Abstract
Behavioral therapies aimed at reducing excess body fat result in limited fat loss after dieting. To understand the causes for maintenance of adiposity, high-fat (HF) diet-induced obese (DIO) mice were switched to a low-fat chow diet, and the effects of chow on histological and molecular alterations of adipose tissue and metabolic parameters were examined. DIO mice reduced and stabilized their body weights after being switched to chow (HF-chow), but retained a greater amount of adiposity than chow-fed mice. Reduction in adipocyte volume, not number, caused the decrease in fat mass. HF-chow mice showed normalized circulating insulin and leptin levels, improved glucose tolerance, and reduced inflammatory status in white adipose tissue (WAT). Circulating leptin levels corrected for fat mass were lower in HF-chow mice. Leptin administration was used to test whether reduced leptin level of HF-chow mice inhibited further fat loss. Leptin treatment led to an additional reduction in adiposity. Finally, HF-HF mice had lower mRNA levels of β3- adrenergic receptor (β3-AR) in epididymal WAT compared to chow-fed mice, and diet change led to an increase in the WAT β3-AR mRNA levels that were similar to the levels of chow-fed mice, suggesting an elevation in sympathetic activation of WAT during diet-switch relative to HF-HF mice leading to the reduced leptin level and pro-inflammatory cytokine content. In summary, HF-chow mice were resistant to further fat loss due to leptin insufficiency. Diet alteration from HF to low-fat improved metabolic state of DIO mice, although their adiposity was defended at a higher level.
Keywords: high-fat diet, adipocyte cellularity, glucose tolerance, adipose tissue inflammation
Introduction
Despite attempts to increase awareness of the deleterious health consequences associated with obesity, there continues to be a significant increase in the obese population worldwide (1). High-fat (HF) content in many western diets is major contributor to human obesity, leading to multiple problems such as hyperglycemia, hyperinsulinemia, and hyperleptinemia, as well as glucose intolerance and insulin resistance (2). The majority of people who lose weight rapidly rebound to their pre-diet body weight and adiposity if proper diet and exercise are not maintained (3). The physiological responses to weight loss include increased food consumption (4–6), enhancement in metabolic efficiency (4), and reduction in resting and non-resting energy expenditure (3; 7; 8). However, the cellular mechanisms underlying limitation in weight loss are not fully understood.
Rodents fed a HF diet, analogous to western diets in terms of fat content and caloric density, develop an obesity syndrome similar to the metabolic syndrome in humans. Like humans, rodents on a HF diet have a generalized expansion in the size of white adipose tissue (WAT) due to both hypertrophy of pre-existing adipocytes and hyperplasia by adipogenesis from the pre-adipocyte population in the stromal tissue (9). In response to weight loss induced by food restriction, the lipid content of adipocytes is readily reduced, yet the increased number of adipocytes is difficult to reverse (9). HF diet-induced obese (DIO) mice lose weight after being switched to a low-fat diet but rarely reach the same lean state as age-matched mice that are never exposed to a HF diet. We hypothesized that the retained excess adiposity was due to an increase in adipocytes induced by the HF diet (10) and would not be easily reversed by diet intervention. Although adiposity is defended at a high level, reduced adiposity suggested that certain metabolic changes caused by a HF diet could be reversed. To investigate this hypothesis, we examined the metabolic effects of a low-fat diet on DIO mice, and the histological and molecular alterations in adipose tissue.
Methods and procedures
Animals
Two cohorts of adult male C57Bl/6J mice obtained from the Jackson Laboratory (Bar Harbor, Maine) were individually housed in micro-isolator cages in pathogen-free, temperature- and humidity-controlled rooms with a 12/12 light/dark cycle. Mice were provided with ad libitum access to pelleted rodent chow (Harlan Teklad, Madison, WI, rodent diet 8604; 12% fat; 3.10 kcal/g) or a HF diet (Research Diets, Inc., New Brunswick, NJ, D12451; 45% fat ; 4.73 kcal/g) and water unless otherwise noted. HF diet-fed mice that gained over 15 grams of body weight after ten weeks of HF diet feeding were divided into two body weight- and body fat-matched groups, and were then either maintained on HF diet (HF-HF) or switched to chow (HF-chow) for another two weeks. The two-week time point was chosen based on our previous unpublished studies showing stabilized body weights and improvement in metabolic profile after two-week diet switch; thus it was important to know what metabolic and molecular events have occurred at this time point. Age-matched chow-fed mice were kept for controls. Quantitative nuclear magnetic resonance (magnetic resonance. nuclear Quantitative nuclear magnetic resonance ((NMR) from EchoMedical Systems (Houston, TX) was used to determine body composition of the mice as previously described (11). All animal experiments were approved by the University of Cincinnati and the Procter & Gamble Co. Institutional Animal Care and Use Committees.
First cohort
Three groups of mice from the first cohort (HF-HF, HF-chow, Chow; n = 10 per group) were used to monitor body weight and body fat, and to evaluate metabolic profile, histology, and gene expression of adipose tissue.
Intraperitoneal glucose tolerance test (ipGTT)
Mice were fasted for four hours and ipGTT was performed during the light phase. All blood samples were obtained from the tip of the tail vein. Following a baseline (0 minute) blood sample withdraw, 0.75 g/kg body weight of 20% D-glucose (Sigma-Aldrich, St. Louis, MO) was injected and the concentration of glucose was measured on duplicate samples at 15, 30, 45, 60, 120, and 180 minutes using One Touch Basic glucometers and One Touch glucose test strips (LifeScan, Milpitas, CA).
Assays for leptin and insulin
All mice were killed by ip administration of FetalPlus (Vortech Pharmaceuticals, Dearborn, MI; 100 mg/kg) at the end of the light phase and cardiac blood samples were collected with cardiac puncture. Blood samples were centrifuged and plasma was stored at −20°C until assay. The concentrations of leptin and insulin were measured using 125I RIA kits (Linco Research, St. Charles, MO). The coefficients of variation within and between leptin assays are 3% and 4%, and the coefficients of variation within and between insulin assays are 5% and 7%. The sensitivity for leptin assay is 0.5 ng/mL and for insulin assay is 3 pmol/L.
Adipose tissue and liver histology
The epididymal WAT (EWAT) pads were dissected and fixed in neutral buffered 10% formalin, embedded in paraffin, sectioned, and stained with hematoxilin and eosin. Microscopic images were digitized from twenty sections across each EWAT depot for assessment of adipocyte cellularity. The size of adipocytes was quantified from the images using a program developed to digitize the cross-sectional area of the cells in a given field. Adipocyte number was derived by dividing the EWAT volume (calculated by multiplying the EWAT mass by 0.92 cm3/g, the approximate density of adipose tissue) by the adipocyte volume. The cell number analysis using this histological method was validated by comparing it to the osmium tetroxide fixation method of Hirsch and Gallian (12). Livers were dissected and fixed in 10% neutral buffered formalin and then 30% sucrose solution before being embedded. Liver sections were stained for lipid with Oil red O to estimate the extent of lipid accumulation.
