Abstract
Many studies have investigated the relationship between serum zinc concentration and prostatic disease, but have shown inconsistent results. Hence, we performed a systematic literature review and meta-analysis to assess the correlation between serum zinc concentration and prostate disease. Systematic literature searches were conducted with PubMed, EMBASE, Science Direct/Elsevier, MEDLINE, CNKI and the Cochrane Library up to June 2015 for studies that involved the relationship between serum zinc concentration and prostate disease. Fourteen studies were identified from the databases. Our results illustrated that the serum zinc concentrations in prostate cancer patients were significantly lower than those in Benign prostatic hyperplasia (BPH) patients and normal controls (SMD (95% CI), −0.94 [−1.57, −0.32]; −1.18 [−1.90, −0.45]). However, the serum zinc concentrations in BPH patients were significantly higher than those in normal controls (SMD (95% CI) 1.77 [0.15, 3.39]). The present study showed that different levels of serum zinc concentrations are correlated with different prostatic disease. Serum zinc concentration may be used as a tool for the diagnosis and screening of prostate disease. But, further studies with well-designed larger sample studies are needed in this field to further clarify the correlation between serum zinc concentration and prostate disease.
Zinc is an important trace element that is relatively nontoxic and plays a very important role in human metabolism. Zinc has been found to be most highly concentrated in the liver, kidney, retina, prostate, and muscle1,2. Prostatic androgen metabolism is modified by the intracellular concentration of Zinc. At high tissue concentrations, this trace element inhibits the transformation of testosterone to dihydrotestosterone and plays an important role in maintaining the physiological function and normal tissue structure of the prostate1,2,3.
Zinc content varies in different organs and is highest in the prostate. Zinc mainly accumulates in the area surrounding prostate epithelial cells. Its content in the prostate is 100 times greater than that in plasma. The high concentration of zinc in the prostate strongly suggests that zinc plays an important role in prostate health. Some studies have reported that the Zn concentration in prostatic tissue is markedly decreased in prostate carcinoma patients and is increased in BPH patients3,4,5,6,7.
Serum zinc concentration is an appropriate biomarker of zinc status, as it has been confirmed to respond to zinc intake and to correctly predict functional responses to zinc interventions8. Zinc concentration is much easier to measure in serum than in the prostate due to the ease of access to serum and can be obtained without causing prostatic damage. Information regarding serum zinc concentration is of obvious interest to improve our understanding of the etiology and pathogenesis of prostatic diseases and also to aid in their diagnosis. However, information about serum zinc concentration in prostatic diseases is lacking and research findings have been highly contradictory.
Studies have investigated the correlation between serum zinc concentration and prostate disease, but their results have been contradictory, with some studies showing a statistically significantly lower serum zinc concentration in prostate cancer patients than in normal controls and others not observing this phenomenon. Several studies have shown that serum zinc concentration in BPH patients is statistically significant higher than that in normal controls, but others did not observe this phenomenon9,10,11,12,13,14,15,16,17,18,19,20,21,22. Therefore, we systematically reviewed the available literature and performed a meta-analysis to evaluate the correlation between serum zinc concentration and prostate disease to obtain valuable insight into the diagnosis and treatment of prostatic disease.
Results
Characteristics of the included studies
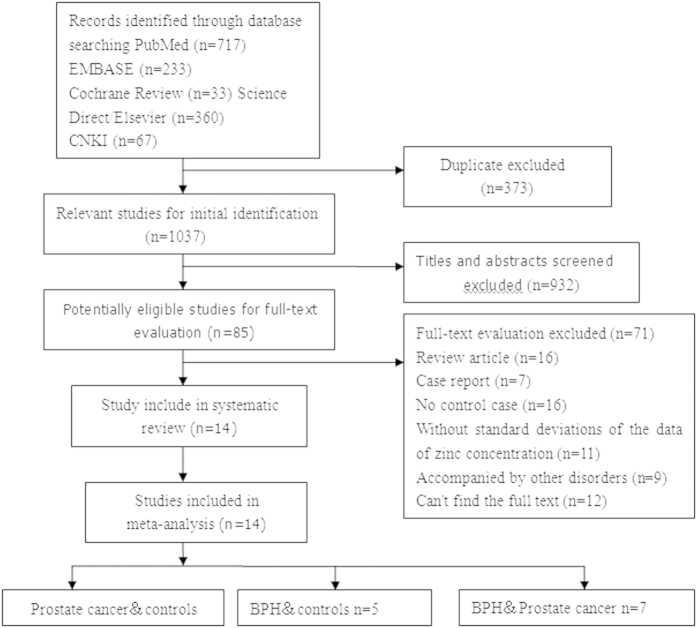
Figure 1 shows the review process in detail. A total of 1037 unduplicated studies were identified, fourteen studies were ultimately selected according to the eligibility criteria. Ten studies investigated the correlation between serum zinc concentration and prostate cancer. Five studies investigated the correlation between serum zinc concentration and BPH, and seven studies investigated the correlation of serum zinc concentration between prostate cancer and BPH. After group discussion, all reviewers were in agreement to include all twelve papers.
