SUMMARY
Astrocytes, once considered passive support cells, are increasingly appreciated as dynamic regulators of neuronal development and function, in part via secreted factors. The extent to which they similarly regulate oligodendrocytes, or proliferation and differentiation of oligodendrocyte progenitor cells (OPCs) is less well understood. Here, we generated astrocytes from human pluripotent stem cells (hiPSC-Astros) and demonstrate that immature astrocytes - as opposed to mature - promoted oligodendrogenesis in vitro. In the PVL mouse model of neonatal hypoxic/ischemic encephalopathy, associated with cerebral palsy in humans, transplanted immature hiPSC-Astros promote myelinogenesis and behavioral outcome. We further identified TIMP-1 as a selectively upregulated component secreted from immature hiPSC-Astros. Accordingly, in the rat PVL model, intranasal administration of conditioned medium from immature hiPSC-Astros promoted oligodendrocyte maturation in a TIMP-1 dependent manner. Our findings suggest stage-specific developmental interactions between astroglia and oligodendroglia, with important therapeutic implications for promoting myelinogenesis.
Keywords: human induced pluripotent stem cells, immature astrocytes, periventricular leukomalacia, oligodendrocytes, myelination
INTRODUCTION
Astrocytes play important roles in organizing and maintaining brain structure and function (Barres, 2008). Astrocytes go through prenatal and protracted postnatal maturation during development and can undergo a spectrum of functional changes associated with development (Molofsky et al., 2012; Pekny and Pekna, 2014), serving stage-specific roles in assisting neuronal development, such as synapse stabilization and elimination (Chung et al., 2013; Molofsky et al., 2012). However, it is unclear how astrocytes, at specific immature and mature stages, may differently regulate development of oligodendrocytes, myelin-producing cells in the CNS.
Human pluripotent stem cells (hPSCs) including human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs) have been efficiently differentiated to astrocytes (Emdad et al., 2011; Jiang et al., 2013b; Krencik et al., 2011; Roybon et al., 2013; Shaltouki et al., 2013). The progenies differentiated from hPSCs are reflective of very early human development (< 6 weeks) (Patterson et al., 2012). Particularly, hPSC-derived astrocytes differentiated by using chemically defined, xeno-free protocols can be maintained at an immature stage in culture (Chen et al., 2014a; Emdad et al., 2011; Jiang et al., 2013b; Krencik et al., 2011; Shaltouki et al., 2013). Moreover, hPSC-derived immature astrocytes can be further differentiated to astrocytes with defined mature phenotypes (Krencik et al., 2011; Roybon et al., 2013). Thus, astroglia derived from hPSCs provides an unprecedented opportunity to investigate the interaction between oligodendroglia and human astrocytes that are at defined immature and mature stages.
Astrocytes are implicated to influence myelination in myelin loss disorders. Prior studies demonstrate that oligodendrocytes preferentially remyelinate axons in areas containing astrocytes (Franklin et al., 1991; Talbott et al., 2005). However, astroglia-based therapy for myelin loss disorders is less studied (Chen et al., 2015), because most of the disorders are associated with profound astrocyte activation and formation of glial scar (Pekny and Pekna, 2014). Scaring astrocytes are regarded as a barrier to regeneration, partly due to secretion of factors that halt survival and differentiation of oligodendroglia progenitor cells (OPCs) (Back et al., 2005; Nash et al., 2011). Recent studies also suggest that in acute phase of injuries, astrogliosis is a defensive reaction. Reactive astrocytes recapitulate numerous processes involved in early development of immature astroglia and exhibit positive effects in the acute phase of injuries (Pekny and Pekna, 2014), but reactivated processes often go awry later, turning on the detrimental effects of the astrocytes on regeneration (Gallo and Deneen, 2014). Recent studies (Jiang et al., 2013b; Noble et al., 2011) demonstrate that transplanted immature astrocytes do not become reactive after CNS injury. Immature but not mature astrocytes are neuroprotective and suppress the activation of endogenous astrocytes and glial scar formation (Chen et al., 2015). Thus, there is a strong rationale to examine whether transplantation of hPSC-derived astrocytes at a defined immature stage could regulate differentiation of endogenous OPCs and promote myelinogenesis.
Perinatally acquired white matter injury, induced by perinatal hypoxia-ischemia and referred to as periventricular leukomalacia (PVL), is the most common cause of brain injury in premature infants. PVL is the leading cause of cerebral palsy and long-term neurological morbidity (Deng, 2010). Currently no effective treatment exists. One of major causes of the white matter injury is that pre-myelinating oligodendrocytes are particularly vulnerable in PVL (Haynes et al., 2003). The decrease of pre-myelinating oligodendrocytes leads to an upstream increase of OPCs, but these OPCs are arrested at the progenitor stage and fail to efficiently differentiate into myelin-producing oligodendrocytes (Fancy et al., 2011; Jablonska et al., 2012; Reid et al., 2012; Segovia et al., 2008). Here we report that immature hiPSC-derived astrocytes (“hiPSC-Astros”), but not mature hiPSC-Astros and mature astrocytes isolated from the human brain, strongly promote proliferation of OPCs and differentiation of OPCs to oligodendrocytes. Moreover, immature hiPSC-Astros promote myelination and recovery of behavioral performance in animal models of PVL injury. Mechanistically, we show that immature hiPSC-Astros regulate OPC differentiation via secreted molecules including tissue inhibitor of metalloproteinase-1 (TIMP-1) both in vitro and in vivo.
RESULTS
Generation of immature and mature hiPSC-Astros
By using our established protocol (Jiang et al., 2013b), we derived astroglia from two hiPSC lines generated from healthy individuals (Chen et al., 2014a) (Fig. S1A and B). These hiPSC-Astros expressed astroglial markers glial fibrillary acidic protein (GFAP) and S100β (Fig. S1C). We compared the regional identities of the hiPSC-Astros with human brain-derived astrocytes (hBrain-Astros) isolated from the cerebral cortex of human brain. Similar to hiPSC-Astros, hBrain-Astros expressed astroglial markers GFAP and S100β (Fig. 1A). We found that the hBrain-Astros mainly expressed the mid/forebrain marker OTX2 (Fig. 1B and G) but not the hindbrain/spinal cord-specific marker HOXB4 (Fig. 1C and G). There were nearly no hBrain-Astros expressing the ventral marker NKX2.1 (< 0.1%, Fig. 1D and G). Similarly, the vast majority of the hiPSC-Astros also showed mid/forebrain identity, as indicated by expressing OTX2 (Fig. 1B and G), but not HOXB4 (< 0.1%, Fig. 1C and G). A small percent of hiPSC-Astros weakly expressed NKX2.1 (Fig. 1C and G).
Figure 1. Differentiation of hiPSC-Astros to human astrocytes with mature phenotypes.
(A) Representatives of GFAP+ and S100β+ hiPSC-Astros and hBrain-Astros.
(B–D) Representatives of OTX2- (B), HOXB4- (C) and NKX2.1-expressing cells (D) in the S100β+ hiPSC-Astros and hBrain-Astros.
(E) Representative images showing that expression of NF1A is abundant in immature hiPSC-Astros, but markedly reduced in mature hiPSC-Astros and hBrain-Astros. Insets, enlarged images from the squared areas.
(F) Representatives of hCD44- and vimentin-expressing cells in hiPSC-Astros, hBrain-Astros and mature hiPSC-Astros.
(G) The percentage of GFAP-, S100β-, OTX2-, HOXB4- and NKX2.1-expressing cells in hiPSC-Astros and hBrain-Astros (n = 4 for each cell line). All quantitative data for hiPSC-Astros are analyses of pooled data collected from hiPSC1-Astros and hiPSC2-Astros.
(H) qPCR analysis of NF1A, hCD44 and vimentin (Vim) mRNA expression in the three groups of human astrocytes (n = 4 from each cell line). One-way ANOVA test, *P < 0.05, **P < 0.01 and ***P < 0.001.
(I) qPCR analysis of EAAT1 and 2 mRNA expression in the three groups of human astrocytes (n = 4 from each cell line). One-way ANOVA test, *P < 0.05.
(J) Glutamate uptake analysis showing that mature hiPSC-Astros and hBrain-Astros exhibit glutamate uptake at a higher rate than immature hiPSC-Astros (n = 4 from each cell line). This uptake capability is Na+-dependent and can be abolished by the Na+-free solution. One-way ANOVA test, *P < 0.05.
