Abstract
Signal transduction via the endothelial receptor for advanced glycation end products (RAGE) plays a key role in vascular inflammation. Recent observations have shown that the myeloperoxidase-H2O2-chloride system of activated phagocytes is highly up-regulated under inflammatory conditions where hypochlorous acid (HOCl) is formed as the major oxidant. Albumin, an in vivo carrier for myeloperoxidase is highly vulnerable to oxidation and a major representative of circulating advanced oxidized proteins during inflammatory diseases. Immunohistochemical studies performed in the present study revealed marked colocalization of HOCl-modified epitopes with RAGE and albumin in sections of human atheroma, mainly at the endothelial lining. We show that albumin modified with physiologically relevant concentrations of HOCl, added as reagent or generated by the myeloperoxidase-H2O2-chloride system, is a high affinity ligand for RAGE. Albumin, modified by HOCl in the absence of free amino acids/carbohydrates/lipids to exclude formation of AGE-like structures, induced a rapid, RAGE-dependent activation of extracellular signal-regulated kinase 1/2 and up-regulation of the proinflammatory mediator monocyte chemoattractant protein-1. Cellular activation could be blocked either by a specific polyclonal anti-RAGE IgG and/or a specific mitogen-activated protein-kinase kinase inhibitor. The present study demonstrates that HOCl-modified albumin acts as a ligand for RAGE and promotes RAGE-mediated inflammatory complications.
Keywords: AOPP, hypochlorous acid, inflammation, myeloperoxidase
Both acute and chronic diseases are associated with increased modification of proteins at sites of pathology. Chronic hyperglycemia promotes formation and accumulation of advanced glycation end products (AGEs) e.g., pentosidine and Nε-(carboxymethyl)lysine (CML), where glucose reacts nonenzymatically with protein amino groups of long-living proteins (predominantly hemoglobin and albumin) (1).
In addition to AGE formation, elevated levels of advanced oxidation protein products (AOPPs) have been identified in subjects with coronary artery disease, diabetic patients, and patients with uremia (2, 3). Plasma concentrations of AOPPs (closely correlating with AGE levels (2, 3)) increase with progression of chronic renal failure; therefore, AOPPs have been considered as novel disease-related markers for oxidative stress. In vitro studies have shown that HOCl generated by the myeloperoxidase(MPO)-H2O2-chloride system of activated phagocytes represents a major pathway for AOPP production (4). Albumin acts as a major in vivo carrier of MPO (5) and is therefore vulnerable to oxidation by HOCl or other reactive oxygen species; Capeillere-Blandin et al. (4) have identified albumin as the main AOPP product in plasma, and both in vivo generated AOPP and in vitro generated HOCl-modified albumin are potent inducers of the oxidative burst in vivo (6) and in vitro (7).
Plasma MPO levels have been reported to correlate with AOPP levels in disease (8) and high plasma concentrations of MPO and MPO-catalyzed oxidation products are indicative for cardiovascular disease (9). MPO is abundantly present on endothelial cells and monocytes/macrophages in human lesions (10, 11), where RAGE, the receptor for advanced glycation end products is also highly expressed (12). RAGE is composed of a short cytosolic tail that is essential for RAGE signaling, a transmembrane domain, and a positively charged extracellular domain (13). RAGE-mediated expression of proinflammatory mediators can be suppressed either by the presence of blocking antibodies to RAGE, soluble RAGE (sRAGE, the extracellular ligand binding domain of RAGE), or by transient transfection of cDNA encoding for the cytosolic tail-deleted RAGE into RAGE-bearing cells (13).
HOCl-modified proteins are present in human and rabbit lesions (14–16), colocalize with MPO on endothelial cells (11), and promote endothelial dysfunction (17). Most importantly, endothelial cells abundantly express RAGE under inflammatory conditions (13). As the extracellular domain of RAGE is positively charged and HOCl-modification increases the net negative charge of proteins, the present study aimed at investigating whether HOCl-modified albumin (also termed AOPP albumin (6)) colocalizes with RAGE on endothelial cells in human atheroma and whether it acts as a RAGE-ligand, thereby triggering proinflammatory intracellular signaling.
MATERIALS AND METHODS
Modification of albumin
HOCl-albumin
Fatty acid-free BSA (low endotoxin, Sigma, St. Louis, MO) was incubated with HOCl (oxidant:protein molar ratio of 25:1, 50:1, and 100:1) in PBS (pH 7.4, 1 h, 4°C) (10) in the absence of free amino acids/carbohydrates/lipids to exclude formation of AGE-like structures. The electrophoretic mobility of HOCl-modified albumin was assessed by agarose gel electrophoresis using the Lipidophor system (18).
MPO-albumin
Modification of albumin by the MPO-H2O2-chloride system was performed in PBS (50 mM, pH 7.4). Briefly, to 0.5 mg BSA/ml PBS additions of 40 µM H2O2 were made at 5-min intervals at 37°C until reaching a total of 11 additions (final concentration 440 µM; assuming quantitative conversion, an HOCl:protein molar ratio of ~50:1 could be expected). MPO (10 nM, Planta Naturstoffe, Wien, Germany) was added at the start and subsequently at every second addition of H2O2. The reaction mixture was incubated for 1 h (37°C).
AGE-albumin
AGE-modified albumin was prepared exactly as described (19). Briefly, 0.5 g of BSA was dissolved with 3.0 g of D-glucose in 10 ml of 500 mM PBS (pH 7.4) containing 0.05% NaN3. The solution was deoxygenated with N2, sterilized by ultrafiltration, and incubated for 90 days at 37°C in the dark.
All modified albumin preparations were passed over a PD10 column (Amersham) immediately before use to remove unreacted HOCl or glucose. Endotoxin levels were measured using the gel-clot method (Charles River Endosafe, Wilmington, MA, USA) and were found to be less than 0.2 ng/mg for all albumin preparations used.
Amino acid analysis
Aliquots of native and modified albumin (500 µg) were lyophilized in 5-ml ampoules and purged with N2 before hydrolysis in constant boiling 6 N HCl (24 h, 120°C). Amino acid analysis was performed on a Biotronics analyzer, as described previously (16).