Figure 1. Flow diagram of selection of eligible studies.
Table 1 summarizes the general data from the fourteen studies. The retrieved studies included a total of 1318 patients with prostate disease and 1413 normal controls. The mean ages of the prostate cancer, BPH and control groups were in the ranges of 59.3–73.6 years, 63.4–76.8 years and 65.9–74.2 years, respectively. The mean ages of the patients and control groups were unavailable for three studies13,16,18. All studies reported exclusion/inclusion criteria9,10,11,12,13,14,15,16,17,18,19,20,21,22. For the ten studies9,10,11,12,13,14,15,16,21,22 that investigated the correlation between serum zinc concentration and prostate cancer, a total of 794 prostate cancer cases and 1359 normal controls were included. For the five studies11,12,13,16,17 that investigated the correlation between serum zinc concentration and BPH, a total of 194 cases and 333 normal controls were included. For the seven studies11,12,13,16,18,19,20 investigated the difference in serum zinc concentration between prostate cancer and BPH patients, a total of 336 prostate cancer patients and 337 BPH patients were included.
Table 1. Characteristics of include studies investigating the serum zinc and prostatic disease.
| Study | Country | Mean age (Cancer/BPH/control) | Control (n) | Cancer | BPH | Assay |
|---|---|---|---|---|---|---|
| Feustel A 1989 | German | 68.6/65.9/NI | 10 | 50 | AAS | |
| Park SY 2013 | America | 68.9 ± 7.2/69.1 ± 7.1/NI | 783 | 392 | AAS | |
| Aydin A 2006 | Turkey | 65.0 ± 6.0/64.3 ± 7.9/67.5 ± 8.8 | 24 | 25 | 36 | AAS |
| Ji K 2007 | China | 70.91 ± 7.99/68.63 ± 7.53/69.11 ± 7.87 | 191 | 76 | 125 | AAS |
| Christudoss P 2011 | India | NI/NI/NI | 20 | 18 | 45 | AAS |
| Li XM 2006 | China | 72 ± 1.62/71.5 ± 0.6/NI | 145 | 29 | AAS | |
| Daragó A 2011 | Poland | 59.30 ± 5.08/70.18 ± 6.30/74.25 ± 5.40 | 20 | 10 | AAS | |
| Yao DH 1977 | China | NI/NI/NI | 28 | 40 | 14 | AAS |
| Liu YY 1993 | China | 64/65/28 | 14 | 15 | AAS | |
| Rahman MT 2012 | Bangladesh | NI/NI/NI | 5 | 13 | AAS | |
| Xu BZ 2002 | China | 71.9/72.2/NI | 13 | 13 | AAS | |
| Zhou ZH 2009 | China | 73.6/71.5/NI | 95 | 91 | AAS | |
| Li XM 2005 | China | 71.9/72.2/NI | 38 | 64 | AAS | |
| Chen J.Q 2015 | China | 65.9 ± 8.4/76.8 ± 12.5/NI | 85 | 90 | AAS | |
| Ogunlewe J.O 1989 | Nigeria | 61.2 ± 6.9/67.3 ± 8.0/65.8 ± 8.1 | 55 | 60 | AAS |
Abbreviations: NI not indicated in studies. AAS, atomic absorption spectrophotometry.
