Abstract
Background
Most studies point to a direct association between social support and better cancer outcomes. This study examined whether baseline social support is associated with better survival and fewer chemotherapy-related adverse events in older, early-stage breast cancer patients.
Methods
This study is part of a pre-planned secondary analysis of CALGB 49907/Alliance A171301, a randomized trial that compared standard adjuvant chemotherapy versus capecitabine in breast cancer patients 65 years of age or older. A subset of patients reported on the extent of their social support.
Results
The median age of this 331-patient cohort was 72 years (range: 65, 90); 179 (55%) were married, and 210 (65%) lived with someone. 145 patients (46%) described a social network of 0-10 people; 110 (35%) of 11-25; and 58 (19%) of 26 or more. The Medical Outcomes Study (MOS) social support survey revealed the median scores (range) for emotional/informational, tangible, positive social interaction, and affectionate social support were 94 (3, 100), 94 (0, 100), 96 (0, 100), and 100 (8, 100), respectively. Social support scores appeared stable over time and higher (more support) than in other cancer settings. No statistically significant associations were observed between social support and the outcomes of survival and adverse events in multivariate analyses. However, married patients had smaller tumors, and those with arthritis reported less social support.
Conclusion
Although social support did not predict survival and adverse events, the exploratory but plausible inverse associations with larger tumors and arthritis suggest social support merits further study in older breast cancer patients.
Introduction
Most published studies point to a direct association between robust social support and improved cancer outcomes, such as more favorable survival and better quality of life [1-4]. Social support is commonly defined as a network of close relatives and friends who can potentially help a cancer patient during illness [5]. In older patients, this support is of value because it helps compensate for the many losses -- loss of spouse/partner, loss of friends, loss of siblings, among others -- that occur at an accelerated pace once individuals have reached an older age. Social support likely enables older cancer patients to attend clinic appointments, to undergo diagnostic testing, to arrive at the chemotherapy unit for cancer treatment, to feel emotionally sustained during cancer therapy, to receive timely surveillance following cancer treatment -- in effect, to procure all the needed benefits of optimal cancer care.
The reasons for the continued study of social support appear at least twofold. First, few previous studies have provided an in depth social support assessment that includes patient-reported perceptions of social support as well as more detailed reporting of marital status, cohabitation status, and number of close friends and family members. Analyzing and reporting both patients' perceived and objective social support should help clarify discrepancies in the published literature on the relationship between social support and clinical outcomes, particularly in older cancer patients. Secondly, the published literature carries potential selection bias. Positive studies are more likely to be submitted for publication -- and to be published -- than negative ones [6]. The large number of positive published studies that speak to the advantages of social support might reflect nothing more than such bias. Thus, further studying social support in older cancer patients and reporting on study results regardless of their findings remains worthwhile.
The current study capitalized on a prospectively-conducted, randomized, adjuvant trial in older breast cancer patients (CALGB 49907/Alliance A171301). It sought to characterize social support within a cohort of older, early-stage breast cancer patients who received adjuvant chemotherapy. Specifically, the current study sought to test the following two hypotheses: 1) objective social support (that is, being married, living with someone, and/or having a large number of friends/family members) at the time of a breast cancer diagnosis has a favorable effect on survival and adverse events in patients 65 years of age or older and 2) older patients' greater perceived social support also has a similar favorable impact on these outcomes.
Methods
Overview
This study is a secondary analysis of CALGB 49907/Alliance A171301, a previously-reported clinical trial that examined adjuvant chemotherapy in early-stage breast cancer patients who were 65 years of age or older as part of a multi-site, National Cancer Institute-funded, cancer cooperative group trial [7]. Briefly, patients were randomly assigned to either standard chemotherapy (cyclophosphamide/methotrexate/fluorouracil for 6 cycles or doxorubicin/cyclophosphamide for 4 cycles) versus capecitabine for 6 cycles. Patients participated in a clinic visit that included an adverse event assessment with the Common Terminology Criteria (CTC, version 2.0) on day 1 of each cycle of chemotherapy followed thereafter by clinic visits every 6 months for 2 years and then annually for 15 years after study entry.
