Abstract
Objective
Clinically amyopathic dermatomyositis (CADM) is a subset of dermatomyositis (DM) presenting with the characteristic rash of DM without objective muscle weakness. Asian studies report that anti–melanoma differentiation–associated gene 5 (anti–MDA-5) autoantibody in CADM is associated with interstitial lung disease (ILD), particularly rapidly progressive ILD (RPILD). These associations have not been established in US myositis patients. The goal of our study was to determine the association of anti–MDA-5 autoantibody with ILD, RPILD, and survival in US patients with CADM and classic DM.
Methods
CADM patients were identified in the University of Pittsburgh Myositis Center Database and matched 1:1 (sex and age) to classic DM controls. Anti–MDA-5 was measured by serum enzyme-linked immunosorbent assay. Kaplan-Meier, log rank, and chi-square tests were used for analysis.
Results
We identified 61 CADM patients (62% women, mean age 48.2 years) and 61 classic DM controls (64% women, mean age 44.8 years). The frequencies of anti–MDA-5-positivity, ILD, and RPILD were similar in the 2 cohorts (MDA-5 positive: CADM 13.1% [8 of 61] and DM 13.1% [8 of 61], ILD positive: CADM 31.1% [19 of 61] and DM 26.2% [16 of 61], and RPILD positive: CADM 8.2% [5 of 61] and DM 5% [3 of 61]; P=1, 0.55, and 0.46, respectively). Anti–MDA-5-positivity was significantly associated with ILD, since 50% of MDA-5–positive subjects (8 of 16) had ILD versus 25.5% of MDA-5–negative subjects (27 of 106; P=0.04). Anti–MDA-5 was strongly associated with RPILD (P < 0.001). Anti–MDA-5–positive patients with ILD had worse baseline pulmonary function testing variables compared to anti–MDA-5–negative patients. Anti–MDA-5-positivity was significantly associated with poor survival (P=0.007).
Conclusion
Anti–MDA-5 antibody is significantly associated with ILD, RPILD, worse pulmonary outcome, and survival in US classic DM and CADM patients.
INTRODUCTION
The idiopathic inflammatory myopathies are a heterogeneous group of systemic autoimmune rheumatic disorders characterized by an immune-mediated attack on skeletal muscle and other organs resulting in muscle weakness and other systemic features. Two major subsets include classic dermatomyositis (DM) and polymyositis (1,2), clinically distinguished by the typical skin rashes of DM. Patients with predominant skin involvement, termed amyopathic DM, manifest the hallmark cutaneous features of DM for 6 months or longer without developing proximal muscle weakness, elevated serum muscle enzymes, or abnormalities on other muscle tests such as electromyography (EMG) or muscle biopsy (3,4). However, another subset of patients with hypomyopathic DM has mild or subclinical evidence of muscle involvement without objective muscle weakness. They may have mildly elevated muscle enzymes, subtle myopathic changes on EMG, or other muscle imaging findings with or without muscle biopsy abnormalities. Therefore, a different term encompasses both amyopathic DM and hypomyopathic DM with the designation clinically amyopathic DM (CADM), referring to a DM subset with pathognomonic rashes with or without subtle myopathic features and no overt muscle weakness (5,6).
Significance & Innovations.
Anti–melanoma differentiation–associated gene 5 (anti–MDA-5) is significantly associated with interstitial lung disease (ILD), rapidly progressive ILD, and worse pulmonary function at diagnosis in US patients with classic dermatomyositis (DM) and clinically amyopathic dermatomyositis (CADM).
Anti–MDA-5 positivity was predictive of poor survival even after controlling for diagnosis, age at diagnosis, sex, ethnicity, smoking, and ILD.
CADM is even more intriguing because there are ethnic and/or geographic differences in the clinical features of these patients. The experience in several Asian countries has been very interesting, as CADM demonstrates an increased frequency of rapidly progressive interstitial lung disease (RPILD), with many patients possessing an autoantibody termed anti-CADM140 (or anti–melanoma differentiation–associated gene 5 [anti–MDA-5]) (7–10). This was originally described in Japan, but subsequently reported in China, with anti–MDA-5 associated with a similarly high prevalence of RPILD (11). These observations have not been seen in the US DM population, and a recent study proposed that anti–MDA-5 positivity was not associated with RPILD (12).
The goal of our study was to determine the association of anti–MDA-5 antibody with ILD, RPILD, and outcome in US patients with CADM compared to classic DM. Our hypothesis was that the anti–MDA-5 autoantibody is indeed associated with ILD, RPILD, and a poor prognosis in US DM patients.