Meta-analysis
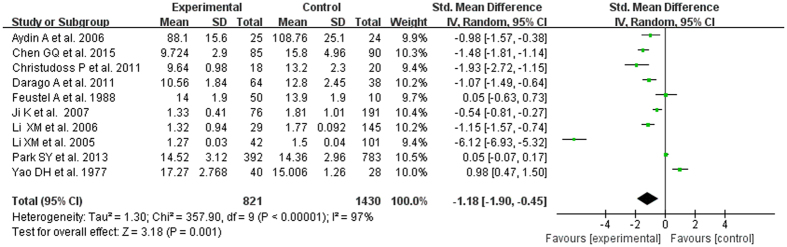
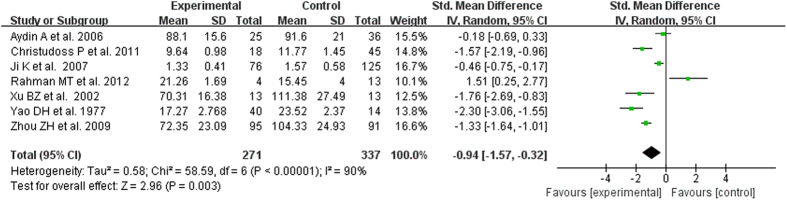
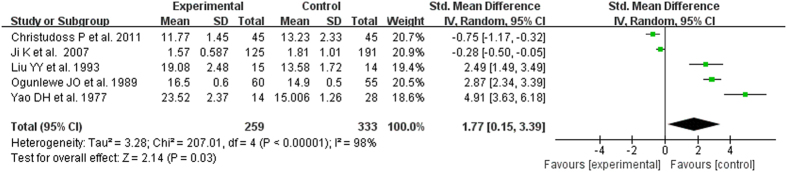
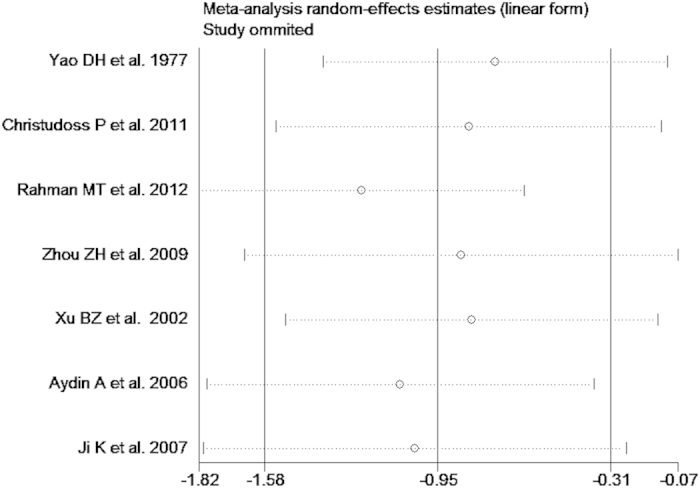
The test of heterogeneity suggested a random effects model, and the meta-analysis revealed that the serum zinc concentration of prostate cancer patients was significantly lower than that of normal controls and BPH patients (SMD (95% CI), −0.94 [−1.57, −0.32]; −1.18 [−1.90, −0.45]) (Figs 2 and 3). However, the serum zinc concentration of BPH patients was significantly higher than that of normal controls (SMD (95% CI) 1.77 [0.15, 3.39]) (Fig. 4). Egger’s regression test indicated little evidence of publication bias (Prostate cancer & controls: t = −2.26 and p = 0.06 > 0.05; BPH & controls; t = 2.07 and p = 0.13 > 0.05; BPH & Prostate cancer: t = −0.21 and p = 0.84 > 0.05) (Table 2). We also conducted a sensitivity analysis of the meta-analysis. We omitted each study sequentially, and the calculated combined SMDs for the remaining studies yielded consistent results. No single study significantly altered the combined results of the overall meta-analysis, which indicated that the results were statistically stable and reliable (Figs 5,6 and 7).
Figure 2. Forest plot showing the meta-analysis outcomes of the correlation between serum zinc concentration and prostate cancer.
Figure 3. Forest plot showing the meta-analysis outcomes of the difference in serum zinc concentration between prostate cancer and BPH patients.
Figure 4. Forest plot showing the meta-analysis outcomes of the correlation between serum zinc concentration and BPH.
Table 2. The Egger’s test of Publication bias.
| Coef. | Bias Std. Err. | t | P > |t| | [95% Conf.Interval] | ||
|---|---|---|---|---|---|---|
| Prostate cancer & controls | −6.35 | 2.80 | −2.26 | 0.06 | −12.82 | 0.13 |
| BPH & controls | 9.50 | 4.60 | 2.07 | 0.13 | −5.12 | 24.12 |
| BPH & Prostate cancer | 0.61 | 2.97 | 0.21 | 0.84 | 8.23 | 6.99 |
Figure 5. Sensitivity analysis plot of serum zinc concentration and prostate cancer.
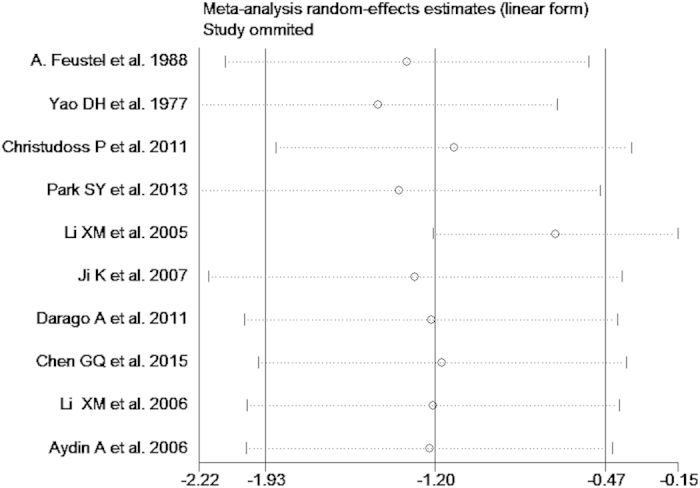
Figure 6. Sensitivity analysis plot of serum zinc concentration and BPH.