Cell culture and overexpression of RAGE
HEK-293 cells were obtained from the American Type Culture Collection (ATCC) (ATCC-No. CRL-1573, USA), maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Carlsbad, CA, USA) supplemented with sodium pyruvate, pyridoxine, 5% FCS (Roche Applied Science, Indianapolis, IN, USA) and glucose (1.0 or 4.5 g/l). For generation of HEK-293 cells, stably expressing RAGE (RAGE-HEK cells), human full-length RAGE cDNA was cloned from a lung cDNA library (Clontech, Palo Alto, CA, USA) (20). The full-length RAGE sequence was amplified by conventional polymerase chain reaction (PCR) techniques using primers exactly as described (20). The full-length rage primers introduced artificial restriction sites for NheI at the N terminus and EcoRI at the C-terminus for subcloning the construct into the expression vector plasmid construct DNA (pcDNA) 3.1+ (Invitrogen) (20).
Expression and isolation of recombinant human sRAGE
Recombinant sRAGE (a C-terminal truncated protein and identical to the extracellular domain of full-length RAGE) containing a C-terminal His-tag was generated and purified as described (21). Briefly, culture supernatant from a HEK-293 clone stably transfected with vector pSecTag2B (Invitrogen) containing the sRAGE cDNA was collected and applied to a Ni2+-charged HiTrap chelating HP column connected to an ÄKTA prime fast protein liquid chromatography (FPLC) system (GE Healthcare). After elution with an imidazole step gradient (200 mM), fractions containing purified sRAGE were identified by SDS-PAGE, pooled, and dialyzed against PBS.
Generation of anti-sRAGE antibody
Polyclonal anti-sRAGE antiserum was raised in rabbits in our own laboratory using purified sRAGE (see above) as the antigen. The specificity of the anti-sRAGE antiserum was checked by SDS-PAGE and Western blot analysis experiments (see below). The polyclonal antiserum was monospecific (identical to monoclonal antibodies (mAbs) clone A11 (22) and clone 9A11 (20) for sRAGE and RAGE).
Labeling of sRAGE
Labeling of sRAGE was performed with Na125I (PerkinElmer Life Sciences) using N-bromosuccinimide as the coupling agent (23). Routinely, 200 µCi of Na125I was used to label 1 mg of protein. This procedure resulted in specific activities between 200 and 400 cpm/ng of protein.
sRAGE binding assay
Microtiter wells (Nunc Maxisorp, VWR) were coated with native albumin, AGE-albumin, HOCl-albumin (at indicated oxidant:protein molar ratio) or MPO-albumin (each 100 µg/ml, 15 mM sodium carbonate, 35 mM sodium bicarbonate, pH 9.6) overnight at 4°C. Subsequently, the wells were washed with washing buffer (ice-cold PBS containing 0.05% Tween-20). In some experiments, adsorbed albumin was oxidized onto the wells by adding 100 µl of HOCl solutions (1–100 µM in PBS, pH 7.4) for 1 h at 4°C. After washing and blocking (2 h at 37°C with PBS containing albumin (10 mg/ml)), the wells were incubated with 125I-sRAGE (100 µl at indicated concentrations; either alone or in the presence of an excess of unlabeled competitors) in DMEM (containing 1 mg albumin/ml) for 2 h at 37°C. Then, the supernatant was removed, and the wells were washed four times with washing buffer. Bound 125I-sRAGE was quantified by γ-counting.
Cell culture experiments
HEK cells were grown to confluence in 6-well plates and serum starved for 18 h before experiments. Cells were treated as indicated by the addition of albumin, AGE-albumin, or HOCl-albumin directly into the medium. In some experiments, cells were preincubated with anti-sRAGE IgG (200 µg/ml) for 2 h followed by a 30-min exposure to the indicated concentrations of albumin, AGE-albumin, or HOCl-albumin. In some experiments, the cells were preincubated with the specific mitogen-activated protein (MAP)-kinase kinase inhibitor PD 98059 (25 µM, Calbiochem, San Diego, CA, USA) for 30 min. The experiments were terminated by aspiration of the medium, and cell lysis was performed on ice for 10 min with 100 µl of lysis buffer (HEPES, 50 mM; NaCl, 150 mM; EDTA, 1 mM; Na4P2O7, 10 mM; Na3VO4, 2 mM; NaF, 10 mM; Triton X-100, 1% (v/v); glycerol, 10% (v/v); PMSF, 1 mM; aprotinin, 10 µg/ml; leupeptin, 2 µg/ml; and pepstatin, 2 µg/ml; pH 7.4). Insoluble cell debris was removed by centrifugation at 13,000 rpm at 4°C for 10 min. Protein content of cell lysates was determined by the Lowry method.
SDS-PAGE and Western blot analysis
SDS-PAGE of albumin preparations, purified sRAGE, or total cell lysates of HEK cells was performed with 3.75–20% gradient or 10% linear PAGE. Aliquots of proteins (5 µg) or cell lysates (80 to 100 µg total protein content) were diluted with sample buffer and incubated at 95°C for 5 min before application to gels (18). After SDS-PAGE, proteins were electrophoretically transferred to nitrocellulose membranes (200 mA, 40 min). The following primary antibodies were used: 1) monoclonal antibody (mAb) clone 2D10G9 (dilution 1:20, specifically recognizing HOCl-modified proteins and not cross-reacting with other protein modifications (18)), 2) polyclonal anti-sRAGE antiserum (dilution 1:5,000) or mAbs anti-sRAGE (clone A11 (22) or 9A11 (20), dilutions of 1:200), 3) phospho-specific mAb anti-phospho extracellular signal-regulated kinase 1/2 (pErk1/2) MAP-kinase antibody (1: 1,000; Cell Signaling Technology, Beverly, MA), and (iv) rabbit polyclonal anti-Erk1/2 (1:2,000, Santa Cruz Biotechnology, Santa Cruz, CA). Peroxidase-conjugated chicken anti-mouse IgG (1:100,000; Pierce, Rockford, IL) or peroxidase-conjugated goat anti-rabbit IgG (1:50,000, Pierce) were used as secondary antibodies. Immunoreactive bands were visualized using the chemoluminescent Super Signal West Pico substrate (Pierce).
