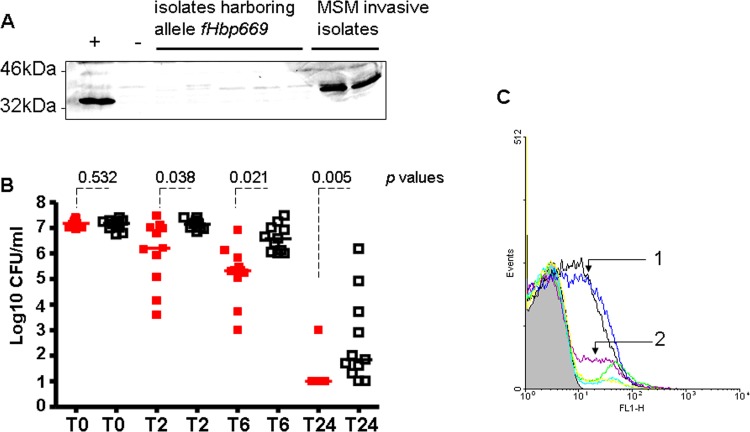
The data presented above suggested that the emergence of the isolates from the outbreak among MSM and from urethritis cases investigated here was associated with AniA expression providing the potential capacity to survive in the urethra. We investigated genomic typing data for differences between invasive isolates from MSM and mucosal isolates from patients with urethritis or proctitis. Comprehensive genotyping revealed that these isolates shared distinct alleles of the factor H binding protein (fHbp) gene that were uncommon to other cc11/ET-15 strains (S1 Table). fHbp binds human factor H (hfH), a negative complement regulator, leading to enhanced bacterial survival in the blood [37]. All urethritis/proctitis isolates, but only a single MSM isolate, possessed fhbp allele 669 containing a frame shift mutation that gives rise to a premature stop codon. Lack of fHbp expression was confirmed in selected isolates by Western blotting using previously described anti-fHbp antibodies (Fig 3A) [27]. This phenotype again resembles that of gonococci, which harbor a homologue of fHbp that is not expressed on the surface due to a defective signal sequence or lipid modification motif [38].
Fig 3.
Impact of fHbp expression of invasive MSM isolates and urethritis isolates on meningococcal pathogenesis (A) Western blotting analysis to detect fHbp. Meningococci from IMD cases in MSM expressing functional fHbp and isolates harboring the fhbp allele 669 (with a pre-mature stop codon) were tested. Meningococcal strain MC58 was used as a positive control (+). (B) Survival of meningococci in transgenic mice expressing human factor H (hfH). Bacterial counts recovered from blood after intraperitoneal challenge with 5x107 colony forming units of meningococcal isolates from urethritis (red) and MSM cases (black). A representative experiment with data representing individual mice is shown. Mice infected with isolates from MSM showed significantly higher bacterial counts 2, 6 and 24 hours after infection compared to those infected with urethritis isolates (p values derived from a Student´s t test are displayed). (C) Flow cytometry analysis of C3b surface deposition on (1) meningococci from IMD cases in MSM expressing fHbp and (2) on meningococci isolated from urethritis cases not expressing fHbp due to an early stop codon. The X axis represents logarithmic scale binding of C3b expressed as the geometric mean of fluorescence. The number of events is displayed on the Y axis. Different coloured lines were used to indicate the isolates for better clarity.