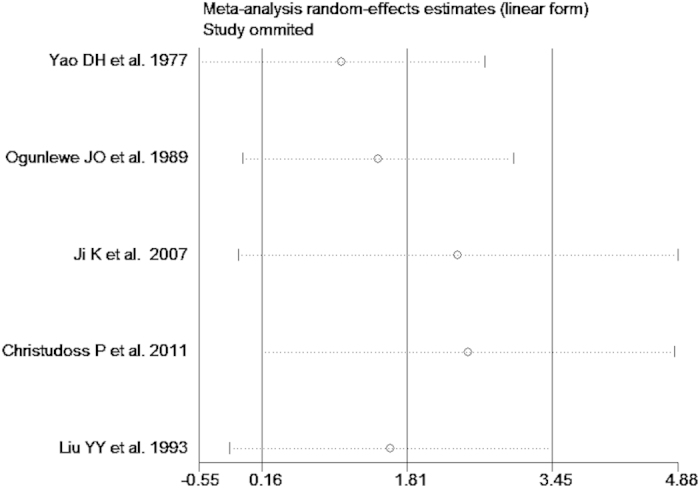
Figure 7. Sensitivity analysis plot of serum zinc concentration in prostate cancer and BPH.
Discussion
In our study, fourteen literatures studied the correlation between serum zinc concentration and prostate disease9,10,11,12,13,14,15,16,17,18,19,20,21,22. Ten of the fourteen literatures9,10,11,12,13,14,15,16,21,22 studied the relationship between the serum zinc concentration and prostate cancer. Seven studies11,12,13,14,15,21,22 reported that the zinc concentration of prostate cancer patients was significantly lower than that of normal controls, while two studies showed a non-statistically significant difference9,10. One study16 reported that the zinc concentration of prostate cancer patients was significantly higher than that of normal controls. In this meta-analysis, the zinc concentration of prostate cancer patients was significant lower than that of normal controls (SMD (95% CI), −0.94 [−1.57, −0.32]). Five literatures11,12,13,16,17 studied serum zinc concentration in BPH patients; three of those studies16,17,23 reported that the zinc concentration of BPH patients was significant higher than that of normal controls, while two studies12,13 reported that the zinc concentration of BPH patients was significantly lower than that of normal controls. In our study, the zinc concentration of BPH patients was significantly higher than that of normal controls (SMD (95% CI), −1.18 [−1.90, −0.45]). Seven literatures11,12,13,16,18,19,20 studied the relationship of serum zinc concentration between prostate cancer and BPH patients. Five studies12,13,16,19,20 reported that the serum zinc concentration in prostate cancer patients was significantly lower than that in BPH patients, and one study18 reported a contrasting result. In this meta-analysis, the serum zinc concentration in prostate cancer patients was significant lower than that in BPH patients (SMD (95% CI), 1.77 [0.15, 3.39]).
Zinc has an important role in human prostatic physiological metabolism. Currently, the understanding of the role of zinc in the prostate are as follows: a. maintenance of physiological function and normal tissue structure (this is especially important for the maintenance of the integrity and stability of the acinar epithelium and ductal epithelium); b. affect the activity of enzymes by acting as a coenzyme, with changes in its concentration greatly influencing the activities of many enzymes1,24,25; c. maintenance of the stability of sperm chromatin in seminal plasma, as indicated by the fact that healthy persons with high zinc content in the seminal plasma had a high percentage of sperm with stable chromatin, while infertile patients had lower zinc content in the seminal plasma and a lower percentage of sperm with stable chromatin26,27; d. killing of common bacteria that cause urogenital infection; e. involvement in the regulation of the growth and apoptosis of prostate epithelial cells28,29. Studies have reported that zinc may be protective against the development and progression of prostate cancer and may be benefit in the patients of with chronic prostatitis30,31,32,33,34,35,36,37,38,39. In prostate cancer, Zinc influences the development of prostate carcinoma. Currently, studies have shown that zinc inhibits prostate carcinoma cell growth, possibly due to the induction of cell-cycle arrest and apoptosis25,40,41. In general, the healthy human prostate accumulates the highest level of zinc in prostate epithelial cells, but this property is lost in prostatic malignancy42,43,44. Indeed, the highest level of zinc in prostate cells diminish early in the course of prostate carcinogenesis, preceding histopathological changes, and continue to decline during progression toward castration-resistant disease25,40,41,42,43,44. In this study, serum zinc concentrations in prostate cancer patients were significantly lower than those in Benign prostatic hyperplasia (BPH) patients and normal controls. This results suggest that serum zinc concentrations diminish may be attenuated prostate accumulates the highest level of zinc and promote tumor development in prostate cancer patients. Oppositely, Some studies have reported that higher concentration of zinc may be the adverse effect of BPH43,45,46,47,48. The serum zinc concentration of BPH patients was significantly higher than that of prostate carcinoma patients and normal controls. This finding contrasts with that in prostate carcinoma, and the mechanism behind the effects of zinc in BPH are not clear. Potentially, Zinc induces a bell-shaped proliferative of smooth muscle cells from benign prostatic hyperplasia48.