(K) Proliferation rate of the three groups of human astrocytes relative to HEK293 cells (n = 4 from each cell line). One-way ANOVA test, * P < 0.05 and ***P < 0.001. Scale bars, 50 μm. Blue, DAPI-stained nuclei.
Nearly all the hiPSC-Astros also expressed human CD44 (hCD44) and vimentin (Fig. S1D), suggesting that these hiPSC-Astros were immature (Liu et al., 2004) (Dahl et al., 1981; Jiang et al., 2013b). We then induced immature hiPSC-Astros to become mature astrocytes by the treatment of fibroblast growth factor 1 (FGF1), leukemia inhibitory factor (LIF) and ciliary neurotrophic factor (CNTF) (Krencik et al., 2011; Roybon et al., 2013). After 30 to 50-day treatment, we first examined expression of an astroglial maturation marker, nuclear factor-1A (NF1A) (Deneen et al., 2006; Roybon et al., 2013), in order to verify the mature phenotypes of these human astrocytes. The expression of NF1A was abundant in the immature hiPSC-Astros, but markedly reduced in the hBrain-Astros and mature hiPSC-Astros (Fig. 1E). Quantitative PCR results showed that the immature hiPSC-Astros had a significantly higher (3 fold) expression level of NF1A than hBrain-Astros and mature hiPSC-Astros (Fig. 1H). We further examined the expression of hCD44 and vimentin in these human astrocytes. Nearly all the hiPSC-Astros, hBrain-Astros and mature hiPSC-Astros were positive for hCD44 and vimentin staining (Fig. 1F). However, qPCR results indicated a significantly higher expression level of hCD44 in the immature hiPSC-Astros, compared to hBrain-Astros (1.7 fold) and mature hiPSC-Astros (2.6 fold) (Fig. 1H). Moreover, qPCR results also showed that immature hiPSC-Astro expressed the highest level of vimentin, which was 2.2 fold and 5.3 fold higher than hBrain-Astros and mature hiPSC-Astros, respectively (Fig. 1H). The expression levels of hCD44 and vimentin were higher in hBrain-Astros than those in mature hiPSC-Astros (Fig. 1H). We further observed that in the human brain tissues derived from normal patients at the age of less than 6 months, human immature astrocytes in situ labeled by GFAP or S100β also expressed hCD44 and vimentin (Fig. S1E and F).
We next quantified expression of mRNAs encoding the astrocyte-specific glutamate transporters excitatory amino acid transporter 1 and 2 (EAAT1 and 2) in all the astroglial preparations. Consistent with the previous study (Roybon et al., 2013), we found that EAAT1 was expressed at a higher level in mature hiPSC-Astros (1.9 fold) and hBrain-Astros (2.3 fold) than in immature hiPSC-Astros (Fig. 1I), while EAAT2 level was not significantly different. The hBrain-Astros expressed EAAT1 and 2 at levels similar to mature hiPSC-Astros. In agreement with the expression of glutamate transporters, hBrain-Astros and mature hiPSC-Astros had 1.6-fold increase in sodium-dependent glutamate transport activity, compared to immature hiPSC-Astros (Fig. 1J). In addition, it is generally accepted that mature astrocytes are not proliferative. We also observed significantly lower proliferation rate of mature hiPSC-Astros and hBrain-Astros than that of immature hiPSC-Astros (Fig. 1K). Together, these data demonstrate that the hiPSC-Astros in this study represent human astrocytes with immature phenotypes, whereas hBrain-Astros and the hiPSC-Astros treated with FGF1 represent human astrocytes with mature phenotypes.
Immature hiPSC-Astros promote proliferation of OPCs
To develop astroglia-based cell therapy for myelin loss disorders, we first investigated how the astroglia differentiated from hiPSCs interact with oligodendroglia, particularly in the presence of neurons. To this end, we fed primary mixed neuron/glia culture at 7 days in vitro (DIV) with astrocyte-conditioned medium (ACM) collected from immature hiPSC-Astros (hiPSC-Astro ACM), from hBrain-Astros (hBrain-Astro ACM) or from mature hiPSC-Astro (mature hiPSC-Astro ACM). We then examined the population of oligodendroglial lineage cells in the culture at DIV 14 (Fig. 2A). At DIV 7, there were βIIITubulin+ neurons, GFAP+ astrocytes and OPCs labeled by NG2+, platelet-derived growth factor receptor alpha (PDGFRα), or Olig2, but no MBP+ mature oligodendrocytes (Fig. 2B). At DIV 14, oligodendroglial lineage cells identified by Olig2 staining were found in control group (Fig. 2C and E), but few of them were proliferating as indicated by not expressing Ki67 (Fig. 2C and E). Compared to control group, more Olig2+ cells and Olig2+/Ki67+ proliferating cells were identified in the group treated with mature hiPSC-Astro ACM and in the group treated with hBrain-Astro ACM (Fig. 2C and E). Notably, compared to mature hiPSC-Astro ACM and hBrain-Astro ACM groups, the group treated with hiPSC-Astro ACM had much more Olig2+ cells and Olig2+/Ki67+ proliferating cells (Fig. 2C and E). For all the groups, the vast majority (> 93.0%) of the Olig2+ cells were labeled by PDGFRα, indicating their nature of OPCs (Fig. 2D and F). Hence, human astrocytes promote proliferation of endogenous OPCs in the primary culture. Immature hiPSC-Astros have much stronger capacity in promoting OPC proliferation than mature hiPSC-Astros and hBrain-Astros.
Figure 2. The effects of immature and mature human astrocytes on OPC proliferation.
(A) A schematic diagram showing that the primary mixed neuron/glia culture at DIV 7 were treated with ACM and the proliferation of OPCs is examined 7 days after treatment of ACM (DIV 14).
(B) Representatives of βIII-tubulin (βIIIT)+ neurons, GFAP+ astrocytes, and OPCs labeled by PDGFRα, NG2 and Olig2 in the mixed culture at DIV 7. There is no MBP+ oligodendrocyte in the culture.
(C) Representatives of Olig2+ oligodendroglial lineage cells and Ki67+ proliferating cells in the control (Cont) culture and cultures fed with hiPSC-Astro ACM, mature hiPSC-Astro ACM and hBrain-Astro ACM.
(D) Representatives of Olig2+ and PDGFRα+ in the cultures fed with the different ACM.
(E) The percentage of Olig2+ and Ki67+/Olig2+ cells in the cultures fed with the different ACM (n = 3 from each cell line). All the quantitative data for hiPSC-Astros and mature hiPSC-Astros are analyses of pooled data collected from hiPSC1-Astros and hiPSC2-Astros, and mature hiPSC1-Astros and hiPSC2-Astros, respectively. One-way ANOVA test, *P < 0.05, **P < 0.01, and ***P < 0.001, comparison between control group versus the groups treated with the different ACM; ##P < 0.01; and NS, not significant.
(F) Quantification of the percentage of PDGFRα+ cells in total Olig2+ cells (n = 3 from each cell line). One-way ANOVA test, P > 0.05. Scale bars, 50 μm.
Immature hiPSC-Astros boost differentiation of OPCs to oligodendrocytes
We next examined the population of MBP+ mature oligodendrocytes at 14 days (DIV 21) after addition of ACM (Fig. 3A). We used Olig1 to label the oligodendroglial cells in the cultures, because Olig2 expression might down-regulate as the OPCs initiate the myelination program (Jiang et al., 2013a). We found that in control group, there were few MBP+ cells (Fig. 3B and D), and the majority of Olig1+ oligodendroglia expressed PDGFRα (Fig. 3C), indicating that OPCs in control culture did not robustly differentiate to oligodendrocytes and were stuck in the progenitor cell stage. Compared to the control group, there were more MBP+ cells in the mature hiPSC-Astro ACM and hBrain-Astro ACM groups. Strikingly, large numbers of MBP+ oligodendrocytes were found in hiPSC-Astro ACM group, suggesting that hiPSC-Astro ACM promoted differentiation of OPCs to oligodendrocytes. Our previous study (Jiang et al., 2013b) reported the generation of two subtypes of human astrocytes from hESCs, NPC-Astros and Olig2PC-Astros. We observed that ACM collected from both of these two subtypes of hESC-derived immature astrocytes also strongly promoted OPC differentiation at DIV 21 (Fig. S2), indicating that the effects on OPC differentiation were common to all the immature astrocytes derived from hiPSCs and hESCs.
