Abstract
Planar cell polarity (PCP) information is a critical determinant of organ morphogenesis. While PCP in bounded epithelial sheets is increasingly well-understood, how PCP is organized in tubular and acinar tissues is not. Drosophila egg chambers (follicles) are an acinus-like ‘edgeless epithelium’ and exhibit a continuous, circumferential PCP that does not depend on pathways active in bounded epithelia; this follicle PCP directs formation of an ellipsoid rather than a spherical egg. Here we apply a novel imaging algorithm to ‘unroll’ the entire 3D tissue surface and comprehensively analyze PCP onset. This approach traces chiral symmetry-breaking to plus-end polarity of microtubules in the germarium, well before follicles form and rotate. PCP germarial microtubules provide chiral information that predicts the direction of whole-tissue rotation as soon as independent follicles form. Concordant microtubule polarity, but not microtubule alignment, requires the atypical cadherin Fat2, which acts at an early stage to translate plus-end bias into coordinated actin-mediated collective cell migration. Because microtubules are not required for PCP or migration after follicle rotation initiates, while dynamic actin and extracellular matrix are, polarized microtubules lie at the beginning of a handoff mechanism that passes early chiral PCP of the cytoskeleton to a supracellular planar polarized extracellular matrix and elongates the organ.
INTRODUCTION
Animal organs undergo development not in isolation, but in the context of global body axes that form in the early embryo. The process of planar cell polarity (PCP) allows organogenesis to occur with reference to these axes. In PCP, cells acquire a common subcellular organization within the plane of the often epithelial tissue. PCP organization involves two features: alignment with respect to a given axis, and a polarized orientation along this axis. This organization allows coordinated morphogenesis with respect to anterior-posterior, dorsal-ventral, or proximal-distal elements of the body plan (Gray et al., 2011; Wallingford, 2012; Zallen, 2007). Our understanding of PCP in morphogenesis comes almost exclusively from tissues that approximate flat sheets, and are generally treated as bounded. In paradigmatic tissues such as Drosophila wing, notum, eye and abdomen, PCP is organized by gradients of factors that specify global directional cues within the epithelium (Goodrich and Strutt, 2011; Singh and Mlodzik, 2012; Vladar et al., 2009). However, many organs contain tubular and acinar structures whose topology can be considered, in part, unbounded, and in which similarly localized sources of cartographic information may not be present. PCP around the circumference of these ‘edgeless epithelia’ is likely to involve distinct mechanisms of organization.
The Drosophila egg chamber (also called the follicle) is an acinar tissue that exhibits a non-canonical form of PCP (reviewed in (Bilder and Haigo, 2012; Cetera and Horne-Badovinac, 2015). This PCP is seen within the ‘follicle epithelium’ that encases the germline. Like paradigmatic PCP tissues, the follicle epithelium exhibits polarized cytoskeletal organization that is coordinated between cells. Basal F-actin filaments align within each follicle cell around the circumference of the tissue, perpendicular to the A-P axis. Alignment of microtubules can also be seen, as well as of fibril-like structures of extracellular matrix in the basement membrane. This organization drives a coordinated collective cell migration that leads the entire tissue to rotate circumferentially in a PCP fashion around the A-P axis. In a further parallel to many PCP tissues, follicle PCP drives A-P tissue elongation, changing shape of the growing egg chamber from an initial sphere to the distinctively ellipsoid egg. However, unlike paradigmatic PCP tissues that are polarized toward specific tissue boundaries, follicle PCP is continuous around the circumference of the tissue. Moreover, follicle PCP does not rely on Fat/Ds or Fz/Vang, the two major signaling pathways that direct PCP in bounded tissues (Bastock and Strutt, 2007; Viktorinova et al., 2009).
While mechanisms controlling tissue rotation continue to be elucidated, how PCP symmetry in the follicle is broken, and how this directs coordinated cellular motility of the tissue remains unclear. In this work we identify the earliest sign of PCP in the follicle and show that chiral polarity of MTs in the follicle precursors, rather than the follicle itself, is regulated by the atypical cadherin Fat2 and sets the template for subsequent PCP organization, tissue rotation, and organ elongation.