The potential mechanisms which zinc concentrations increased in BPH but decreased in prostate cancer is unclear. Serum zinc level variety is a consequence of nutritional status, dietary, tumor, chronic stress, chronic diseases, aging or of a combination of all these effects30,44,49. Chronic stress and inflammation is a common hallmark of cancer could lead to redistribution of zinc between body compartments which zinc importer (ZIP), zinc exporter (ZnT), and metallothionein may be involved and thus may be important mechanism of serum zinc level decrease30,41,42,44,49. Previous studies have shown that nutritional status, diet, race and clinical conditions may also influence the serum zinc concentration1,49,50,51,52. In our study, clinical conditions, nutritional status, diet and race of patients may be a crucial factor in serum level of zinc. In this study, the serum zinc concentration of patients with both prostatic carcinoma and metastases was decreased in comparison to control and BPH9,10,12,13,14,15,16,23. But, in this meta analysis, due to the reasons for the research design form the published literature, study on correlation between serum zinc concentration with tumor grade, stage and histological type is unable to assess. In addition, in this meta analysis, we included different countries and races of patients9,10,11,12,13,14,15,16,21,22. This will lead to different diet habits, nutritional status and lifestyle. These factors may be affect the serum zinc concentration of the included patients. But, in this meta analysis, because the research design and small sample size form the published literature, study on correlation between serum zinc concentration with nutritional status, diet and race is still unable to assess. Therefore, further studies with a larger sample of well-designed studies are needed to illuminate the relationship between serum zinc concentration and nutritional status, diet, race and clinical conditions.
There are some limitations that need to be taken into consideration when interpreting the results of this meta-analysis. First, the sample sizes of all included studies were relatively small, with a total of 1318 disease patients and 1413 normal controls in all twelve studies. Secondly, several studies related to the subject of interest were excluded due to a lack of control data (means and standard deviations) or unavailability of the full text. Thirdly, although this meta-analysis showed that serum zinc concentration was decreased in prostate cancer patients and increased in BPH patients, it was not clear whether prostatic disease caused the change in serum zinc concentration or the change in the serum zinc concentration led to prostatic disease. The result are not sufficient to evaluate causality. Fourth, serum zinc concentration has a limited predictive value because it is a specific intracellular ion that fluctuates with the circadian rhythm53. Therefore, it is necessary that through averaging the testing values of repeated measurements and precise sampling time at 8.00 which may be the best time. Therefore, this meta-analysis just access the real results of the zinc and prostate disease, and this phenomenon may benefit for the diagnostic of prostate disease, but further studies is warranted to confirm these findings.
In summary, the present study showed that serum zinc concentration was significantly lower in prostate cancer patients than in normal controls. Additionally, serum zinc concentration was significantly higher in BPH patients than in prostate cancer patients and normal controls. Serum zinc concentration may be used as a tool for the diagnosis and screening of prostate disease. But, further studies with well-designed larger sample studies are needed in this field to further clarify the correlation between serum zinc concentration and prostate disease.
Methods
Literature search
This meta-analysis was restricted to published studies that investigated the difference in serum zinc concentration between prostatic disease patients and normal controls. Two independent reviewers searched PubMed, EMBASE, Science Direct/Elsevier, Medline CNKI and the Cochrane Library from inception to June 2015, without language or study type restriction. The search terms combined text words and MeSH terms. For example, the search terms for serum zinc concentration were ‘serum zinc concentration’, ‘serum zinc content’, ‘zinc concentration’, ‘zinc content’, ‘serum zinc level’, ‘zinc level’, ‘zinc status’ and ‘trace element’ those for prostatic cancer and BPH were ‘prostatic cancer’, ‘prostatic tumour’, ‘prostate malignancies’, ‘prostate carcinoma’, ‘Prostate malignant diseases’, ‘Benign Prostatic Hyperplasia’, ‘BPH’, ‘prostatic benign diseases’, and ‘prostatic hyperplasia’. All related articles and abstracts were retrieved. In addition, references cited within the relevant articles and abstracts were retrieved manually, but only references that were full articles were reviewed for eligibility.
Eligibility criteria
Inclusion criteria
Studies were included their study population included patients diagnosed with prostatic carcinoma and benign prostatic hyperplasia. The controls were healthy human males with no history or evidence of andrologic or urologic disease. Zinc was detected in the serum by the atomic absorption spectrophotometry (AAS) test. Available data were extracted from the eligible articles, including means and standard deviations of serum zinc concentrations in all case-control groups.