Meningococci specifically bind hfH, but not murine fH [39]. Upon binding of fH to fHbp on the bacterial surface complement activation is downregulated and bacterial survival enhanced. fH inactivates C3b and inhibits binding of factor B to C3b and hence reduces the production of C3 convertase [40]. We therefore constructed transgenic mice expressing hfH to test whether isolates that caused invasive disease in MSM have a survival advantage compared to urethritis/proctitis isolates due to fHbp expression. Mice were infected via the intraperitoneal route with invasive isolates from MSM or with isolates from urethral or anal sites. After 2, 6 and 24 hours of infection, invasive isolates from MSM showed significantly higher survival in the blood of infected mice than isolates from urethritis or proctitis (Fig 3B). Human fH binds to fHbp on the bacterial surface and subsequently inactivates the complement component C3b, [40] thereby enhancing meningococcal survival. In the mouse infection model, lower C3b deposition was observed on meningococcal isolates expressing fHbp (from MSM with IMD) than on those isolates harbouring fHbp allele 669 with a premature stop codon (urethritis /proctitis isolates) (Fig 3C). Blood samples from mice infected by urethritis /proctitis isolates expressing the fHbp allele 669 were plated on GCB medium and colonies (about 5000 colonies per plate) were transferred onto nitrocellulose membrane by colony blotting for detection by anti-fHbp antibodies (about 50.000 colonies were screened). No fHbp-positive colonies were detected suggesting that genotype switches in vivo, if possible, occurs at too low frequency to be detected. Taken together, these results suggest that invasive isolates from MSM with functional fHbp expression are more virulent than urethritis/proctitis isolates.
Discussion
Our results demonstrate the power of combining laboratory infection surveillance, genomics and proteomics technologies and transgenic animal models to unravel the molecular basis of meningococcal evolution that lead to short-term changes in the epidemiology of meningococcal disease. Our data also suggest that genomic plasticity of meningococci permits a rapid generation of variants with increased fitness for alternative/novel niches. The AniA+, fHbp- phenotype seems to be associated with urethral and rectal colonization, leading to the clinical manifestation of urethritis/proctitis as well as the capacity for direct sexual transmission. These findings further highlight the link between metabolic processes and virulence.
Our finding that cc11/ET-15 meningococci adapted to a gonococcus-like lifestyle suggests that the variant may be widely distributed in the MSM community. This should be further investigated by meningococci C carriage studies in MSM. As suggested by our results, reversion to a hypervirulent phenotype (AniA+, fHbp+) is possible through the reacquisition of functional fHbp that enhances bacterial survival in the blood. The spontaneous reversion to fHbp+ state may occur but at low frequency (less than 5x10-4). Alternatively, transformation and recombination during mixed carriage and/or mixed urethral infection may be responsible for this reversion [15].
The impact of the acquired capacity for sexual transmission on meningococcal evolution remains to be followed. If persistent wide-spread transmission in the MSM community is confirmed, time-limited vaccination strategy specifically targeting MSM implemented thus far mainly in areas affected by the outbreaks should be reconsidered [11, 41], since more widespread vaccination of MSM could limit international spread in the MSM community.
Supporting Information
(DOC)
(DOC)
(DOC)
Acknowledgments
The French Reference Laboratory is funded by the Institut de Veille Sanitaire and the Institut Pasteur. The German Reference Laboratory is funded by the Robert Koch Institute, Berlin, Germany. The genomics work was funded by the European Community's Seventh Framework Program [grant number FP7/2007-2013 to DH] under Grant Agreement N° 278864 in the framework of the EU PathoNGenTrace project. The animal work was also supported by a grant from the ERA-NET PathoGenomics (ANR-08-PATH-003-01). The proteome work was funded by Bundesministerium für Bildung und Forschung grant Medizinische Infektionsgenomik: Proteomics von Meningokokken und Pneumokokken, Teilprojekt Würzburg, Foerderkennzeichen 0315828D, to U.V. and 0315828A to D.B. This publication made use of the Neisseria Multilocus Sequence Typing website (http://pubmlst.org/neisseria/) developed by Keith Jolley and sited at the University of Oxford. The development of this site has been funded by the Wellcome Trust and European Union. We thank the Centre d’Ingénierie Génétique Murine at the Institut Pasteur for their technical support in the construction of the transgenic mice.
Data Availability
The mass spectrometry based proteomics data (Thermo raw files, dta-select-filter.txt) have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository.22. The dataset ID is PXD001498 (http://proteomecentral.proteomexchange.org/cgi/GetDataset?ID=PXD000181).
