Abstract
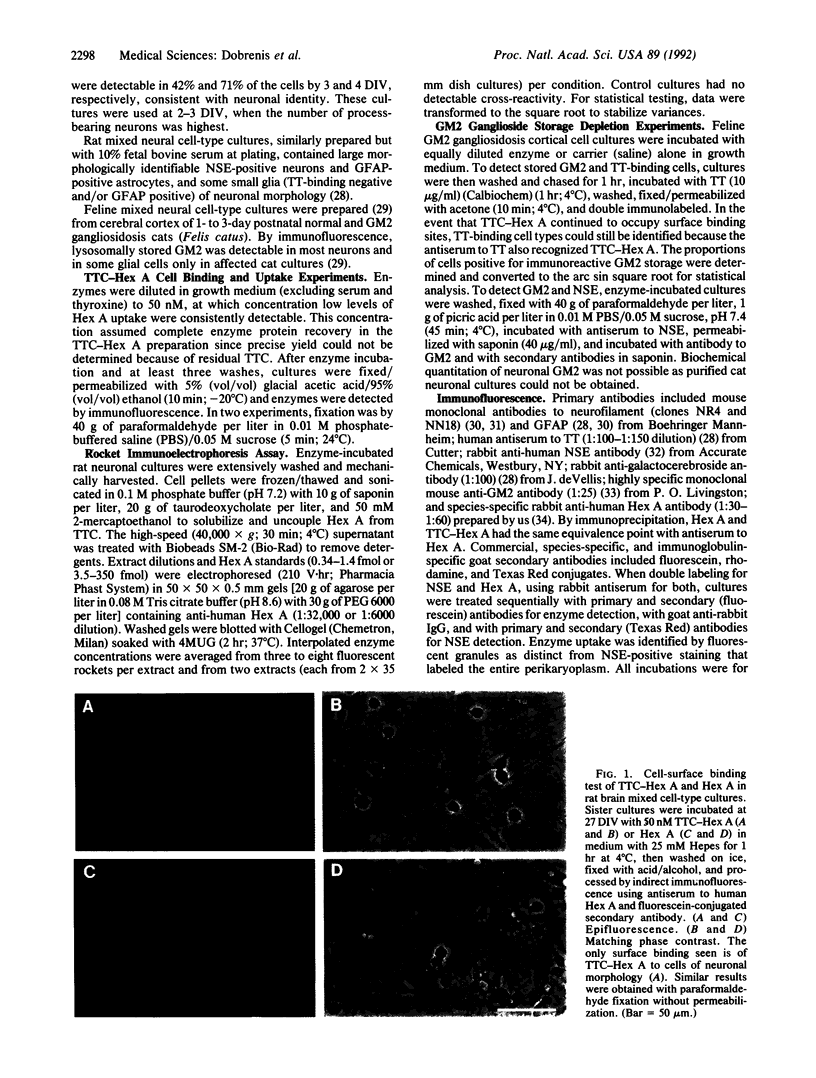
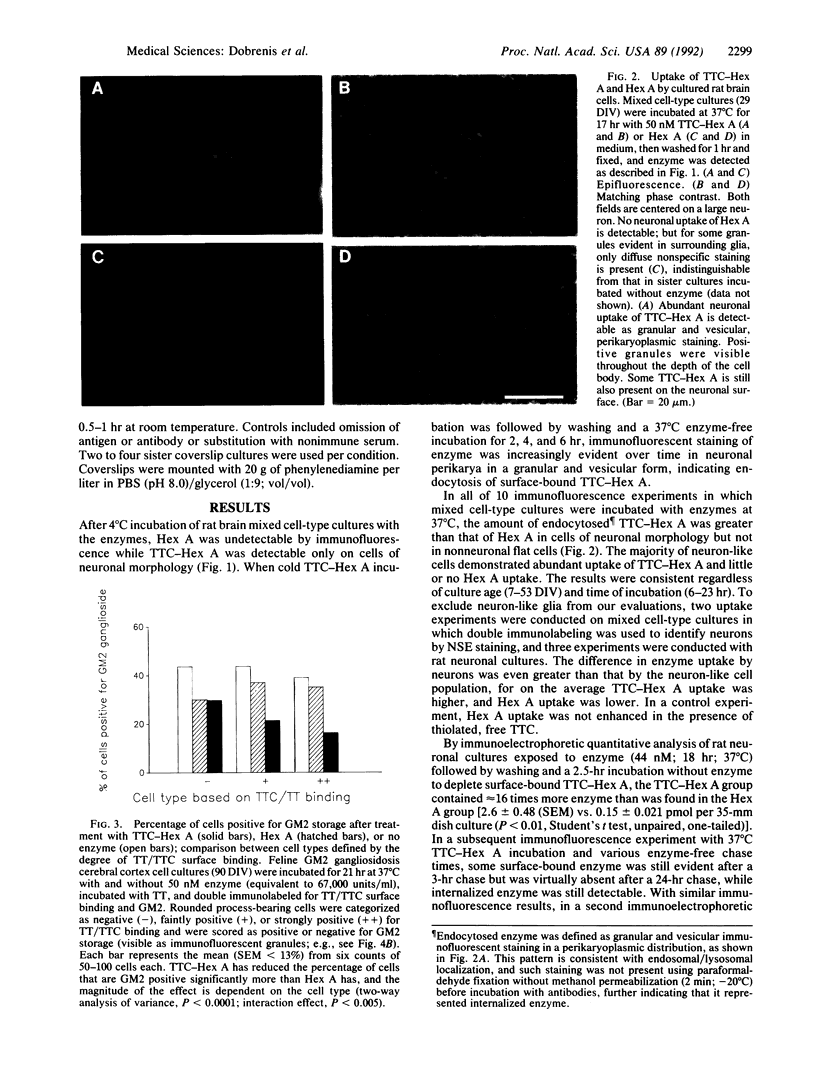
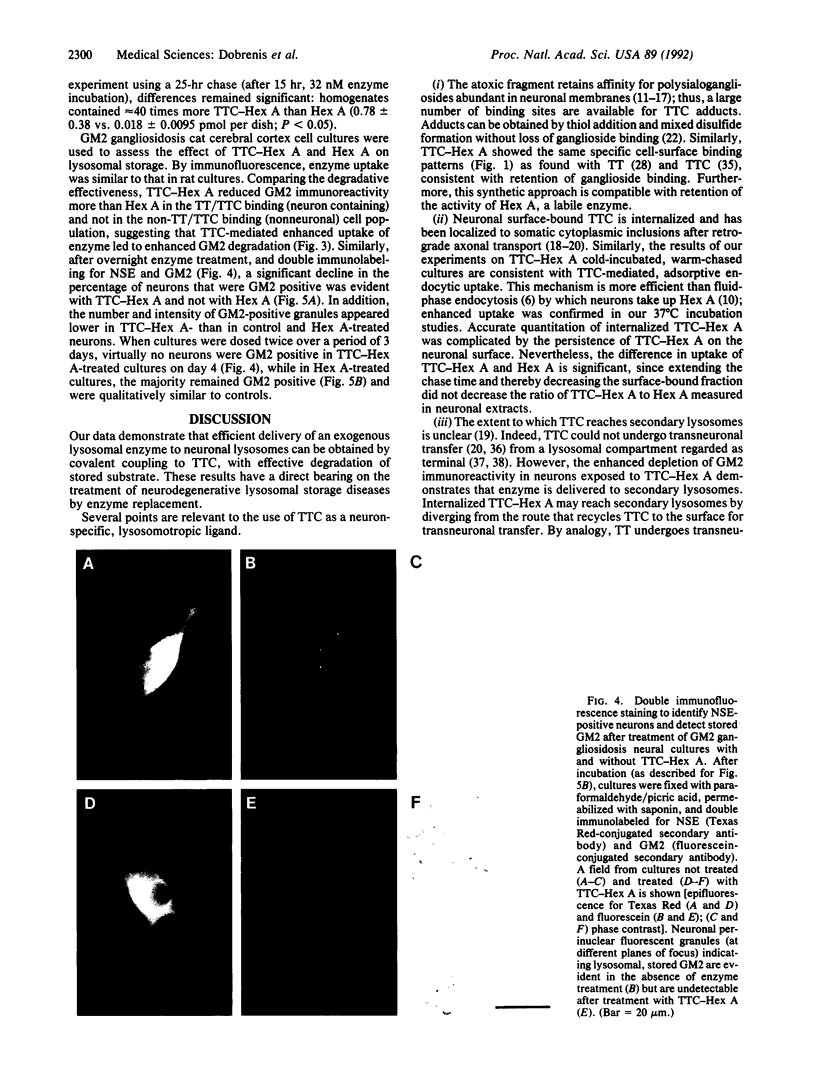
Development of a strategy for efficient delivery of exogenous enzyme to neuronal lysosomes is essential to achieve enzyme replacement in neurodegenerative lysosomal storage diseases. We tested whether effective lysosomal targeting of the human enzyme beta-N-acetylhexosaminidase A (Hex A; beta-N-acetyl-D-hexosaminide N-acetylhexosaminohydrolase, EC 3.2.1.52) can be obtained by coupling it via disulfide linkage to the atoxic fragment C of tetanus toxin (TTC) that is bound avidly by neuronal membrane. TTC-Hex A conjugation resulted in neuronal surface binding and enhanced endocytosis of enzyme as observed in immunofluorescence studies with rat brain cultures. In immunoelectrophoretic quantitative uptake studies, rat neuronal cell cultures contained 16- and 40-fold greater amounts of enzyme after incubation with TTC-Hex A than with nonderivatized Hex A. In cerebral cortex cell cultures from a feline model of human GM2 gangliosidosis (Tay-Sachs and Sandhoff diseases), binding and uptake patterns of the enzymes were similar to those in the rat brain cell cultures. After exposure to extracellular concentrations of enzyme attainable in vivo, lysosomal storage of immunodetectable GM2 ganglioside was virtually eliminated in neurons exposed to TTC-Hex A, whereas a minimal effect was observed with Hex A. These findings demonstrate the usefulness of TTC adducts for effective neuronal lysosomal enzyme replacement.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen-Beckh B., Binz T., Kurazono H., Mayer T., Eisel U., Niemann H. Expression of tetanus toxin subfragments in vitro and characterization of epitopes. Infect Immun. 1989 Nov;57(11):3498–3505. doi: 10.1128/iai.57.11.3498-3505.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton N. W., Brady R. O., Dambrosia J. M., Di Bisceglie A. M., Doppelt S. H., Hill S. C., Mankin H. J., Murray G. J., Parker R. I., Argoff C. E. Replacement therapy for inherited enzyme deficiency--macrophage-targeted glucocerebrosidase for Gaucher's disease. N Engl J Med. 1991 May 23;324(21):1464–1470. doi: 10.1056/NEJM199105233242104. [DOI] [PubMed] [Google Scholar]