RESULTS
PCP rotation begins immediately following follicle budding
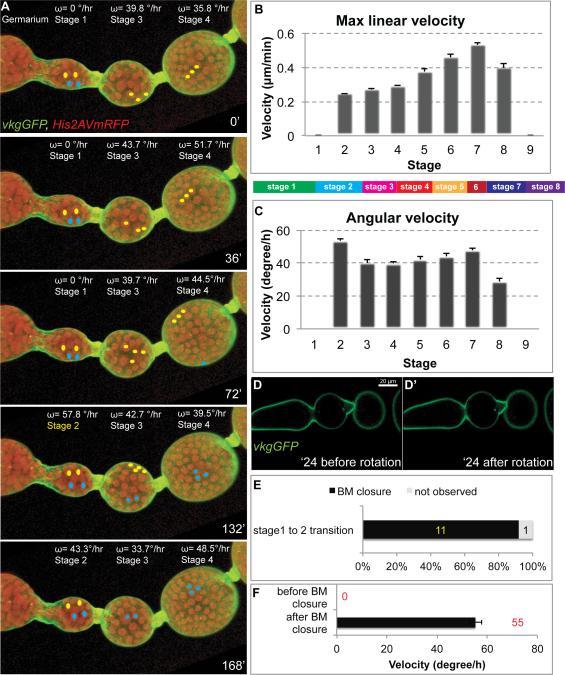
In order to investigate the origins of follicle PCP, we sought to examine its initial stages. Follicles form in region 3 of the germarium, where ~30 progeny of somatic stem cells residing in region 2b surround a germline cyst and establish a contiguous epithelium (Supplemental Fig. 1) (Nystul and Spradling, 2010). This structure, also called a stage 1 follicle, then progresses through 13 additional developmental stages to create a mature egg (Spradling, 1993). Our previous work described robust rotation at stage 5 but did not identify the time when it initiates (Haigo and Bilder, 2011). Live imaging demonstrates that follicles rotate, perpendicular to the A-P axis and with a stable chirality, shortly after they are formed (Fig. 1A; Supplemental Movie 1). Cetera et al., (2014) were first to report early follicle rotation, placing it at st. 1; we do not detect consistent axial movement at this stage. However, rotation was seen reliably at stage 2, after the stalk completes budding of the follicle from the germarium (Fig. 1A-F). We live-imaged the stage 1 to stage 2 transition and compared the timing of rotation initiation to the production of the follicle basement membrane (BM) over which the epithelial cells move. BM surrounds the stage 1 follicle at all regions except the anterior pole; BM is deposited in this region during stage 2 to isolate the follicle from the anterior stalk cells (Fig. 1D). Follicles in which Collagen IV (assessed by an α chain (Flybase:Vkg)-GFP fusion protein) was not detected between the epithelium and the anterior stalk did not progressively rotate, but 92% (n=12) of follicles with continuous anterior Collagen IVGFP rotated (Fig. 1D-F). Once initiated, the absolute speed of rotation increased over time, from 0.2 μm/min at stage 2 to 0.5 μm/min at stage 6 (measured at the equator, Fig. 1B). Interestingly, while rotation speed increased, the angular velocity of rotation was much less changed, approximating 40 degrees/hour from stage 2 to stage 6 (Fig. 1C). As follicles rotate with a determined alignment and orientation from the time that they first become isolated from external cells, the PCP symmetry-breaking that directs rotation must originate prior to follicle formation.
Figure 1. PCP rotation initiates during follicle budding.