Figure 3. The effects of immature and mature human astrocytes on OPC differentiation.
(A) A schematic diagram showing that the differentiation of OPCs to oligodendrocytes in the primary mixed neuron/glia culture is examined after 14-day treatment of ACM (DIV 21).
(B) Representatives of MBP+ oligodendrocytes and MAP2+ neurons in Cont culture and the cultures fed with hiPSC-Astro ACM, mature hiPSC-Astro ACM and hBrain-Astro ACM. The squared areas are enlarged.
(C) Representatives of Olig1+ oligodendroglial lineage cells and PDGFRα+ OPCs in the mix cultures fed with the different ACM.
(D) Quantification of pooled data showing the percentage of MBP+, Olig1+ and Olig1+/ PDGFRα+ cells in the cultures fed with the different ACM (n = 3 from each cell line). One-way ANOVA test, *P < 0.05 and ***P < 0.001, comparison between control group versus the groups treated with the different ACM; ###P < 0.001; and NS, not significant.
(E) qPCR analysis of Olig1, Mbp, Plp, Cnp and Pdgfra expression in the cultures fed with the different ACM (n = 3 from each cell line). One-way ANOVA test, **P < 0.01 and ***P < 0.001, comparison between control group versus the groups treated with the different ACM; ##P < 0.01 and ###P < 0.001 and NS, not significant. Scale bars, 50 μm in the original and enlarged images. Blue, DAPI-stained nuclei.
To examine the population of OPCs in the culture at DIV 21, we double stained Olig1 with PDGFRα. As expected, we found that there were significantly more Olig1+ oligodendroglia in hiPSC-Astro ACM group (Fig. 3C and D), compared to control group, mature hiPSC-Astro ACM group and hBrain-Astro ACM group. Moreover, significantly more Olig1+/PDGFRα+ OPCs were found in hiPSC-Astro ACM group (Fig. 3C and D) than in control group, mature hiPSC-Astro ACM group and hBrain-Astro ACM group. To further confirm these findings, we did qPCR to examine the gene expression of Olig1, mature oligodendroglial markers Mbp, Plp, and Cnp, and OPC marker Pdgfra. We consistently observed that hiPSC-Astro ACM group had the highest expression of Olig1 (Fig. 3E). Gene transcripts encoding mature oligodendrocyte markers were also highly expressed in hiPSC-Astro ACM group. In particular, Mbp expression was 16.6 fold higher in hiPSC-Astro ACM group than that in control group. The expression Plp and Cnp was respectively 9.2 fold and 2.1 fold higher in hiPSC-Astro ACM group than those in control group. The expression of Pdgfra was lower (0.5 fold) in mature hiPSC-Astro ACM and hBrain-Astro ACM groups, compared to control and hiPSC-Astro ACM groups. Altogether, these data indicate that compared to the addition of mature hiPSC-Astro ACM or hBrain-Astros ACM, the addition of hiPSC-Astro ACM not only replenishes the OPCs pool by robustly promoting OPC proliferation, but also strongly boosts the differentiation of OPCs to oligodendrocytes.
Immature hiPSC-Astros regulate OPC differentiation partly via release of TIMP-1
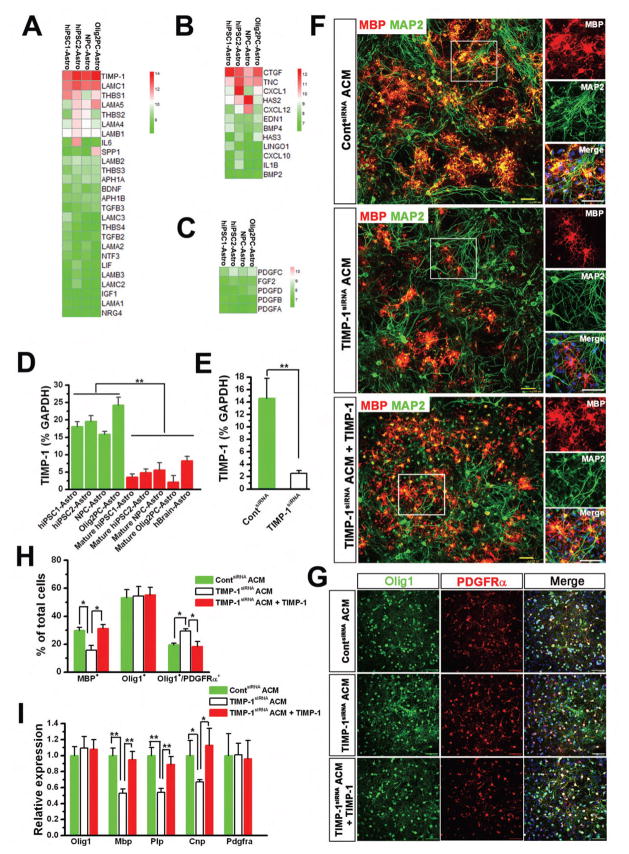
To explore the possible mechanisms underlying the effects of immature hiPSC-Astros on OPCs, we added the hiPSC-Astro ACM to purified culture of primary mouse OPCs, and found that hiPSC-Astro ACM similarly promoted proliferation and differentiation of OPCs in the purified culture (Fig. S3), suggesting that hiPSC-Astro ACM had direct effects on OPCs. Then, we performed global gene expression microarray and analyzed the data obtained from hiPSC1-Astros, hiPSC2-Astros and hESC-derived astrocytes, including NPC-Astros and Olig2PC-Astros (Chen et al., 2014a; Jiang et al., 2013b). We focused on analyzing the genes encoding astrocyte-secreted factors that were reported to directly regulate OPC proliferation and differentiation. The detailed information of these genes is shown in Table S1. Notably, immature astrocytes expressed gene transcripts encoding factors that both promote myelination, such as TIMP-1, laminin and TSPs (Fig. 4A), and inhibit myelination, such as CTGF, TnC and CXCL1 (Fig. 4B). Moreover, immature astrocytes expressed gene transcripts encoding PDGF and FGF2 (Fig. 4C), which are OPC mitogens. Therefore, the gene expression of secreted factors with various effects on OPCs may collectively account for the compositive effects of immature astrocytes on OPCs.
Figure 4. The role of TIMP-1 in the effects of hiPSC-Astros on OPC differentiation.
(A–C) Gene expression analysis of immature hiPSC-Astros (hiPSC1-Astro and hiPSC2-Astro) and hESC-Astros (NPC-Astros and Olig2PC-Astros). Heat maps focusing on gene transcripts encoding factors that are secreted by astrocytes and are involved in promoting OPC differentiation (A), inhibiting OPC differentiation (B), and increasing OPC proliferation (C). High expressions relative to mean are colored red. Low expressions are colored green.
(D) qPCR analysis of TIMP-1 expression in immature hiPSC-Astros and hESC-Astros, and in mature hiPSC-Astros, hESC-Astros and hBrain-Astros (n = 4 for each cell line). One-way ANOVA test, **P < 0.01.
(E) qPCR analysis of pooled data showing the expression of TIMP-1 in hiPSC-Astros at 48 h after transfection with control (Cont) and TIMP-1 siRNA (n = 3–5 from each line). Student’s t test, **P < 0.01.
(F) Representatives of MBP+ oligodendrocytes and MAP2+ neurons in the primary mixed neuron/glia cultures fed with ContsiRNA ACM, TIMP-1siRNA ACM or TIMP-1siRNA ACM supplemented with TIMP-1. The squared areas are enlarged.
(G) Representatives of Olig1+ and PDGFRα+ cells in the mix cultures fed with the different ACM.
(H) Quantification of pooled data showing the percentage of MBP+, Olig1+ and Olig1+/ PDGFRα+ cells in the cultures fed with the different ACM (n = 3 from each line). One-way ANOVA test, *P < 0.05.
(I) qPCR analysis of pooled data showing Olig1, Mbp, Plp, Cnp and Pdgfra expression in the cultures fed with the different ACM (n = 3 from each line). One-way ANOVA test, *P < 0.05 and **P < 0.01. Scale bars, 50 μm. Blue, DAPI-stained nuclei.