Exclusion criteria
Studies were excluded if they were case reports or review articles. Studies involving patients with prostatic disease accompanied by other disorders of the urogenital system, prostate metastatic carcinoma patients, and patients who were ongoing zinc supplementation therapy were also excluded.
Study selection and validity assessment
Two independent reviewers screened the titles and abstracts of all citations from the literature search. All relevant studies that appeared to meet the eligibility criteria were retrieved. If it was unclear whether the study was eligible for inclusion based on the review of the title and abstract, the full text was examined. The full texts of all potentially eligible studies were reviewed to confirm their eligibility. Disagreements were resolved by consensus or by a third reviewer. Two reviewers completed quality assessments of the included studies according to the primary criteria of the Newcastle-Ottawa Quality Assessment scale (NOS) for assessing the quality of nonrandomized and observational studies in meta-analyses.
Data extraction and statistical analysis
Data, including demographic data (authors, year of publication, country, number and mean age of participants, and assay method) and the outcome data of serum zinc concentration in all case-control studies were extracted by three reviewers. Disagreements were resolved by consensus. A quantitative meta-analysis was performed by two reviewers using Review Manager (RevMan) software (version 5.2, The Nordic Cochrane Centre, The Cochrane Collaboration, 2012, Copenhagen) and Stata software (version 12.0, College Station, Texas, USA). Available data were analyzed in the meta-analysis.
We pooled the standard mean differences (SMD) of the serum zinc concentrations of the case-control groups, which were identified with 95% confidence intervals (95% CI). Heterogeneity was assessed by the P-value and the I-square statistic (I2) in the pooled analyses, which represent the percentage of total variation across studies. If the P-value was less than 0.1 or the I2-value was greater than 50%, the summary estimate was analyzed by a random-effects model. Otherwise, a fixed-effects model was applied. To estimate the stability of the Meta-analysis, we conducted a sensitivity analysis. In addition, publication bias was detected by the Egger’s test, and a P-value of less than 0.05 indicated the presence of publication bias.
Additional Information
How to cite this article: Zhao, J. et al. Comparative study of serum zinc concentrations in benign and malignant prostate disease: A Systematic Review and Meta-Analysis. Sci. Rep. 6, 25778; doi: 10.1038/srep25778 (2016).
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 81500580, 81230017 and 81170704).
Footnotes
Author Contributions J.Z. and L.K.L. designed the research; J.Z., X.Y.D., X.Y.H., L.W. and Q.J.W. conducted the studies; J.Z., Q.L. and Z.L. analyzed the data and prepared the manuscript; J.Z. and L.K.L. guided experiments and edited the paper. All authors read and approved the manuscript.
References
- Wu X., Tang J. & Xie M. Serum and hair zinc levels in breast cancer: a meta-analysis. Sci Rep 16, 12249 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chyan W., Zhang D. Y., Lippard S. J. & Radford R. J. Reaction-based fluorescent sensor for investigating mobile Zn2+ in mitochondria of healthy versus cancerous prostate cells. Proc Natl Acad Sci USA 111, 143–8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feustel A. & Wennrich R. Zinc and cadmium in cell fractions of prostatic cancer tissues of different histological grading in comparison to BPH and normal prostate. Urol Res 12, 147–50 (1984). [DOI] [PubMed] [Google Scholar]
- Feustel A., Wennrich R., Steiniger D. & Klauss P. Zinc and cadmium concentration in prostatic carcinoma of different histological grading in comparison to normal prostate tissue and adenofibromyomatosis (BPH). Urol Res 10, 301–3 (1982). [DOI] [PubMed] [Google Scholar]
- Feustel A., Wennrich R. & Dittrich H. Zinc, cadmium and selenium concentrations in separated epithelium and stroma from prostatic tissues of different histology. Urol Res 15, 161–3 (1987). [DOI] [PubMed] [Google Scholar]
- Shilstein S. S. et al. Prostatic Zn determination for prostate cancer diagnosis. Talanta 70, 914–21 (2006). [DOI] [PubMed] [Google Scholar]
- Vartsky D. et al. Prostatic zinc and prostate specific antigen: an experimental evaluation of their combined diagnostic value. J Urol 170, 2258–62 (2003). [DOI] [PubMed] [Google Scholar]