Funding Statement
The French Reference Laboratory is funded by the Institut de Veille Sanitaire and the Institut Pasteur. The German Reference Laboratory is funded by the Robert Koch Institute, Berlin, Germany. The genomics work was funded by the European Community's Seventh Framework Program [grant number FP7/2007-2013 to DH] under Grant Agreement N° 278864 in the framework of the EU PathoNGenTrace project. The animal work was also supported by a grant from the ERA-NET PathoGenomics (ANR-08-PATH-003-01). The proteome work was funded by Bundesministerium für Bildung und Forschung grant Medizinische Infektionsgenomik: Proteomics von Meningokokken und Pneumokokken, Teilprojekt Würzburg, Foerderkennzeichen 0315828D, to UV and 0315828A to DB. This publication made use of the Neisseria Multilocus Sequence Typing website (http://pubmlst.org/neisseria/) developed by Keith Jolley and sited at the University of Oxford. The development of this site has been funded by the Wellcome Trust and European Union. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Cohn AC, MacNeil JR, Harrison LH, Hatcher C, Theodore J, Schmidt M, et al. Changes in Neisseria meningitidis disease epidemiology in the United States, 1998–2007: implications for prevention of meningococcal disease. Clin Infect Dis. 2010;50(2):184–91. 10.1086/649209 [DOI] [PubMed] [Google Scholar]
- 2.ECDC. Surveillance of invasive bacterial diseases in Europe 2008/2009 Stockholm: ECDC, 2011. [Google Scholar]
- 3.Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. N Engl J Med. 2001;344(18):1378–88. [DOI] [PubMed] [Google Scholar]
- 4.Maiden MC. Multilocus sequence typing of bacteria. Annu Rev Microbiol. 2006;60:561–88. Epub 2006/06/16. 10.1146/annurev.micro.59.030804.121325 [DOI] [PubMed] [Google Scholar]
- 5.Urra E, Alkorta M, Sota M, Alcala B, Martinez I, Barron J, et al. Orogenital transmission of Neisseria meningitidis serogroup C confirmed by genotyping techniques. Eur J Clin Microbiol Infect Dis. 2005;24(1):51–3. Epub 2004/12/16. 10.1007/s10096-004-1257-7 [DOI] [PubMed] [Google Scholar]
- 6.Marcus U, Vogel U, Schubert A, Claus H, Baetzing-Feigenbaum J, Hellenbrand W, et al. A cluster of invasive meningococcal disease in young men who have sex with men in Berlin, October 2012 to May 2013. Euro Surveill. 2013;18(28). [DOI] [PubMed] [Google Scholar]
- 7.Schmink S, Watson JT, Coulson GB, Jones RC, Diaz PS, Mayer LW, et al. Molecular epidemiology of Neisseria meningitidis isolates from an outbreak of meningococcal disease among men who have sex with men, Chicago, Illinois, 2003. J Clin Microbiol. 2007;45(11):3768–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aubert L, Taha M, Boo N, Le Strat Y, Deghmane AE, Sanna A, et al. Serogroup C invasive meningococcal disease among men who have sex with men and in gay-oriented social venues in the Paris region: July 2013 to December 2014. Euro Surveill. 2015;20(3). [DOI] [PubMed] [Google Scholar]
- 9.Brehony C, Trotter CL, Ramsay ME, Chandra M, Jolley KA, van der Ende A, et al. Implications of differential age distribution of disease-associated meningococcal lineages for vaccine development. Clin Vaccine Immunol. 2014;21(6):847–53. 10.1128/CVI.00133-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashton FE, Ryan JA, Borczyk A, Caugant DA, Mancino L, Huang D. Emergence of a virulent clone of Neisseria meningitidis serotype 2a that is associated with meningococcal group C disease in Canada. J Clin Microbiol. 1991;29(11):2489–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss D, Varma J. Control of recent community-based outbreaks of invasive meningococcal disease in men who have sex with men in Europe and the United States. Euro Surveill. 2013;18(28). [DOI] [PubMed] [Google Scholar]
- 12.Parent du Châtelet I, Taha M-K, Lepoutre A, Maine C, Deghmane AE, Lévy-Bruhl D . Les infections invasives à méningocoques en France en 2011. Bull Epidemiol Hebd. 2012:569–73. [Google Scholar]