Quantitative PCR (Q-PCR) analyses of gene expression of adipose tissues
Monocyte chemoattractant protein (MCP-1), a macrophage-specific marker CD68, and β3-adrenergic receptor (β3-AR) gene expressions were measured in EWAT. β3-AR and uncoupling protine-1 (UCP-1) gene expressions were measured in the interscapular brown adipose tissues (IBAT). Briefly, RNA was isolated from EWAT or IBAT using TRI-Reagent (MRC Inc., Cincinnati, OH). Following DNAase treatment (Ambion, Austin, TX), cDNA was synthesized using an iScript kit (BioRad, Hercules, CA). L-32 was used as an endogenous control to indicate relative quantification of gene expression from every sample. The sequences selected primers were from GenBank, and the primers were selected using Primer3 web-based primer design (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi; Table 1). Q-PCR was performed using a BioRad iCycler and the iQ SYBRGreen Supermix (BioRad, Hercules, CA) with 2-step amplification at 95° C for 10 seconds and annealing temperature of 61.2°C (L32, MCP-1, β3-AR and UCP-1) or 64.3°C (CD68) for 30 seconds for 40 cycles. The difference of threshold cycle (ΔCT) between control gene L32 and interested gene was calculated. For relative quantification, the ΔCT was averaged for the HF-HF group and was then subtracted from the ΔCT of each HF-chow sample to calculate the approximate fold difference (Applied Biosystems manufacturer’s instructions).
Table 1.
Oligonucleotides used for quantitative RT-PCR amplifications
| Target gene | Accession No. | Sequence |
|---|---|---|
| MCP-1 | J04467 | forward primer: 5′-CCCAATGAGTAGGCTGGAGA reverse primer: 5′-TCTGGACCCATTCCTTCTTG |
| CD68 | P31996 | forward primer: 5′-TTCTGCTGTGGAAATGCAAG reverse primer: 5′-AGAGGGGCTGGTAGGTTGAT |
| β3 -AR | X72862 | forward primer: 5′-TCGACATGTTCCTCCACAAA reverse primer: 5′-GATGGTCCAAGATGGTGCTT |
| UCP-1 | U63418 | forward primer: 5′-GGGCCCTTGTAAACAACAAA reverse primer: 5′-GTCGGTCCTTCCTTGGTGTA |
β3-AR, β3-adrenergic receptor; CD68, a macrophage-specific marker; MCP-1, monocyte chemoattractant protein-1; RT, reverse transcriptase; UCP-1, uncoupling protein-1.
Second cohort
Data from the first cohort demonstrated that leptin levels of HF-chow and chow mice were identical although HF-chow mice contained significantly greater adiposity; the leptin level was lower in the HF-chow mice when corrected for the total adiposity (Table 2). It is possible that the suppressed leptin level of HF-chow mice leads to resistance in further body fat loss. In this cohort, we tested whether HF-chow mice were leptin sensitive and whether administration of exogenous leptin would lead to further reduction in body weight and fat.
Table 2.
Body weight, body fat, and concentrations of plasma leptin and insulin of chow-fed, HF-chow, and HF-HF mice
| Diet | BW (g) |
Total fat (g) |
Leptin (ng/ml) |
Leptin/body fat (ng/ml/g) |
Insulin (ng/ml) |
Insulin/body fat (ng/ml/g) |
|---|---|---|---|---|---|---|
| Chow | 28.50 ± 0.24 | 2.68 ± 0.15 | 8.02 ± 1.68 | 2.77 ± 0.52 | 2.66 ± 1.16 | 0.91 ± 0.37 |
| HF-chow | 33.30 ± 0.90* | 5.28 ± 0.59* | 7.14 ± 1.39 | 1.55 ± 0.22* | 2.64 ± 0.94 | 0.43 ± 0.11 |
| HF-HF | 40.88 ± 0.94*, † | 13.46 ± 0.51*, † | 25.57 ± 0.40* | 1.92 ± 0.06 | 13.29 ± 0.48* | 1.00 ± 0.05 |
BW, body weight.
p < 0.05 comparing to chow mice.
p < 0.05 comparing to HF-chow mice. n = 10.
Leptin sensitivity test
HF-HF and HF-chow mice were divided into three groups that were matched with body weight and body fat (n = 10 per group). All mice were injected intraperitoneally twice daily with either phosphate buffered saline (PBS) or leptin (10 or 20 mg/kg; R&D systems, Milwaukee, WI) for seven days based on a published paradigm (13). Leptin injection of 10 mg/kg is effective in decreasing food intake in chow-fed and four day HF diet-fed mice, but not in sixteen-day HF diet-fed mice (13). Dose-dependent responses in the current ten-week HF DIO mice may exist; thus a higher dose of leptin (20 mg/kg) was also used. Injections were performed immediately prior to the onset of dark period and six hours afterwards. Body weight and food intake of the mice were monitored daily. On the eighth day, mice were injected with PBS or leptin before the onset of darkness; then all mice were killed and concentrations of circulating leptin levels were measured using same method as mentioned above.
Statistical analysis
Data were expressed as mean ± standard error mean (SEM). Comparisons between multiple groups were made using one-way or two-way analysis of variance (ANOVA) when appropriated. Post-hoc tests of individual groups were made using Tukey’s tests (SigmaStat 3.1, San Rafael, CA). Significance was set at P < 0.05.
Results
First cohort
HF-chow mice maintained excess adiposity
Two weeks after their diet switch, HF-chow mice showed a significant reduction in body weight and body fat. HF-HF mice weighed an average of over 40 grams and had over 30% body fat, whereas HF-chow mice weighed an average of 33 grams and had approxiamtely15% body fat. Chow-fed mice weighed less than 30 grams and had less than 10 % body fat on average. Total adiposity of HF-chow mice was 50% less than that of HF-HF mice; however adiposity of HF-chow mice was twice that of chow-fed mice (Fig. 1A and Table 2). Total body fat, and body fat % were all significantly different among three groups (Fig. 1A and Table 2).
Figure 1. HF-chow mice lost weight but retained excess body adiposity compared to chow-fed mice. HF-chow mice had reduced fat cell size but not fat cell number.