PATIENTS AND METHODS
Study design
The University of Pittsburgh Myositis Center Database in the division of rheumatology includes prospectively collected clinical, laboratory, and serologic data with a matching serum repository for nearly 3 decades. In our university, rheumatology, dermatology, and neurology have strong clinic collaborations, since many patients first presenting to dermatology and neurology are referred to rheumatology and enrolled in the myositis database. CADM patients were selected consecutively from the patients enrolled in our database from January 1985 to July 2013 (n=450), with CADM defined by one of the typical DM rashes without objective muscle weakness for at least 6 months after rash onset and no or minimal abnormalities of serum muscle enzymes (<3 × upper limit of normal), EMG, or muscle biopsy (i.e., minimal histologic changes not significant enough to make a conclusive diagnosis). The DM patients were selected similarly from the patients enrolled in our database from January 1985 to July 2013 and matched 1:1 (sex and age ±5 years) to CADM patients. The CADM and classic DM cohorts were further dichotomized as MDA-5 positive versus negative and assessed for clinical outcomes. Pulmonary outcomes such as functional studies (pulmonary function tests [PFTs]), imaging (chest radiograph and high-resolution computerized tomography [HRCT]), oxygen requirement, and death or transplant were assessed using our myositis database as well as the electronic medical record system when necessary. ILD was defined as pulmonary fibrosis seen on chest radiography or HRCT. RPILD was defined as acute and progressive worsening of dyspnea secondary to ILD requiring hospitalization, supplementary oxygen, or respiratory failure requiring intubation within 3 months of the diagnosis of ILD. For the patients with ILD/ RPILD, composite pulmonary outcomes, including PFT worsening (defined as ≥10% decline in baseline forced vital capacity [FVC] or ≥15% decline in baseline diffusing capacity for carbon monoxide) at any time or development of RPILD, were evaluated. Survival outcomes such as time to death or time to RPILD were assessed and cause of death was ascertained. Patients with an unknown status or indeterminate cause of death (n=11) were submitted to the National Death Index and the resultant cause of death codes, along with independent chart review by one physician (SM-K) and Social Security Death Index, were used to determine the primary cause of death. Cumulative and event-free survival (event was defined as death) were evaluated. Survival outcomes were assessed as time to death due to ILD.
Anti–MDA-5 autoantibody was measured by a commercially available enzyme-linked immunosorbent assay (ELISA) kit (MBL) in the CADM and classic DM cohorts using serum stored during the first outpatient visit of the patient to the University of Pittsburgh Myositis Center (or hospital). The ELISA kit utilized a recombinant protein encompassing the entire amino acid sequence of MDA-5, which was expressed and purified using a baculovirus expression system, in accordance with a previous report (13).
Statistical analysis
Chi-square test and Student’s t-test were used to assess the association of demographic features, ILD, RPILD, and anti–MDA-5 with CADM compared to classic DM, and to assess differences in pulmonary outcomes between DM/ILD and CADM/ILD. Similarly, all outcomes were compared between MDA-5–positive patients and MDA-5–negative patients as well as CADM and classic DM. Four subgroups (MDA-5–positive CADM, MDA-5–positive DM, MDA-5–negative CADM, and MDA-5–negative DM) were also compared for outcomes. Kaplan-Meier curve with log rank test was used for survival analyses (time to death in all patients and time to pulmonary outcomes in those with ILD) between the various groups. Cox proportional hazards model was used to compare survival and pulmonary outcomes after controlling for confounding factors (sex, ethnicity, smoking, diagnosis, age at diagnosis, and ILD).
RESULTS
We identified 61 CADM patients and 61 age- and sex-matched classic DM controls. There were 62% and 64% women and 92% and 87% whites, with a mean±SD age of 48.2±16.9 and 44.8±17.6 years, in the classic DM and CADM cohorts, respectively. The frequency of anti–MDA-5 positivity was similar in both the CADM (8 [13.1%] of 61) and classic DM (8 [13.1%] of 61) cohorts (P=1) (Table 1). Among the 16 MDA-5–positive patients, 14 (87.5%) were white and the remaining 2 (12.5%) were African American. Among the 106 MDA-5–negative patients, 95 (89.6%) were white, 5 (4.7%) were African American, 2 (1.9%) were Asian, 1 (0.9%) was Hispanic, and 3 (2.8%) were unknown. Therefore, interestingly, none of our MDA-5–positive patients were Asian. The frequencies of ILD (31% CADM [19 of 61] versus 26%classic DM [16 of 61]) and RPILD (8% CADM [5 of 61] versus 5% classic DM [3 of 61]) were also similar in both cohorts (P=0.55 and 0.46, respectively) (Table 1).