The above trial included a preplanned quality of life substudy, as described in detail by Kornblith and others [8]. To enroll in the substudy, patients had to be English- or Spanish-speaking with adequate cognitive and psychological function. Patients were consecutively approached after enrollment to the parent chemotherapy trial until a substudy sample size of 350 eligible patients was reached.
Social Support Assessment
The study reported here explored the implications of social support in this cohort, focusing on patients who completed questions on objective social support as well as on the previously-validated, 20-item, Medical Outcome Study (MOS) Social Support Survey [9]. This questionnaire includes a 4-domain scale of social support: emotional/informational support, tangible support, positive social interaction support, and affectionate support. These domains are for the most part self-explanatory, particularly in the context of the actual survey questions [10]. Social support was graded with a 100-point scale with higher scores denoting the highest degree of social support. Patients were asked to complete the questionnaire at baseline, mid-chemotherapy, 1 month post-chemotherapy, and then at 12, 18, and 24 months from their initial baseline assessment.
The MOS was especially advantageous because it not only includes 19 questions that captured patients' subjective feelings about social support but it also includes a question that allowed patients to report more objectively on the size of their social support network. This question was phrased, “About how many close friends and close relatives do you have now (people you feel at ease with and can talk to about what is on your mind)?” Furthermore, at study entry, patients were asked to complete two questions which also provided more objective measures of social support. One question was phrased, “What is your marital status?” with 5 choices that captured potential responses. The other was phrased, “With whom do you live?” and prompted patients to mark all that applied, including spouse/partner, children aged 18 years or younger, parents/parents-in-law, other relative, live alone, and other (specify). Thus, CALGB 49907/Alliance A171301 uniquely enabled patients to report on both subjective and objective measures of social support.
Data Analyses
Demographic and baseline social support data are presented descriptively. Because no salient differences in social support were observed among the treatment arms, all analyses were performed using the entire cohort. Comparative tests, as specified within each table, were used to examine associations between measures of social support and clinically relevant outcomes. Such analyses were adjusted for age when it was thought such clinical outcomes might vary at the extremes of the age spectrum. Relationships were explored between baseline social support (both objective and perceived) and other endpoints, such as overall survival, adverse events, and other exploratory endpoints of interest, such as patient morbidity. With respect to survival and subjective social support, MOS scores were dichotomized based on a perfect score of 100 versus any other score; a Cox proportional hazards model incorporated these dichotomized MOS scores along with study arm and tumor burden, which was characterized by tumor size and extent of lymph node involvement. A two-sided p-value of <0.05 is considered statistically significant. All analyses were performed with SAS, version 9 (Cary, North Carolina USA).
Results
Demographics
As noted, a total of 350 women were eligible for the quality of life, questionnaire portion of CALGB49907/Alliance A171301. Of these, 331 completed the baseline questionnaires of interest in this study and are the focus of this report. No demographic differences were observed between those who did and did not complete the social support questionnaires. The median age of this cohort was 72 years (range: 65, 90). Baseline demographics are summarized in Table 1.
Table 1. Baseline Characteristics; n=331*.
| CHARACTERISTIC** | |
|---|---|
|
| |
| Median age, in years (range) | 72 (65, 90) |
|
| |
| Study arm | |
| cyclophosphamide/methotrexate/5-fluorouracil or doxorubicin/cyclophosphamide | 171 (52) |
| capecitabine | 160 (48) |
|
| |
| Marital status | |
| Married | 179 (55) |
| Not married | 146 (45) |
|
| |
| Cohabitation | |
| With at least one person | 210 (65) |
| Alone | 115 (35) |
|
| |
| Size of support group | |
| 0-10 people | 145 (46) |
| 11-25 people | 110 (35) |
| 26+ people | 58 (19) |
|
| |
| Emotional/informational support score, median (range) | 94 (3.1, 100) |
|
| |
| Tangible support score, median (range) | 94 (0, 100) |
|
| |
| Social interaction support score, median (range) | 96 (0, 100) |
|
| |
| Affectionate support score, median (range) | 100 (8.3, 100) |
Numbers in parentheses refer to percentages unless otherwise specified.