Among the gene transcripts encoding factors that promote myelination, we found that the top highly expressed gene was TIMP-1, which has been previously reported to critically regulate oligodendrocyte development in mice (Moore et al., 2011). We then verified the expression of TIMP-1 in all human astrocytes by qPCR. The result showed that TIMP-1 expression was abundant in immature hiPSC-Astros and hESC-Astros (Fig. 4D), whereas its expression was significantly decreased in mature hiPSC-Astros, mature hESC-Astros and hBrain-Astros (Fig. 4D). To further investigate the role TIMP-1 in the effects of immature hiPSC-Astros on OPC differentiation, we inhibited TIMP-1 expression in hiPSC-Astros by small interfering RNA (siRNA). qPCR analysis showed that, at 48 h after transfection of TIMP-1 siRNA, TIMP-1 expression was significantly reduced (5.8 fold), compared to hiPSC-Astros transfected with control siRNA (Fig. 4E). Next, we fed the primary mixed neuron/glia culture at DIV7 with conditioned medium collected from hiPSC-Astros transfected with TIMP-1 siRNA (TIMP-1siRNA ACM), control conditioned medium collected from hiPSC-Astros transfected with control siRNA (ContsiRNA ACM), and TIMP-1siRNA ACM supplemented with TIMP-1 (10 ng/ml). Then, we examined OPC differentiation at DIV 21. There were less MBP+ oligodendrocytes in TIMP-1siRNA ACM group than those in ContsiRNA ACM group (Fig. 4F and H). Adding TIMP-1 to TIMP-1siRNA ACM increased the percentage of MBP+ oligodendrocytes. The total number of Olig1+ cells was not significantly different among groups (Fig. 4G and H), suggesting that TIMP-1 knockdown did not change the effects on increasing OPC proliferation. We also observed that there were more Olig1+/PDGFRα+ OPCs in TIMP-1siRNA ACM group, compared to ContsiRNA and TIMP-1siRNA plus TIMP-1 ACM groups (Fig. 4G and H). Our qCPR results further confirmed these observations. Olig1 expression was not significantly different among the three groups (Fig. 4I). TIMP-1siRNA ACM group had significantly lower expression of mature oligodendrocyte genes, Mbp (~ 0.5 fold), Plp (~0.5 fold) and Cnp (~0.6 fold), compared to ContsiRNA and TIMP-1siRNA plus TIMP-1 ACM groups. Together, these data indicate that TIMP-1 secreted by immature hiPSC-Astros contributes to their effects on promoting OPC differentiation, but not to their effects on increasing OPC proliferation.
Transplanted immature hiPSC-Astros promote myelination in a mouse model of PVL
Considering the effects of immature hiPSC-Astros on OPCs in vitro, we hypothesized that transplantation of immature hiPSC-Astros might promote myelination after PVL injury. To facilitate survival of transplanted cells, we induced insults in P6 Rag1−/− immunodeficient mice using unilateral carotid ligation followed with hypoxia, which resulted in selective injury to the subcortical white matter, without detectable injury to cortical neurons (Liu et al., 2011a; Shen et al., 2010). Consistent with our previous studies (Liu et al., 2011a; Shen et al., 2010), we observed reactive astrogliosis at 4 days after induction of PVL injury selectively in the ipsilateral side of the brain (Fig. S4A–C). The contralateral side of the brain was not significantly changed after PVL injury and was used as control. Due to the extremely low proliferation rate of mature hiPSC-Astros and hBrain-Astros (Fig. 1K) and low engraftment efficiency commonly seen from transplantation of terminally differentiated mature cells, it was technically difficult to collect ample mature human astrocytes and perform transplantation experiments with a good cell survival rate. Thus, we only transplanted immature hiPSC-Astros into the mouse brain at P7 when we started to see reactive astrocytes (Fig. 5A). Immature hiPSC-Astros were grafted to the periventricular area adjacent to the corpus callosum (CC) where hypo-myelination was observed (Liu et al., 2011a; Shen et al., 2010) (Fig. 5B). No tumor formation or overgrowth of transplanted cells was observed throughout the experiments. Transplanted hiPSC-Astros identified by human nuclei (hN) staining survived in the mouse brains at 4 days after transplantation (P11) (Fig. 5C). The majority of the transplanted cells were found located close to the hippocampus and the lateral ventricle. Similar to our previous transplantation studies (Jiang et al., 2013b), a small percentage of the hiPSC-Astros expressed GFAP in vivo at 4 days after transplantation, as indicated by the double-labeling of GFAP and hN (Fig. 5C, 9.2 ± 1.4% of the hN+ cells were GFAP+, n = 4). The transplanted cells did not differentiate to oligodendrocytes, as indicated by expressing hCD44 but lack of MBP and Olig2 (Fig. 5C and Fig. S4D). Next, we examined the number of oligodendroglial lineage cells in the CC. Consistent with previous studies (Jablonska et al., 2012; Reid et al., 2012; Segovia et al., 2008), in vehicle control group, the number of Olig2+ oligodendroglia were increased in ipsilateral side, compared to contralateral side (Fig. 5D and E). Transplantation of hiPSC-Astros further increased the number of Olig2+ cells in the CC of the ipsilateral side, but did not significantly affect the contralateral side (Fig. 5D and E). Similar to our previous studies (Liu et al., 2011a), we observed severe hypo-myelination selectively in the ipsilateral side at 4 days after PVL insult, as indicated by MBP staining (Fig. 5D and F). Quantification of fluorescence intensity of MBP staining revealed that there was higher MBP immuno-positivity in ipsilateral side in hiPSC-Astro group than those in vehicle control group (Fig. 5D and F). We further examined the number of immature and mature oligodendroglia, respectively identified by Olig2+/CC1− and Olig2+/CC1+, in the ipsilateral sides of vehicle and hiPSC-Astro groups. There were significantly more immature and mature oligodendroglia cells in hiPSC-Astro group than in vehicle group (Fig. 5G). To further confirm that transplanted immature hiPSC-Astros promoted myelination, we examined the node of Ranvier and the density of myelinated axons in the ipsilateral side brains of the vehicle and hiPSC-Astro groups. Consistently, there were more nodes of Ranvier, identified by β IV spectrin+ staining flanked by Caspr+ staining, in the hiPSC-Astro group, compared to vehicle group (Fig. S4E). Moreover, the electron microscopic analyses demonstrated that the density of myelinated axons in hiPSC-Astro group was significantly higher than that in vehicle group (Fig. S4F). In addition, the majority of transplanted hiPSC-Astros abundantly expressed TIMP-1 at this time point (Fig. 5H). We also transplanted immature TIMP-1siRNA hiPSC-Astros, in which TIMP-1 expression was inhibited. MBP immuno-positivity in ipsilateral sides at P11 in TIMP-1siRNA hiPSC-Astro and vehicle control groups was not significantly different (Fig. S4G), suggesting that TIMP-1 secreted by transplanted hiPSC-Astros crucially contribute to the maturation of endogenous OPCs.
Figure 5. Transplantation of immature hiPSC-Astros into Rag1−/− mouse brains subjected to PVL.
(A) A schematic diagram showing the timeline for the in vivo experiments from postnatal day (P) 6 to 60.
(B) Left, a cresyl violet-stained coronal brain section at the level of the hippocampus, where analyses occurred. The red box outlines the white matter area used for immunohistochemical analyses of PVL insult. Right, a diagram showing the cell transplantation site, which is adjacent to the injured white matter area.
(C) Representative images showing that grafted hiPSC-Astros are identified by human nuclei (hN) staining at P11. The transplanted hiPSC-Astros are also identified by hCD44, but negative for MBP staining. The squared areas in the middle panels are enlarged in the bottom panels. Arrowheads indicate the hN+/GFAP+ cells. CC, corpus callosum; LV, lateral ventricle; and HIP, hippocampus.
(D) Upper panels, representative images showing that there are more Olig2+ oligodendroglial cells in the ipsilateral (Ipsi) side than in the contralateral (Contra) side brain in both vehicle and hiPSC-Astro groups. Transplantation of hiPSC-Astros further increases the expansion of Olig2+ cells in the Ipsi side. Lower panels, representative images showing that in vehicle group, MBP expression in the CC is only decreased substantially in the Ipsi side, with no significant changes in the Contra side. Transplantation of hiPSC-Astros promotes myelination in the Ipsi side and has no significant effect on the uninjured Contra side.
(E) The number of Olig2+ cells in the analyzed area (n = 4 for each group). Student’s t test, **P < 0.01, and ***P < 0.001, comparison between the Ipsi side versus the Contra side; and ##P < 0.01.
