Abstract
Previous work has shown that red blood cells (RBCs) reduce nitrite to NO under conditions of low oxygen. Strong support for the ability of red blood cells to promote nitrite bioactivation comes from using platelet activation as a NO-sensitive process. Whereas addition of nitrite to platelet rich plasma in the absence of RBCs has no effect on inhibition of platelet activation, when RBCs are present platelet activation is inhibited by an NO-dependent mechanism that is potentiated under hypoxia. In this paper, we demonstrate that nitrite bioactivation by RBCs is blunted by physiologically-relevant concentrations of nutrients including glucose and the important signaling amino acid leucine. Our mechanistic investigations demonstrate that RBC mediated nitrite bioactivation is largely dependent on nitrosation of RBC surface proteins. These data suggest a new expanded paradigm where RBC mediated nitrite bioactivation not only directs blood flow to areas of low oxygen but also to areas of low nutrients. Our findings could have profound implications for normal physiology as well as pathophysiology in a variety of diseases including diabetes, sickle cell disease, and arteriosclerosis.
Abbreviations: RBC, red blood cell; Hb, hemoglobin; PRP, platelet rich plasma; Hct, hematocrit; CPTIO, 2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt; Lat1, l-type amino acid transporter; CSNO, S-nitrosocysteine; SNAP, S-Nitroso-N-acetylpenicillamine; DTNB, 5,5′-Dithiobis(2-nitrobenzoic acid)
Keywords: Nitric oxide, Nitrite, Red blood cells, Hemoglobin, Leucine, Glucose
Graphical abstract
Highlights
-
•
Nitrite bioactivation by red blood cells inhibits platelet activation.
-
•
The mechanism appears to involve modification of red blood cell surface thiols.
-
•
Glucose and leucine inhibit nitrite bioactivation by red blood cells.
-
•
A paradigm involving targeted delivery of these nutrients is proposed.
1. Introduction
Although nitrite was once considered to be inert in human physiology [1], several studies have shown that, in fact, nitrite acts as a vasodilator under hypoxic conditions, protects against ischemic reperfusion injury, and reduces platelet activation along with other biological and therapeutic actions [2], [3], [4], [5], [6], [7], [8], [9], [10]. In 2003, we and others showed that slightly supraphysiological levels of infused nitrite increased forearm blood flow [2]. Importantly, the increases in blood flow were potentiated during exercise suggesting that nitrite is preferentially bioactivated under hypoxic and mildly acidic conditions so that nitrite bioactivation helps direct blood flow to where it is needed [2]. We hypothesized that nitrite-mediated vasodilation was due to deoxygenated hemoglobin (Hb) in the red blood cell (RBC) which reduces nitrite to NO as worked out by Doyle and others [11],
| (1) |
A major challenge to our hypothesis is that NO rapidly reacts with oxygenated Hb to form nitrate or binds deoxygenated Hb so that NO export from the RBC is theoretically extremely inefficient or impossible [2]. Thus, several alternative pathways have been suggested where intermediate or alternate species, such as a nitrosothiol or N2O3, are formed in the reaction of nitrite and Hb that could carry NO activity from the RBC to the smooth muscle or other targets [12], [13], [14], [15], [16].
Several other enzymatic and non-enzymatic systems have been proposed to account for nitrite bioactivity that do not involve the RBC [17], [18], [19], [20], [21]. However, studies conducted using aortic rings showing potentiation of vessel relaxation when nitrite is combined with deoxygenated RBCs or Hb support a role of the RBC and deoxygenated Hb in nitrite's ability to effect vasodilation [2], [22], [23]. In addition, strong support for a role of RBC in nitrite bioactivation has come from the work of Schechter and colleagues where it was shown that nitrite alone in platelet rich plasma (PRP) does not affect platelet activation or aggregation, but when RBC were present, platelet activity was inhibited [7]. The effect of RBC was enhanced upon deoxygenation and the effect was abrogated in the presence of an NO scavenger [7]. These results suggest RBC are capable of producing NO activity from nitrite which reduces platelet activity via the established pathway of NO-mediated soluble guanylate cyclase activation and cGMP signaling [24], [25], [26], [27]. Although other RBC associated enzymes have been suggested to be responsible for nitrite bioactivation [18], [19], [28], we have recently provided evidence that Hb is indeed the main erythrocytic nitrite reductase [29].
In this paper, we use nitrite-mediated inhibition of platelet activation to demonstrate a role for glucose and leucine in modulating erythrocytic bioactivation of nitrite. We further explore the mechanism of erythrocytic bioactivation of nitrite and have explored the role of cell-surface protein nitrosation in RBC.
2. Materials and methods
2.1. Materials
Blood was drawn from volunteers after providing informed consent under a protocol approved by the Wake Forest University School of Medicine Internal Review Board. EZ-Link Maleimide-PEG2-Biotin, micro BSA reagent and C18 spin columns were purchased from Thermo Scientific. Pre-made 4–20% linear gradient SDS gels, Avidin-HRP and Avidin-HRP substrate were purchased from Bio-Rad. Protease inhibitor cocktail was purchased from Sigma. Glucose colorimetric assay kit was purchased from Cayman chemicals. Pac-1 and CD 61 monoclonal antibodies were purchased from Becton Dickinson Immunocytometry systems.
2.2. Platelet activation
Platelet activation was measured as described previously using PAC-1 and CD61 antibodies with data collected using a BD Sciences FACSCalibur flow cytometer [29]. Inhibition of platelet activation by deoxygenated RBCs and nitrite was evaluated by adding the deoxygenated RBCs and nitrite to PRP as described previously [29].