Table 1.
Frequency of anti–MDA-5 antibody, ILD, and RPILD in CADM compared to classic DM*
| CADM (n=61) | Classic DM (n=61) | P | |
|---|---|---|---|
| Anti–MDA-5 | 8 (13.1) | 8 (13.1) | 1 |
| ILD | 19 (31.1) | 16 (26.2) | 0.55 |
| RPILD | 5 (8.2) | 3 (5) | 0.46 |
Values are the number (percentage). Anti–MDA-5=anti–melanoma differentiation–associated gene 5; ILD=interstitial lung disease; RPILD=rapidly progressive ILD; CADM=clinically amyopathic dermatomyositis; DM = dermatomyositis.
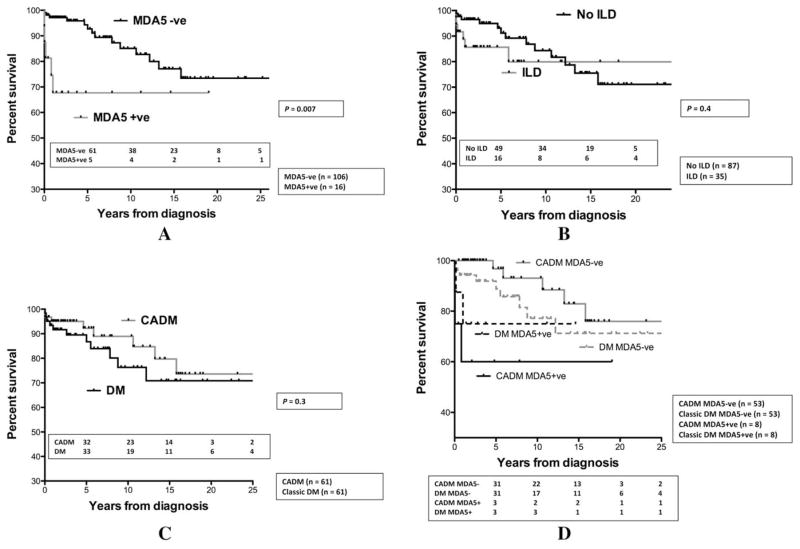
Anti–MDA-5-positivity was significantly associated with ILD, as 50% of MDA-5–positive subjects (8 of 16) had ILD compared to only 25.5% of MDA-5–negative subjects (27 of 106; P=0.04) (Table 2). Anti–MDA-5 was strongly associated with RPILD, as 87.5% of MDA-5–positive patients with ILD (7 of 8) had RPILD compared to only 3.7% of MDA-5–negative patients with ILD (1 of 27) having RPILD (P< 0.001) (Table 2). Early death was observed in 5 of the 7 anti–MDA-5–positive patients with RPILD (3 dying within 1 month and 2 within 1 year), whereas the singleMDA-5–negative patient with RPILD died 5.8 years after diagnosis. Among the 8 anti–MDA-5–positive patients with ILD, baseline PFT variables were only available in 3, as the severity of RPILD precluded testing in the remaining patients. The FVC was 48%, 30%, and 41% and the forced expiratory volume in 1 second (FEV1) was 57%, 37%, and 54% in these 3 patients compared to the anti–MDA-5–negative cohort with ILD (n=18), collectively demonstrating a mean FVC of 79% and a mean FEV1 of 84% at baseline. Also, among the 7 MDA-5–positive patients with RPILD, 5 were hospitalized for respiratory failure requiring intubation and 3 died within a few weeks. The respiratory status stabilized in 2 MDA-5–positive patients with RPILD who were followed after diagnosis (1 year in one patient and 2 years in the other patient), and they are still alive. Conversely, the MDA-5–negative patients with ILD generally did well, as their median declines from baseline to last FVC and FEV1 were 3% (interquartile range [IQR] 2–35%) and 14% (IQR 3–36%), respectively, in the 18 subjects with available PFTs. The composite pulmonary outcome of RPILD or decline in PFT was seen in 7 of 8 MDA-5–positive patients with ILD versus only 7 of 27 MDA-5–negative patients (P=0.002). Anti–MDA-5 positivity was significantly associated with poor survival (67% at both 5 and 10 years) compared to the MDA-5–negative cohort (92% at 5 years and 85% at 10 years; P=0.007) (Figure 1A). These differences are not believed to be due to a higher frequency of ILD in MDA-5–positive patients, since patients with ILD had similar survival compared to patients without ILD (Figure 1B). However, RPILD definitely led to poor survival among MDA-5–positive patients. The CADM cohort had a similar survival to classic DM patients (Figure 1C). The MDA-5–positive CADM and classic DM patients had a similar survival to the MDA-5–negative CADM and classic DM patients. As one might expect, among the 4 groups, the MDA-5–negative CADM cohort had the best survival (Figure 1D).