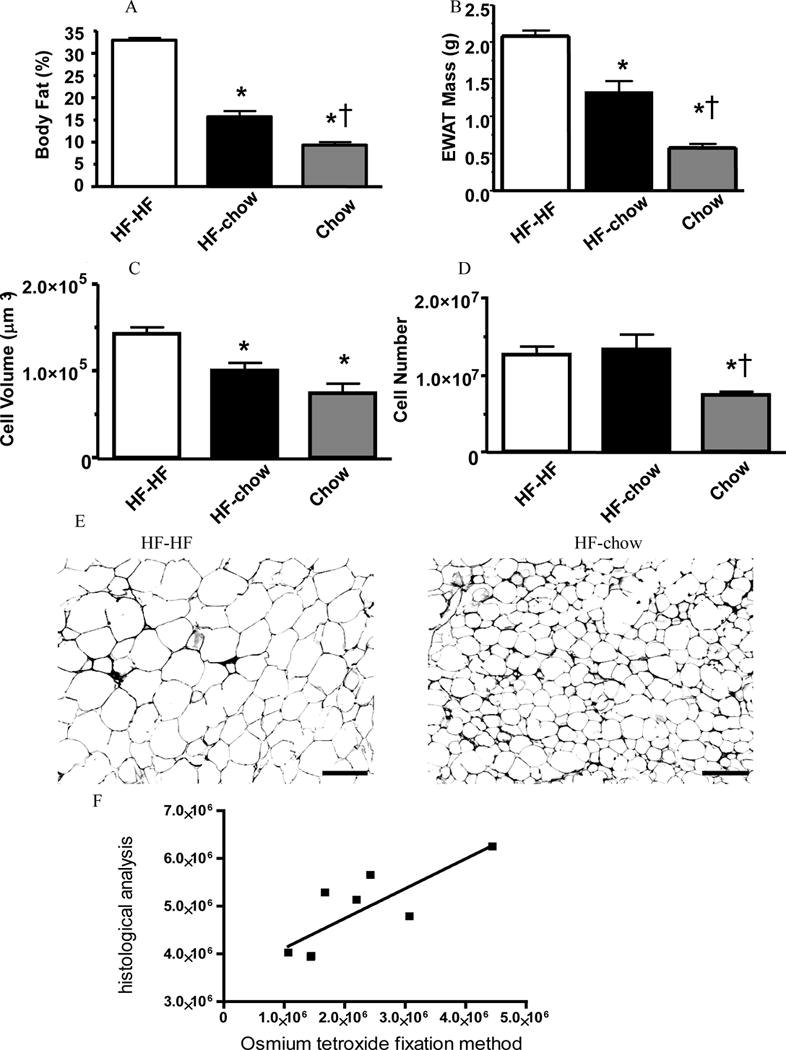
Body fat percentage (A), epididymal white adipose tissue (EWAT) mass (B), fat cell volume (C), and fat cell number (D) of HF-HF, HF-chow, and chow-fed mice. Representative images of EWAT of HF-HF and HF-chow mice (E). Scale bar = 100 μm. Linear regression of cellularity between histological prepared and osmium tetroxide fixed samples (F).
* p < 0.05 comparing to HF-HF mice. †p < 0.05 comparing to HF-chow mice.
EWAT mass of HF-chow mice was analogously reduced, being significantly less than that of HF-HF mice but greater than that of chow-fed mice (Fig. 1B). The cell number analysis using the histological method was validated by comparing it to the osmium tetroxide fixation method of Hirsch and Gallian (12). There was a significant correlation between two methods (R2 = 0.620, P = 0.036; Fig. 1F). HF-HF mice had enlarged and increased number of adipocytes comparing to chow-fed mice. Diet switch in HF-chow mice did not change the number of fat cells but decreased fat cell size by 1/3, proportionally to the decrease in EWAT mass (Fig. 1C–E). The sizes of adipocytes of HF-chow mice and chow-fed mice were not significantly different, yet the number of adipocytes of HF-chow mice was approximately twice that of the chow-fed group (Fig. 1C and 1D). Therefore diet switch from HF to chow reduced adipocyte volume with no apparent changes in adipocyte number of EWAT.
HF-chow mice improved steototic livers and metabolic profile
The livers of HF-HF mice were steototic as assessed by oil red O staining in liver sections. In contrast, after the DIO mice had been switched to chow for as little as two weeks, the steotosis decreased and histology of livers was indistinguishable between HF-chow and chow-fed mice (Fig. 2A–C). The rapid reversal in steotosis may be indicative of changes in other metabolic endpoints therefore we analyzed the levels of leptin and insulin in the serum. Circulating leptin and insulin levels were significantly reduced in HF-chow mice compared to HF-HF mice; and these levels were equivalent in HF-chow and chow-fed mice, despite the greater adiposity of HF-chow mice (Table 2). In addition, when the concentrations of circulating leptin and insulin were corrected for the total amount of body fat, leptin level, but not insulin level, of HF-chow mice was significantly lower than that of chow-fed mice (Table 2).
Figure 2. HF-chow mice improved steototic livers and glucose tolerance.
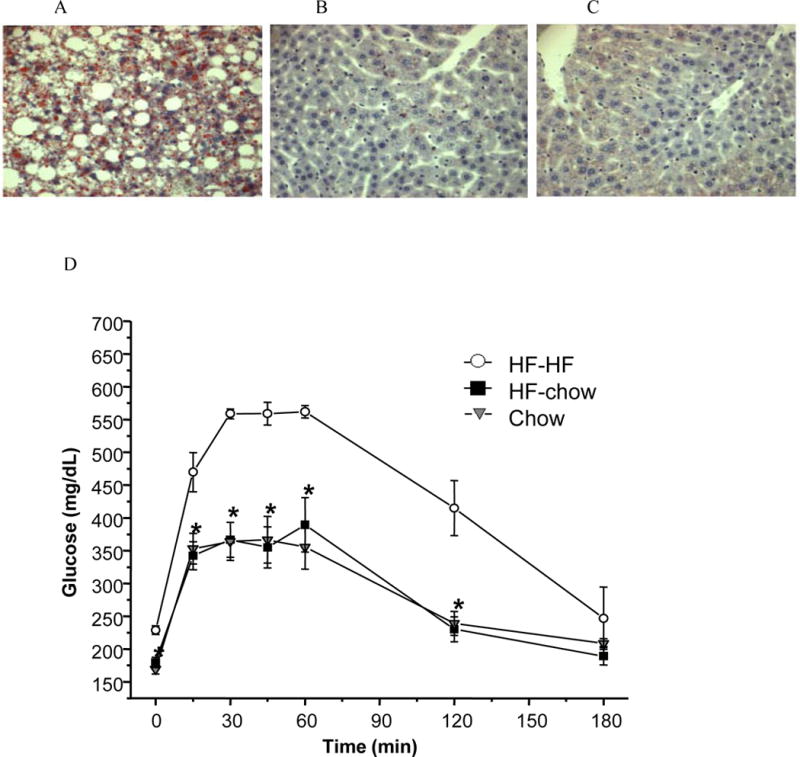
HF-HF mice had fatty liver that was recovered in HF-chow DIO mice. Liver histological analysis of HF-HF (A), HF-chow (B), and chow-fed (C) mice using oil red O staining. HF-HF mice had impaired whereas HF-chow mice had normal glucose tolerance similar as chow-fed mice (D). *p < 0.05 comparing to HF-HF mice.