2.3. Time resolved absorption spectroscopy
Time resolved absorption was performed similarly to that described previously [29]. Briefly, samples were prepared in 1 cm quartz, septum capped cuvettes, purged with nitrogen, and filled with 4 mL of 100 µM deoxygenated Hb in the presence or absence of 1 mM leucine or 10 mM glucose. Nitrite (500 µM final concentration) was added to each cuvette to initiate the reaction. Spectra were taken every 180 s at 37° C in a Cary 100 UV–vis spectrometer. Half-lives were determined by when deoxygenated Hb fell to 50% of its initial value.
2.4. RBC nitrite uptake kinetics
Fresh blood was centrifuged at 1000 g for 5 min at 25 °C at least three times until the supernatant was clear, and RBCs were resuspended in PBS at a stock concentration of 60% hematocrit (Hct) and then deoxygenated using nitrogen. Deoxygenated RBCs at 15% Hct were incubated with 1 mM leucine, 10 mM glucose, or neither at 37 °C for 15 min before adding nitrite. Nitrite (100 µM final concentration) was injected in each sample. Every 15 min in the first hour and every 30 min in the second hour, a 500 µL aliquot was taken out and spun at 1000 g for 1 min. Supernatant (5 µL) was injected to measure extracellular nitrite by a Sievers 280i Nitric Oxide Analyzer. Nitrite consumption was calculated by the percentage of nitrite remaining at each time-point divided by the original nitrite concentration.
2.5. Plasma glucose measurement
Plasma glucose was measured in our assays by glucose colorimetric assay kit according to the manufacturer's recommendation using micro plate reader.
2.6. Measurement of S-Nitrosohemoglobin (SNO-Hb)
SNO-Hb was measured using the modified 2 C assay as described previously [30]. All mixtures containing nitrosothiols included metal chelation with diethylene triamine pentaacetic acid and protected from light.
3. Results
3.1. Nitrite-mediated inhibition of platelet activation by RBCs involves NO or nitrosothiols
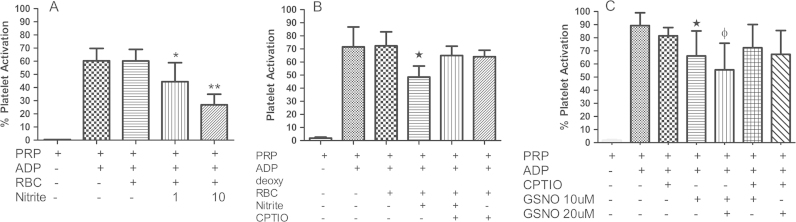
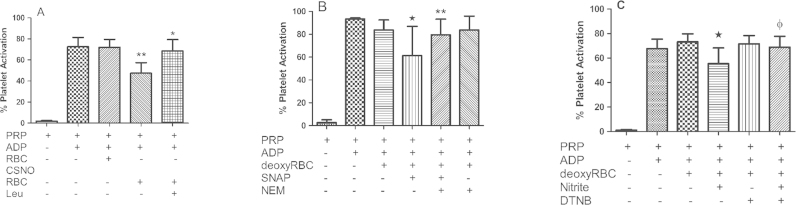
Consistent with previously published work [7], we show that nitrite combined with deoxygenated RBCs reduces platelet activation (Fig. 1(A)) and this action is blunted by the NO scavenger 2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt (CPTIO) (Fig. 1(B)). Note that there is substantial variance in the inter-individual response in platelet activation for a given amount of the agonist ADP (see for example error bar in Fig. 1(A) for PRP + ADP). There will also be variability in nitrite response. The percentage inhibition by 10 μM nitrite in Fig. 1(A) vs. B is 52±4% vs. 31±2%, calculated as [(PRP+ADP)−(PRP+ADP+Nitrite)]/(PRP+Nitrite)]. Despite these variances, these data strongly suggest that bioactivation of nitrite by RBCs involves NO itself. However, previous work has shown that nitrosothiol-mediated inhibition of platelet activation is also inhibited by NO scavengers including hemoglobin [31]. Nitrosothiols are reduced to NO at the surface of, or inside RBCs [31], [32]. In Fig. 1(C), we show that CPTIO significantly blunts GSNO-mediated inhibition of platelet activation. Importantly, the effects of 10 μM nitrite are comparable to those of GSNO shown in Fig. 1(C) where the percentage inhibition was 24±11% and 31±19% for 10 and 20 μM GSNO respectively. These data suggest that RBC RSNO formation cannot be ruled out as at least part of the nitrite bioactivation mechanism based on effects of CPTIO and that the effect of nitrite is comparable to other compounds that have been considered as anti-platelet agents.
Fig. 1.
Nitrite and NO mediated Inhibition of Platelet Activation. (A) Deoxygenated (deoxy) RBC in presence of nitrite inhibits platelet activation. Human PRP and deoxy erythrocytes (15% hematocrit) were incubated at 37 °C in presence of 1 μM ADP ±1 μM or 10 μM nitrite, *p<0.05 and **p<0.01 compared to deoxyRBC+PRP. Values are means±SD (n=3). (B) ADP induced platelet activation is inhibited by nitrite+deoxy erythrocytes. Human PRP+deoxy RBC (15% hematocrit) were incubated with 10 μM nitrite and 1 μM ADP in the presence and absence of 500 μM CPTIO for ten minutes, *p<0.01 comparing to deoxyRBC+PRP+nitrite and deoxyRBC+PRP+nitrite+CPTIO. Experiments were performed at 37 °C. Data values are means±SD (n=3). (C) CPTIO inhibits the effect of GSNO on platelet activation. Platelet activation was induced by 10 μM ADP in presence or absence of 200 μM CPTIO, 10 and 20 μM GSNO. 200 μM CPTIO abrogates the GSNO mediated effect on platelets, *p<0.04 compared with PRP+CPTIO+10 μM GSNO and ɸp<0.01compared with PRP+CPTIO+20 μM GSNO. All experiments were performed at 37 °C. Data values are means±SD (n=6).