- 13.Hayakawa K, Itoda I, Shimuta K, Takahashi H, Ohnishi M. Urethritis caused by novel Neisseria meningitidis serogroup W in man who has sex with men, Japan. Emerg Infect Dis. 2014;20(9):1585–7. 10.3201/eid2009.140349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcus U, Bremer V, Hamouda O, Kramer MH, Freiwald M, Jessen H, et al. Understanding recent increases in the incidence of sexually transmitted infections in men having sex with men: changes in risk behavior from risk avoidance to risk reduction. Sex Transm Dis. 2006;33(1):11–7. [DOI] [PubMed] [Google Scholar]
- 15.Janda WM, Morello JA, Lerner SA, Bohnhoff M. Characteristics of pathogenic Neisseria spp. isolated from homosexual men. J Clin Microbiol. 1983;17(1):85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke SC, Diggle MA, Edwards GF. Semiautomation of multilocus sequence typing for the characterization of clinical isolates of Neisseria meningitidis. J Clin Microbiol. 2001;39(9):3066–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diggle MA, Clarke SC. Rapid assignment of nucleotide sequence data to allele types for multi-locus sequence analysis (MLSA) of bacteria using an adapted database and modified alignment program. J Mol Microbiol Biotechnol. 2002;4(6):515–7. [PubMed] [Google Scholar]
- 18.Maiden MC, Bygraves JA, Feil E, Morelli G, Russell JE, Urwin R, et al. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci U S A. 1998;95(6):3140–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sullivan CB, Jefferies JM, Diggle MA, Clarke SC. Automation of MLST using third-generation liquid-handling technology. Mol Biotechnol. 2006;32(3):219–26. [DOI] [PubMed] [Google Scholar]
- 20.Thompson EA, Feavers IM, Maiden MC. Antigenic diversity of meningococcal enterobactin receptor FetA, a vaccine component. Microbiology. 2003;149(Pt 7):1849–58. [DOI] [PubMed] [Google Scholar]
- 21.Urwin R, Russell JE, Thompson EA, Holmes EC, Feavers IM, Maiden MC. Distribution of surface protein variants among hyperinvasive meningococci: implications for vaccine design. Infect Immun. 2004;72(10):5955–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jolley KA, Maiden MC. BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics. 2010;11:595 10.1186/1471-2105-11-595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leopold SR, Goering RV, Witten A, Harmsen D, Mellmann A. Bacterial whole-genome sequencing revisited: portable, scalable, and standardized analysis for typing and detection of virulence and antibiotic resistance genes. J Clin Microbiol. 2014;52(7):2365–70. 10.1128/JCM.00262-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–9. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vogel U, Szczepanowski R, Claus H, Junemann S, Prior K, Harmsen D. Ion torrent personal genome machine sequencing for genomic typing of Neisseria meningitidis for rapid determination of multiple layers of typing information. J Clin Microbiol. 2012;50(6):1889–94. 10.1128/JCM.00038-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cardinale JA, Clark VL. Determinants of nitric oxide steady-state levels during anaerobic respiration by Neisseria gonorrhoeae. Mol Microbiol. 2005;58(1):177–88. [DOI] [PubMed] [Google Scholar]
- 27.Hong E, Giorgini D, Deghmane AE, Taha MK. Functional impacts of the diversity of the meningococcal factor H binding protein. Vaccine. 2012;31:183–9. 10.1016/j.vaccine.2012.10.072 [DOI] [PubMed] [Google Scholar]
- 28.Elias J, Vogel U. IS1301 fingerprint analysis of Neisseria meningitidis strains belonging to the ET-15 clone. J Clin Microbiol. 2007;45(1):159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lappann M, Otto A, Becher D, Vogel U. Comparative proteome analysis of spontaneous outer membrane vesicles and purified outer membranes of Neisseria meningitidis. J Bacteriol. 2013;195(19):4425–35. 10.1128/JB.00625-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonn F, Bartel J, Buttner K, Hecker M, Otto A, Becher D. Picking vanished proteins from the void: how to collect and ship/share extremely dilute proteins in a reproducible and highly efficient manner. Anal Chem. 2014;86(15):7421–7. 10.1021/ac501189j [DOI] [PubMed] [Google Scholar]