(F) Fluorescence intensity (FI) of MBP staining (n = 4 for each group). Data represent the FI value normalized to the Contra side brain of vehicle group. Student’s t test, *P < 0.05 and ***P < 0.001, comparison between Ipsi side versus Contra side; and #P < 0.05.
(G) Representatives and quantification of Olig2+/CC1+ and Olig2+/CC1− cells in the Ipsi side brains of vehicle and hiPSC-Astro groups. Arrows indicate Olig2+/CC1+ cells and arrowheads indicate Olig2+/CC1− cells. Student’s t test, **P < 0.01, and ***P < 0.001, n = 4 for each group.
(H) Representatives and quantification showing that the majority of hN+ transplanted hiPSC-Astros are positive for human TIMP-1 (hTIMP-1) staining at P11. Scar bars, 50 μm. n = 4. Scale bars, 50 μm. Blue, DAPI-stained nuclei.
Transplanted immature hiPSC-Astros promote recovery of behavioral performance and myelination
To examine the behavioral performance of the animals, three groups of animals were used: sham group in which animals received sham PVL surgery and no vehicle PBS injection or cell transplantation, vehicle group in which animals received PVL surgery and PBS injection after the surgery, and hiPSC-Astro group in which animals received PVL surgery and hiPSC-Astro transplantation after the surgery. Consistent with our previous study, we observed no significant difference in motor functions in climbing on the wall with a slope angle of 45º (Liu et al., 2011a) from P11 to P21 among the three groups of animals. Myelin mass changes are highly associated with the higher functions of the brain (Liu et al., 2012). Accumulative studies have shown that myelination deficiency contributes to the development of long-term deficits in learning and memory in both mouse and rat models of PVL (Cengiz et al., 2011; Huang et al., 2009). We thus examined learning and memory ability of P60 adult mice in Morris water maze. All mice were able to swim normally and locate the hidden platform during training trials in Morris water maze test. As expected, mice in vehicle group had worse performance and required more time to find the platform than those in sham group. Analysis of escape latency revealed significant differences among sham, vehicle and hiPSC-Astro groups (Fig. 6A and C (1)–(3)). On navigation days 3 to 5, mice in sham group showed less escape latency compared to mice in vehicle group. Notably, on navigation day 4 and 5, mice in hiPSC-Astro group showed better performance compared to mice in vehicle group. In the probe trials, mice in sham and hiPSC-Astro groups showed better performance and spent significantly more time than vehicle group in the quadrant where the platform had been (Fig. 6B and C (4)–(6)). Moreover, we examined the distribution of transplanted hiPSC-Astros in the P60 animals. hN+ cells were found close to the lateral ventricle and integrated into the hippocampus, close to the CA3 region (Fig. S5A). These transplanted cells maintained their astroglial lineage properties, as indicated by over half of them expressing GFAP (58.7 ± 3.4%, n = 7). However, at this time point, expression of TIMP-1 was undetectable in these transplanted hiPSC-Astros (Fig. S5B). We did not observe any abnormal cellular hypertrophy and massive proliferation of the vast majority of transplanted hiPSC-Astros, indicating that the transplanted hiPSC-Astro did not become reactive astrocytes.
Figure 6. The effects of transplanted immature hiPSC-Astros on behavioral recovery and myelin ultrastructure.
(A) Morris water-maze task is performed at P60 to test the spatial learning ability of sham (n = 7), vehicle (n = 8), and hiPSC-Astro (n = 10) transplanted groups, as shown by the time (escape latency) to find the submerged platform at navigation day 1 to 5. Two-way ANOVA test, *P < 0.05, comparison between vehicle group versus sham group; and #P < 0.05, comparison between vehicle group versus hiPSC-Astro group.
(B) Probe trials are performed 4 h after the last maze trails on navigation day 5, monitored by relative radial-quadrant occupancy (time spent in the target radial-quadrant) (n = 7 10). One way ANOVA test, *P < 0.05, comparison between vehicle group versus sham group; #P < 0.05, comparison between vehicle group versus hiPSC-Astro group; and NS, not significant.
(C) Representative sample paths from the maze trials ((1)–(3)) and the search patterns on the probe trials ((4)–(6)). (1),(4): sham group; (2),(5): vehicle group; (3),(6): hiPSC-Astro group.
(D) Low magnification electron micrographs showing a portion of corpus callosum from the animals in sham, vehicle and hiPSC-Astro groups. Scale bars, 2 μm.
(E) A enlarged image from vehicle group showing axons that have no compact myelin sheath (nma) among axons with myelin (ma). Scale bars, 500 nm.
(F) The density of myelinated axons of each group (n = 3 mice per group). One-way ANOVA test, *P < 0.05, comparison between vehicle group versus sham group; and # P < 0.05 comparison between vehicle group versus hiPSC-Astro group. NS, not significant.
(G) Representative electron micrographs in high magnification. Line “A” indicates diameter of a myelinated axon fiber and line “a” indicates diameter of axonal caliber. Scale bars, 0.25 μm.
(H) A scatter plot graph showing the relationship between g ratio values and axon diameters in the three groups (n = 3 mice per group).
(I) Mean g ratio of the three groups (n = 3 mice per group). One-way ANOVA test, **P < 0.01, comparison between vehicle group versus sham group; and ##P < 0.01, comparison between vehicle group versus hiPSC-Astro group, and NS, not significant.
Next, to further explore the differences in myelination that might contribute to the changes in behavioral performance, we first examined MBP expression in vehicle and hiPSC-Astro groups at P60. We found that there was no significant difference in MBP immuno-positivity between contralateral and ipsilateral sides from both vehicle and hiPSC-Astro groups (Fig. S5C and D). Previous studies have demonstrated that cellular recovery in oligodendrocytes does not correlate with proper axonal myelination (Jablonska et al., 2012). We thus used electron microscopy to determine whether in our model, neonatal hypoxic-ischemic injury caused abnormalities in ultrastructure of myelinated axons at P60. We found that unmyelinated axons were distributed among myelinated axons in vehicle group (Fig. 6D and E). Compared to sham group, the density of myelinated axons in vehicle group was significantly lower (Fig. 6F). The density of myelinated axons in hiPSC-Astro group was similar to that in sham group and was significantly higher than that in vehicle group (Fig. 6F). Under higher magnification, some myelinated axons exhibited thinner myelin sheath in vehicle group, compared to sham and hiPSC-Astro groups (Fig. 6G). Scatter plot graph of g ratio analysis in Fig. 6H demonstrated that g ratio values from vehicle group were mostly between 0.8–0.9; however, g ratio values from sham and hiPSC-Astro groups were largely overlapped and were mostly between 0.7–0.8, with some being around 0.6. Notably, the majority of low caliber axons were unmyelinated in vehicle group (Fig. 6D) and thus many myelinated axons with large diameters were included for g ratio analysis (Fig. 6H). Compared to vehicle group, sham and hiPSC-Astro groups had significantly lower g ratio values (Fig. 6I). The effects of transplanted hiPSC-Astros on both the density of myelinated axons and g ratio may indicate their impact on axons, in addition to their impact on oligodendroglia myelination. Moreover, the significant but small differences in the density of myelinated axons among the groups may suggest that in all conditions, oligodendrocytes are able to mature into myelin-forming cells but the maturation is accelerated in sham and hiPSC-Astro groups, compared with vehicle group.