(A) Stills (opacity projections) from live imaging of germarium through stage 4 follicles, with angular velocity (ω) of each given above. His2AV-mRFP (red) and Vkg-GFP (green) show nuclei and basement membrane respectively; colored dots mark tracked nuclei. The st. 1 follicle initially does not rotate, but between 72 and 132 minutes transitions to st. 2 and begins rotation (B, C) Maximum linear and angular velocity of rotating follicles (n>5 for each stage, bars represent SEM). Color-coded bar indicates relative time length of stages of oogenesis. (D) BM closure during st. 1-2 transition, shown in single confocal section from live imaging. In the non-rotating st. 1 follicle, BM at the anterior is non-continuous (arrowheads). Rotation initiates after BM becomes continuous around the entire follicle. (E, F) Quantitation of relationship between BM completion and follicle rotation (n=11, bars represent SEM).
Alignment of microtubules and actin precede PCP rotation
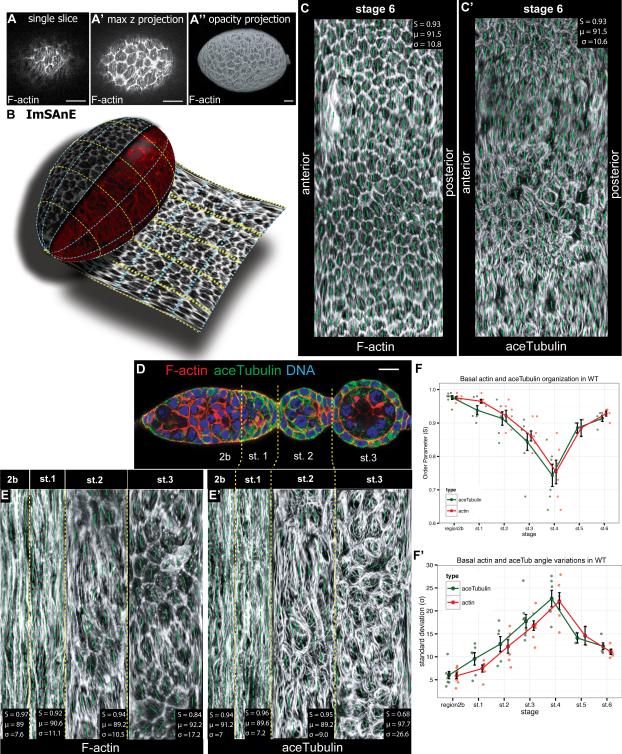
To identify the earliest molecular sign of follicle PCP, we examined the germarium, including stage 1 follicles, for proteins known to show PCP organization at later stages. An obstacle to comprehensive quantitative analysis of the germarium is its architecture. Like many tubular and acinar tissues, the germarium and early follicle have highly curved surfaces. Confocal sections of such tissues capture only the tangential fraction of the epithelial plane, while volumetric projections provide 3D visualization but not quantification along the curved surface; moreover, these approaches overrepresent the equator and miss the poles (Fig. 2A). To comprehensively and accurately assess PCP in the follicle epithelium, we employed a newly-developed imaging algorithm that can accurately analyze the surfaces of contoured objects. This algorithm, ImSAnE (Imaging Surface Analysis Environment), extracts the plane of the epithelium from three-dimensional stacks and projects it two-dimensionally; projections can be chosen to maintain area or orientation, while geometric observables, generally distorted in projections, can be correctly quantified using built-in correction methods (Fig. 2B) (Heemskerk and Streichan, 2015). Nematic order parameters (S, see Experimental Procedures), as well as angle of orientation to axes with standard deviations can then be automatically assessed. Values for PCP elements along the entire basal surfaces of the large stage 6 follicles correspond to those previously published (Fig. 2C) (Cetera et al., 2014; Viktorinová and Dahmann, 2013). Using ImSAnE, we confirmed the high degree of F-actin alignment in region 2b first reported by Frydman (Fig. 2D-F) (Frydman and Spradling, 2001). Notably, MTs are also strongly aligned in this region, with order parameters that parallel those of F-actin (Fig. 2E-F). Alignment of both actin and MTs is reduced but not lost as the follicle forms. Nematic order parameters, which are > 0.9 at stage 1, drop below 0.8 during stage 4 before recovering to 0.9 at stage 5; the standard deviation of nematic angles increases when order parameters drop. By contrast to cytoskeletal elements, we were unable to detect polarized organization of Rab10, Msn, or Ena in the germarium (Cetera et al., 2014; Lerner et al., 2013; Lewellyn et al., 2013). These data emphasize that coordinate alignment of the cytoskeleton precedes PCP rotation as well as PCP organization of several other molecular markers.