IpGTT was performed in HF-HF, HF-chow, and chow-fed mice to test whether the normalized circulating insulin level and improved hepatic steatosis led to improved glucose tolerance in the HF-chow mice. While HF-HF mice showed high baseline glucose level (229 ± 7 mg/dl) and poor glucose tolerance, HF-chow mice showed normal baseline glucose level (HF-chow: 180 ± 8 mg/dl; chow: 170 ± 7 mg/dl) and normal glucose tolerance indistinguishable from chow-fed mice, with lowered glucose levels comparing to HF-HF mice at all time points during ipGTT (Fig. 2D). Therefore, despite maintaining a higher level of adiposity, HF-chow mice had similar metabolic profiles and glucose tolerance compared to chow-fed mice.
HF-chow mice have reduced macrophage markers in EWAT
The improved glucose tolerance in HF-chow mice could be due to improved inflammatory status and reduced macrophage content in adipose tissue. Using quantitative PCR analysis, there was a 70% decrease in transcript level of the chemoattractant MCP-1 and the macrophage-specific marker CD68 in EWAT of HF-chow mice compared to that of HF-HF mice (Table 3). In addition, the EWAT mRNA levels of MCP-1 and CD68 were not different between HF-chow and chow-fed mice. This likely indicates that HF-chow mice had improved inflammatory status and reduced macrophage infiltration in adipose tissue, which was normalized to the same levels as chow-fed lean mice.
Table 3.
Percentages of EWAT and IBAT gene expression of HF-HF, HF-chow, and chow-fed mice
| % change | HF-HF | HF-chow | Chow |
|---|---|---|---|
| EWAT MCP-1 | 100.00 ± 15.27 | 32.92 ± 7.16* | 26.28 ± 4.56* |
| EWAT CD68 | 100.00 ± 27.12 | 34.64 ± 12.83* | 45.32 ± 8.29* |
| EWAT β-3 AR | 100.00 ± 18.76 | 232.09 ± 50.23* | 338.19 ± 79.70* |
| IBAT β-3 AR | 100.00 ± 8.94 | 50.76 ± 8.69* | 66.96 ± 12.83 |
| IBAT UCP-1 | 100.00 ± 8.36 | 50.25 ± 10.13* | 30.09 ± 2.52* |
β3-AR, β3-adrenergic receptor; CD68, a macrophage-specific marker; EWAT, epididymal white adipose tissue; IBAT, interscapular brown adipose tissue; MCP-1, monocyte chemoattractant protein-1; UCP-1, uncoupling protein-1.
p < 0.05 comparing to HF-HF mice.
There is no difference between HF-chow and Chow-fed mice.
β3-AR expression is increased in EWAT but decreased in IBAT in HF-chow mice
The finding that circulating leptin is reduced when corrected for total body fat content suggests that leptin secretion is inhibited. Inhibition of leptin secretion (14–16) and pro-inflammatory cytokine production suggests possible sympathetic activation in the WAT (17). Diet switch from HF to chow increased β3-AR expression in EWAT but decreased in IBAT relative to the HF-HF mice (Table 3). In addition, the mRNA levels of β3-AR and UCP-1 of IBAT were equivalently reduced in HF-chow mice and were comparable to the mRNA levels of chow-fed mice (Table 3). There was no difference in β3-AR expression between HF-chow and chow-fed mice. The lowered levels of both β3-AR and UCP-1 mRNA in IBAT and the increased β3-AR expression in EWAT relative to HF-HF mice but similar to chow-fed mice suggest that HF-chow mice might maintain a lower sympathetic activation in IBAT but an elevated sympathetic drive to EWAT than HF-HF mice did, and HF-chow mice had similar sympathetic drive in IBAT and EWAT as chow-fed lean mice.
Second cohort
Leptin is secreted in direct proportion to body adiposity – as adipose tissue increases so does the circulating level of leptin (18). The data from the first cohort data suggests this may not be the case. HF-chow mice have greater amount of adipose tissue than chow-fed mice, yet they have similar circulating leptin levels (Table 2). When the concentration of circulating leptin is corrected for adiposity, the HF-chow mice have a lower leptin level than that of chow-fed mice (Table 2), suggesting that a leptin insufficient state exists in HF-chow mice. The suppressed leptin level may resist further body fat loss in HF-chow mice. Therefore, we tested the effects of exogenous leptin administration in HF-chow mice using a second cohort.
Body weights of HF-HF and HF-chow mice before leptin treatment
HF diet induced obese mice were switched to the chow diet and body weight monitored for seven weeks prior to leptin injection. During the initial week, the body weights of the HF-HF and HF-chow mice were significantly more than chow-fed control mice. By the end of the second week, the body weights of HF-chow and chow-fed mice were no longer significantly different (Fig. 3A). Importantly, total fat tissue mass of the HF-chow mice was significantly different from the HF-HF and chow-fed mice (HF-HF mice: 15.81 ± 0.48 g; HF-chow mice: 7.19 ± 0.38 g; Chow mice: 2.41 ± 0.21 g). HF-HF mice but not HF-chow mice weighed significantly greater than chow-fed mice between week 2 and week 7. There was no further weight decrease in HF-chow mice from 2 – 7 weeks suggesting that the body weight of HF-chow mice stabilized 2 weeks after the diet switch (Fig. 3A).
Figure 3. HF-chow but not HF-HF mice were leptin sensitive.