Quantification of mRNA by reverse transcriptase-PCR
The relative amounts of mRNA encoding human monocyte chemotactic protein-1 (MCP-1) and β-actin were measured by reverse transcriptase (RT)-PCR. Total RNAs were isolated using the Qiagen RNeasy system. Reverse transcription was performed as described by Strauss et al. (24). PCR amplifications for MCP-1 and β-actin were performed in parallel reactions. Gene-specific oligonucleotide primers based on sequences published in GenBank human DNA database were from Invitrogen: for MCP-1, forward primer, 5′-CAGCCAGATGCAATCAATGC-3′, and reverse primer, 5′-GTGGTCCATGGAATCCTGAA-3′; and for β-actin, forward primer, 5′-GACTACCTCATGAAGATC-3′ and reverse primer, 5′-GATCCACATCTGCTGGAA-3′. PCR mixtures of 50 µl contained 2.5 µl of the completed RT-reaction as template, 0.2 mM dNTPs, appropriate primers at 10 µM, 1 × PCR buffer and 1 U of Hot Fire DNA polymerase (Solis, BioDyne, Tartu, Estonia). The reaction mixtures were heated to 96°C for 10 min, and amplification was carried out as follows: β-actin (20 cycles): 94°C, 30 s, 55°C, 30 s, 72°C 60 s; MCP-1 (30 cycles): 94°C, 45 s, 55°C, 45 s, 72°C 45 s. PCR products (16 µl) were separated by electrophoresis through 1.8% agarose at 60 V for 60 min in Tris acetate/EDTA buffer containing 0.05 µl/ml ethidium bromide (10 mg/ml). The relative amounts of MCP-1 and β-actin mRNA were determined by densitometry. Results are expressed as the ratio of the intensity of the MCP-1 band to that corresponding to β-actin using the EDAS 290 photodocumentation system (Kodak) and EasyWin software (Herolab).
Double immunofluorescence and confocal laser scanning microscopy
Aortae and aortae abdominalis of four autopsy cases with different histological classification of atherosclerosis (lesion type II and IV) who died of cerebral hemorrhage were used (16). The autopsy material (kindly provided by Dr. G. Höfler, Institute of Pathology, Medical University of Graz) was taken 12 h after death.
Immunohistochemistry was carried out on tissues embedded in tissue freezing medium (16). For double immunofluorescence, the sections were thawed, fixed in acetone at 22°C (5 min), and rehydrated in PBS. After blocking (10 min with human AB serum, diluted 1:10 in blocking solution; Dako, Copenhagen, Denmark), the sections were incubated with 1) biotinylated mAb (clone 2D10G9, isotype IgG2bκ, dilution 1:10 (18)) for 30 min at 22°C, followed by an incubation step with cyanine (Cy)-3 (red) labeled streptavidin (1:300, Amersham Biosciences) for 30 min at 22°C, or 2) a specific endothelial marker (mAb anti-human CD141, 1:20, clone QBEND/40, isotype IgG2α, Dako), followed by a Cy-3 labeled goat anti-mouse IgG (1:300; Jackson Dianova, Hamburg, Germany), respectively. For the second antibody-staining rabbit polyclonal anti-sRAGE antiserum (1:25) or rabbit anti-human albumin antiserum (1:100, Abcam, Cambridge, MA) was applied for 30 min at 22°C, followed by Cy-2 labeled goat anti-rabbit IgG (1:300, Jackson Dianova). PBS was used for washing sections between the different incubation steps. Sections were mounted with Moviol (Calbiochem-Novabiochem) and analyzed in sequential mode on a confocal laser-scanning microscope (Leica SP2, Leica Lasertechnik GmbH, Heidelberg, Germany) using 488 nm and 543 nm for excitation of Cy-2 (green) for Cy-3 (red), respectively (16). Control experiments encompassed immunofluorescence 1) without primary antibodies, 2) with nonimmune antibodies as primary antibodies, 3) preabsorbtion of the primary antibodies (5-fold molar excess with sRAGE or 15-fold molar excess with HOCl-albumin), and 4) without secondary antibodies.
RESULTS
1. Immunofluorescence
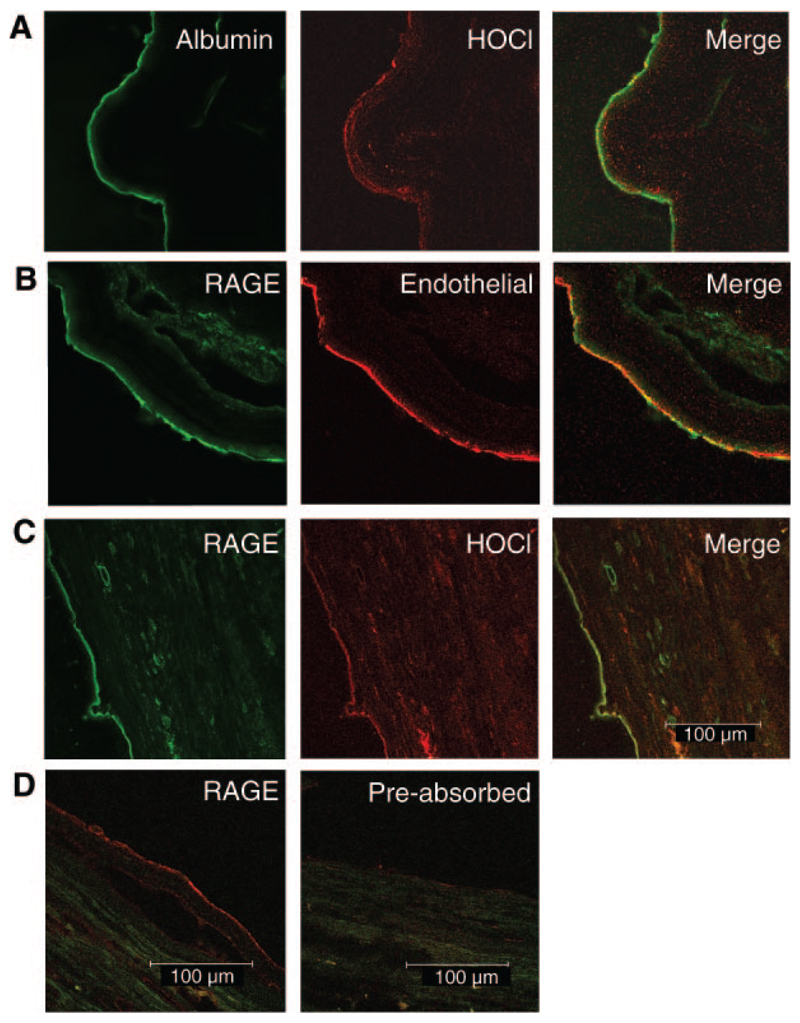
We previously reported colocalization of MPO with HOCl-modified epitopes on the endothelial layer in tissue sections of autopsy material (11). Immunohistochemical analysis performed during the present study shows that HOCl-modified epitopes colocalize with albumin (Fig. 1A). Pronounced staining for RAGE is present on the endothelial layer lining the blood vessel (Fig. 1B) and colocalization was observed with HOCl-modified proteins (Fig. 1C). Preabsorbtion of the primary polyclonal anti-sRAGE antibody with purified sRAGE prevented antibody binding (Fig. 1D). Omission of the primary mAb (clone 2D10G9) or preabsorbtion of mAb 2D10G9 with HOCl-albumin prevented antibody binding (data not shown).