Missing response data account for a sum of less than 331 at times.
Social Support
Objective measures showed that 179 patients (55%) were married. In response to the question, “With whom do you live?” 210 patients (65%) responded that they lived with at least one other person, and 115 (35%) lived alone (Table 1). The objective query on “close friends and close relatives” showed that the median size of patients' networks was 12 people (range: 0, 824). One hundred forty-five patients (46%) described a network that included 0-10 people; 110 (35%) patients described 11-25 people; and 58 (19%) patients described 26 or more people.
As per the MOS Social Support Questionnaire, patients reported that their baseline median (range) for the domains of emotional/informational, tangible, positive social interaction, and affectionate social support were 94 (3, 100), 94 (0, 100), 96 (0, 100), and 100 (8, 100), respectively (Table 1). Social support remained stable over time (Figure 1).
Figure 1.
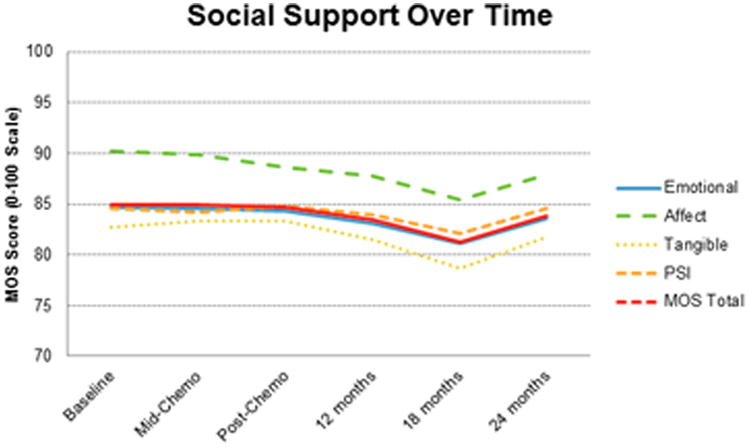
Social support remained stable over time, as indicated by mean values of MOS scores. The slight dip at 18 months was not statistically significant. Of note, the y-axis has been condensed.
In an exploratory manner, we examined whether baseline social support was associated with tumor characteristics. Patients who were married had smaller tumors than those not married, and, similarly, living alone was associated with a trend towards larger tumors (Table 2). In contrast, MOS scores were not associated with tumor size. No statistically significant associations were observed between lymph node tumor involvement and any of the social support variables (data not shown).
Table 2. Social Support and Tumor Size.
| TUMOR SIZE | 1-2 centimeters | > 2-5 centimeters | >5 centimeters | P-VALUE* |
|---|---|---|---|---|
|
| ||||
| size of social support group | ||||
| 0-10 | 58 (40) | 79 (55) | 7 (5) | 0.887 |
| 11-25 | 50 (45) | 55 (50) | 5 (5) | |
| 26+ | 24 (41) | 30 (52) | 4 (7) | |
|
| ||||
| Living status | ||||
| alone | 39 (34) | 69 (60) | 7 (6) | 0.053 |
| not alone | 100 (48) | 99 (47) | 10 (5) | |
|
| ||||
| Marital status | ||||
| married | 88 (49) | 85 (48) | 5 (3) | 0.008 |
| not married | 51 (35) | 83 (57) | 12 (8) | |
|
| ||||
| Emotional/informational support (mean (SD)) | 86 (19) | 84 (20) | 85 (18) | 0.52 |
|
| ||||
| Tangible support (mean (SD)) | 85 (21) | 80 (24) | 88 915) | 0.16 |
|
| ||||
| Social interaction support (mean (SD)) | 87 (19) | 82 (22) | 89 (18) | 0.10 |
|
| ||||
| Affectionate support (mean (SD)) | 91 (18) | 90 (18) | 90 (18) | 0.4 |
Chi square test or Kruskall Wallis used for analyses as appropriate.