Figure 2. Germarial PCP revealed by ‘unrolling’ algorithm.
(A) Phalloidin-stained st. 6 follicle, shown in (A) single confocal section at basal surface, (A’) maximum intensity projection of confocal stack, and (A”) 3D opacity projections. (B) Diagram of ImSAnE unrolling of the basal surface of the follicle. (C) ImSAnE unrolling of stage 6 follicle stained with phalloidin and (C’) anti-acTub. Nematic order parameters (quantitated in upper left) demonstrate PCP organization. (D) Confocal cross-section of germarium stained with phalloidin (red) and anti-acTub (green). (E) ImSAnE unrolling of region 2b through stage 3 basal surface of ovariole in D. (F) Nematic order quantitation of MTs demonstrate dynamics of PCP organization in the germarium and early follicle. Data for Actin and acetylated Tubulin is shown to the immediate left and right (respectively) of each stage. Scale bars: 10μm.
MTs are required in the germarium prior to PCP rotation
To assess the functional role of early cytoskeletal organization in follicle PCP, we employed genetic and chemical approaches. A forward genetic screen using transgenic RNAi expression specifically within the somatic gonad (unpublished results) identified several cytoskeletal regulators whose knockdown induced defects in follicle elongation. As expected from actin's well-established role in cell motility, these included regulators of actin organization. Disrupting dynamic actin by knocking down the SCAR complex regulators Hem (also known as Kette/NAP1) or Abi throughout follicle development prevents rotation and leads to round eggs (Fig. 3E, Supplemental Fig. 2) (Cetera et al., 2014; Chen et al., 2014). Sra-1 depletion had a similar effect, although it displayed a more general disruption of cortical F-actin that hindered analyses of molecular PCP. Acute chemical inhibition using the Arp2/3 inhibitor CK-666 demonstrated that Arp2/3-initiated actin polymerization is required during stages 1-5 for PCP follicle rotation (Fig. 3E) (Cetera et al., 2014).
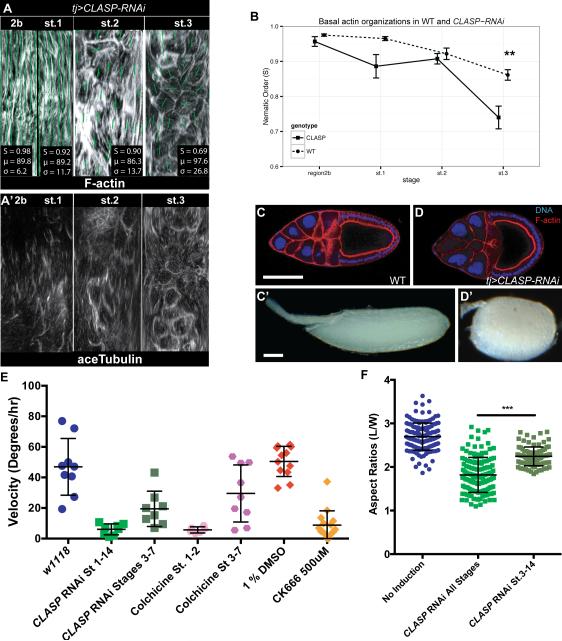
Figure 3. PCP MTs in the germarium direct rotation.
(A) ImSAnE unrolling of basal surface of CLASP-depleted ovariole, showing regions 2b through st. 3 stained with phalloidin and anti-acTub. (B) Nematic order shows intact actin alignment when MTs are disrupted. (n≥5 for each stages, **p<0.01) (C, D) Control stage 9 follicle and mature eggs are elongated, while CLASP-RNAi stage 9 follicles and mature eggs are round. (E) Rotation speeds of st. 6-7 follicles, depleted at specified times using tj> CLASP RNAi tubGAL80ts, or treated with the MT polymerization inhibitor colchicine or the Arp2/3 inhibitor CK-666. (F) Quantitation of tj> CLASP RNAi tubGAL80ts egg shape.