Figure 1.
Immunofluorescence and double immunofluorescence for RAGE staining. 5 µm frozen sections of abdominal aorta (type II) were incubated with polyclonal anti-human albumin antiserum (A), polyclonal anti-human-sRAGE antiserum (prepared as described in the methods section) (B–D), mAb clone 2D10G9 (recognizing HOCl-modified epitopes) (A, C), and mAb anti-CD141 (an endothelial marker; B) as primary antibodies. Preabsorbtion of anti-sRAGE IgG was performed with a 5-fold molar excess of purified sRAGE for 30 min (D). Cy-2/-3 labeled secondary antibodies were used.
2. HOCl-albumin/RAGE interaction
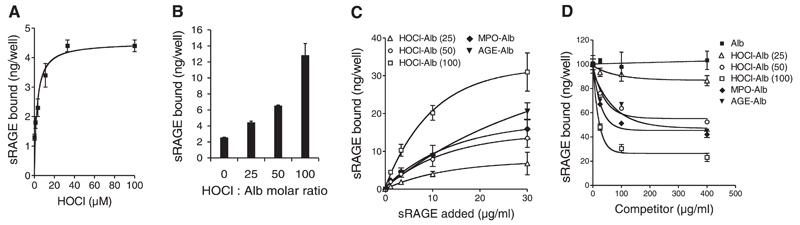
Prompted by immunofluorescence double-labeling experiments (Fig. 1A–C), we investigated to what extent the degree of HOCl modification affects interaction of albumin with RAGE. For these studies, a microplate-based, cell-free assay was used (25); albumin was coated to the plates and then directly modified by increasing HOCl concentrations in the absence of free amino acids, lipids, or carbohydrates to avoid formation of AGE-like structures. Fig. 2A shows that binding of 125I-sRAGE (3 µg/ml) to albumin increased as a function of increasing HOCl-mediated protein modification. Only weak interaction of 125I-sRAGE with native albumin could be observed. To exclude that HOCl added to the plates would undergo localized reactions that may interfere with RAGE binding, albumin was first modified with increasing HOCl concentrations and then directly coated to the plates. Figure 2B confirms results from Fig. 2A demonstrating that binding of 125I-sRAGE (3 µg/ml) to albumin increased as a function of increasing HOCl-modification.
Figure 2.
sRAGE binding to HOCl-modified albumin. A) Albumin was immobilized onto wells of plastic dishes and modified by indicated HOCl-concentrations. After washing and blocking of excess binding sites with native albumin, 125I-sRAGE (3 µg/ml) was added to coated wells. Subsequently, wells were washed and radioactivity was counted. B) Native albumin (Alb) and freshly prepared HOCl-albumin (oxidant:protein molar ratio of 25:1, 50:1, and 100:1) was immobilized onto wells of plastic dishes. After washing and blocking of excess binding sites with native albumin, 125I-sRAGE (3 µg/ml) was added to coated wells. Subsequently, wells were washed, and radioactivity was counted. C) Albumin (Alb), AGE-Alb, HOCl-Alb (oxidant:protein molar ratio of 25:1, 50:1, and 100:1) and MPO-Alb (modified by the MPO-H2O2-chloride system; oxidant:protein molar ratio of 50:1) were immobilized onto wells of plastic dishes. After washing and blocking of unspecific binding sites, indicated concentrations of 125I-sRAGE (1, 3, 10, and 30 µg/ml) were added to coated wells. Subsequently, wells were washed and radioactivity was counted. Binding of 125I-sRAGE to control albumin-coated wells (nonspecific binding) was subtracted from binding values to modified-albumin to calculate specific binding. Only specific data are shown. D) Dose-response curves for 125I-sRAGE (5 µg/ml) binding inhibition to AGE-BSA-coated microtiter wells by albumin (Alb), AGE-Alb, HOCl-Alb (oxidant:protein molar ratio of 25:1, 50:1, and 100:1) and MPO-Alb (modified by the MPO-H2O2-chloride system; H2O2:Alb molar ratio of 50:1). Results represent mean ± sd (n=3) of one experiment out of four.
To study the binding capacity/affinity of various ligands toward sRAGE, the wells were coated with native and modified albumin preparations (characterization of all modified albumin preparations is listed in the supplement, Table I, Fig. I), and increasing concentrations of 125I-sRAGE (1 to 30 µg/ml) were added. Binding capacity of indicated 125I-sRAGE concentrations to albumin preparations increased as a function of increasing HOCl:protein molar ratio (Fig. 2C). Notably, binding affinity of 125I-sRAGE to albumin modified with an oxidant:protein molar ratio of 100:1 was significantly higher (Table 1) than that observed for AGE-albumin, a preferential ligand for RAGE (26). 125I-sRAGE binding to the modified albumin preparations was saturable as Bmax values could be calculated as seen in Table 1. Binding of 125I-sRAGE to MPO-albumin was similar to that observed for HOCl-albumin (50:1).
TABLE 1.
Binding constants for albumin: RAGE interaction
|
Kd (µg/ml) |
Bmax (ng/well) |
IC50 (µg/ml) |
|
|---|---|---|---|
| Alb | n.d. | n.d. | n.d. |
| HOCl-Alb (25:1) | 15.7 ± 1.2 | 10.5 ± 0.9 | n.d. |
| HOCl-Alb (50:1) | 14.6 ± 0.3 | 20.3 ± 1.6 | 29.0 ± 5.1 |
| HOCl-Alb (100:1) | 10.6 ± 0.6 | 42.8 ± 5.2 | 14.4 ± 3.5 |
| MPO-Alb | 16.1 ± 1.1 | 24.6 ± 1.7 | 20.1 ± 3.8 |
| AGE-Alb | 46.9 ± 4.6 | 52.6 ± 4.2 | 51.9 ± 15.2 |
Kd and Bmax values for 125I-soluble receptor for advanced glycation end products (RAGE) binding to microtiter wells coated with the indicated albumin preparations are listed. IC50 values for 125I-sRAGE binding inhibition to AGE-albumin (AGE-Alb)-coated microtiter wells by albumin (Alb), HOCl-Alb (at indicated oxidant:protein molar ratio), MPO-Alb (calculated HOCl:protein molar ratio of 50:1), and AGE-Alb are given. Calculations were performed by nonlinear regression analysis (GraphPad Prism). n.d. = non determined.