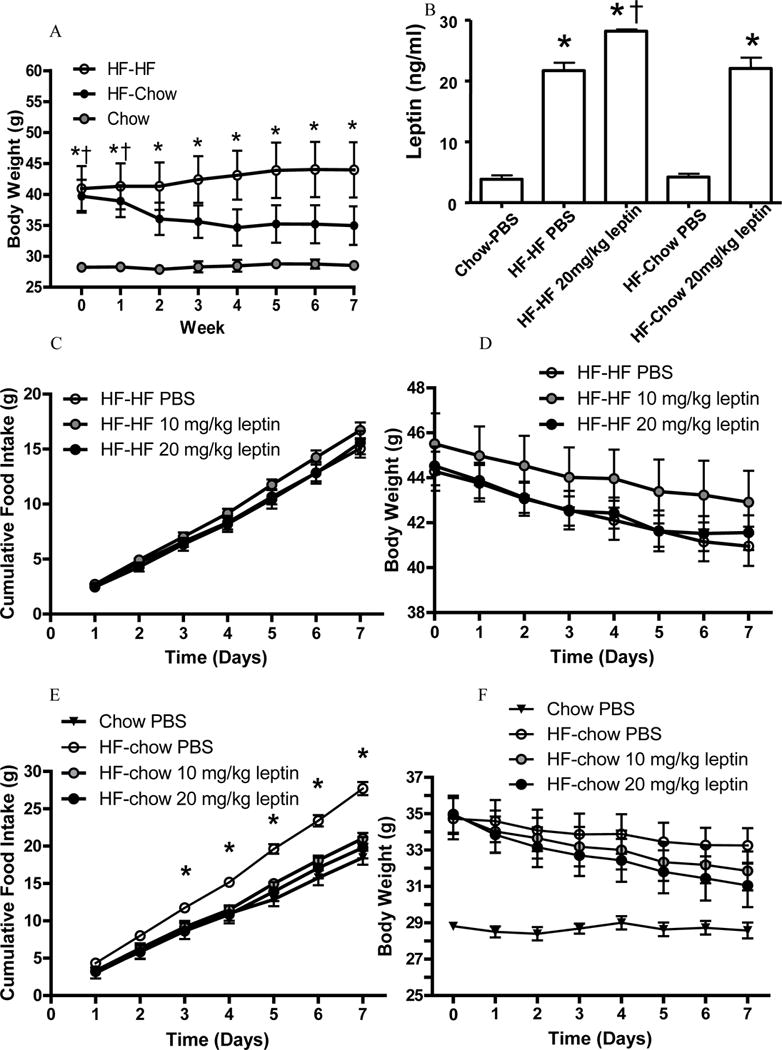
Body weight curves after diet switch and before leptin administration (A).
* p < 0.05 HF-HF mice comparing to chow-fed mice. †p < 0.05 HF-chow mice comparing to chow-fed mice.
Plasma leptin levels following leptin treatment (B).
* p < 0.05 comparing to chow-fed PBS mice. †p < 0.05 comparing to HF-HF PBS mice.
Food intake (C) and body weight (D) of vehicle (PBS)- or leptin-treated HF-HF mice.
Food intake (E) and body weight (F) of vehicle (PBS)- or leptin-treated HF-chow mice and PBS-treated chow-fed mice. *p < 0.05 comparing to chow-fed mice.
Circulating leptin levels of treated mice
Consistent with data from the first cohort, HF-chow mice had similar circulating leptin level as chow-fed mice, and significantly lower leptin level than HF-HF mice (Fig. 3B). HF-chow mice treated with the high-dose of leptin (20 mg/kg) had increased circulating leptin levels that are comparable to the PBS-treated HF-HF mice (Fig. 3B).
HF-chow mice were leptin sensitive
Consistent with previous reports we confirmed that DIO mice are leptin resistant (13). HF-HF mice treated with 10 or 20 mg/kg leptin showed no reduction in food intake or body weight compared to PBS treated HF-HF mice (Fig. 3C and 3D). Food intake of the PBS-treated HF-chow mice was greater than that of PBS-treated chow-fed mice (Fig. 3E). Treatment of HF-chow mice with 10 mg/kg or 20 mg/kg leptin led to a reduction in food intake, comparable to the food intake of chow-fed mice (Fig. 3E). Leptin-treated HF-chow mice also reduced their body weights though not to the level of chow fed mice (Fig. 3F). Thus, unlike HF-HF mice, HF-chow mice were sensitive to exogenous leptin administration.
Leptin-treated HF-chow mice further decreased body fat and adipocyte number
All HF-chow mice showed a reduction in adiposity during the treatment period (Fig. 4A). Importantly, the reduction in body weight was dose dependent, and with increasing concentration of leptin, there was a more pronounced decrease in body fat. Both the 10 and 20 mg/kg leptin-treated HF-chow mice showed a significantly greater reduction in body fat than PBS-treated HF-chow mice (Fig. 4A). Leptin treatment of 20 mg/kg also led to a significant decrease in EWAT mass, but it did not reach the level of chow-fed mice (Fig. 4B). EWAT adipocyte sizes of all groups were similar (Fig. 4C), however, adipocyte number was significantly reduced in the 20 mg/kg leptin-treated mice and was similar to that of the chow-fed mice (Fig. 4D). Therefore, leptin treatment of HF-chow mice leads to a further decrease in fat mass by reducing the number of adipocytes.
Figure 4. HF-chow mice decreased total body fat, epididymal white adipose tissue (EWAT) mass and fat cell number after leptin treatment.
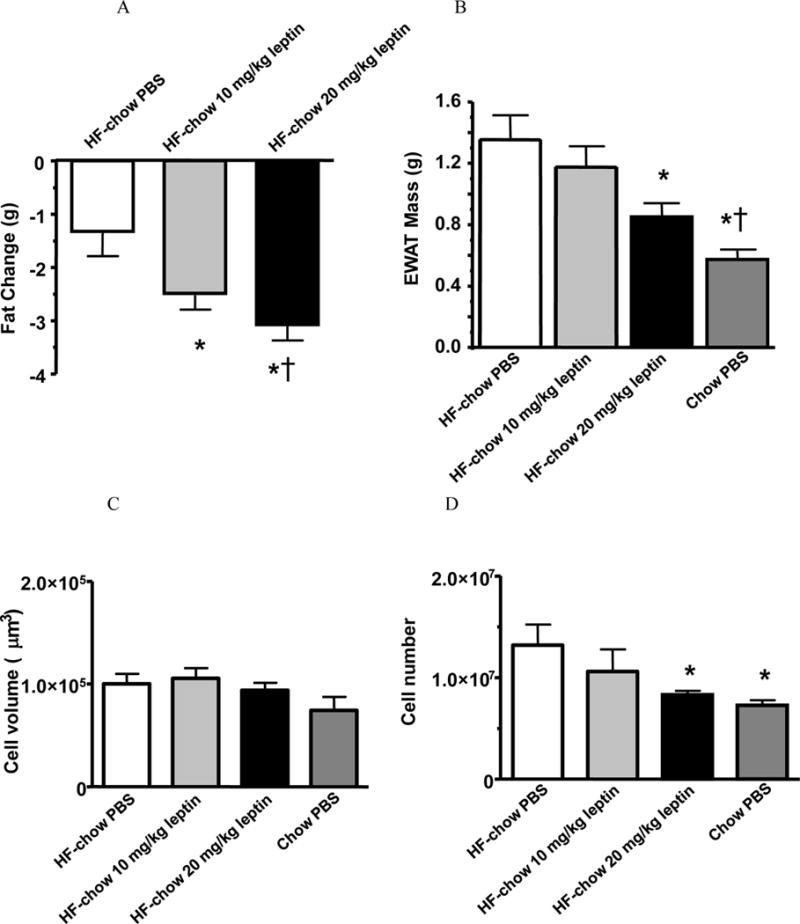
Body fat change (A), EWAT mass (B), and fat cell size (C) and fat cell number (D) of vehicle (PBS)- or leptin-treated HF-chow mice.