3.2. Nitrite-mediated inhibition of platelet activation by RBCs is inhibited by leucine and glucose
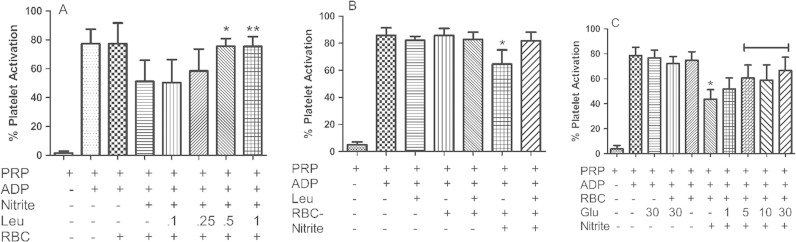
The l-type amino acid transporter 1 (LAT1) system has been shown to modulate S-nitrosothiol transport, which is inhibited by leucine [33], [34], [35], [36]. Fig. 2(A) shows the effect of different concentrations of leucine on RBC-nitrite dependent inhibition of platelet activation. Leucine prevented RBC and nitrite-dependent inhibition of platelet aggregation at the highest (500 µM, p=0.006) dose tested, with trends towards significant observed a lower (250 μM) doses. Leucine had no effect on platelet activation with or without RBCs in the absence of nitrite (Fig. 2(B)). As leucine is an important signaling molecule that regulates amino acid and glucose uptake [37], [38], we investigated whether glucose itself would modulate nitrite bioactivation by RBCs. Fig. 2(C) shows that as little as 1 mM added glucose shows a trend in blunting erythrocytic nitrite bioactivation (p=0.07), that was significant at higher tested glucose concentrations. Glucose alone did not affect platelet activation in the absence of nitrite (Fig. 2(C)). Importantly, we found that in our assays glucose in PRP is lowered to 3.7 mM, so that addition of 1 mM glucose in these assays raises glucose up to physiologic levels. These data show that physiological or slightly supraphysiological levels of nutrients blunt nitrite bioactivation by RBCs.
Fig. 2.
Nutrient effect on Nitrite-mediated inhibition of Platelet Activation. (A) Leucine abrogates platelet inhibition mediated by nitrite and deoxyRBC. Different concentrations of leucine were preincubated with 10 μM nitrite, PRP and deoxy RBC (15% HCT) at 37 °C for ten minutes, platelets were further activated by 1 μM ADP at 37 °C for 10 min. Leucine concentrations 0.5 and 1 mM significantly inhibits nitrite mediated platelet inhibition. *p<0.006 and **p<0.01 compared to nitrite+deoxyRBC. Data values are means±SD (n=3). (B) Human PRP+deoxyRBCs were pre-incubated with and without nitrite and leucine for 10 min at 37 °C. After pre-incubation platelets were activated by 1 μM ADP for ten minutes at 37 °C. Leucine alone has no effect on platelet activation. *p<0.001 compared to deoxyRBCs+nitrite+leucine. Data values are means±SD (n=3). (C) Glucose dose dependently abrogates platelet inhibition mediated by nitrite and deoxyRBCs. Different concentrations of glucose were preincubated with 10 μM nitrite, PRP and deoxy RBC (15% HCT) at 37 °C for 15 min, platelets were further activated by 1 μM ADP at 37 °C for 10 min. Glucose at 5, 10 and 30 mM significantly inhibits nitrite mediated platelet inhibition, p<0.002 compared to nitrite+deoxyRBC. Data values are means±SD (n=4).
3.3. Effects of leucine and glucose are not due to uptake by RBCs or reaction with Hb
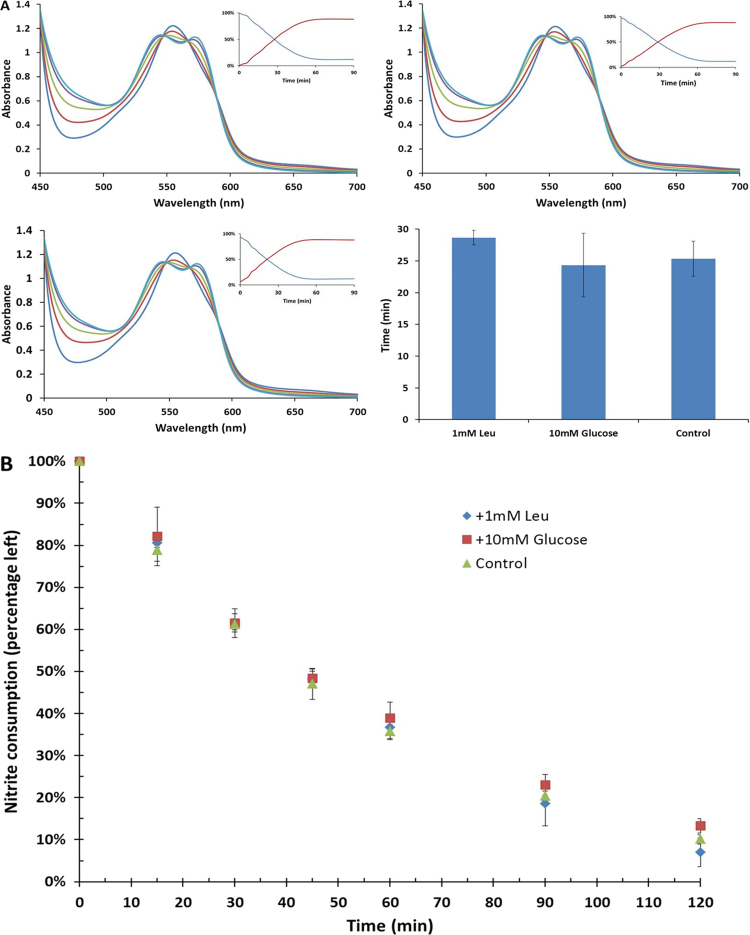
One potential mechanism for the observed effects of glucose and leucine on nitrite bioactivation could be direct effects of these nutrients on the rate of nitrite reduction by deoxygenated Hb. However, time resolved absorption experiments revealed no difference in the kinetics of the deoxygenated Hb (100 µM)/nitrite (500 µM) reaction with half-lives of the reaction being 29±1 min, 24±5 min, and 25±3 min for control, plus 1 mM leucine and plus10 mM glucose respectively (Fig. 3(A)). In addition, leucine and glucose had no effect on nitrite uptake by red blood cells (Fig. 3(B)).