- 31.Maiden MC, van Rensburg MJ, Bray JE, Earle SG, Ford SA, Jolley KA, et al. MLST revisited: the gene-by-gene approach to bacterial genomics. Nat Rev Microbiol. 2013;11(10):728–36. 10.1038/nrmicro3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jünemann S, Sedlazeck FJ, Prior K, Albersmeier A, John U, Kalinowski J, et al. Updating benchtop sequencing performance comparison. Nat Biotechnol. 2013;31(4):294–6. 10.1038/nbt.2522 [DOI] [PubMed] [Google Scholar]
- 33.Vizcaino JA, Cote RG, Csordas A, Dianes JA, Fabregat A, Foster JM, et al. The PRoteomics IDEntifications (PRIDE) database and associated tools: status in 2013. Nucleic Acids Res. 2013;41(Database issue):D1063–9. 10.1093/nar/gks1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Householder TC, Belli WA, Lissenden S, Cole JA, Clark VL. cis- and trans-acting elements involved in regulation of aniA, the gene encoding the major anaerobically induced outer membrane protein in Neisseria gonorrhoeae. J Bacteriol. 1999;181(2):541–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stefanelli P, Colotti G, Neri A, Salucci ML, Miccoli R, Di Leandro L, et al. Molecular characterization of nitrite reductase gene (aniA) and gene product in Neisseria meningitidis isolates: is aniA essential for meningococcal survival? IUBMB Life. 2008;60(9):629–36. 10.1002/iub.95 [DOI] [PubMed] [Google Scholar]
- 36.Ku SC, Schulz BL, Power PM, Jennings MP. The pilin O-glycosylation pathway of pathogenic Neisseria is a general system that glycosylates AniA, an outer membrane nitrite reductase. Biochem Biophys Res Commun. 2009;378(1):84–9. 10.1016/j.bbrc.2008.11.025 [DOI] [PubMed] [Google Scholar]
- 37.Vu DM, Shaughnessy J, Lewis LA, Ram S, Rice PA, Granoff DM. Enhanced bacteremia in human factor H transgenic rats infected by Neisseria meningitidis. Infect Immun. 2012;80(2):643–50. 10.1128/IAI.05604-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jongerius I, Lavender H, Tan L, Ruivo N, Exley RM, Caesar JJ, et al. Distinct binding and immunogenic properties of the gonococcal homologue of meningococcal factor h binding protein. PLoS Pathog. 2013;9(8):e1003528 10.1371/journal.ppat.1003528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Granoff DM, Welsch JA, Ram S. Binding of complement factor H (fH) to Neisseria meningitidis is specific for human fH and inhibits complement activation by rat and rabbit sera. Infect Immun. 2009;77(2):764–9. 10.1128/IAI.01191-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zipfel PF, Skerka C, Hellwage J, Jokiranta ST, Meri S, Brade V, et al. Factor H family proteins: on complement, microbes and human diseases. Biochem Soc Trans. 2002;30(Pt 6):971–8. [DOI] [PubMed] [Google Scholar]
- 41.Robert Koch-Institut. Empfehlung des Berliner Impfbeirates zur Impfung gegen Meningokokken-Erkrankungen bei Männern, die Sex mit Männern haben. Epidemiologisches Bulletin. 2013;30:281. [Google Scholar]
- 42.Tettelin H, Saunders NJ, Heidelberg J, Jeffries AC, Nelson KE, Eisen JA, et al. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science. 2000;287(5459):1809–15. [DOI] [PubMed] [Google Scholar]
- 43.Bentley SD, Vernikos GS, Snyder LA, Churcher C, Arrowsmith C, Chillingworth T, et al. Meningococcal genetic variation mechanisms viewed through comparative analysis of serogroup C strain FAM18. PLoS Genet. 2007;3(2):e23 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(DOC)
Data Availability Statement
The mass spectrometry based proteomics data (Thermo raw files, dta-select-filter.txt) have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository.22. The dataset ID is PXD001498 (http://proteomecentral.proteomexchange.org/cgi/GetDataset?ID=PXD000181).