Knockdown of the MT stabilizing protein CLASP (Flybase: Chromosome Bows) caused a potent loss of MT bundles, with minor effects on the formation of F-actin filaments, in both germaria and stage 1 follicles (Fig. 3A, B). Strikingly, CLASP-depleted follicles failed to rotate and gave rise to round follicles with mispolarized F-actin, as well as later to round eggs (Fig. 3B-D). Disrupting MTs by overexpressing the MT-severing protein Spastin also induced round eggs (not shown). Moreover, conditional RNAi expression revealed that CLASP knockdown after stage 3 slows but does not prevent rotation (Fig. 3E) and leads to only mildly round eggs (Fig. 3F). We confirmed these data by using chemical inhibitors. When monitoring follicles from colchicine-fed flies, we found that no stage 2 follicles initiated rotation (Fig. 3E). However, follicles that were at stage 3 and later when colchicine feeding commenced continued to rotate on-axis, albeit at a slower rate (Fig. 3E) (Viktorinová and Dahmann, 2013). These data suggest that, while MTs are not required for cell motility driving follicle rotation per se, they are required at stage 1-2 to initiate it.
Germarial Fat2 regulates both rotation initiation and maintenance but not cytoskeletal alignment
Since MTs are required to initiate PCP follicle rotation, we considered potential regulators of germarial PCP organization. The atypical cadherin Fat2 is required to initiate rotation (Cetera et al., 2014) and for egg elongation and cytoskeletal PCP, but its role in the latter has been largely inferred from phenotypes seen ~16 hours after rotation has initiated (Viktorinova et al., 2009; 2011). We identified RNAi lines that knockdown fat2 either strongly or mildly; the strong knockdown line phenocopies the fat2 null phenotype with 100% penetrance (Supplemental Fig. 3). We examined strongly fat2-depleted germaria and found that they failed to rotate at stage 2, despite stalk and basement membrane morphogenesis comparable to WT, and shortly after this stage, PCP organization fails to align (Fig. 4A-B, Supplemental Movie S2). To define the requirements for Fat2 in initiation and maintenance of follicle rotation, we employed conditional knockdown, with the limitation that we were unable to directly assess the completeness of Fat2 depletion. When follicles raised at permissive conditions and initiating rotation were shifted to knockdown fat2 at st. 3 and following, they slowed rotation within 8 hours and arrested after 12 hours; the resultant eggs showed substantial elongation defects. If fat2 was instead knocked down prior to stage 3, follicles always failed to rotate and gave rise to round eggs, even when shifted to permissive conditions that terminated fat2 knockdown for the remaining 40 hours of oogenesis (Fig. 4C,D). These results indicate an absolute requirement for Fat2 in both rotation initiation as well as maintenance, as well as elongation, following st. 2. Despite this, we found no significant changes in the circumferential alignment of either actin or MT in fat2-depleted or null as compared to WT germaria (Fig. 4A, B). Thus, Fat2 regulates rotation by a mechanism that is independent of germarial cytoskeletal alignment.
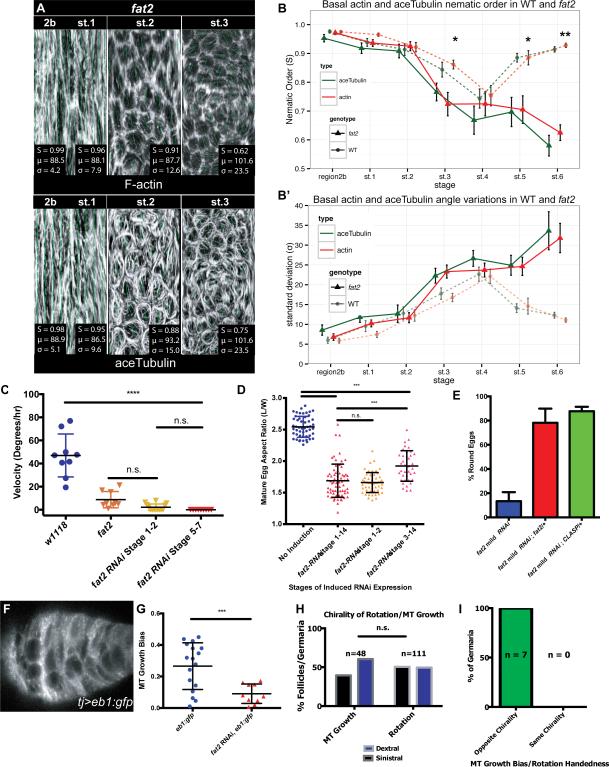
Figure 4. Fat2 regulates rotation initiation and MT chirality.