Fig. 3.
Nutrient effects on Hb/nitrite reactivity and RBC uptake. (A) Effect of nutrients on nitrite reduction by Hb. Time resolved absorbance spectra after mixing deoxy hemoglobin (100 µM) and nitrite (500 µM) in the presence of sodium dithionite (10 mM) with Leucine (1 mM, left top, T1/2=29 min), Glucose (10 mM, right top, T1/2=24 min) and buffer as control (left bottom, T1/2=25 min). Insets show the percentage of deoxyHb (blue) and HbNO (red) as a function of time. Bar graph shows the average of half-life of three samples (n=3). Spectra were recorded at 37 °C every 180 s, and all samples were prepared in PBS buffer, pH 7.4. Neither leucine (p=0.09) or glucose (p=0.53) significantly affected the kinetics of the reaction compared to control (paired t-test). (B) Effect on nitrite uptake by red blood cells. Deoxygenated RBCs (15% Hct) were mixed with nitrite (100 mM) in the presence of Leucine (1 mM, blue diamond), Glucose (10 mM, red square), and buffer as control (green triangle). The nitrite consumption was calculated by the percentage of nitrite concentration at every time-point over the initial value (n=3). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4. Effects of leucine are not due to nitrosothiol transport
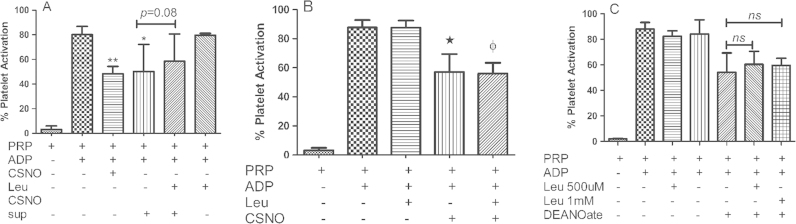
Another possible mechanism for the blunting of RBC mediated nitrite bioactivation by leucine involves S-nitrosation within the RBC due to the nitrite/Hb reaction along with inhibition of S-nitrosothiol transport either out of the RBC or into the platelet. To test whether leucine inhibits S-nitrosocysteine (CSNO) export from RBCs, we loaded RBCs with CSNO (1 mM) and washed extensively so that only 40 nM CSNO remained in the supernatant (which is less than that required to affect platelet activation in our experiments (data not shown)). After washing, we incubated the SNO-loaded cells in buffer with and without leucine (1 mM). We then examined the ability of the supernatant (which would contain CSNO exported from the RBC) to inhibit platelet activation. As shown in Fig. 4(A), leucine shows a trend in blunting CSNO export from the RBC as demonstrated by the ability of the supernatant to inhibit platelet activation, but the effect is weak and did not reach statistical significance (P=0.08). Thus, inhibition of CSNO export from RBCs by leucine is not likely a major factor in the blunting of inhibition of platelet activation by nitrite. We next evaluated whether leucine inhibits CSNO uptake by platelets. In Fig. 4(B), we show that l-leucine does not have an effect on nitrite-mediated inhibition of platelet activation by S-nitrosocysteine or NO produced by an NO donor, DEANONOate (Fig. 4(C)).
Fig. 4.
Potential role of uptake and export in Leucine Effects. (A) Leucine does not inhibit CSNO export from erythrocytes. RBC were treated with 1 mM CSNO for an hour, washed CSNO treated RBC were further incubated with and without 1 mM leucine for 45 min at room temperature, supernatant from these RBCs were collected and used in the platelet activation assay. CSNO treated RBC supernatant and 2 μM CSNO significantly inhibits ADP (1 μM) induced platelet activation (**p<0.0001), *p<0.002 compared to PRP+ADP. Leucine and CSNO treated RBC supernatant did not significantly blunt the platelet inhibition (p=0.08). Data values are ±SD (n=4). (B) Leucine does not abrogate CSNO mediated platelet inhibition. CSNO (20 μM) was added to the PRP in presence and absence of 1 mM leucine and preincubated at 37 °C for 5 min. Platelets were activated with ADP at 37 °C for 10 min. CSNO show statistically significant inhibition of platelets (*p<0.004), leucine with and without CSNO has no effect on platelet activation (ɸp=0.84). Data values are ±SD (n=3). (C) Leucine does not inhibit platelet inhibition by the NO donor DEANOate. Platelets were preincubated for 5 min at 37 °C in presence and absence of 5 μM DEANOate and 0.5 and 1 mM leucine. After preincubation platelets were activated by ADP for 10 min at 37 °C. The NO donor DEANOate significantly inhibits platelet activation, but leucine fails to blunt DEANOate mediated platelet inhibition. Data values are ±SD (n=3).