- Bayleran J., Hechtman P., Saray W. Synthesis of 4-methylumbelliferyl-beta-D-N-acetylglucosamine-6-sulfate and its use in classification of GM2 gangliosidosis genotypes. Clin Chim Acta. 1984 Nov 15;143(2):73–89. doi: 10.1016/0009-8981(84)90215-8. [DOI] [PubMed] [Google Scholar]
- Beaude P., Delacour A., Bizzini B., Domuado D., Remy M. H. Retrograde axonal transport of an exogenous enzyme covalently linked to B-IIb fragment of tetanus toxin. Biochem J. 1990 Oct 1;271(1):87–91. doi: 10.1042/bj2710087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzini B., Akert K., Glicksman M., Grob P. Preparation of conjugates using two tetanus toxin derived fragments: their binding to gangliosides and isolated synaptic membranes and their immunological properties. Toxicon. 1980;18(5-6):561–572. doi: 10.1016/0041-0101(80)90083-5. [DOI] [PubMed] [Google Scholar]
- Bizzini B., Grob P., Glicksman M. A., Akert K. Use of the B-IIb tetanus toxin derived fragment as a specific neuropharmacological transport agent. Brain Res. 1980 Jul 7;193(1):221–227. doi: 10.1016/0006-8993(80)90959-2. [DOI] [PubMed] [Google Scholar]
- Bizzini B., Stoeckel K., Schwab M. An antigenic polypeptide fragment isolated from tetanus toxin: chemical characterization, binding to gangliosides and retrograde axonal transport in various neuron systems. J Neurochem. 1977 Mar;28(3):529–542. doi: 10.1111/j.1471-4159.1977.tb10423.x. [DOI] [PubMed] [Google Scholar]
- Cabot J. B., Mennone A., Bogan N., Carroll J., Evinger C., Erichsen J. T. Retrograde, trans-synaptic and transneuronal transport of fragment C of tetanus toxin by sympathetic preganglionic neurons. Neuroscience. 1991;40(3):805–823. doi: 10.1016/0306-4522(91)90014-f. [DOI] [PubMed] [Google Scholar]
- Conzelmann E., Sandhoff K. Purification and characterization of an activator protein for the degradation of glycolipids GM2 and GA2 by hexosaminidase A. Hoppe Seylers Z Physiol Chem. 1979 Dec;360(12):1837–1849. doi: 10.1515/bchm2.1979.360.2.1837. [DOI] [PubMed] [Google Scholar]
- DEDUVE C. FROM CYTASES TO LYSOSOMES. Fed Proc. 1964 Sep-Oct;23:1045–1049. [PubMed] [Google Scholar]
- Debus E., Weber K., Osborn M. Monoclonal antibodies specific for glial fibrillary acidic (GFA) protein and for each of the neurofilament triplet polypeptides. Differentiation. 1983;25(2):193–203. doi: 10.1111/j.1432-0436.1984.tb01355.x. [DOI] [PubMed] [Google Scholar]