(A) ImSAnE unrolling of basal surface of fat2-depleted ovariole, showing regions 2b through st. 3 stained with phalloidin and anti-acTub. (B) Nematic order shows MT and actin alignment resemble WT until st. 2, but become disrupted following st. 3. (C) Rotation speeds at st. 7-8 of follicles depleted of fat2 by RNAi at specified stages. Follicles depleted of fat2 either prior to or following st. 3 fail to rotate. (D) Aspect ratios of eggs from conditional depletion of fat2, showing strong early and late requirements for egg shape. (E) Egg shapes from fat2-RNAi-mild genetic interaction tests. Heterozygosity for either fat2 or CLASP enhances the round egg phenotype. (F) Still frame from live imaging of MT +end growth in WT st. 1 follicle. (G) Quantification of EB1 growth bias in WT and fat2-depleted st. 1 follicle (0=unbiased direction of growth, 1=fully concordant direction of growth), shows that significant MT growth bias in WT is lost when fat2 is depleted. (H) Population-level MT growth biases at st. 1 and follicle rotation directions at st. 6 show similar proportions of chiralities. (I) Live imaging of EB1-GFP expressing follicles during st.1 to st. 2 transition reveals rotation with a chirality opposite to that of MT growth bias.
Fat2-regulated microtubule polarity in the germarium predicts follicle PCP chirality
To explore this mechanism, we took advantage of the mild fat2 RNAi line to test genetic interactions. Knockdown of fat2 using the mild line gives a moderately penetrant round egg phenotype: ~20% of laid eggs are round (Fig. 4E). This is a sensitized background, as heterozygosity for fat2 enhances this phenotype to over 80%. Remarkably, heterozygosity for CLASP causes a similar degree of enhancement: over 80% of eggs laid by such females are round. This result suggests that Fat2 regulates follicle elongation through close functional ties to the MT cytoskeleton.
The intimate and essential roles of both Fat2 and MTs in initiating follicle rotation prompted us to examine early MT organization more closely. Since Fat2 did not regulate germarial MT alignment (Fig. 4A, B), we instead evaluated the polarized organization of the MTs. MTs have an intrinsic polarity, with a fast-growing + end and a slow-growing – end. We used the + end tracking protein EB1 to assess the polarity of the aligned MTs within live stage 1 follicles (Fig. 4F, Supplemental Movie S3), and calculated a bias parameter in which 0 represents unbiased MT+ end growth, while 1 represents all MT+ ends growing in the same direction. Interestingly, this analysis revealed a significant (p<0.003) bias in the chirality of MT + end growth, and nearly 2/3 of germaria displayed a bias of 0.2 or greater (Fig. 4G). Moreover, the chirality of this MT bias was not identical in all stage 1 follicles. With respect to the ovariole A-P axis, the ratio of dextral (clockwise) to sinistral (counter-clockwise) MT +end biases seen in follicles prior to rotation was ~50%(Fig. 4H). This corresponds to the ratio of chiralities of rotation seen in later stage follicles (Fig. 4H). To determine the relationship between MT bias at stage 1 and chirality of rotation once it initiates at stage 2, we carried out live imaging on EB1-expressing budding follicles. Of follicles that remained healthy throughout imaging, 7 of 8 showed a, germarial MT bias exceeding that of fat2, and 100% of these later rotated with a chirality opposite to their earlier +-end bias (n=7; p<0.01) (Fig. 4I, Supplemental Movie S4). Strikingly, despite the fact that MT bundles in fat2 mutant follicles retain PCP alignment at stage 1, their biased orientation was lost, with MT + end organization randomized (Fig. 4G). These results indicate that Fat2 acts in the germarium to organize MT chirality, and suggest that polarity of MT in the developing proto-follicle is the symmetry-breaking event that sets subsequent PCP including the direction of rotation after organ formation.