3.5. Nitrite-mediated inhibition of platelet activation by RBCs is dependent on RBC surface nitrosation
We next evaluated whether the RBCs themselves were involved in leucine-inhibitable, RBC mediated nitrite bioactivation. We resuspended the pellet containing CSNO-loaded RBCs and observed that these cells inhibit platelet activation and leucine blunts the inhibition (Fig. 5(A)). These data suggest that bioactivation of nitrite by RBCs may be due to red cell membrane surface nitrosation. To further investigate a possible role for surface nitrosation of RBCs, we employed S-Nitroso-N-acetylpenicillamine (SNAP), a cell membrane-impermeable nitrosating agent, to surface nitrosate red blood cells and test whether these cells can inhibit platelet activation. This experiment was designed to test whether surface nitrosation of RBCs can result in platelet inhibition (if not, then RBC-mediated nitrite bioactivation is not likely to involve RBC surface nitrosation). We confirmed that SNAP would only nitrosate surface thiols when RBCs are exposed to SNAP vs. CSNO (which enters cells) by incubating these with RBCs and measuring SNO-Hb. When RBCs were incubated with 500 μM CSNO for one hour, 2.2±0.6 μM SNO-Hb was formed. When RBCs were treated with 500 μM SNAP for one hour, only 0.09±0.03 μM SNO-Hb was detected (n=3). It should be noted that we found trans-nitrosation from SNAP and CSNO to Hb have similar efficiencies outside the RBC. We found that when 50 μM CSNO or SNAP was incubated with 1 mM Hb for one hour, 0.28±0.15 μM and 0.26±0.003 μM SNO-Hb was produced, respectively (n=3). These data are consistent with previous studies examining cellular uptake of SNAP [39], [40]. We thus incubated red blood cells with SNAP, thoroughly washed the cells, and tested their ability to inhibit platelet activation. Indeed, these surface nitrosated cells inhibited platelet activation and their activity was abrogated with prior treatment with the thiol blocking agent N-Ethylmaleimide (NEM, Fig. 5(B)). In addition, we found that when red cells were pre-incubated with the cell-impermeable surface thiol blocking agent 5,5′-Dithiobis(2-nitrobenzoic acid) (DTNB) and thoroughly washed, nitrite-mediated inhibition of platelet activation by red blood cells was abrogated (Fig. 5(C)). These data strongly support the notion that incubation of RBCs with nitrite leads to cell-surface nitrosation and this action plays a major role in nitrite bioactivation by RBCs.
Fig. 5.
Role of RBC surface nitrosation in nitrite bioactivation. (A) Leucine abrogates CSNO loaded RBCs mediated nitrite bioactivation. RBCs were treated with 1 mM CSNO as described in Fig. 3(A). Washed CSNO treated RBCs were pre-incubated with 1 mM leucine for 10 min at 37 °C. After pre-incubation platelets were activated by ADP at 37 °C for another 10 min. Leucine blunts the CSNO RBC mediated platelet inhibition. *p<0.03 compared to PRP+CSNO RBCs. **p<0.001 compared to deoxyRBCs+PRP. Data values are means±SD (n=3). (B) RBC thiol nitrosation inhibited platelet activation. RBCs were incubated with 200 μM of the nitrosating agent SNAP ±10 mM NEM for an hour at room temperature. After incubation, RBCs were washed extensively. Washed SNAP±NEM RBCs were pre-incubated with PRP at 37 °C for 10 min. After pre-incubation platelets were activated by ADP at 37 °C for 10 min. *p<0.02 compared with PRP+RBCs and **p<0.04 compared with SNAP RBC. Data values are means±SD (n=3). (C) RBC surface thiol oxidation abrogates nitrite mediated platelet inhibition. RBCs were pre-incubated with 2.5 mM DTNB for an hour at room temperature. DTNB treated RBCs were extensively washed and deoxygenated. 10 μM nitrite was added to the deoxy DTNB RBCs in presence of PRP and incubated for 5 min at 37 °C. Platelets were activated by ADP at 37 °C for 10 min. *p<0.0001 compared to PRP+deoxyRBCs and ɸp=0.27 compared to PRP+deoxyRBCs+DTNB. Data values are means±SD (n=7).
4. Discussion
The biggest discovery resulting from our work reported above is that leucine and glucose blunt nitrite bioactivation by red blood cells (Fig. 2). Whole blood levels of leucine after overnight fast are about 130 μM [41], but can increase 2–3 fold after a meal [42]. Thus, based on the data shown in Fig. 2(A), physiological levels of leucine are likely to modulate nitrite bioactivation by RBCs. Likewise, as physiological levels of blood glucose range from 4 to 6 mM, and we found levels of glucose in our PRP after RBC addition to be about 3.7 mM, our data (Fig. 2(C)) show that physiological levels of glucose modulate nitrite bioactivation by RBCs. The extent of modulation by these nutrients requires further study, particularly at lower pH which occurs in active tissue and also in which reduction of nitrite by Hb is facilitated [43].