Intranasal administration of immature hiPSC-Astro ACM promotes OPC maturation after PVL injury
To further compare the effects of immature and mature human astrocytes on OPC maturation after PVL injury and to investigate the role of TIMP-1, we intranasally applied the total concentrated factors collected from ACM. Similar to the mouse PVL model, unilateral carotid ligation followed by hypoxia (6% O2 for 1 hr) in rats at P7 also resulted in selective white matter injury without evident injury to cortical neurons (Follett et al., 2000). Here we used the rat model of PVL to facilitate intranasal administration, because neonatal rats have more easily visible nares than neonatal mice. Starting from P8, we applied control (Cont) medium that had not been exposed to cells, concentrated mature hiPSC-Astro ACM, as well as ContsiRNA ACM and TIMP-1siRNA ACM, collected from immature hiPSC-Astros transfected with control and TIMP-1 siRNA, respectively (Fig. 7A). The ELISA assay confirmed the higher abundance of human TIMP-1 in ContsiRNA ACM than that in TIMP-1siRNA ACM or mature hiPSC-Astro ACM (Fig. S6A). The intranasal route allowed effective delivery directly to the brain from the nasal mucosa, as indicated by ELISA assay of the brain tissues collected at 1 hr after the last intranasal administration (Fig. S6B). At P11, we found that administration of both ContsiRNA and TIMP-1siRNA ACM significantly increased the number of Olig2+ oligodendroglia not only in the ipsilateral side, but also in the contralateral side of the brain, compared to administration of Cont medium and mature hiPSC-Astro ACM (Fig. 7B and E). There were also more proliferating oligodendroglial cells, identified by Ki67+ and Olig2+, in both ipsilateral and contralateral sides in ContsiRNA and TIMP-1siRNA ACM groups than those in Cont medium and mature iPSC-Astro ACM groups (Fig. 7B and F). MBP staining revealed that there was higher MBP immuno-positivity in ipsilateral side brain from ContsiRNA ACM group than in Cont medium group (Fig. 7C and G), indicating that ACM from immature hiPSC-Astro promoted OPC maturation. Administration of TIMP-1siRNA ACM or mature hiPSC-Astro ACM was not able to promote OPC maturation after PVL injury (Fig. 7C and G). Since there was an increased number of Olig2+ cells but not MBP immuno-positivity in the ipsilateral side from TIMP-1siRNA ACM group, we further investigated whether these cells stayed at a progenitor stage. In the CC of the ipsilateral side, more Olig2+ cells expressing OPC marker NG2 were found in TIMP-1siRNA ACM group than in ContsiRNA ACM group (Fig. 7D and H). Together, these results demonstrate that released factors such asTIMP-1, mediate the effects of immature hiPSC-Astros on promoting OPC maturation after PVL injury.
Figure 7. Intranasal administration of ACM in a rat model of PVL.
(A) A schematic diagram showing the timeline for intranasal administration of ACM from P8 to P11.
(B) Representatives of Olig2+ oligodendroglial cells and Olig2+/Ki67+ proliferating oligodendroglial cells, in Ipsi and Contra side brains from the rats received intranasal administration of control (Cont) medium, ContsiRNA ACM, TIMP-1siRNA ACM and mature hiPSC-Astro ACM. CC, corpus callosum. Arrowheads indicate the Olig2+/Ki67+ cells.
(C) Representatives of MBP expression in the CC of both Contra and Ipsi side brains from the four groups.
(D) Representatives of Olig2+ and NG2+ cells in the CC of the Ipsi side brains from ContsiRNA ACM and TIMP-1siRNA ACM groups. Arrowheads indicate the Olig2+/NG2+ cells.
(E and F) The number of Olig2+ cells (E) and Olig2+/Ki67+ cells (F) in the analyzed area (n = 4 for each group). Student’s t test, *P < 0.05, and ***P < 0.01, comparison between Ipsi side versus Contra side within the different groups; and ##P < 0.01 and ###P< 0.001, comparison between the Ipsi or Contra side from Cont medium group versus that from the other groups.
(G) Fluorescence intensity (FI) of MBP staining (n = 4 for each group). Data represent the FI value normalized to the Contra side brain of Cont medium group. Student’s t test, *P < 0.05 and ***P < 0.001, comparison between Ipsi side versus Contra side within the different groups; and ##P < 0.01, comparison between the Ipsi or Contra side from Cont medium group versus that from the other groups.
(H) The number of Olig2+/NG2+ cells in the CC of the Ipsi side brains from ContsiRNA ACM and TIMP-1siRNA ACM group (n = 4 for each group). Student’s t test, **P < 0.01. Scar bars, 50 μm.
DISCUSSION
By differentiating hPSCs to astrocytes with defined immature and mature phenotypes, we report a novel regulation of oligodendrocyte development by astrocytes at a previously understudied immature stage. We demonstrate that hiPSC-derived immature astroglial transplant or ACM promotes myelinogenesis and improves behavioral outcome in animal models of PVL. These results implicate a novel strategy for promoting myelinogenesis by hiPSC-derived immature astroglia.
Due to a lack of efficient methods for purifying and maintaining astrocytes with defined immature and mature phenotypes in culture (Foo et al., 2011), no specific studies have been performed to examine how astrocytes at specific developmental stages interact with oligodendrocytes. Krencik et al. (2011) show that hPSC-Astros become mature in a serum-free condition after a long period of time (180 days). Roybon et al. (2013) report that hPSC-Astros cultured in a serum-containing medium (1% fetal bovine serum) exhibit mature phenotypes after a short exposure to FGF1 (7 days). Here, we promote maturation of hiPSC-Astros in a serum-free medium containing FGF1. We find that after 30 to 50-day culture, immature hiPSC-Astros become mature as indicated by increased EAAT1 expression and glutamate uptake, and decreased expression of NF1A, hCD44 and vimentin, consistent with the observations of astrocyte maturation in human tissue (Bjorklund et al., 1984; Girgrah et al., 1991; Yamada et al., 1992). Moreover, immature and mature hiPSC-Astros also possess forebrain identity similar to hBrain-Astros. Thus, using our protocol, immature and mature human astrocytes could be efficiently derived from hPSCs, providing new opportunities to study human astrocyte development and developmental interactions between astroglia and oligodendroglia.
Our microarray gene analyses show that immature human astrocytes express gene transcripts encoding OPC mitogens and factors that inhibit OPC maturation to myelinating oligodendrocytes. We propose that the inhibitory factors and OPC mitogens work synergistically to promote OPC proliferation. Moreover, immature astrocytes express gene transcripts encoding factors that promote OPC maturation and myelination. By a combination of qPCR, siRNA knockdown and intranasal ACM administration experiments, we provide both in vitro and in vivo evidence that TIMP-1 partially but critically mediates the effects of immature astrocytes on OPC differentiation, but not the effects on OPC proliferation. Hence, the effects of hiPSC-Astros on oligodendroglia could depend on which stage the oligodendroglia cells are at and which astrocyte-secreted factors the oligodendroglia cells are exposed to. The gene expression of secreted factors with multifaceted effects on OPCs may indicate finely regulatory effects of the immature astrocytes on oligodendroglial lineage progression. In addition, by analyzing a transcriptome database of mouse astrocytes (Cahoy et al., 2008), we consistently find that mouse immature astrocytes also express gene transcripts encoding factors that promote or inhibit myelination, and promote OPC proliferation (Fig. S7A–D). Interestingly, Tgfb2, encoding transforming growth factor β2 (TGFβ2), but not Timp-1, is prominently expressed in immature mouse astrocytes (Fig. S7B and E). Previous studies have demonstrated that TGFβ signaling pathway critically promotes oligodendrocyte differentiation (Palazuelos et al., 2014). Thus, it would be interesting to investigate whether immature human and mouse astrocytes regulate oligodendrocyte development through different mechanisms. The iPSC technology provides new opportunities to obtain human glial cells and to study their interactions. With further advancement and development in purification and separation of human astroglia and oligodendroglia (Sim et al., 2011), future studies are warranted to provide deep insights into human glial cell interactions.
Our in vitro findings indicate that immature hiPSC-Astros not only promote the OPC proliferation but also robustly boost OPC differentiation to oligodendrocytes. We further demonstrate that forebrain immature hiPSC-derived astroglial transplants rescue the hypo-myelination in a hypoxic/ischemic mouse model of PVL. We find that TIMP-1 expression is abundant in transplanted hiPSC-Astros at 4 days after transplantation and is undetectable at about 2 months after transplantation, which is consistent with our in vitro observation (Fig. 4D) and previously reported developmental regulation of TIMP-1 expression in astrocytes (Ulrich et al., 2005). Furthermore, our study of intranasal administration of ContsiRNA and TIMP-1siRNA ACM pinpoint the role of TIMP-1 in mediating the effects of ACM on promoting OPC differentiation in vivo. Taken together, through released factors such as TIMP-1, hiPSC-Astros promote myelination and behavioral recovery.
Intracerebral cell transplantation during the neonatal period is not ideal in clinical settings. We demonstrate that direct application of hiPSC-Astro ACM via intranasal administration promotes myelination after PVL injury. This study thus puts forward a notion of hiPSC-based cell-free therapy. It is tempting to suggest that this approach may be particularly useful in myelin disorders such as multiple sclerosis, where inflamed environment significantly compromises survival of transplanted cells. Administration of the cell-free, concentered factors that are released from human immature astrocytes may be effective in promoting remyelination (Chen et al., 2014b). Future secretomic studies may help identify more comprehensively all key factors released from hiPSC-Astros and further promote the translational potential of hiPSC-based cell-free therapy.