Table 2.
Frequency of ILD and RPILD in anti–MDA-5–positive and anti–MDA-5–negative DM patients*
| Anti–MDA-5 positive (n=16) | Anti–MDA-5 negative (n=106) | P | |
|---|---|---|---|
| ILD | 8 (50) | 27 (25.5) | 0.043 |
| RPILD | 7 (87.5) | 1 (3.7) | < 0.001 |
Values are the number (percentage) of the total clinically amyopathic dermatomyositis (DM) and DM patients (n=122). ILD=interstitial lung disease; RPILD=rapidly progressive ILD; anti–MDA-5=anti–melanoma differentiation–associated gene 5.
Figure 1.
Kaplan-Meier survival curves for A, melanoma differentiation–associated gene 5 (MDA-5) positive versus MDA-5 negative, B, interstitial lung disease (ILD) versus no ILD, C, clinically amyopathic dermatomyositis (CADM) versus classic dermatomyositis (DM), and D, MDA-5–positive CADM versus MDA-5–positive DM versus MDA-5–negative CADM versus MDA-5–negative DM. Anti–MDA-5 positivity was significantly associated with poor survival. CADM and ILD were not predictive of survival.
RPILD had a very poor prognosis, with a hazard ratio (HR) of 28 (95% confidence interval [95% CI] 9–86, P < 0.001). Multivariate analysis suggested that anti–MDA-5 positivity predicted survival (HR 7 [95%CI 2–23], P=0.001) even after controlling for diagnosis (CADM versus classic DM), age at diagnosis, sex, ethnicity, smoking, and ILD (P=0.002).
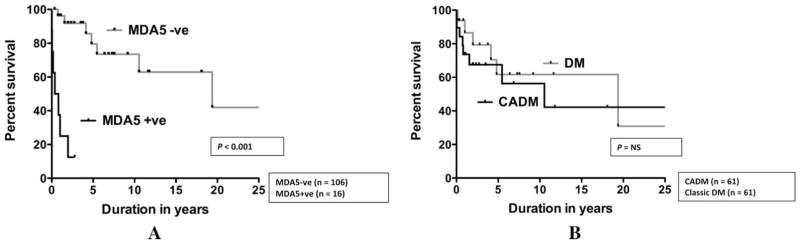
Analyzing the patients with ILD, anti–MDA-5 positivity was significantly associated with a poor pulmonary outcome using the aforementioned composite pulmonary outcome (P < 0.001) (Figure 2). CADM, however, was not predictive of a poor pulmonary outcome.
Figure 2.
Kaplan-Meier pulmonary outcome curves for interstitial lung disease patients with A, melanoma differentiation–associated gene 5 (MDA-5) positive versus MDA-5 negative, and B, clinically amyopathic dermatomyositis (CADM) versus classic dermatomyositis (DM). Anti–MDA-5 positivity was significantly associated with poor pulmonary outcome. CADM was not predictive of poor pulmonary outcome. NS=not significant.
DISCUSSION
ILD in myositis is an under-recognized and deadly complication of this rare autoimmune disease. In classic DM and CADM, this is a particularly perplexing and tragic complication worldwide, most notably in several Asian countries (7–11). Studies on CADM and the role of anti–MDA-5 as a potential biomarker of ILD in the US population have been limited.