We found that the mechanism of the observed effects of glucose and leucine is not due to modulation of the kinetics of the Hb/nitrite reaction or nitrite uptake by RBCs (Fig. 3). We also found that leucine does not affect CSNO mediated inhibition of platelet activation either by direct interaction with the platelets or through export of CSNO from the RBC (Fig. 4). We did, however, find that leucine blunts inhibition of platelet activation by CSNO-loaded red cells themselves (Fig. 5(A)), leading us to explore whether RBC membrane surface nitrosation may be involved. We found that surface nitrosated red cells do indeed blunt inhibition of platelet activation and that oxidation of surface thiols abrogates nitrite-mediated inhibition of platelet activation by red blood cells.
5. Conclusions
In conclusion, our results suggest an expansion of the paradigm that we and others put forth in 2003 whereby low oxygen tension leads to Hb deoxygenation bioactivating nitrite so that NO activity is exported to induce vasodilation and increased blood flow to low oxygen tissue where it is needed [2]. Our data suggest that RBC mediated nitrite bioactivation is also targeted to areas of low nutrients, further facilitating blood flow to where it is needed, in metabolically active muscle and other tissues. It is well known that glucose is absorbed by active muscle and is a very important fuel for endurance exercise [44], [45]. Leucine is an important signaling amino acid that facilitates insulin-associated glucose uptake [38], and is a major factor in regulating protein synthesis and increasing muscle mass [37], [42]. Our findings thus have important implications for regulation of blood flow during exercise in normal physiology and in pathological conditions that involve poor peripheral circulation such as peripheral artery disease, heart failure with preserved ejection, diabetes, and sickle cell disease.
Conflicts of interest
RPP, and DBK-S are co-authors on a patent entitled Use of Nitrite Salts for the Treatment of Cardiovascular Conditions. The authors declare no other conflict of interest.
Acknowledgments
We thank Peter Perlegas for his art work (graphical abstract). We also thank Victor Darley-Usmar and Mark T Gladwin for helpful discussions. This work was supported by the National Institute of Health (Grant numbers HL058091, CA121291-37, P30CA012197). We acknowledge services provided by the Flow Cytometry Core Laboratory of the Comprehensive Cancer Center, supported in part by NCI, National Institutes of Health, and Wake Forest Baptist Comprehensive Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Lauer T., Preik M., Rassaf T., Strauer B.E., Deussen A., Feelisch M., Kelm M. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc. Natl. Acad. Sci. USA. 2001;98:12814–12819. doi: 10.1073/pnas.221381098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gladwin M.T., Cosby K., Partovi K.S., Patel R.P., Crawford J.H., Kim-Shapiro D.B., Schechter A.N., Cannon R.O. Novel function of human hemoglobin as a nitrite reductase regulates nitric oxide homeostasis and hypoxic vasodilation. Blood. 2003;102 255a-255a. [Google Scholar]
- 3.Zweier J.L., Wang P.H., Samouilov A., Kuppusamy P. Enzyme-independent formation of nitric-oxide in biological tissues. Nat. Med. 1995;1:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 4.Modin A., Bjorne H., Herulf M., Alving K., Weitzberg E., Lundberg J.O.N. Nitrite-derived nitric oxide: a possible mediator of 'acidic-metabolic' vasodilation. Acta Physiol. Scand. 2001;171:9–16. doi: 10.1046/j.1365-201X.2001.00771.x. [DOI] [PubMed] [Google Scholar]
- 5.Duranski M.R., Greer J.J.M., Dejam A., Jaganmohan S., Hogg N., Langston W., Patel R.P., Yet S.F., Wang X.D., Kevil C.G., Gladwin M.T., Lefer D.J. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J. Clin. Investig. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Webb A.J., Patel N., Loukogeorgakis S., Okorie M., Aboud Z., Misra S., Rashid R., Miall P., Deanfield J., Benjamin N., MacAllister R., Hobbs A.J., Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srihirun S., Sriwantana T., Unchern S., Kittikool D., Noulsri E., Pattanapanyasat K., Fucharoen S., Piknova B., Schechter A.N., Sibmooh N. Platelet inhibition by nitrite is dependent on erythrocytes and deoxygenation. PLoS One. 2012;7:e30380. doi: 10.1371/journal.pone.0030380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lundberg J.O., Gladwin M.T., Ahluwalia A., Benjamin N., Bryan N.S., Butler A., Cabrales P., Fago A., Feelisch M., Ford P.C., Freeman B.A., Frenneaux M., Friedman J., Kelm M., Kevil C.G., Kim-Shapiro D.B., Kozlov A.V., Lancaster J.R., Lefer D.J., McColl K., McCurry K., Patel R.P., Petersson J., Rassaf T., Reutov V.P., Richter-Addo G.B., Schechter A., Shiva S., Tsuchiya K., van Faassen E.E., Webb A.J., Zuckerbraun B.S., Zweier J.L., Weitzberg E. Nitrate and nitrite in biology, nutrition and therapeutics. Nat. Chem. Biol. 2009;5:865–869. doi: 10.1038/nchembio.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Velmurugan S., Kapil V., Ghosh S.M., Davies S., McKnight A., Aboud Z., Khambata R.S., Webb A.J., Poole A., Ahluwalia A. Antiplatelet effects of dietary nitrate in healthy volunteers: involvement of cGMP and influence of sex. Free Radic. Biol. Med. 2013;65:1521–1532. doi: 10.1016/j.freeradbiomed.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park J.W., Piknova B., Huang P.L., Noguchi C.T., Schechter A.N. Effect of blood nitrite and nitrate levels on murine platelet function. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doyle M.P., Pickering R.A., Deweert T.M., Hoekstra J.W., Pater D. Kinetics and mechanism of the oxidation of human deoxyhemoglobin by nitrites. J. Biol. Chem. 1981;256:12393–12398. [PubMed] [Google Scholar]