Binding affinity was also determined in a different experimental setup by measuring the ability of modified albumin to inhibit 125I-sRAGE binding to microtiter wells coated with AGE-albumin. Dose-response curves show that HOCl-albumin (50:1 and 100:1) or MPO-albumin preparations effectively blocked 125I-sRAGE binding to AGE-albumin dependent on the oxidant:protein molar ratio (Fig. 2D). Again, the affinity of 125I-sRAGE was higher for MPO-albumin or HOCl-albumin compared to AGE-albumin. The corresponding IC50 values are listed in Table 1.
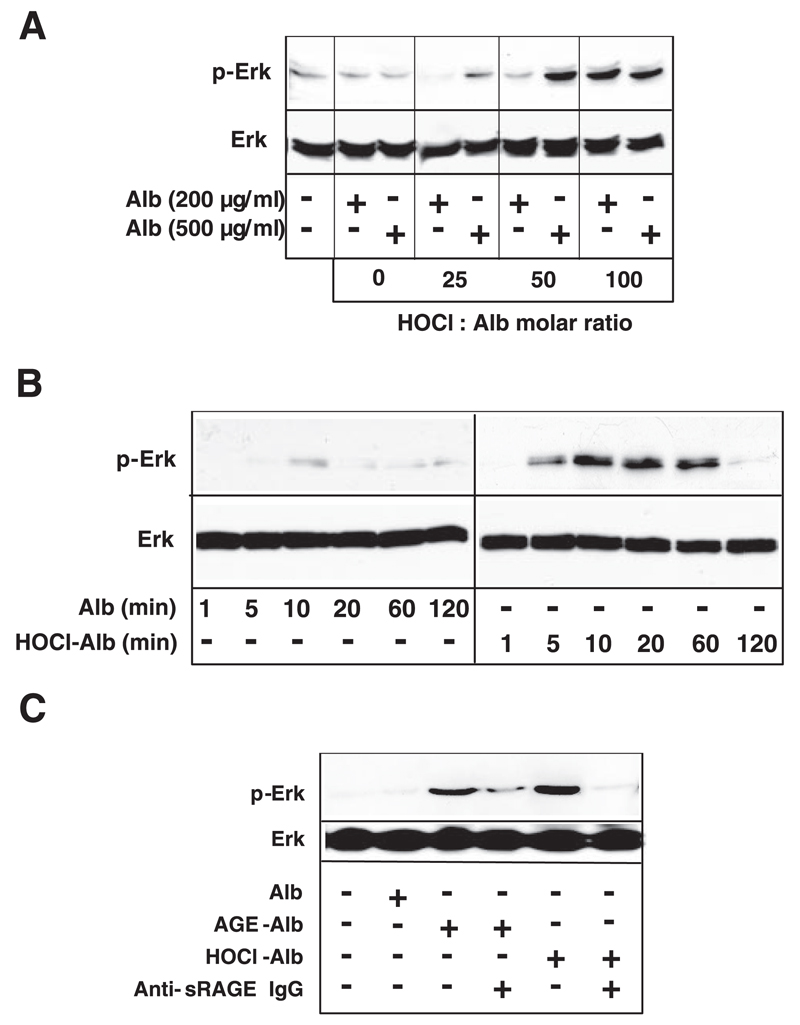
3. HOCl-albumin binds to RAGE and activates downstream signaling
As the RAGE-ligand axis may induce activation of key cell signaling pathways, including MAP kinases (26), HEK cells overexpressing full-length RAGE were used. To avoid cell activation by MPO (27) that was further reported to bind to albumin (5), the experiments described below were performed only with albumin modified by HOCl added as reagent and not generated by the MPO-H2O2-chloride system. HOCl-albumin induced activation of Erk1/2 in an oxidant:protein molar ratio-dependent manner (Fig. 3A). Activation of Erk1/2 increased up to 20 min and returned to baseline levels after 2 h (Fig. 3B). The involvement of RAGE is confirmed by the observation that the monospecificpolyclonal anti-sRAGE IgG (raised against the extracellular domain of RAGE) inhibited or completely abolished Erk1/2 activation induced by HOCl-albumin or AGE-albumin (Fig. 3C). No activation of Erk1/2 could be observed in control HEK cells (data not shown). The specific anti-sRAGE IgG effectively blocked binding of HOCl-albumin and AGE-albumin to RAGE (Supplement, Fig. II).
Figure 3.
RAGE-dependent Erk1/2 MAP-kinase activation. A) RAGE-HEK cells were incubated with indicated concentrations of albumin (Alb) or HOCl-Alb (oxidant:protein molar ratio of 25:1, 50:1, and 100:1) for 20 min. B) RAGE-HEK cells were incubated at indicated time periods with 200 µg/ml Alb or HOCl-Alb (oxidant:protein molar ratio of 100:1). C) RAGE-HEK cells were incubated with 1 mg/ml Alb, 1 mg/ml AGE-Alb, or 200 µg/ml HOCl-Alb (oxidant:protein molar ratio of 100:1) for 20 min in the absence or presence of 200 µg/ml polyclonal anti-sRAGE IgG. Anti-sRAGE IgG was added to the cells 2 h before addition of AGE-Alb or HOCl-Alb. After cell lysis, SDS-PAGE, and Western blot analysis, phosphorylation of Erk1/2 (pErk1/2) was determined by use of phospho-specific Erk1/2-kinase specific antibodies. Blots were reprobed with anti-Erk antibody to ensure equivalence of loading. A typical experiment out of three is shown.
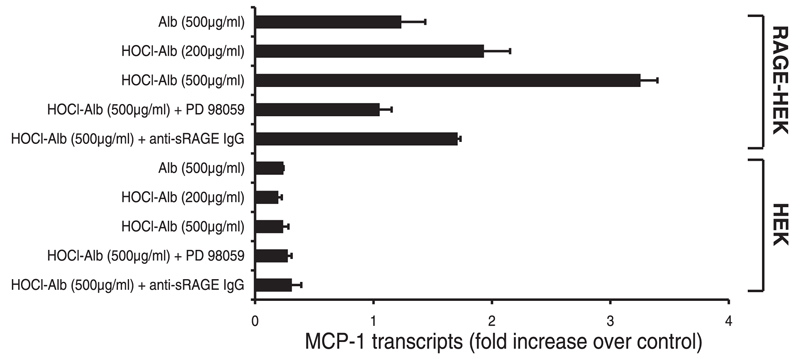
4. Role of Erk1/2 in HOCl-albumin-induced expression of monocyte chemoattractant protein-1 (MCP-1)
To determine the functional significance of HOCl-albumin-mediated Erk1/2 activation, expression of the chemokine MCP-1, an early and important mediator in the recruitment of monocytes (28), was analyzed. RT-PCR analysis of RNA extracted from RAGE-HEK cells and control HEK cells revealed that HOCl-albumin, in contrast to native albumin, potently induced expression of MCP-1 transcription (Fig. 4). HOCl-albumin-induced expression of MCP-1 transcripts could be inhibited by the monospecific anti-sRAGE IgG and was also attenuated by the specific MAP-kinase kinase inhibitor PD 98059. Thus HOCl-albumin-induced expression of MCP-1 critically depends on Erk1/2 phosphorylation. Control HEK cells showed only minimal expression of MCP-1 transcripts.