In our experience, anti–MDA-5 was significantly associated with ILD, RPILD, and poor survival in US DM patients. However, there were no differences between classic DM and CADM in terms of pulmonary involvement or survival in an age- and sex-matched cohort. This observation is contrary to findings from other US centers. A retrospective study by Morganroth et al showed that the ILD prevalence was not different between patients with classic DM and CADM (14). This was the first systematic comparison of CADM and classic DM in the US, but the investigators did not assess the autoantibody profile. A study of 77 DM patients seen at a dermatology clinic at Stanford University found that anti–MDA-5 is associated with ILD and a unique cutaneous phenotype consisting of tender palmar papules and/or skin ulceration (15). The association between anti–MDA-5 and ILD is consistent with our findings, but the investigators did not assess RPLID. Another report in US patients showed that anti–MDA-5–positive patients were more likely to have features seen with the antisynthetase syndrome, but without RPILD (12). Our findings are clearly different and demonstrate a strong association between anti–MDA-5 and RPILD in a US cohort of classic DM and CADM patients. Previously, anti–MDA-5 autoantibody had been suggested as a risk factor for RPILD in Asian patients with inflammatory myopathies, and in a recent single-center cohort of 64 consecutive Chinese patients with myositis, anti–MDA-5 was detected in 26 patients with classic DM or CADM (11). Anti–MDA-5–positive patients showed a higher prevalence of RPILD compared to anti–MDA-5–negative patients (P=0.001). Therefore, our findings mirror the observations of Asian cohorts of CADM in that our US patients with CADM (or classic DM) possessing the anti–MDA-5 autoantibody have similarly severe RPILD leading to high mortality. Due to the lack of overt muscle weakness, such patients may not be diagnosed and treated in a timely manner, particularly if the DM skin rash is subtle or if there are other poorly recognized features of an autoimmune illness.
A poor pulmonary outcome (based on the composite metric of RPILD or a decline in PFT) was seen in 7 (87.5%) of 8 MDA-5–positive patients with ILD compared to 7 (25.9%) of 27 MDA-5–negative patients (P=0.002), suggesting that one-quarter of patients without MDA-5 antibody have chronic progressive ILD. It is also interesting to note that although anti–MDA-5 positivity was significantly associated with poor pulmonary outcomes, the subset of CADM itself was not associated with anti–MDA-5, ILD, RPILD, or poor pulmonary outcomes in US patients compared to classic DM. This is in contrast to that observed in Asian patients, where in a recent cohort of 114 Japanese patients with polymyositis/DM/CADM–ILD, the diagnosis of CADM was found to be a significant predictor of poor prognosis and overall mortality (16). However, there may still be an interaction between anti–MDA-5 status and disease subset (CADM versus classic DM) that this study was underpowered to detect. Among the 4 groups of MDA-5–negative CADM, MDA-5–negative classic DM, MDA-5–positive CADM, and MDA-5–positive classic DM, the MDA-5–negative CADM cohort had the best survival and the MDA-5–positive CADM cohort had the worst survival. Both classic DM groups had intermediate survival. It appears that anti–MDA-5 positivity had a larger effect on survival in the CADM patients than in the classic DM patients.
In our experience, anti–MDA-5 had a similar frequency in both CADM and classic DM. In a total of 233 patients with anti–MDA-5 antibody derived from 16 studies, Japanese patients had a higher frequency of CADM compared to non-Japanese patients (74.7% versus 39.2%; P=not significant) (16). Therefore, the distribution of CADM and classic DM in patients with anti–MDA-5 may vary among different ethnic groups, assuming the ascertainment of muscle disease is similar among reported cohorts. Therefore, anti–MDA-5 may not exclusively segregate to CADM, but rather is associated with a phenotype consisting of ILD and RPILD in US patients.
In this retrospective cohort, selection bias was a limitation to the study. We attempted to minimize selection bias by selecting the CADM and classic DM groups without knowing the survival or pulmonary outcome. Additionally, most data (except missing data) are prospectively collected in our myositis database. However, since the patients were collected from a single center, the selection bias cannot be completely ruled out.
We conclude that anti–MDA-5 is significantly associated with ILD, RPILD, and poor survival in US DM patients. It is important to expand our understanding of this serious problem in the white population, including the characterization and study of additional patients as well as assessing the role of anti–MDA-5 as a potential biomarker of CADM and associated ILD.
Footnotes
Drs. Sato and Kuwana hold a patent on an anti-MDA5 antibody-measuring kit.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Aggarwal had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Moghadam-Kia, Oddis, Sato, Kuwana, Aggarwal.
Acquisition of data. Moghadam-Kia, Oddis, Sato, Kuwana, Aggarwal.
Analysis and interpretation of data. Moghadam-Kia, Oddis, Kuwana, Aggarwal.