- 12.Basu S., Grubina R., Huang J., Conradie J., Huang Z., Jeffers A., Jiang A., He X., Azarov I., Seibert R., Mehta A., Patel R., King S.B., Hogg N., Ghosh A., Gladwin M.T., Kim-Shapiro D.B. Catalytic generation of N2O3 by a concerted nitrite reductase and anhydrase activity of hemoglobin. Nat. Chem. Biol. 2007;3:785–794. doi: 10.1038/nchembio.2007.46. [DOI] [PubMed] [Google Scholar]
- 13.Nagababu E., Ramasamy S., Abernethy D.R., Rifkind J.M. Active nitric oxide produced in the red cell under hypoxic conditions by deoxyhemoglobin-mediated nitrite reduction. J. Biol. Chem. 2003;278:46349–46356. doi: 10.1074/jbc.M307572200. [DOI] [PubMed] [Google Scholar]
- 14.Luchsinger B.P., Rich E.N., Gow A.J., Williams E.M., Stamler J.S., Singel D.J. Routes to S-nitroso-hemoglobin formation with heme redox and preferential reactivity in the beta subunits. Proc. Natl. Acad. Sci. USA. 2003;100:461–466. doi: 10.1073/pnas.0233287100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angelo M., Singel D.J., Stamler J.S. An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate. Proc. Natl. Acad. Sci. USA. 2006;103:8366–8371. doi: 10.1073/pnas.0600942103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roche C.J., Cassera M.B., Dantsker D., Hirsch R.E., Friedman J.M. Generating S-nitrosothiols from hemoglobin mechanisms, conformational deendencem and physioligical relevance. J. Biol. Chem. 2013;288:22408–22425. doi: 10.1074/jbc.M113.482679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H., Cui H., Kundu T.K., Alzawahra W., Zweier J.L. Nitric oxide production from nitrite occurs primarily in tissues not in the blood: critical role of xanthine oxidase and aldehyde oxidase. J. Biol. Chem. 2008;283:17855–17863. doi: 10.1074/jbc.M801785200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh S.M., Kapil V., Fuentes-Calvo I., Bubb K.J., Pearl V., Milsom A.B., Khambata R., Maleki-Toyserkani S., Yousuf M., Benjamin N., Webb A.J., Caulfield M.J., Hobbs A.J., Ahluwalia A. Enhanced vasodilator activity of nitrite in hypertension critical role for erythrocytic xanthine oxidoreductase and translational potential. Hypertension. 2013;61:1091–1102. doi: 10.1161/HYPERTENSIONAHA.111.00933. [DOI] [PubMed] [Google Scholar]
- 19.Webb A.J., Milsom A.B., Rathod K.S., Chu W.L., Qureshi S., Lovell M.J., Lecomte F.M.J., Perrett D., Raimondo C., Khoshbin E., Ahmed Z., Uppal R., Benjamin N., Hobbs A.J., Ahluwalia A. Mechanisms underlying erythrocyte and endothelial nitrite reduction to nitric oxide in hypoxia role for xanthine oxidoreductase and endothelial nitric oxide synthase. Circ. Res. 2008;103 doi: 10.1161/CIRCRESAHA.108.175810. 957-U114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Totzeck M., Hendgen-Cotta U.B., Luedike P., Berenbrink M., Klare J.P., Steinhoff H.-J., Semmler D., Shiva S., Williams D., Kipar A., Gladwin M.T., Schrader J., Kelm M., Cossins A.R., Rassaf T. Nitrite regulates hypoxic vasodilation via myoglobin-dependent nitric oxide generation. Circulation. 2012;126:325. doi: 10.1161/CIRCULATIONAHA.111.087155. (-+) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ormerod J.O.M., Ashrafian H., Maher A.R., Arif S., Steeples V., Born G.V.R., Egginton S., Feelisch M., Watkins H., Frenneaux M.P. The role of vascular myoglobin in nitrite-mediated blood vessel relaxation. Cardiovasc. Res. 2011;89:560–565. doi: 10.1093/cvr/cvq299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crawford J.H., Isbell T.S., Huang Z., Shiva S., Chacko B.K., Schechter A.N., Darley-Usmar V.M., Kerby J.D., Lang J.D., Jr, Kraus D., Ho C., Gladwin M.T., Patel R.P. Hypoxia, red blood cells and nitrite regulate NO-dependent hypoxic vasodilatation. Blood. 2006;107:566–574. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isbell T.S., Gladwin M.T., Patel R.P. Hemoglobin oxygen fractional saturation regulates nitrite-dependent vasodilation of aortic ring bioassays. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H2565–H2572. doi: 10.1152/ajpheart.00759.2007. [DOI] [PubMed] [Google Scholar]
- 24.Loscalzo J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ. Res. 2001;88:756–762. doi: 10.1161/hh0801.089861. [DOI] [PubMed] [Google Scholar]
- 25.Wollny T., Iacoviello L., Buczko W., DeGaetano G., Donati M.B. Prolongation of bleeding time by acute hemolysis in rats: a role for nitric oxide. Am. J. Physiol. Heart Circ. Physiol. 1997;272:H2875–H2884. doi: 10.1152/ajpheart.1997.272.6.H2875. [DOI] [PubMed] [Google Scholar]