- Gibson R. S., Hess S. Y., Hotz C. & Brown K. H. Indicators of zinc status at the population level: a review of the evidence. Br J Nutr 99, S14–23 (2008). [DOI] [PubMed] [Google Scholar]
- Feustel A., Wennrich R. & Schmidt B. Serum-Zn-levels in prostatic cancer. Urol Res 17, 41–2 (1989). [DOI] [PubMed] [Google Scholar]
- Park S. Y. et al. Serum zinc and prostate cancer risk in a nested case-control study: The multiethnic cohort. Prostate 73, 261–6 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin A. et al. Oxidative stress and antioxidant status in non-metastatic prostate cancer and benign prostatichyperplasia. Clin Biochem 39, 176–9 (2006). [DOI] [PubMed] [Google Scholar]
- Ji K. et al. Significance of trace elements copper and zinc change in the serum of patients with prostate cancer. Chinese Journal of Andrology 21, 9–11 (2007). [Google Scholar]
- Christudoss P., Selvakumar R., Fleming J. J. & Gopalakrishnan G. Zinc status of patients with benign prostatic hyperplasia and prostate carcinoma. Indian J Urol 27, 14–8 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. M. et al. Serumzinc improves the performance of prostate cancer. Chin J Lab Diagn 10, 132–135 (2006). [Google Scholar]
- Daragó A. et al. The correlation between zinc and insulin-like growth factor 1 (IGF-1), its binding protein (IGFBP-3) and prostate-specific antigen (PSA) in prostate cancer. Clin Chem Lab Med 49, 1699–705 (2011). [DOI] [PubMed] [Google Scholar]
- Yao D. H. et al. The content of serum zinc concentration in prostate disease. International Journal of Surgery 4, 225–227 (1977). [Google Scholar]
- Liu Y. Y. et al. The change of zinc, androgen metabolism in patients with benign prostatic hyperplasia. Chin J Endocrinol Metab 9, 30–311 (1993). [Google Scholar]
- Rahman M. T., Mumu M. A., Kabir Y., Choudhury M. M. & Saiedullah M. Serum zinc level and prostatic lesion. Mymensingh Med J 21, 679–83 (2012). [PubMed] [Google Scholar]
- Xu B. Z. et al. Value of Zinc Concentration in Differentiating Benign or Malignant Prostatic Diseases. Fudan Univ J Med Sci 29, 406–408 (2002). [Google Scholar]
- Zhou Z. H. et al. The changes in the whole Blood zinc concentration in prostate carcinoma and the corresponding diagnostic value. Chinese. Journal. of. Clinical. Oncology 36, 1391–1393 (2009). [Google Scholar]
- Li X. M. et al. Measurement of serum zinc improves prostate cancer detection efficiency in patients with PSA levels between 4 ng/mL and 10 ng/mL. Asian J Androl 7, 323–8 (2015). [DOI] [PubMed] [Google Scholar]
- Chen J. Q. et al. content changes of zinc and cadmius in serum of patients with prostate cancer and its clinical significance. J Clin Urology (China) 5, 439–431 (2015). [Google Scholar]
- Ogunlewe J. O. & Osegbe D. N. Zinc and cadmium concentrations in indigenous blacks with normal, hypertrophic, and malignant prostate. Cancer 63, 1388–92 (1989). [DOI] [PubMed] [Google Scholar]
- Ong C. L., Walker M. J. & McEwan A. G. Zinc disrupts central carbon metabolism and capsule biosynthesis in Streptococcus pyogenes. Sci Rep 5, 10799 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie A. R., Hall T. & Whitmore W. F. Jr. Zinc content of expressed human prostatic fluid. Nature 193, 72–3 (1962). [DOI] [PubMed] [Google Scholar]
- Gmeiner W. H. et al. The cytotoxic and pro-apoptotic activities of the novel fluoropyrimidine F10 towards prostate cancer cells are enhanced by Zn(2+) -chelation and inhibiting the serine protease Omi/HtrA2. Prostate 75, 360–9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellberg S., Björndahl L. & Kvist U. Sperm chromatin stability and zinc binding properties in semen from men in barren unions. Int J. Androl 15, 103–13 (1992). [DOI] [PubMed] [Google Scholar]
- Untergasser G. et al. High levels of zinc ions induce loss of mitochondrial potential and degradation of antiapoptotic Bcl-2 protein in in vitro cultivated human prostate epithelial cells. Biochem. Biophys. Res Commun 279, 607–14 (2000). [DOI] [PubMed] [Google Scholar]
- Ku J. H., Seo S. Y., Kwak C. & Kim H. H. The role of survivin and Bcl-2 in zinc-induced apoptosis in prostate cancer cells. Urol Oncol 30, 562–8 (2012). [DOI] [PubMed] [Google Scholar]
- Costello L. C., Franklin R. B., Feng P., Tan M. & Bagasra O. Zinc and prostate cancer: a critical scientific, medical, and public interest issue (United States). Cancer Causes Control 16, 901–15 (2005). [DOI] [PubMed] [Google Scholar]