* p < 0.05 comparing to PBS-treated HF-chow mice. †p < 0.05 comparing to 10 mg/kg leptin-treated HF-chow mice.
Discussion
High-fat diet feeding in mice leads to an increase in adiposity due to hypertrophy and hyperplasia of adipocytes. The mice develop an obesity syndrome that includes hyperleptinemia, hyperinsulinemia, and impaired glucose tolerance. HF-DIO mice switched to a low-fat diet lose body weight and adiposity, and exhibited improved metabolic profile, normal glucose tolerance, and increased leptin sensitivity. Surprisingly, HF-chow mice retained the increased adipocytes accumulated during the high-fat feeding, which limited their overall weight loss. Concomitant with the weight reduction was decreases in the content of macrophage and pro-inflammatory factors in white adipose tissue. In addition, the level of the β3-AR mRNA was elevated in white adipose tissue relative to HF-HF mice suggesting an increase in sympathetic tone following the diet change, which was similar to the level of lean control mice.
Leptin is produced principally by white adipocytes and secreted in proportion to the amount of body fat (19). Leptin levels are also influenced by meal consumption and short-term swings in energy balance such as fasting or overfeeding; and we recently reported that circulating levels of male mice dynamically changed during caloric restriction and re-feeding (11). Interestingly, circulating leptin levels in chow-fed and HF-chow mice were nearly identical despite greater body adiposity in HF-chow mice. When corrected for body fat, the leptin/adiposity ratio of HF-chow mice was significantly lower than that of chow-fed mice. This indicates that HF-chow mice are in a state of leptin insufficiency, leading to greater food intake of PBS-treated HF-chow mice compared to PBS-treated chow-fed mice and maintenance of greater adiposity in HF-chow mice (Fig. 3E). The leptin insufficiency of HF-chow mice can be overcome by exogenous leptin. Treament of HF-chow mice led to a further reduction in fat by decreasing the number of adipocytes, in marked contrast to the reduction of adipocyte size by just the low-fat diet. Therefore, reduced leptin level underlies the resistance of further body fat reduction in HF-chow mice. Injections of leptin corrected the leptin insufficient state in HF-chow mice and led to further body fat reduction. Leptin plays important roles in the regulation of energy balance and inflammation (20). In the current study, adipose gene expression was not measured in leptin-treated animals because changes of gene expression in adipose tissue are secondary to the loss of body fat and change of adipocyte cellularity after seven-day leptin treatment.
DIO mice have impaired glucose tolerance and are leptin resistant. Both conditions are resolved after reducing weight in DIO mice using a low-fat diet. Reduction in fat cell content is associated with improvements in metabolic parameters including glucose tolerance. Indeed, one of the consequences of treatment with the antidiabetic thiazolidinediones is an increase in number of small adipocytes, suggesting reduced adipocyte size contributes to the improvement in insulin sensitivity (21). Therefore, weight loss that induces a reduction in adipocyte size would cause the most improvement in the inflammatory status and glucose handling in the obese mice. Our findings are consistent with other observations that enlarged adipocytes are strongly correlated with obesity related inflammation (22). Reduction in fat content leads to a regain in glucose tolerance and leptin sensitivity (23). In contrast, increased fat cell number following HF diet induced obesity is not easily reduced. In related studies, long-term diet restriction and exercise training decrease fat cell volume without a significant change in fat cell number in human (24) and rats (25). Leptin treatment however, decreases fat cell numbers in rats (26) and in mice from the current study. This is not surprising, as leptin has been shown to induce adipocyte apoptosis in vivo (27) and inhibit maturation of preadipocytes in vitro 3T3-L1 adipocytes (28).
We focused on EWAT because it is the adipose tissue with the most existing data in the literature and more importantly because it had the highest levels of expression of inflammation-related genes such as MCP-1 and CD68 in HF DIO mice (29). In addition, EWAT contains less connective tissue than subcutaneous inguinal WAT and fewer blood vessels than perirenal or mesenteric WAT, assuring accuracy in analysis of cellularity and gene expressions. EWAT also has the distinction of having a large influence on glucose tolerance. For example, surgical removal of epididymal white adipose tissue caused the largest improvements in glucose tolerance when compared with surgical removal of equal amounts from varying fat depots (30).
The current study suggests that sympathetic activity in EWAT of HF-chow mice is elevated two weeks after switch to chow relative to the HF-HF mice and was normalized to the lean controls, as evidenced by an increased of β3-AR gene expression compared to HF-HF mice that was returned to the level of chow-fed lean mice. β3-AR, the primary postsynaptic sympathetic adrenoceptor subtype in rodent WAT, is an important sympathetically related receptor in the control of adiposity (31). β3-AR gene expression in WAT is significantly greater in HF-chow mice relative to HF-HF mice and is similar to lean mice. Although it might be expected that the diet switch-induced increase in sympathetic drive on adipose tissues would down-regulate β3-ARs or promote post-receptor desensitization (32), the β3-AR has been reported to be regulated primarily at the transcriptional level and its expression associated with increased norepinephrine (NE) level (33). This contrasts with the β1- and β2-ARs that are desensitized after sustained stimulation (33). The greater β3-AR mRNA in WAT of HF-chow mice compared to HF-HF mice from the current study is consistent with the increase in sympathetic stimulation observed previously in DIO rats that were switched to chow diet assessed by NE level and NE turnover rate in WAT (34). In contrast to the putative sympathetic stimulation in WAT, sympathetic activity in the brown adipose tissue was lower in HF-chow and lean mice relative to HF-HF mice – in all likelihood – due to a decrease in diet-induced thermogenesis (35). Direct measurement requires an assay of norepinephrine turnover rates in individual tissues. While we did not directly measure sympathetic activation, the correlation between sympathetic activity and β3-AR gene expression is well established in many physiological models. For example, sympathetic drive to white adipose tissue is activated during fasting and suppressed during re-feeding, and β3-AR mRNA level in white adipose tissue correspondingly increases during fasting and decreases during re-feeding (36).
Levin and colleagues reported a general increase in sympathetic activity in DIO Sprague-Dawley rats after switching to regular chow from HF diet (34). They found a significant increase of sympathetic activity in brown fat. This was accompanied by a slight but non-significant increase of sympathetic activity in epididymal white fat three days after DIO rats were switched to chow (34). While those observations are not consistent with the current data, the variation may be due to the different time points when sympathetic activity was assessed. In Levin’s study, adipose tissues were harvested three days after the diet was switched from HF to chow. At this early time point, the animals are losing weight so it is not surprising that they have high sympathetic activity in the brown fat. In contrast, by two weeks, our findings indicate that the DIO mice reached a new stable body weight and a concomitant decrease in sympathetic innervation of brown fat.