Figure 4.
RAGE-dependent MCP-1 transcript expression by HOCl-albumin. Control HEK and RAGE-HEK cells were incubated for 3 h with indicated concentrations of albumin (Alb) and HOCl-Alb (oxidant:protein molar ratio of 100:1) in the absence or presence of polyclonal anti-sRAGE IgG (200 µg/ml) or the specific MAP-kinase kinase inhibitor PD 98059 (25 µM). MCP-1 transcripts were determined by RT-PCR as described in the Methods section. Results are the average of duplicates of one representative experiment out of three, and error bars represent the ranges of the measurements. MCP-1 expression of nontreated RAGE-HEK cells were used as controls.
DISCUSSION
RAGE has been demonstrated to play a key role in vascular inflammation (13). Most compelling evidence comes from animal models where antagonisms of RAGE reduced the consequences of diabetes, hypercholesterolemia, or physical injury to the vasculature(29). The present study provides several lines of evidence that HOCl-albumin could act as an important player in RAGE-mediated vascular inflammation. First, in human atherosclerotic lesions, HOCl-modified epitopes colocalize with albumin and RAGE expressed on endothelial cells. Second, the binding affinity of HOCl-albumin to RAGE markedly increases with increasing degree of HOCl-modification. Third, binding of HOCl-albumin to RAGE evokes downstream signaling via the MAP-kinase pathway and the expression of the highly proinflammatory mediator MCP-1 independent of AGE-like structures.
A common characteristic of all known RAGE ligands is a net negative charge at physiological pH. Polyanionic molecules such as heparin, fucoidan, and dextran sulfate have been shown to block the interaction between AGEs and RAGE. When preparing different AGE-albumin preparations and characterizing them for reactive free amine and carbonyl content, as well as for the presence for AGEs, e.g., pentosidine and CML, only the extent of modified amine groups robustly correlated with RAGE binding affinity (25). In line with this finding, modification of albumin-lysine residues by HOCl (leading to an increase of negative charge and increased electrophoretic mobility) markedly affects albumin interaction with RAGE (Table 1).
CML-modifications of proteins have been shown to be an active component of AGE-modified proteins and engage cellular RAGE, thereby activating key cell signaling pathways, such as NF-κB and modulating gene expression in response to activation of the MAP-kinase pathway (26, 30). Thus, CML-RAGE interaction triggers processes intimately linked to accelerate vascular and inflammatory complications. A previous study suggested that CML-modifications may be formed directly by the MPO-H2O2-halide system but only in the presence of free amino acids, e.g., serine, providing a mechanism for conversion of hydroxy amino acids into glycoaldehydes, leading to formation of CML (6, 31). However, in our experimental setup, modification of albumin by HOCl was performed in the absence of free amino acids, and therefore no AGE-like structures are formed (6). HPLC analysis confirmed that the AGE content (i.e., pentosidine and CML) in HOCl-albumin (AOPP-albumin) is not increased compared with native albumin (6).
A property of RAGE is its involvement in chronic diseases such as diabetic complications, atherosclerosis, systemic amyloidosis and Alzheimer disease (13, 32–34). Abundant staining for either RAGE or MPO in human lesions provides strong evidence for both proteins to be involved in the pathogenesis of atherosclerosis (12). The colocalization of immunoreactive HOCl-modified epitopes with albumin and RAGE on the endothelial layer (Fig. 1) is of further support that the MPO-H2O2-chloride system contributes to oxidative damage of albumin in vivo (4) and that HOCl-modified proteins are likely physiological ligands for RAGE.
A key inflammatory mediator stimulated by HOCl-albumin via the RAGE signaling pathway is MCP-1, a chemotactic cytokine involved in the recruitment of leukocytes to inflammatory sites (28). The involvement of MCP-1 in atherogenesis has been suggested by its strong chemotactic effect on monocytes, both in vitro and in vivo (35). Furthermore, mice deficient in MCP-1 are protected from the development of vascular lesions (36). Thus, MCP-1 generated via interaction of HOCl-modified proteins with RAGE could strongly impact vascular inflammation, e.g., activation of cells contributing to the development of atherosclerosis. Cholesterol feeding of rabbits led to expression of MPO in activated phagocytes and colocalization of MPO with HOCl-modified epitopes in serial sections of rabbit lesions (15, 37). Most importantly, intravenous infusion of HOCl-albumin in hypercholesterolemic rabbits significantly increased macrophage infiltration and smooth muscle cell proliferation in atherosclerotic plaques compared with animals treated with albumin (6); an effect independent of AGEs that could be mediated—at least in part—via RAGE-triggered MCP-1 expression by endothelial cells. In addition, macrophages and smooth muscle cells have been reported to express MCP-1 even during initial stages of atherosclerosis in rabbits in response to diet-induced hypercholesterolemia (38). Of note, taurine, the most important in vivo scavenger for HOCl suppresses development of atherosclerotic lesion formation in these animals (39), apparently by inhibiting NF-kB-dependent expression of MCP-1 (40).
Summarizing, we show that modification of albumin by HOCl (added as reagent or generated by the MPO-H2O2-chloride system) generates a proinflammatory protein that specifically binds to RAGE; this interaction may amplify RAGE-dependent macrovascular complications as observed in diabetes, atherosclerosis, and end-stage renal disease.
Supplementary Material
Acknowledgments
The expert technical assistance of Monika Sundl and Michaela Tritscher is appreciated. This work was supported by grants from the Austrian Science Fund (FWF, P17013-B05 and P19074-B05), the Austrian National Bank (OENB 9962), the Kamillo Eisner Stiftung (Switzerland), and the Austrian Forschungsförderungsgesellschaft (Bridge 810994) to A.H., W.S., and E.M.