- Desnick R. J., Dean K. J., Grabowski G., Bishop D. F., Sweeley C. C. Enzyme therapy in Fabry disease: differential in vivo plasma clearance and metabolic effectiveness of plasma and splenic alpha-galactosidase A isozymes. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5326–5330. doi: 10.1073/pnas.76.10.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimpfel W., Huang R. T., Habermann E. Gangliosides in nervous tissue cultures and binding of 125I-labelled tetanus toxin, a neuronal marker. J Neurochem. 1977 Aug;29(2):329–334. doi: 10.1111/j.1471-4159.1977.tb09626.x. [DOI] [PubMed] [Google Scholar]
- Fishman P. S., Savitt J. M., Farrand D. A. Enhanced CNS uptake of systemically administered proteins through conjugation with tetanus C-fragment. J Neurol Sci. 1990 Sep;98(2-3):311–325. doi: 10.1016/0022-510x(90)90272-o. [DOI] [PubMed] [Google Scholar]
- Fishman P. S., Savitt J. M. Transsynaptic transfer of retrogradely transported tetanus protein-peroxidase conjugates. Exp Neurol. 1989 Nov;106(2):197–203. doi: 10.1016/0014-4886(89)90094-0. [DOI] [PubMed] [Google Scholar]
- Goldberg R. L., Costa T., Habig W. H., Kohn L. D., Hardegree M. C. Characterization of fragment C and tetanus toxin binding to rat brain membranes. Mol Pharmacol. 1981 Nov;20(3):565–570. [PubMed] [Google Scholar]
- Halpern J. L., Habig W. H., Neale E. A., Stibitz S. Cloning and expression of functional fragment C of tetanus toxin. Infect Immun. 1990 Apr;58(4):1004–1009. doi: 10.1128/iai.58.4.1004-1009.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helting T. B., Zwisler O., Wiegandt H. Structure of tetanus toxin. II. Toxin binding to ganglioside. J Biol Chem. 1977 Jan 10;252(1):194–198. [PubMed] [Google Scholar]
- Hoffman W. L., Strucely P. D., Jump A. A., Smiley J. D. A restricted human antitetanus clonotype shares idiotypic cross-reactivity with tetanus antibodies from most human donors and rabbits: reactivity with antibodies of widely differing electrophoretic mobility. J Immunol. 1985 Dec;135(6):3802–3807. [PubMed] [Google Scholar]