Leptin secretion is inhibited by conditions which promote increased sympathetic drive on WAT (37) and these changes do not necessarily reflect adjustments in adiposity. Sympathetic activation with adrenergic agonists, in particular those specific for the β3-AR, inhibit ob gene expression and leptin secretion in vitro (38–40), and in vivo in mice (41; 42), rats (43) and humans (44). The suppressed leptin levels in the HF-chow mice may initiate hypothalamic changes that resist further weight loss by biasing the animal towards greater food intake and/or decreased energy expenditure. For example, Enriori et al. recently demonstrated altered leptin sensitivity of pro-opiomelanocortin and neuropeptide Y/agouti-related peptide neurons in the arcuate nucleus of the hypothalamus in HF DIO mice (23).
Our data supports the concept that DIO mice resist weight loss due to defense of adipose tissue and more notably the conservation of fat cell number in adipose tissue. We suggest that it is the leptin insufficient state, which leads to a drive to defend the excess fat cells accumulated during the weight gain phase on a HF diet. Although adiposity is defended at a high level, reduced adiposity caused by diet switch from high-fat to low-fat improved metabolic profiles.
Acknowledgments
We thank Ms. Jennifer Waldrop for her comments on this manuscript and Proctor & Gamble for their generous gift of leptin. This work was partially supported by National Research Service Award DK-75255 to H. Shi.
Footnotes
Disclosure statement
SA, JCB, and KMH are P&G stock shareholders. JCB and KMH are current P&G
References
- 1.Yach D, Stuckler D, Brownell KD. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat Med. 2006;12:62. doi: 10.1038/nm0106-62. [DOI] [PubMed] [Google Scholar]
- 2.Lazar MA. How Obesity Causes Diabetes: Not a Tall Tale. Science. 2005;307:373–375. doi: 10.1126/science.1104342. [DOI] [PubMed] [Google Scholar]
- 3.Levin BE. Why some of us get fat and what we can do about it. J Physiol. 2007;583:425–430. doi: 10.1113/jphysiol.2007.135434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacLean PS, Higgins JA, Johnson GC, Fleming-Elder BK, Donahoo WT, Melanson EL, Hill JO. Enhanced metabolic efficiency contributes to weight regain after weight loss in obesity-prone rats. Am J Physiol. 2004;287:R1306–1315. doi: 10.1152/ajpregu.00463.2004. [DOI] [PubMed] [Google Scholar]
- 5.Cornier MA, Grunwald GK, Johnson SL, Bessesen DH. Effects of short-term overfeeding on hunger, satiety, and energy intake in thin and reduced-obese individuals. Appetite. 2004;43:253–259. doi: 10.1016/j.appet.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Doucet E, Imbeault P, St-Pierre S, Almeras N, Mauriege P, Richard D, Tremblay A. Appetite after weight loss by energy restriction and a low-fat diet-exercise follow-up. Int J Obes Relat Metab Disord. 2000;24:906–914. doi: 10.1038/sj.ijo.0801251. [DOI] [PubMed] [Google Scholar]
- 7.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 8.Ravussin E, Burnand B, Schutz Y, Jequier E. Energy expenditure before and during energy restriction in obese patients. Am J Clin Nutr. 1985;41:753–759. doi: 10.1093/ajcn/41.4.753. [DOI] [PubMed] [Google Scholar]
- 9.Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obes Rev. 2001;2:239–254. doi: 10.1046/j.1467-789x.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- 10.Faust IM, Johnson PR, Stern JS, Hirsch J. Diet-induced adipocyte number increase in adult rats: A new model of obesity. American Journal of Physiology. 1978;235:E279–E286. doi: 10.1152/ajpendo.1978.235.3.E279. [DOI] [PubMed] [Google Scholar]
- 11.Shi H, Strader AD, Woods SC, Seeley RJ. Sexually dimorphic responses to fat loss after caloric restriction or surgical lipectomy. Am J Physiol. 2007;293:E316–326. doi: 10.1152/ajpendo.00710.2006. [DOI] [PubMed] [Google Scholar]
- 12.Hirsch J, Gallian E. Methods for the determination of adipose cell size in man and animals. J Lipid Res. 1968;9:110–119. [PubMed] [Google Scholar]
- 13.Van Heek M, Compton DS, France CF, Tedesco RP, Fawzi AB, Graziano MP, Sybertz EJ, Strader CD, Davis HR. Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest. 1997;99:385–390. doi: 10.1172/JCI119171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trayhurn P, Duncan JS, Rayner DV. Acute cold-induced suppression of ob (obese) gene expression in white adipose tissue of mice: mediation by the sympathetic system. Biochem J. 1995;311:729–733. doi: 10.1042/bj3110729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gettys TW, Harkness PJ, Watson PM. The beta 3-adrenergic receptor inhibits insulin-stimulated leptin secretion from isolated rat adipocytes. Endocrinology. 1996;137:4054–4057. doi: 10.1210/endo.137.9.8756584. [DOI] [PubMed] [Google Scholar]
- 16.Ricci MR, Lee MJ, Russell CD, Wang Y, Sullivan S, Schneider SH, Brolin RE, Fried SK. Isoproterenol decreases leptin release from rat and human adipose tissue through posttranscriptional mechanisms. Am J Physiol Endocrinol Metab. 2005;288:E798–804. doi: 10.1152/ajpendo.00446.2004. [DOI] [PubMed] [Google Scholar]
- 17.Calcagni E, Elenkov I. Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Ann N Y Acad Sci. 2006;1069:62–76. doi: 10.1196/annals.1351.006. [DOI] [PubMed] [Google Scholar]
- 18.Benoit SC, Clegg DJ, Seeley RJ, Woods SC. Insulin and Leptin as Adiposity Signals. Recent Prog Horm Res. 2004;59:267–285. doi: 10.1210/rp.59.1.267. [DOI] [PubMed] [Google Scholar]
- 19.Lonnqvist F, Arner P, Nordfors L, Schalling M. Overexpression of the obest (ob) gene in adipost tissue of human obese subjects. Nature Medicine. 1995;1:950–953. doi: 10.1038/nm0995-950. [DOI] [PubMed] [Google Scholar]