We also explored relationships between social support and certain categories of morbidity that we thought might potentially influence social support. We found no consistent associations with cognitive function, number of comorbid conditions, glaucoma, and circulatory issues (data not shown). However, patients with arthritis reported less social support across all four domains of the MOS compared to patients without arthritis with mean scores (standard deviations) of 82.4 (20.4) and 88.1 (17.5) for emotional/informational (p=0.006); 79.1 (23.8) and 87.7 (19.3) for tangible(p=0.0001); 81.4 (22.2) and 89.1 (17.8) for positive social interaction (p=0.0002); and 88.4 (19) and 92.7 (15.6) for affectionate (p=0.01), respectively.
Social Support, Survival, and Adverse Events
The median survival for the cohort has not yet been attained. At the time of this report, 107 deaths had occurred. Although univariate analyses suggested that being married and not living alone were associated with better survival, in multivariate analyses, no statistically significant differences in survival were observed based on extent of social support, regardless of whether comparisons centered on size of support group, marital status, cohabitation status, or MOS score (Table 3).
Table 3. Social Support and Survival.
| SOCIAL SUPPORT MEASURE | HAZARD RATIO (95% confidence interval) | P-VALUE | ADJUSTED* HAZARD RATIO (95% confidence interval) | P-VALUE |
|---|---|---|---|---|
|
| ||||
| size of social support group | ||||
| 0-10 versus 26+ | 1.53 (0.90, 2.61) | 0.12 | 1.30 (0.75, 2.23) | 0.35 |
| 11-25 versus 26+ | 0.95 (0.53, 1.69) | 0.95 | 0.87 (0.48, 1.57) | 0.63 |
|
| ||||
| Living status | ||||
| alone versus not | 1.49 (1.01, 2.20) | 0.04 | 1.25 (0.85, 1.86) | 0.26 |
|
| ||||
| Marital status | ||||
| married versus not | 0.69 (0.47, 1.00) | 0.05 | 0.84 (0.57, 1.26) | 0.40 |
|
| ||||
| Emotional/informational support | 1.02 (0.68, 1.52) | 0.92 | 1.00 (0.66, 1.50) | 0.99 |
|
| ||||
| Tangible support | 0.98 (0.66, 1.45) | 0.91 | 1.07 (0.72, 1.59) | 0.75 |
|
| ||||
| Social interaction support | 0.79 (0.54, 1.16) | 0.23 | 0.80 (0.54, 1.20) | 0.28 |
|
| ||||
| Affectionate support | 0.97 (0.65, 1.43) | 0.87 | 0.95 (0.64, 1.42) | 0.81 |
In the multivariate analyses, adjustments were made for tumor burden (a function of tumor size and lymph node status) and number of tumor-positive nodes.
Of note, 189 patients (57%) suffered one or more severe adverse events. However, no statistically significant relationships were observed between extent of social support and the development of severe adverse events (Table 4). Additionally, baseline social support, regardless of how it was assessed, was not significantly associated with whether a patient completed all her chemotherapy on protocol (data not shown).
Table 4. Social Support and Severe Adverse Events.
| SOCIAL SUPPORT MEASURE** | SEVERE ADVERSE EVENT | NO SEVERE ADVERSE EVENT | P-VALUE* |
|---|---|---|---|
|
| |||
| size of social support group | |||
| 0-10 | 75 (52) | 70 (48) | |
| 11-25 | 67 (61) | 43 (39) | 0.230 |
| 26+ | 36 (62) | 22 (38) | |
|
| |||
| Living status | |||
| alone | 61 (53) | 54 (47) | 0.225 |
| not alone | 126 (60) | 84 (40) | |
|
| |||
| Marital status | |||
| married | 106 (59) | 73 (41) | 0.498 |
| not married | 81 (56) | 65 (45) | |
|
| |||
| Emotional/informational support (mean (standard deviation (SD))) | 84(19) | 86 (18) | 0.466 |
|
| |||
| Tangible support (mean (SD)) | 83 (22) | 83 (23) | 0.976 |
|
| |||
| Social interaction support (mean (SD)) | 85 (22) | 85 (20) | 0.666 |
|
| |||
| Affectionate support (mean (SD)) | 89 (19) | 91 (16) | 0.293 |
t-test
Missing response data account for a sum of less than 331 at times.