References
- 1.Wautier JL, Schmidt AM. Protein glycation. A firm link to endothelial cell dysfunction. Circ Res. 2004;95:233–238. doi: 10.1161/01.RES.0000137876.28454.64. [DOI] [PubMed] [Google Scholar]
- 2.Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, Jungers P, Descamps-Latscha B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304–1313. doi: 10.1038/ki.1996.186. [DOI] [PubMed] [Google Scholar]
- 3.Witko-Sarsat V, Friedlander M, Nguyen Khoa T, Capeillere-Blandin C, Nguyen AT, Canteloup S, Dayer JM, Jungers P, Drueke T, Descamps-Latscha B. Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J Immunol. 1998;161:2524–2532. [PubMed] [Google Scholar]
- 4.Capeillere-Blandin C, Gausson V, Descamps-Latscha B, Witko-Sarsat V. Biochemical and spectrophotometric significance of advanced oxidized protein products. Biochim Biophys Acta. 2004;1689:91–102. doi: 10.1016/j.bbadis.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Tiruppathi C, Naqvi T, Wu Y, Vogel SM, Minshall RD, Malik AB. Albumin mediates the transcytosis of myeloperoxidase by means of caveolae in endothelial cells. Proc Natl Acad Sci U S A. 2004;101:7699–7704. doi: 10.1073/pnas.0401712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu SX, Hou FF, Guo ZJ, Nagai R, Zhang WR, Liu ZQ, Zhou ZM, Zhou M, Xie D, Wang GB, et al. Advanced oxidation protein products accelerate atherosclerosis through promoting oxidative stress and inflammation. Arterioscler Thromb Vasc Biol. 2006;26:1156–1162. doi: 10.1161/01.ATV.0000214960.85469.68. [DOI] [PubMed] [Google Scholar]
- 7.Witko-Sarsat V, Gausson V, Nguyen AT, Touam M, Drueke T, Santangelo F, Descamps-Latscha B. AOPP-induced activation of human neutrophil and monocyte oxidative metabolism: a potential target for N-acetylcysteine treatment in dialysis patients. Kidney Int. 2003;64:82–91. doi: 10.1046/j.1523-1755.2003.00044.x. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Ayala E, Anderstam B, Suliman ME, Seeberger A, Heimburger O, Lindholm B, Stenvinkel P. Enhanced RAGE-mediated NF-κB stimulation in inflamed hemodialysis patients. Atherosclerosis. 2005;180:333–340. doi: 10.1016/j.atherosclerosis.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Zheng L, Nukuna B, Brennan ML, Sun M, Goormastic M, Settle M, Schmitt D, Fu X, Thomson L, Fox PL, et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114:529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malle E, Waeg G, Schreiber R, Gröne E, Sattler W, Gröne HJ. Immunohistochemical evidence for the myeloperoxidase/H2O2/halide system in human atherosclerotic lesions: colocalization of myeloperoxidase and hypochlorite-modified proteins. Eur J Biochem. 2000;267:4495–4503. doi: 10.1046/j.1432-1327.2000.01498.x. [DOI] [PubMed] [Google Scholar]
- 11.Malle E, Marsche G, Panzenboeck U, Sattler W. Modification of low-density lipoprotein by myeloperoxidase-derived oxidants and reagent hypochlorous acid. Arch Biochem Biophys. 2006;445:245–255. doi: 10.1016/j.bbalip.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 12.Cipollone F, Iezzi A, Fazia M, Zucchelli M, Pini B, Cuccurullo C, De Cesare D, De Blasis G, Muraro R, Bei R, et al. The receptor RAGE as a progression factor amplifying arachidonate-dependent inflammatory and proteolytic response in human atherosclerotic plaques: role of glycemic control. Circulation. 2003;108:1070–1077. doi: 10.1161/01.CIR.0000086014.80477.0D. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949–955. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazell LJ, Arnold L, Flowers D, Waeg G, Malle E, Stocker R. Presence of hypochlorite-modified proteins in human atherosclerotic lesions. J Clin Invest. 1996;97:1535–1544. doi: 10.1172/JCI118576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malle E, Wäg G, Thiery J, Sattler W, Gröne HJ. Hypochlorite-modified (lipo)proteins are present in rabbit lesions in response to dietary cholesterol. Biochem Bio-phys Res Commun. 2001;289:894–900. doi: 10.1006/bbrc.2001.6074. [DOI] [PubMed] [Google Scholar]
- 16.Marsche G, Hammer A, Oskolkova O, Kozarsky KF, Sattler W, Malle E. Hypochlorite-modified high density lipoprotein, a high affinity ligand to scavenger receptor class B, type I, impairs high density lipoprotein-dependent selective lipid uptake and reverse cholesterol transport. J Biol Chem. 2002;277:32172–32179. doi: 10.1074/jbc.M200503200. [DOI] [PubMed] [Google Scholar]
- 17.Marsche G, Heller R, Fauler G, Kovacevic A, Nuszkowski A, Graier W, Sattler W, Malle E. 2-chlorohexadecanal derived from hypochlorite-modified high-density lipoprotein-associated plasmalogen is a natural inhibitor of endothelial nitric oxide biosynthesis. Arterioscler Thromb Vasc Biol. 2004;24:2302–2306. doi: 10.1161/01.ATV.0000148703.43429.25. [DOI] [PubMed] [Google Scholar]
- 18.Malle E, Hazell L, Stocker R, Sattler W, Esterbauer H, Waeg G. Immunologic detection and measurement of hypochlorite-modified LDL with specific monoclonal antibodies. Arterioscler Thromb Vasc Biol. 1995;15:982–989. doi: 10.1161/01.atv.15.7.982. [DOI] [PubMed] [Google Scholar]
- 19.Takata K, Horiuchi S, Araki N, Shiga M, Saitoh M, Morino Y. Endocytic uptake of nonenzymatically glycosylated proteins is mediated by a scavenger receptor for aldehyde-modified proteins. J Biol Chem. 1988;263:14819–14825. [PubMed] [Google Scholar]
- 20.Demling N, Ehrhardt C, Kasper M, Laue M, Knels L, Rieber EP. Promotion of cell adherence and spreading: a novel function of RAGE, the highly selective differentiation marker of human alveolar epithelial type I cells. Cell Tissue Res. 2006;323:475–488. doi: 10.1007/s00441-005-0069-0. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh HL, Schafer BW, Weigle B, Heizmann CW. S100 protein translocation in response to extracellular S100 is mediated by receptor for advanced glycation endproducts in human endothelial cells. Biochem Biophys Res Commun. 2004;316:949–959. doi: 10.1016/j.bbrc.2004.02.135. [DOI] [PubMed] [Google Scholar]