- 20.Lago R, Gómez R, Lago F, Gómez-Reino J, Gualillo O. Leptin beyond body weight regulation–Current concepts concerning its role in immune function and inflammation. Cellular Immunology. 2008;252:139. doi: 10.1016/j.cellimm.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Boden G, Cheung P, Mozzoli M, Fried SK. Effect of thiazolidinediones on glucose and fatty acid metabolism in patients with type 2 diabetes. Metabolism. 2003;52:753–759. doi: 10.1016/s0026-0495(03)00055-6. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 23.Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, Grove KL, Cowley MA. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007;5:181–194. doi: 10.1016/j.cmet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Tremblay A, Després JP, Bouchard C. The effects of exercise-training on energy balance and adipose tissue morphology and metabolism. Sports Med. 1985;2:223–233. doi: 10.2165/00007256-198502030-00005. [DOI] [PubMed] [Google Scholar]
- 25.Bailey JW, Barker RL, Beauchene RE. Age-Related Changes in Rat Adipose Tissue Cellularity Are Altered by Dietary Restriction and Exercise. J Nutr. 1993;123:52–58. doi: 10.1093/jn/123.1.52. [DOI] [PubMed] [Google Scholar]
- 26.Chen G, Koyama K, Yuan X, Lee Y, Zhou YT, O’Doherty R, Newgard CB, Unger RH. Disappearance of body fat in normal rats induced by adenovirus-mediated leptin gene therapy. Proceedings of the National Academy of Science, USA. 1996;93:14795–14799. doi: 10.1073/pnas.93.25.14795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian H, Azain MJ, Compton MM, Hartzell DL, Hausman GJ, Baile CA. Brain Administration of Leptin Causes Deletion of Adipocytes by Apoptosis. Endocrinology. 1998;139:791–794. doi: 10.1210/endo.139.2.5908. [DOI] [PubMed] [Google Scholar]
- 28.Ambati S, Kim H-K, Yang J-Y, Lin J, Della-Fera MA, Baile CA. Effects of leptin on apoptosis and adipogenesis in 3T3-L1 adipocytes. Biochemical Pharmacology. 2007;73:378. doi: 10.1016/j.bcp.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi H, Strader AD, Woods SC, Seeley RJ. The effect of fat removal on glucose tolerance is depot specific in male and female mice. Am J Physiol. 2007;293:E1012–1020. doi: 10.1152/ajpendo.00649.2006. [DOI] [PubMed] [Google Scholar]
- 31.Tavernier G, Galitzky J, Valet P, Remaury A, Bouloumie A, Lafontan M, Langin D. Molecular mechanisms underlying regional variations of catecholamine-induced lipolysis in rat adipocytes. Am J Physiol Endocrinol Metab. 1995;268:E1135–E1142. doi: 10.1152/ajpendo.1995.268.6.E1135. [DOI] [PubMed] [Google Scholar]
- 32.Harden TK. Agonist-induced desensitization of the beta-adrenergic receptor-linked adenylate cyclase. Pharmacol Rev. 1983;35:5–32. [PubMed] [Google Scholar]
- 33.Carpene C, Galitzky J, Collon P, Esclapez F, Dauzats M, Lafontan M. Desensitization of beta-1 and beta-2, but not beta-3, adrenoceptor-mediated lipolytic responses of adipocytes after long-term norepinephrine infusion. J Pharmacol Exp Ther. 1993;265:237–247. [PubMed] [Google Scholar]
- 34.Levin BE, Triscari J, Sullivan AC. Altered sympathetic activity during development of diet-induced obesity in rat. Am J Physiol. 1983;244:R347–355. doi: 10.1152/ajpregu.1983.244.3.R347. [DOI] [PubMed] [Google Scholar]
- 35.Lowell BB, Bachman ES. Beta-Adrenergic receptors, diet-induced thermogenesis, and obesity. J Biol Chem. 2003;278:29385–29388. doi: 10.1074/jbc.R300011200. [DOI] [PubMed] [Google Scholar]
- 36.Bertile F, Criscuolo F, Oudart H, Le Maho Y, Raclot T. Differences in the expression of lipolytic-related genes in rat white adipose tissues. Biochem Biophys Res Commun. 2003;307:540–546. doi: 10.1016/s0006-291x(03)01196-3. [DOI] [PubMed] [Google Scholar]
- 37.Rayner DV, Trayhurn P. Regulation of leptin production: sympathetic nervous system interactions. J Mol Med. 2001;79:8–20. doi: 10.1007/s001090100198. [DOI] [PubMed] [Google Scholar]
- 38.Deng C, Moinat M, Curtis L, Nadakal A, Preitner F, Boss O, Assimacopoulos-Jeannet F, Seydoux J, Giacobino JP. Effects of beta-adrenoceptor subtype stimulation on obese gene messenger ribonucleic acid and on leptin secretion in mouse brown adipocytes differentiated in culture. Endocrinology. 1997;138:548–552. doi: 10.1210/endo.138.2.4922. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell SE, Rees WD, Hardie LJ, Hoggard N, Tadayyon M, Arch JR, Trayhurn P. ob gene expression and secretion of leptin following differentiation of rat preadipocytes to adipocytes in primary culture. Biochem Biophys Res Commun. 1997;230:360–364. doi: 10.1006/bbrc.1996.5964. [DOI] [PubMed] [Google Scholar]
- 40.Hardie LJ, Guilhot N, Trayhurn P. Regulation of leptin production in cultured mature white adipocytes. Horm Metab Res. 1996;28:685–689. doi: 10.1055/s-2007-979878. [DOI] [PubMed] [Google Scholar]
- 41.Trayhurn P, Duncan JS, Rayner DV, Hardie LJ. Rapid inhibition of ob gene expression and circulating leptin levels in lean mice by the beta 3-adrenoceptor agonists BRL 35135A and ZD2079. Biochem Biophys Res Commun. 1996;228:605–610. doi: 10.1006/bbrc.1996.1704. [DOI] [PubMed] [Google Scholar]
- 42.Mantzoros CS, Qu D, Frederich RC, Susulic VS, Lowell BB, Maratos-Flier E, Flier JS. Activation of beta(3) adrenergic receptors suppresses leptin expression and mediates a leptin-independent inhibition of food intake in mice. Diabetes. 1996;45:909–914. doi: 10.2337/diab.45.7.909. [DOI] [PubMed] [Google Scholar]
- 43.Moinat M, Deng C, Muzzin P, Assimacopoulos-Jeannet F, Seydoux J, Dulloo AG, Giacobino JP. Modulation of obese gene expression in rat brown and white adipose tissues. FEBS Lett. 1995;373:131–134. doi: 10.1016/0014-5793(95)01030-i. [DOI] [PubMed] [Google Scholar]
- 44.Pinkney JH, Coppack SW, Mohamed-Ali V. Effect of isoprenaline on plasma leptin and lipolysis in humans. Clin Endocrinol. 1998;48:407–411. doi: 10.1046/j.1365-2265.1998.00480.x. [DOI] [PubMed] [Google Scholar]