Discussion
This study is one of many to examine the implications of social support in patients with cancer [1-4]. We sought to test the hypothesis that objective social support has a favorable effect on survival and adverse events in early-stage breast cancer patients 65 years of age or older and found this was not the case. We also sought to test whether older patients' greater perceived social support has a favorable impact on survival and adverse events and observed it does not.
In contrast to our findings, Lutgendorf and others examined 168 ovarian cancer patients, who admittedly were contending with a more advanced and lethal malignancy [2]. These investigators reported that a more robust subjective measure of social support, as assessed by means of patient-completed questionnaires, was associated with a lower likelihood of death [2]. Similarly, Deiperink and others examined 337 patients with T1-T3 prostate cancer and, although they did not report on adverse events, they did report on quality of life. They observed that, although cancer stage and dose of radiation had no impact on quality of life, patients who described living alone, an objective measure of social support, described inferior quality of life [3]. To our knowledge, few prior studies have measured both objective and subjective social support. In our study, we found that neither demonstrated a statistically significant association with survival nor with adverse events from chemotherapy.
It remains unclear why so many other studies have identified an association between greater social support and better cancer outcomes and why this study did not. In addition to the explanations advanced earlier, another reason for this discrepancy is that the current study was a companion trial to a prospectively-conducted clinical trial in early-stage, potentially curable patients and may therefore have selected patients with a greater degree of baseline social support and better functional status. To be able to enroll in a clinical trial, to commit to extra testing (including extensive questionnaire completion), and to remain willing to participate in a well-defined plan of follow up post-chemotherapy is more likely to be possible in a cancer patient who has greater social support. Indeed, in the current study, the median size of a patient's network of people was 12, a number that seems substantial enough to be able to provide a patient the extra help she may need to participate in a clinical trial. Moreover, a recent study from Leung and others in breast cancer patients, who were not clinical trial participants, showed that the MOS questionnaire yielded overall lower social support scores within their cohort than what we observed in ours [11]. Thus, it seems possible that the overall high degree of social support in our cohort precluded our ability to discern major differences in clinical outcomes based on extent of social support, and it seems plausible that clinical trial participation selects for patients who have a higher degree of social support at baseline and who are healthier than the cancer population as a whole.
A second explanation for these absent associations may involve sample size. The sample size of the current study is relatively modest when compared to a few of the other studies that observed social support had a positive impact on cancer outcomes [1,4]. It is conceivable that the favorable impact of social support is subtle and that detecting this impact requires a much larger sample size than what was used in our analyses. These two explanations that social support might facilitate trial participation and that a large sample size might be necessary to detect the impact of social support are in fact interrelated and provide potential explanations for why the current study did not find that social support was associated with better cancer outcomes.
Yet a third explanation for the missing associations observed here may involve our multivariate analyses. Although we did observe some direct associations between social support and survival in univariate analyses – with patients living with someone or being married showing trends in favor of living longer –these associations lost their statistical significance in multivariate analyses. Thus, it is possible that many of the previous studies that observed notable direct relationships between social support and survival did not adjust for the same factors that we did.
Nonetheless, we do report three interesting observations. First, patients who were married were diagnosed with smaller breast tumors. This observation is the result of a post hoc exploratory analysis, but it appears plausible. Having a spouse does likely lead to patients' seeking healthcare more readily. Perhaps a spouse or cohabiting individual is more likely to urge a patient to seek healthcare sooner after the initial detection of a breast mass or perhaps even to be more adherent to routine cancer screening. This last observation is in keeping with what others have reported on the relationship between social support and early cancer diagnosis [12,13]. Second, it also appears plausible that arthritis symptoms have a negative impact on social interactions because of compromised mobility, thereby restricting a patient's social support network. Indeed, in a recent analysis that examined the social implications of low back pain, Froud and others commented on how patients “struggle to meet social expectations and obligations” and on how some ultimately “withdraw” because of their inability to meet social demands [14]. Third, we observed stable social support over time per MOS scores. To our knowledge, this observation has not been previously reported, particularly over a long span of 2 years, as shown in our data. This observation might be viewed as reassuring within an older cohort who, with aging, appears more vulnerable for suffering from a decline in social support. Although these three observations were generated in an exploratory fashion, they seem noteworthy.