- Singh C. K., Pitschmann A. & Ahmad N. Resveratrol-zinc combination for prostate cancer management. Cell Cycle 13, 1867–74 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P., Pang Y., Dong J. & Berg A. H. Identification and characterization of membrane androgen receptors in the ZIP9 zinc transporter subfamily: II. Role of human ZIP9 in testosterone-induced prostate and breast cancer cell apoptosis. Endocrinology 155, 4250–65 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodarzi D., Cyrus A., Baghinia M. R., Kazemifar A. M. & Shirincar M. The efficacy of zinc for treatment of chronic prostatitis. Acta Med Indones 45, 259–64 (2013). [PubMed] [Google Scholar]
- Gómez Y. et al. Zinc levels in prostatic fluid of patients with prostate pathologies. Invest Clin 48, 287–94 (2007). [PubMed] [Google Scholar]
- Zaichick V. Y., Sviridova T. V. & Zaichick S. V. Zinc concentration in human prostatic fluid: normal, chronic prostatitis, adenoma and cancer. Int Urol Nephrol 28, 687–94 (1996). [DOI] [PubMed] [Google Scholar]
- Colleen S., Mårdh P. A. & Schytz A. Magnesium and zinc in seminal fluid of healthy males and patients with non-acute prostatitis with and without gonorrhoea. Scand J Urol Nephrol 9, 192–7 (1975). [DOI] [PubMed] [Google Scholar]
- Deng C. H., Zheng B. & She S. F. A clinical study of biological zinc for the treatment of male infertility with chronic prostatitis. Zhonghua Nan Ke Xue 11, 127–9 (2005). [PubMed] [Google Scholar]
- Boström K. & Andersson L. Creatine phosphokinase relative to acid phosphatase, lactate dehydrogenase, zinc and fructose in human semen with special reference to chronic prostatitis. Scand J Urol Nephrol 5, 123–32 (1971). [DOI] [PubMed] [Google Scholar]
- Zhao H. et al. Changes of seminal parameters, zinc concentration and antibacterial activity in patients with non-inflammatory chronic prostatitis/chronic pelvic pain syndrome. Zhonghua Nan Ke Xue 14, 530–2 (2008). [PubMed] [Google Scholar]
- Makhov P. et al. Docetaxel-mediated apoptosis in myeloid progenitor TF-1 cells is mitigated by zinc: potential implication for prostate cancer therapy. Prostate 71, 1413–9 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P. et al. Zinc induces mitochondria apoptogenesis in prostate cells. Mol Urol 4, 31–6 (2000). [PubMed] [Google Scholar]
- Gonzalez A., Peters U., Lampe J. W. & White E. Zinc intake from supplements and diet and prostate cancer. Nutr Cancer 61, 206–15 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristal A. R. et al. Dietary patterns, supplement use, and the risk of symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. Am J Epidemiol 167, 925–34 (2008). [DOI] [PubMed] [Google Scholar]
- Kolenko V., Teper E., Kutikov A. & Uzzo R. Zinc and zinc transporters in prostate carcinogenesis. Nat Rev Urol 10, 219–26 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyad M. A. Zinc for prostate disease and other conditions: a little evidence, a lot of hype, and a significant potential problem. Urol. Nurs 24, 49–52 (2004). [PubMed] [Google Scholar]
- Espinosa G. Nutrition and benign prostatic hyperplasia. Curr Opin Urol 23, 38–41 (2013). [DOI] [PubMed] [Google Scholar]
- Tavani A. et al. Intake of selected micronutrients and the risk of surgically treated benign prostatic hyperplasia: a case-control study from Italy. Eur Urol 50, 549–54 (2006). [DOI] [PubMed] [Google Scholar]
- Adolfsson P. I., Bloth B., Hägg S. & Svensson S. P. Zinc induces a bell-shaped proliferative dose-response effect in cultured smooth muscle cells from benign prostatic hyperplasia. Urology 85, 704.e15-9 (2015). [DOI] [PubMed] [Google Scholar]
- Gumulec J. et al. Serum and tissue zinc in epithelial malignancies: a meta-analysis. Plos One 9, e99790 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad A. S. Discovery of human zinc deficiency and studies in an experimental human model. Am J Clin Nutr 53, 403–12 (1991). [DOI] [PubMed] [Google Scholar]
- Paz-Tal O. et al. Effect of changes in food groups intake on magnesium, z-inc, copper, and selenium serum levels during 2 years of dietary intervention. J Am Coll Nutr 34, 1–14 (2015). [DOI] [PubMed] [Google Scholar]
- Vallee B. L. The biochemistry, physiology and pharmacology of zinc. Physiol Rev 39, 443–458 (1959). [DOI] [PubMed] [Google Scholar]
- Couturier E., Onderbergen A., Bosson D. & Neve J. Circadian variations in plasma zinc and cortisol in man. J Trace Elem Electrolytes Health 2, 245–249 (1988). [PubMed] [Google Scholar]