DISCUSSION
In this work we identify a central symmetry-breaking role for microtubule polarity in PCP of an ‘edgeless’ epithelial organ. MTs are the earliest PCP molecule during follicle development, germarial MT polarity predicts the chirality of subsequent follicle PCP events, and disruption of either MT alignment or polarity in the germarium prevents all subsequent aspects of follicle PCP, including the coordinated cell motility that initiates follicle rotation. These requirements for MTs are not due to secondary effects on actin, which retains its organization in germaria with disrupted MTs. Importantly, unlike actin, which is required acutely and constantly for collective cell migration, MTs are not strictly required for follicle cell motility once rotation has initiated. We therefore propose that MTs provide the initial source of PCP information in the early follicle, and that actin, through its role in promoting tissue rotation, serves to amplify and propagate PCP.
Our data demonstrate that the MT polarity bias in the forming follicle predicts the chirality of PCP tissue rotation that initiates ~10 hours later. It was recently reported that, in st. 4 follicles, MT + end orientation anticorrelates with rotation direction at st. 7 (Viktorinová and Dahmann, 2013). However, in that work, stage 4 and earlier follicles were thought to represent pre-rotation stages, contrary to our identification here of rotation initiation at st. 2 and that of Cetera et al. (2014) who place it at st. 1. Since both MT alignment and polarity are present in the germarium, the st. 5 correlation is not predictive and reflects pre-existing PCP information rather than revealing its source. In the absence of an independent and direct manipulation of MT + end orientation, we cannot conclusively state that the MT polarity that we document in the germarium is instructive for rotational direction. Nevertheless, the strong correlation between this chirality and the subsequent direction of follicle rotation, along with disruption of both in the absence of fat2, point to a model in which coordination of MT polarity in cells across the follicle is required for a unidirectional consensus amongst individual motile cells to initiate productive rotation.
Our data thus suggest that the atypical cadherin Fat2, a key regulator of follicle PCP, rotation, and elongation, acts through effects on early MT polarity. Through analyses of middle stages of follicle development, it has been argued that Fat2 regulates global PCP alignment of the cytoskeleton, as does the canonical PCP regulator Fat in the developing Drosophila wing (Harumoto et al., 2010; Matis et al., 2014; Olofsson et al., 2014; Viktorinova et al., 2009; 2011). However, in fat2 germaria, actin and MT alignment are maintained; it is coordinated MT polarity that is lost. These phenotypes, along with its strong genetic interaction with CLASP, argue that Fat2 promotes rotation initiation and follicle PCP via its effects on MT polarity. We have not excluded an additional role in actin regulation other than polarized alignment; the requirement for Fat2 and actin but not MTs to maintain rotation, as well as the direct binding of actin regulators by vertebrate Fat1, a possible ortholog of Drosophila Fat2 (Moeller et al., 2004; Tanoue and Takeichi, 2004), suggests such a role. Moreover, while this work was in revision, Squarr et al. (2016) showed that Fat2 can directly influence the actin cytoskeleton via binding to the WAVE complex.