References
- 1.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292:344–7. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 2.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts) N Engl J Med. 1975;292:403–7. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 3.Euwer RL, Sontheimer RD. Amyopathic dermatomyositis (dermatomyositis sine myositis). Presentation of six new cases and review of the literature. J Am Acad Dermatol. 1991;24:959–66. [PubMed] [Google Scholar]
- 4.Pearson C. Arthritis and allied conditions: a textbook of rheumatology. 9. Philadelphia (PA): Lea & Febiger; 1979. [Google Scholar]
- 5.Sontheimer RD. Would a new name hasten the acceptance of amyopathic dermatomyositis (dermatomyositis sine myositis) as a distinctive subset within the idiopathic inflammatory dermatomyopathies spectrum of clinical illness? J Am Acad Dermatol. 2002;46:626–36. doi: 10.1067/mjd.2002.120621. [DOI] [PubMed] [Google Scholar]
- 6.Gerami P, Schope JM, McDonald L, Walling HW, Sontheimer RD. A systematic review of adult-onset clinically amyopathic dermatomyositis (dermatomyositis sine myositis): a missing link within the spectrum of the idiopathic inflammatory myopathies. J Am Acad Dermatol. 2006;54:597–613. doi: 10.1016/j.jaad.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 7.Sato S, Hirakata M, Kuwana M, Suwa A, Inada S, Mimori T, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum. 2005;52:1571–6. doi: 10.1002/art.21023. [DOI] [PubMed] [Google Scholar]
- 8.Gono T, Kawaguchi Y, Satoh T, Kuwana M, Katsumata Y, Takagi K, et al. Clinical manifestation and prognostic factor in anti–melanoma differentiation–associated gene 5 antibody-associated interstitial lung disease as a complication of dermatomyositis. Rheumatology (Oxford) 2010;49:1713–9. doi: 10.1093/rheumatology/keq149. [DOI] [PubMed] [Google Scholar]
- 9.Hamaguchi Y, Kuwana M, Hoshino K, Hasegawa M, Kaji K, Matsushita T, et al. Clinical correlations with dermatomyositis-specific autoantibodies in adult Japanese patients with dermatomyositis: a multicenter cross-sectional study. Arch Dermatol. 2011;147:391–8. doi: 10.1001/archdermatol.2011.52. [DOI] [PubMed] [Google Scholar]
- 10.Nagata K, Tomii K, Nanjo S, Kubota M, Tachikawa R, Nishio M. Four cases of interstitial pneumonia associated with amyopathic dermatomyositis characterized by the anti-CADM-140 antibody. Nihon Kokyuki Gakkai Zasshi. 2011;49:30–6. In Japanese. [PubMed] [Google Scholar]
- 11.Chen Z, Cao M, Plana MN, Liang J, Cai H, Kuwana M, et al. Utility of anti–melanoma differentiation–associated gene 5 antibody measurement in identifying patients with dermatomyositis and a high risk for developing rapidly progressive interstitial lung disease: a review of the literature and a metaanalysis. Arthritis Care Res (Hoboken) 2013;65:1316–24. doi: 10.1002/acr.21985. [DOI] [PubMed] [Google Scholar]
- 12.Hall JC, Casciola-Rosen L, Samedy LA, Werner J, Owoyemi K, Danoff SK, et al. Anti–melanoma differentiation–associated protein 5–associated dermatomyositis: expanding the clinical spectrum. Arthritis Care Res (Hoboken) 2013;65:1307–15. doi: 10.1002/acr.21992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato S, Hoshino K, Satoh T, Fujita T, Kawakami Y, Fujita T, et al. RNA helicase encoded by melanoma differentiation–associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: association with rapidly progressive interstitial lung disease. Arthritis Rheum. 2009;60:2193–200. doi: 10.1002/art.24621. [DOI] [PubMed] [Google Scholar]
- 14.Morganroth PA, Kreider ME, Okawa J, Taylor L, Werth VP. Interstitial lung disease in classic and skin-predominant dermatomyositis: a retrospective study with screening recommendations. Arch Dermatol. 2010;146:729–38. doi: 10.1001/archdermatol.2010.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiorentino D, Chung L, Zwerner J, Rosen A, Casciola-Rosen L. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J Am Acad Dermatol. 2011;65:25–34. doi: 10.1016/j.jaad.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujisawa T, Hozumi H, Kono M, Enomoto N, Hashimoto D, Nakamura Y, et al. Prognostic factors for myositis-associated interstitial lung disease. PLoS One. 2014;9:e98824. doi: 10.1371/journal.pone.0098824. [DOI] [PMC free article] [PubMed] [Google Scholar]