- Kelly R. B. The cell biology of the nerve terminal. Neuron. 1988 Aug;1(6):431–438. doi: 10.1016/0896-6273(88)90174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S., Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- Mahuran D., Lowden J. A. The subunit and polypeptide structure of hexosaminidases from human placenta. Can J Biochem. 1980 Apr;58(4):287–294. doi: 10.1139/o80-038. [DOI] [PubMed] [Google Scholar]
- Morris N. P., Consiglio E., Kohn L. D., Habig W. H., Hardegree M. C., Helting T. B. Interaction of fragments B and C of tetanus toxin with neural and thyroid membranes and with gangliosides. J Biol Chem. 1980 Jul 10;255(13):6071–6076. [PubMed] [Google Scholar]
- Natoli E. J., Jr, Livingston P. O., Pukel C. S., Lloyd K. O., Wiegandt H., Szalay J., Oettgen H. F., Old L. J. A murine monoclonal antibody detecting N-acetyl- and N-glycolyl-GM2: characterization of cell surface reactivity. Cancer Res. 1986 Aug;46(8):4116–4120. [PubMed] [Google Scholar]
- Neuwelt E. A., Goldman D. L., Dahlborg S. A., Crossen J., Ramsey F., Roman-Goldstein S., Braziel R., Dana B. Primary CNS lymphoma treated with osmotic blood-brain barrier disruption: prolonged survival and preservation of cognitive function. J Clin Oncol. 1991 Sep;9(9):1580–1590. doi: 10.1200/JCO.1991.9.9.1580. [DOI] [PubMed] [Google Scholar]
- O'Neil D. C., Bartholomew W. R., Rattazzi M. C. Antigenic homology of feline and human beta-hexosaminidase. Biochim Biophys Acta. 1979 Sep 29;580(1):1–9. doi: 10.1016/0005-2795(79)90191-0. [DOI] [PubMed] [Google Scholar]
- Ohata K., Marmarou A., Povlishock J. T. An immunocytochemical study of protein clearance in brain infusion edema. Acta Neuropathol. 1990;81(2):162–177. doi: 10.1007/BF00334505. [DOI] [PubMed] [Google Scholar]
- Parton R. G., Ockleford C. D., Critchley D. R. A study of the mechanism of internalisation of tetanus toxin by primary mouse spinal cord cultures. J Neurochem. 1987 Oct;49(4):1057–1068. doi: 10.1111/j.1471-4159.1987.tb09994.x. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Fields K. L., Hakomori S. I., Mirsky R., Pruss R. M., Winter J. Cell-type-specific markers for distinguishing and studying neurons and the major classes of glial cells in culture. Brain Res. 1979 Oct 5;174(2):283–308. doi: 10.1016/0006-8993(79)90851-5. [DOI] [PubMed] [Google Scholar]
- Rattazzi M. C. Enzyme therapy in lysosomal storage diseases: current approaches. Prog Clin Biol Res. 1982;103(Pt B):573–587. [PubMed] [Google Scholar]