- 22.Srikrishna S, Huttunen HJ, Johansson L, Weigle B, Yamaguchi Y, Rauvala H, Freeze HH. N-Glycans on the receptor for advanced glycation end products influence amphoterin binding and neurite outgrowth. J Neurochem. 2002;80:998–1008. doi: 10.1046/j.0022-3042.2002.00796.x. [DOI] [PubMed] [Google Scholar]
- 23.Marsche G, Levak-Frank S, Quehenberger O, Heller R, Sattler W, Malle E. Identification of the human analog of SR-BI and LOX-1 as receptors for hypochlorite-modified high-density lipoprotein on human umbilical venous endothelial cells. FASEB J. 2001;15:1095–1097. doi: 10.1096/fj.00-0532fje. [DOI] [PubMed] [Google Scholar]
- 24.Strauss JG, Hayn M, Zechner R, Levak-Frank S, Frank S. Fatty acids liberated from high-density lipoprotein phospholipids by endothelial-derived lipase are incorporated into lipids in HepG2 cells. Biochem J. 2003;371:981–988. doi: 10.1042/BJ20021437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valencia JV, Stephen CW, Douglas Q, Geesje HK, DeGroot J, TeKoppele JM, Hughes TE. Advanced glycation end product ligands for the receptor for advanced glycation end products: biochemical characterization and formation kinetics. Anal Biochem. 2004;324:68–78. doi: 10.1016/j.ab.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Lander HM, Tauras JM, Ogiste JS, Hori O, Moss RA, Schmidt AM. Activation of the receptor for advanced glycation end products triggers a p21(ras)-dependent mitogen-activated protein kinase pathway regulated by oxidant stress. J Biol Chem. 1997;272:17810–17814. doi: 10.1074/jbc.272.28.17810. [DOI] [PubMed] [Google Scholar]
- 27.Lau D, Mollnau H, Eiserich JP, Freeman BA, Daiber A, Gehling UM, Brummer J, Rudolph V, Munzel T, Heitzer T, et al. Myeloperoxidase mediates neutrophil activation by association with CD11b/CD18 integrins. Proc Natl Acad Sci U S A. 2005;102:431–436. doi: 10.1073/pnas.0405193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters W, Charo IF. Involvement of chemokine receptor 2 and its ligand, monocyte chemoattractant protein-1, in the development of atherosclerosis: lessons from knockout mice. Curr Opin Lipidol. 2001;12:175–180. doi: 10.1097/00041433-200104000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Naka Y, Bucciarelli LG, Wendt T, Lee LK, Rong LL, Ramasamy R, Yan SF, Schmidt AM. RAGE axis: Animal models and novel insights into the vascular complications of diabetes. Arterioscler Thromb Vasc Biol. 2004;24:1342–1349. doi: 10.1161/01.ATV.0000133191.71196.90. [DOI] [PubMed] [Google Scholar]
- 30.Kislinger T, Fu C, Huber B, Qu W, Taguchi A, Du Yan S, Hofmann M, Yan SF, Pischetsrieder M, Stern D, et al. Nε-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol Chem. 1999;274:31740–31749. doi: 10.1074/jbc.274.44.31740. [DOI] [PubMed] [Google Scholar]
- 31.Anderson MM, Requena JR, Crowley JR, Thorpe SR, Heinecke JW. The myeloperoxidase system of human phagocytes generates Nε-(carboxymethyl)lysine on proteins: a mechanism for producing advanced glycation end products at sites of inflammation. J Clin Invest. 1999;104:103–113. doi: 10.1172/JCI3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ritthaler U, Deng Y, Zhang Y, Greten J, Abel M, Sido B, Allenberg J, Otto G, Roth H, Bierhaus A, et al. Expression of receptors for advanced glycation end products in peripheral occlusive vascular disease. Am J Pathol. 1995;146:688–694. [PMC free article] [PubMed] [Google Scholar]
- 33.Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ, Jr, Chow WS, Stern D, Schmidt AM. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med. 1998;4:1025–1031. doi: 10.1038/2012. [DOI] [PubMed] [Google Scholar]
- 34.Yan SF, Ramasamy R, Naka Y, Schmidt AM. Glycation, inflammation, and RAGE: a scaffold for the macrovascular complications of diabetes and beyond. Circ Res. 2003;93:1159–1169. doi: 10.1161/01.RES.0000103862.26506.3D. [DOI] [PubMed] [Google Scholar]
- 35.Yoshimura T, Yuhki N, Moore SK, Appella E, Lerman MI, Leonard EJ. Human monocyte chemoattractant protein-1 (MCP-1): full-length cDNA cloning, expression in mitogen-stimulated blood mononuclear leukocytes, and sequence similarity to mouse competence gene JE. FEBS Lett. 1989;244:487–493. doi: 10.1016/0014-5793(89)80590-3. [DOI] [PubMed] [Google Scholar]
- 36.Gosling J, Slaymaker S, Gu L, Tseng S, Zlot CH, Young SG, Rollins BJ, Charo IF. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Invest. 1999;103:773–778. doi: 10.1172/JCI5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bräsen JH, Hakkinen T, Malle E, Beisiegel U, Yla-Herttuala S. Patterns of oxidized epitopes, but not NF-κB expression, change during atherogenesis in WHHL rabbits. Atherosclerosis. 2003;166:13–21. doi: 10.1016/s0021-9150(02)00130-2. [DOI] [PubMed] [Google Scholar]
- 38.Chen YL, Chang YJ, Jiang MJ. Monocyte chemotactic protein-1 gene and protein expression in atherogenesis of hypercholesterolemic rabbits. Atherosclerosis. 1999;143:115–123. doi: 10.1016/s0021-9150(98)00285-8. [DOI] [PubMed] [Google Scholar]
- 39.Murakami S, Kondo Y, Sakurai T, Kitajima H, Nagate T. Taurine suppresses development of atherosclerosis in Watanabe heritable hyperlipidemic (WHHL) rabbits. Atherosclerosis. 2002;163:79–87. doi: 10.1016/s0021-9150(01)00764-x. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Quinn MR. Chemokine production by rat alveolar macrophages is inhibited by taurine chloramine. Immunol Lett. 2002;80:27–32. doi: 10.1016/s0165-2478(01)00291-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.