- 26.Azuma H., Ishikawa M., Sekizaki S. Endothelium-dependent inhibition of platelet aggregation. Br. J. Pharmacol. 1986;88:411–415. doi: 10.1111/j.1476-5381.1986.tb10218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schafer A., Wiesmann F., Neubauer S., Eigenthaler M., Bauersachs J., Channon K. Rapid regulation of platelet activation in vivo by nitric oxide. Circulation. 2004;109:1819–1822. doi: 10.1161/01.CIR.0000126837.88743.DD. [DOI] [PubMed] [Google Scholar]
- 28.Aamand R., Dalsgaard T., Jensen F.B., Simonsen U., Roepstorff A., Fago A. Generation of nitric oxide from nitrite by carbonic anhydrase: a possible link between metabolic activity and vasodilation. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H2068–H2074. doi: 10.1152/ajpheart.00525.2009. [DOI] [PubMed] [Google Scholar]
- 29.Liu C., Wajih N., Liu X., Basu S., Janes J., Marvel M., Keggi C., Helms C.C., Lee A.N., Belanger A.M., Diz D.I., Laurienti P.J., Caudell D.L., Wang J., Gladwin M.T., Kim-Shapiro D.B. Mechanisms of human erythrocytic bioactivation of nitrite. J. Biol. Chem. 2015;290:1281–1294. doi: 10.1074/jbc.M114.609222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basu S., Wang X.D., Gladwin M.T., Kim-Shapiro D.B. Chemiluminescent detection of S-nitrosated proteins: comparison of tri-iodide, copper/CO/cysteine, and modified copper/cysteine methods in nitric oxide, Part F: oxidative and nitrosative stress in redox regulation of cell signaling. Methods Enzymol. 2008;440:137–156. doi: 10.1016/S0076-6879(07)00808-7. [DOI] [PubMed] [Google Scholar]
- 31.Radomski M.W., Rees D.D., Dutra A., Moncada S. S-nitroso-glutathione inhibits platelet acrtivation in vitro and in vivo. Br. J. Pharmacol. 1992;107:745–749. doi: 10.1111/j.1476-5381.1992.tb14517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bell S.E., Shah C.M., Gordge M.P. Protein disulfide-isomerase mediates delivery of nitric oxide redox derivatives into platelets. Biochem. J. 2007;403:283–288. doi: 10.1042/BJ20061146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y.H., Hogg N. The mechanism of transmembrane S-nitrosothiol transport. Proc. Natl. Acad. Sci. USA. 2004;101:7891–7896. doi: 10.1073/pnas.0401167101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandmann J., Schwedhelm K.S., Tsikas D. Specific transport of S-nitrosocysteine in human red blood cells: implications for formation of S-nitrosothiols and transport of NO bioactivity within the vasculature. FEBS Lett. 2005;579:4119–4124. doi: 10.1016/j.febslet.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 35.Li S., Whorton A.R. Identification of stereoselective transporters for s-nitroso-l-cysteine. J. Biol. Chem. 2005;280:20102–20110. doi: 10.1074/jbc.M413164200. [DOI] [PubMed] [Google Scholar]
- 36.Li S., Whorton A.R. Functional characterization of two S-nitroso-l-cysteine transporters, which mediate movement of NO equivalents into vascular cells. Am. J. Physiol. Cell. 2007;292:C1263–C1271. doi: 10.1152/ajpcell.00382.2006. [DOI] [PubMed] [Google Scholar]
- 37.Dodd K.M., Tee A.R. Leucine and mTORC1: a complex relationship. Am. J. Physiol. Endocrinol. Metab. 2012;302:E1329–E1342. doi: 10.1152/ajpendo.00525.2011. [DOI] [PubMed] [Google Scholar]
- 38.Liu H., Liu R., Xiong Y.F., Li X., Wang X.L., Ma Y., Guo H.L., Hao L.P., Yao P., Liu L.G., Wang D., Yang X.F. Leucine facilitates the insulin-stimulated glucose uptake and insulin signaling in skeletal muscle cells: involving mTORC1 and mTORC2. Amino Acids. 2014;46:1971–1979. doi: 10.1007/s00726-014-1752-9. [DOI] [PubMed] [Google Scholar]
- 39.Mallis R.J., Thomas J.A. Effect of S-nitrosothiols on cellular glutathione and reactive protein sulfhydryls. Arch. Biochem. Biophys. 2000;383:60–69. doi: 10.1006/abbi.2000.2048. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y.H., Hogg N. S-nitrosothiols: cellular formation and transport. Free Radic. Biol. Med. 2005;38:831–838. doi: 10.1016/j.freeradbiomed.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 41.Lewis A.M., Waterhouse C., Jacobs L.S. Whole-blood and plasma amino-acid-analysis – gas-liquid and cation-exchange chromatography compared. Clin. Chem. 1980;26:271–276. [PubMed] [Google Scholar]
- 42.Norton L.E., Layman D.K. Leucine regulates translation initiation of protein synthesis in skeletal muscle after exercise. J. Nutr. 2006;136:533S–537S. doi: 10.1093/jn/136.2.533S. [DOI] [PubMed] [Google Scholar]
- 43.Huang Z., Shiva S., Kim-Shapiro D.B., Patel R.P., Ringwood L.A., Irby C.E., Huang K.T., Ho C., Hogg N., Schechter A.N., Gladwin M.T. Enzymatic function of hemoglobin as a nitrite reductase that produces Nitric oxide under allosteric control. J. Clin. Investig. 2005;115:2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richter E.A., Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev. 2013;93:993–1017. doi: 10.1152/physrev.00038.2012. [DOI] [PubMed] [Google Scholar]
- 45.Chauveau M.A., Kaufmann M. Experiences pour la determination du coefficient de l’activite nutritive et respiratoire des muscles en repos et en travail. Comptes Rendus Hebd. Des. séances De. l’Académie Des. Sci. 1887;104:1126–1132. [Google Scholar]