EXPERIMENTAL PROCEDURES
Culture of hiPSC
The two hiPSC lines, hiPSC1 and hiPSC2, were reprogrammed from healthy individuals’ fibroblasts by using retroviruses encoding OCT4, SOX2, KLF4 and c-MYC (Fig. S1A) (Chen et al., 2014a). All experiments conducted on hPSCs adhered to approved Stem Cell Research Oversight Committee at University of California, Davis.
Differentiation and culture of human astrocytes
Embryoid body-based differentiation procedure was used for astroglial differentiation of hiPSCs (Fig. S1B). The hBrain-Astros were isolated from the cerebral cortex of fetal human brain (ScienCell; Catalog number: 1800).
Generation of astrocyte-conditioned medium
ACM was concentrated 50-fold using centrifugal concentrators (Millipore). Protein concentration was determined by BCA assay (Thermo Scientific) and ACM was fed to primary mixed neuron/glia culture at 100 μg/ml. TIMP-1siRNA ACM supplemented with TIMP-1 (10 ng/ml; Peprotech) was also fed to the primary culture.
Immunostaining
Cells fixed with 4% paraformaldehyde and brain sections (18 μm thick) from P11–P60 mice and rats were processed for immunofluorescence staining (Liu et al., 2011b). The information for primary antibodies and dilutions were listed in Table S2. Images were captured using a Nikon Eclipse C1 or Nikon A1 confocal laser-scanning microscope.
Microarray analysis and heat maps
Illumina bead array was performed for gene expression analysis (Liu et al., 2006). Array data were processed using Illumina GenomeStudio software (Illumina).
PVL animal model
Animal experiments were performed following protocols approved by the Animal Care and Use Committees at the University of California, Davis. By unilateral carotid ligation (UCL) followed with hypoxia, we induced hypoxic/ischemic insults in P6 mouse pups of Rag1−/− immunodeficient mice (B6.129S7-Rag1tm1Mom on a C57BL/6 background, Jackson Lab) or P7 Long-Evans rat pups (Charles Rivers laboratories) (Follett et al., 2000).
Cell transplantation
One day after induction of hypoxic/ischemic injury in mice (P7), cell transplantation was performed (Fig. 5A). Hamilton syringe and needle were used to deliver cells by inserting directed through the skull into the target site (Chen et al., 2014a).
Intranasal administration
One day after induction of hypoxic/ischemic injury in rats, intranasal administration of concentrated ACM was performed (Fig. 7A). Control medium or ACM was administered every 12 h from P8 to P11.
Electron microscopy
Brain tissues were fixed and sectioned as previously described (Jiang et al., 2013a). EM images were captured using a high resolution CCD camera (Gatan, Pleasanton, CA). Images were processed using DigitalMicrograph (Gatan). EM images were analyzed using ImageJ software.
Morris water-maze test
The Morris water-maze test was performed with mice at P60 (Jiang et al., 2013b).
Data analysis
For all experiments, analysis was derived from at least three independent experiments. All data are represented as mean ± s.e.m. The escape latency in the behavioral training tests was determined by two-way repeated measures analysis of variance (ANOVA). All other assessments were analyzed using Student’s t test when only two groups were compared or one-way ANOVA when three or more groups were compared.
Supplementary Material
Acknowledgments
We thank Dr. Shenglan Li for assisting with gene expression analysis. This work was in part supported by grants from National Institutes of Health (R01NS061983, R01ES015988 and R01HD087566, to W.D.), the National Multiple Sclerosis Society (to W.D.) and Shriners Hospitals for Children (to W.D.). Y.L. was supported by California Institute of Regenerative Medicine (RT1-011071), the Memorial Hermann Foundation (Staman Ogilvie Fund) and the Bentsen Stroke Center. C.C. was supported by a postdoctoral fellowship from California Institute of Regenerative Medicine.
Footnotes
Author Contributions
PJ and WD designed experiments and interpreted data. PJ and CC carried out most of experiments with technical assistance from XL and YL. XL performed the electron microscopy analysis. YL performed the gene expression analysis. DEP provided critical input to the research design. WD directed the study. PJ and WD wrote the paper with input from all co-authors.
Competing Financial Interests
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Back SA, Tuohy TM, Chen H, Wallingford N, Craig A, Struve J, Luo NL, Banine F, Liu Y, Chang A, et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11:966–972. doi: 10.1038/nm1279. [DOI] [PubMed] [Google Scholar]
- Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Bjorklund H, Eriksdotter-Nilsson M, Dahl D, Olson L. Astrocytes in smears of CNS tissues as visualized by GFA and vimentin immunofluorescence. Med Biol. 1984;62:38–48. [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cengiz P, Uluc K, Kendigelen P, Akture E, Hutchinson E, Song C, Zhang L, Lee J, Budoff GE, Meyerand E, et al. Chronic neurological deficits in mice after perinatal hypoxia and ischemia correlate with hemispheric tissue loss and white matter injury detected by MRI. Dev Neurosci. 2011;33:270–279. doi: 10.1159/000328430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Chan A, Wen H, Chung SH, Deng W, Jiang P. Stem and Progenitor Cell-Derived Astroglia Therapies for Neurological Diseases. Trends Mol Med. 2015;21:715–729. doi: 10.1016/j.molmed.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Jiang P, Xue H, Peterson SE, Tran HT, McCann AE, Parast MM, Li S, Pleasure DE, Laurent LC, et al. Role of astroglia in Down’s syndrome revealed by patient-derived human-induced pluripotent stem cells. Nat Commun. 2014a;5:4430. doi: 10.1038/ncomms5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Coleman R, Leang R, Tran H, Kopf A, Walsh CM, Sears-Kraxberger I, Steward O, Macklin WB, Loring JF, et al. Human neural precursor cells promote neurologic recovery in a viral model of multiple sclerosis. Stem Cell Rep. 2014b;2:825–837. doi: 10.1016/j.stemcr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504:394–400. doi: 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl D, Rueger DC, Bignami A, Weber K, Osborn M. Vimentin, the 57 000 molecular weight protein of fibroblast filaments, is the major cytoskeletal component in immature glia. Eur J Cell Biol. 1981;24:191–196. [PubMed] [Google Scholar]
- Deneen B, Ho R, Lukaszewicz A, Hochstim CJ, Gronostajski RM, Anderson DJ. The transcription factor NFIA controls the onset of gliogenesis in the developing spinal cord. Neuron. 2006;52:953–968. doi: 10.1016/j.neuron.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Deng W. Neurobiology of injury to the developing brain. Nat Rev Neurol. 2010;6:328–336. doi: 10.1038/nrneurol.2010.53. [DOI] [PubMed] [Google Scholar]
- Emdad L, D’Souza SL, Kothari HP, Qadeer ZA, Germano IM. Efficient differentiation of human embryonic and induced pluripotent stem cells into functional astrocytes. Stem Cells Dev. 2011;21:404–410. doi: 10.1089/scd.2010.0560. [DOI] [PubMed] [Google Scholar]
- Fancy SP, Harrington EP, Yuen TJ, Silbereis JC, Zhao C, Baranzini SE, Bruce CC, Otero JJ, Huang EJ, Nusse R, et al. Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat Neurosci. 2011;14:1009–1016. doi: 10.1038/nn.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follett PL, Rosenberg PA, Volpe JJ, Jensen FE. NBQX attenuates excitotoxic injury in developing white matter. J Neurosci. 2000;20:9235–9241. doi: 10.1523/JNEUROSCI.20-24-09235.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo LC, Allen NJ, Bushong EA, Ventura PB, Chung WS, Zhou L, Cahoy JD, Daneman R, Zong H, Ellisman MH, et al. Development of a method for the purification and culture of rodent astrocytes. Neuron. 2011;71:799–811. doi: 10.1016/j.neuron.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJ, Crang AJ, Blakemore WF. Transplanted type-1 astrocytes facilitate repair of demyelinating lesions by host oligodendrocytes in adult rat spinal cord. J Neurocytol. 1991;20:420–430. doi: 10.1007/BF01355538. [DOI] [PubMed] [Google Scholar]