In conclusion, despite a lack of statistically significant findings with respect to our main endpoints, we believe there continues to be a strong impetus to study social support in older cancer patients, to understand the factors that contribute to social support, and to better understand its clinical implications in patients with a variety of cancer types. Our reported associations between social support and tumor size as well as arthritis symptoms suggest that the former can impact the health of older patients. Future studies should perhaps focus on other endpoints, in addition to survival and adverse events, and test ways to compensate for the health disadvantages that appear to occur in older patients with more limited social support.
Acknowledgments
The research for CALGB 49907 and Alliance A171301 was supported, in part, by grants National Cancer Institute of the National Institutes of Health under Award Numbers: CA31946 and CA180821 to the Alliance for Clinical Trials in Oncology (Monica M. Bertagnolli, M.D., Chair) and CA33601 and CA180882 to the Alliance Statistics and Data Center (Daniel J. Sargent, Ph.D.). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
References
- 1.Aizer AA, Chen MH, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31:3869–3876. doi: 10.1200/JCO.2013.49.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lutgendorf SK, De Geest K, Bender D, et al. Social influences on clinical outcomes of patients with ovarian cancer. J Clin Oncol. 2012;30:2885–2890. doi: 10.1200/JCO.2011.39.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deiperink KB, Hansen S, Wagner L, et al. Living alone, obesity, and smoking: important factors for quality of life after radiotherapy and androgen deprivation for prostate cancer. Acta Oncol. 2012;51:722–729. doi: 10.3109/0284186X.2012.682627. [DOI] [PubMed] [Google Scholar]
- 4.Cavalli-Bjorkman N, Qvortrup C, Sebjornsen S, et al. Lower treatment intensity and poorer survival in metastatic colorectal cancer patients who live alone. Br J Cancer. 2012;107:189–194. doi: 10.1038/bjc.2012.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sjolander C, Ahlstrom G. The meaning and validation of social support networks for close family of persons with advanced cancer. BMC Nursing. 2012;11:17. doi: 10.1186/1472-6955-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stern JM, Simes RJ. Publication bias: evidence of delayed publication in a cohort study of clinical research projects. BMJ. 1997;315:640–645. doi: 10.1136/bmj.315.7109.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muss HB, Berry DA, Cirrincione CT, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360:2055–2065. doi: 10.1056/NEJMoa0810266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kornblith AB, Lan L, Archer L, et al. Quality of life of older patients with early-stage breast cancer receiving adjuvant chemotherapy: a companion study to Cancer and Leukemia Group B 49907. J Clin Oncol. 2011;29:1022–1028. doi: 10.1200/JCO.2010.29.9859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 10.http://www.rand.org/health/surveys_tools/mos.html; last accessed May 26, 2014.
- 11.Leung J, Pachana NA, McLaughlin D. Social support and health-related quality of life in women with breast cancer: a longitudinal study. Psychooncology. 2014 Apr 3; doi: 10.1002/pon.3523. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Ekberg M, Callender M, Hamer H, Rogers S. Exploring the decision to participate in the National Heatlh Service Bowel Cancer Screening Programme. Eur J Cancer Prev. 2014 Jan 10; doi: 10.1097/CEJ.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 13.Chatwin J, Sanders C. The influence of social factors on help-seeking for people with lung cancer. Eur J Cancer Care. 2013;22:709–713. doi: 10.1111/ecc.12078. [DOI] [PubMed] [Google Scholar]
- 14.Froud R, Patterson S, Eldridge S, et al. A systematic review and meta-synthesis of the impact of low back pain on people's lives. BMC Musculoskeletal Disore. 2014 Feb 21; doi: 10.1186/1471-2474-15-50. [DOI] [PMC free article] [PubMed] [Google Scholar]