- Schwab M. E., Suda K., Thoenen H. Selective retrograde transsynaptic transfer of a protein, tetanus toxin, subsequent to its retrograde axonal transport. J Cell Biol. 1979 Sep;82(3):798–810. doi: 10.1083/jcb.82.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secchi J., Lecaque D., Cousin M. A., Lando D., Legault-Demare L., Raynaud J. P. Detection and localization of 14-3-2 protein in primary cultures of embryonic rat brain. Brain Res. 1980 Feb 24;184(2):455–466. doi: 10.1016/0006-8993(80)90812-4. [DOI] [PubMed] [Google Scholar]
- Shaw G., Banker G. A., Weber K. An immunofluorescence study of neurofilament protein expression by developing hippocampal neurons in tissue culture. Eur J Cell Biol. 1985 Nov;39(1):205–216. [PubMed] [Google Scholar]
- Shen W. C., Ryser H. J., LaManna L. Disulfide spacer between methotrexate and poly(D-lysine). A probe for exploring the reductive process in endocytosis. J Biol Chem. 1985 Sep 15;260(20):10905–10908. [PubMed] [Google Scholar]
- Simpson L. L. Pharmacological experiments on the binding and internalization of the 50,000 dalton carboxyterminus of tetanus toxin at the cholinergic neuromuscular junction. J Pharmacol Exp Ther. 1985 Jul;234(1):100–105. [PubMed] [Google Scholar]
- Skaper S. D., Adler R., Varon S. A procedure for purifying neuron-like cells in cultures from central nervous tissue with a defined medium. Dev Neurosci. 1979;2(5):233–237. doi: 10.1159/000112485. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983 Jan;96(1):1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager J. M., Hamers M. N., Schram A. W., Van den Bergh F. A., Rietra P. J., Loonen C., Koster J. F., Slee R. An appraisal of human trials in enzyme replacement therapy of genetic diseases. Birth Defects Orig Artic Ser. 1980;16(1):343–359. [PubMed] [Google Scholar]
- Vladutiu G. D., Rattazzi M. C. Excretion-reuptake route of beta-hexosaminidase in normal and I-cell disease cultured fibroblasts. J Clin Invest. 1979 Apr;63(4):595–601. doi: 10.1172/JCI109341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Specht B. U., Geiger B., Arnon R., Passwell J., Keren G., Goldman B., Padeh B. Enzyme replacement in Tay-Sachs disease. Neurology. 1979 Jun;29(6):848–854. doi: 10.1212/wnl.29.6.848. [DOI] [PubMed] [Google Scholar]