- Gallo V, Deneen B. Glial development: the crossroads of regeneration and repair in the CNS. Neuron. 2014;83:283–308. doi: 10.1016/j.neuron.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgrah N, Letarte M, Becker LE, Cruz TF, Theriault E, Moscarello MA. Localization of the CD44 glycoprotein to fibrous astrocytes in normal white matter and to reactive astrocytes in active lesions in multiple sclerosis. J Neuropathol Exp Neurol. 1991;50:779–792. doi: 10.1097/00005072-199111000-00009. [DOI] [PubMed] [Google Scholar]
- Haynes RL, Folkerth RD, Keefe RJ, Sung I, Swzeda LI, Rosenberg PA, Volpe JJ, Kinney HC. Nitrosative and oxidative injury to premyelinating oligodendrocytes in periventricular leukomalacia. J Neuropathol Exp Neurol. 2003;62:441–450. doi: 10.1093/jnen/62.5.441. [DOI] [PubMed] [Google Scholar]
- Huang Z, Liu J, Cheung PY, Chen C. Long-term cognitive impairment and myelination deficiency in a rat model of perinatal hypoxic-ischemic brain injury. Brain Res. 2009;1301:100–109. doi: 10.1016/j.brainres.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Jablonska B, Scafidi J, Aguirre A, Vaccarino F, Nguyen V, Borok E, Horvath TL, Rowitch DH, Gallo V. Oligodendrocyte regeneration after neonatal hypoxia requires FoxO1-mediated p27Kip1 expression. J Neurosci. 2012;32:14775–14793. doi: 10.1523/JNEUROSCI.2060-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P, Chen C, Liu XB, Selvaraj V, Liu W, Feldman DH, Liu Y, Pleasure DE, Li RA, Deng W. Generation and characterization of spiking and nonspiking oligodendroglial progenitor cells from embryonic stem cells. Stem Cells. 2013a;31:2620–2631. doi: 10.1002/stem.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P, Chen C, Wang R, Chechneva OV, Chung SH, Rao MS, Pleasure DE, Liu Y, Zhang Q, Deng W. hESC-derived Olig2+ progenitors generate a subtype of astroglia with protective effects against ischaemic brain injury. Nat Commun. 2013b;4:2196. doi: 10.1038/ncomms3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krencik R, Weick JP, Liu Y, Zhang ZJ, Zhang SC. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat Biotechnol. 2011;29:528–534. doi: 10.1038/nbt.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, Vialou V, Lobo MK, Dietz DM, Nestler EJ, et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nature Neurosci. 2012;15:1621–1623. doi: 10.1038/nn.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Shen Y, Plane JM, Pleasure DE, Deng W. Neuroprotective potential of erythropoietin and its derivative carbamylated erythropoietin in periventricular leukomalacia. Exp Neurol. 2011a;230:227–239. doi: 10.1016/j.expneurol.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Han SS, Wu Y, Tuohy TM, Xue H, Cai J, Back SA, Sherman LS, Fischer I, Rao MS. CD44 expression identifies astrocyte-restricted precursor cells. Dev Biol. 2004;276:31–46. doi: 10.1016/j.ydbio.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jiang P, Deng W. OLIG gene targeting in human pluripotent stem cells for motor neuron and oligodendrocyte differentiation. Nat Prot. 2011b;6:640–655. doi: 10.1038/nprot.2011.310. [DOI] [PubMed] [Google Scholar]
- Liu Y, Shin S, Zeng X, Zhan M, Gonzalez R, Mueller FJ, Schwartz CM, Xue H, Li H, Baker SC, et al. Genome wide profiling of human embryonic stem cells (hESCs), their derivatives and embryonal carcinoma cells to develop base profiles of U.S. Federal government approved hESC lines. BMC Dev Biol. 2006;6:20. doi: 10.1186/1471-213X-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Krencik R, Ullian EM, Tsai HH, Deneen B, Richardson WD, Barres BA, Rowitch DH. Astrocytes and disease: a neurodevelopmental perspective. Genes Dev. 2012;26:891–907. doi: 10.1101/gad.188326.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CS, Milner R, Nishiyama A, Frausto RF, Serwanski DR, Pagarigan RR, Whitton JL, Miller RH, Crocker SJ. Astrocytic tissue inhibitor of metalloproteinase-1 (TIMP-1) promotes oligodendrocyte differentiation and enhances CNS myelination. J Neurosci. 2011;31:6247–6254. doi: 10.1523/JNEUROSCI.5474-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash B, Thomson CE, Linington C, Arthur AT, McClure JD, McBride MW, Barnett SC. Functional duality of astrocytes in myelination. J Neurosci. 2011;31:13028–13038. doi: 10.1523/JNEUROSCI.1449-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble M, Davies JE, Mayer-Proschel M, Proschel C, Davies SJ. Precursor cell biology and the development of astrocyte transplantation therapies: lessons from spinal cord injury. Neurotherapeutics. 2011;8:677–693. doi: 10.1007/s13311-011-0071-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazuelos J, Klingener M, Aguirre A. TGFbeta signaling regulates the timing of CNS myelination by modulating oligodendrocyte progenitor cell cycle exit through SMAD3/4/FoxO1/Sp1. J Neurosci. 2014;34:7917–7930. doi: 10.1523/JNEUROSCI.0363-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson M, Chan DN, Ha I, Case D, Cui Y, Van Handel B, Mikkola HK, Lowry WE. Defining the nature of human pluripotent stem cell progeny. Cell Res. 2012;22:178–193. doi: 10.1038/cr.2011.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M, Pekna M. Astrocyte Reactivity and Reactive Astrogliosis: Costs and Benefits. Physiol Rev. 2014;94:1077–1098. doi: 10.1152/physrev.00041.2013. [DOI] [PubMed] [Google Scholar]
- Reid MV, Murray KA, Marsh ED, Golden JA, Simmons RA, Grinspan JB. Delayed myelination in an intrauterine growth retardation model is mediated by oxidative stress upregulating bone morphogenetic protein 4. J Neuropathol Exp Neurol. 2012;71:640–653. doi: 10.1097/NEN.0b013e31825cfa81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roybon L, Lamas NJ, Garcia-Diaz A, Yang EJ, Sattler R, Jackson-Lewis V, Kim YA, Kachel CA, Rothstein JD, Przedborski S, et al. Human stem cell-derived spinal cord astrocytes with defined mature or reactive phenotypes. Cell Rep. 2013;4:1035–1048. doi: 10.1016/j.celrep.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia KN, McClure M, Moravec M, Luo NL, Wan Y, Gong X, Riddle A, Craig A, Struve J, Sherman LS, et al. Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Annals Neurol. 2008;63:520–530. doi: 10.1002/ana.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaltouki A, Peng J, Liu Q, Rao MS, Zeng X. Efficient Generation of Astrocytes From Human Pluripotent Stem Cells in Defined Conditions. Stem Cells. 2013;31:941–952. doi: 10.1002/stem.1334. [DOI] [PubMed] [Google Scholar]
- Shen Y, Plane JM, Deng W. Mouse models of periventricular leukomalacia. J Vis Exp. 2010;39 doi: 10.3791/1951. pii: 1951. http://www.jove.com/index/Details.stp?ID=1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim FJ, McClain CR, Schanz SJ, Protack TL, Windrem MS, Goldman SA. CD140a identifies a population of highly myelinogenic, migration-competent and efficiently engrafting human oligodendrocyte progenitor cells. Nat Biotechnol. 2011;29:934–941. doi: 10.1038/nbt.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott JF, Loy DN, Liu Y, Qiu MS, Bunge MB, Rao MS, Whittemore SR. Endogenous Nkx2.2+/Olig2+ oligodendrocyte precursor cells fail to remyelinate the demyelinated adult rat spinal cord in the absence of astrocytes. Exp Neurol. 2005;192:11–24. doi: 10.1016/j.expneurol.2004.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich R, Gerhauser I, Seeliger F, Baumgartner W, Alldinger S. Matrix metalloproteinases and their inhibitors in the developing mouse brain and spinal cord: a reverse transcription quantitative polymerase chain reaction study. Dev Neurosci. 2005;27:408–418. doi: 10.1159/000088455. [DOI] [PubMed] [Google Scholar]
- Yamada T, Kawamata T, Walker DG, McGeer PL. Vimentin immunoreactivity in normal and pathological human brain tissue. Acta Neuropathologica. 1992;84:157–162. doi: 10.1007/BF00311389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.