As with PCP in the developing Drosophila wing disc (Matis et al., 2014; Olofsson et al., 2014; Shimada et al., 2006), the initial PCP bias provided by MT polarity within the early follicle precursors is mild, but becomes more robust as organogenesis progresses. A mechanism for amplification in the follicle involves whole-tissue rotation. Preventing rotation by disrupting actin or integrins causes a rapid loss of all PCP organization primed in the germarium (Bilder and Haigo, 2012; Cetera and Horne-Badovinac, 2015). Interestingly, just as MTs are largely dispensable for PCP after actin PCP becomes established (Figs. 3 and 4), actin PCP is dispensable after circumferentially aligned ECM becomes established (Cetera et al., 2014). Hence, PCP transitions from highly dynamic and intracellular MTs, to longer-lasting and sometimes juxtacellular actin filaments, and then finally to the durable ECM fibrils that span multiple cells. PCP information in the follicle is therefore passed along by a ‘handoff’ mechanism, to increasingly stable as well as larger scale components that can ultimately exert force to shape the organ.
Non-centrosomal microtubule arrays, and in particular their regulated polarized organization, have previously been implicated as central governors of global PCP in tissues such as Drosophila wings, zebrafish gastrulae, and mammalian airway epithelia (reviewed in Galic and Matis, 2015; Sepich et al., 2011). In ‘edged’ PCP tissues in Drosophila, a ‘global PCP’ module molecularly controlled by Fat is thought to use gradients of positional information from specific sources to bring individual cell PCP in alignment with overall body axes (Goodrich and Strutt, 2011; Singh and Mlodzik, 2012; Vladar et al., 2009). In the circumferential ‘edgeless’ PCP axis of the follicle epithelium, where no such graded information is known, Fat2 seems to similarly coordinate the PCP of individual cells. That both contexts involve important roles for polarized MTs and are controlled by related protocadherins raises the possibility of ancient links between the modes of epithelial PCP organization.
EXPERIMENTAL PROCEDURES
RNAi-mediated depletion in follicle cells used a Gal4 insertion in tj (Hayashi et al., 2002; Li et al., 2003) with UAS-RNAi transgenes. Conditional depletion was carried out in combination with tubGAL80ts, using 18° as restrictive temperature and 29° as permissive temperature. Live follicles were cultured ex vivo under previously described conditions (Haigo and Bilder, 2011), and imaged on a Zeiss 700 confocal microscope. EB1-GFP comets were analyzed using Fiji (Schindelin et al., 2012). ImSAnE analysis was executed on full stacks of confocal sections, and nematic order was analyzed as described in Supplemental Experimental Procedures, where additional genetic and imaging details are provided.
Supplementary Material
Acknowledgements
We thank Christian Dahmann, Nasser Rusan, Nina Tang Sherwood and Jeff Axelrod for sending reagents, and are grateful to Boris Shraiman and the Bilder lab, especially Justin Crest, for helpful discussions. We also acknowledge Lin Yuan, who first documented stage 2 follicle rotation. We thank the TRiP at Harvard Medical School (NIH/NIGMS R01-GM084947) and the Bloomington Stock Center for providing transgenic RNAi fly stocks used in this study. KRL is an American Heart Association graduate fellow. This work was supported by NIH RO1 GM068675 to DB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
DYC, KRL, and YD conducted the experiments; SJS developed analytical tools; DYC, KRL, and DB designed the experiments and wrote the paper.
SUPPLEMENTARY FIGURES AND FILES
Supplemental Experimental Procedures and Figures (separate file)
REFERENCES
- Bastock R, Strutt D. The planar polarity pathway promotes coordinated cell migration during Drosophila oogenesis. Development. 2007;134:3055–3064. doi: 10.1242/dev.010447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder D, Haigo SL. Expanding the morphogenetic repertoire: perspectives from the Drosophila egg. Dev Cell. 2012;22:12–23. doi: 10.1016/j.devcel.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetera M, Horne-Badovinac S. Round and round gets you somewhere: collective cell migration and planar polarity in elongating Drosophila egg chambers. Curr. Opin. Genet. Dev. 2015;32:10–15. doi: 10.1016/j.gde.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetera M, Ramirez-San Juan GR, Oakes PW, Lewellyn L, Fairchild MJ, Tanentzapf G, Gardel ML, Horne-Badovinac S. Epithelial rotation promotes the global alignment of contractile actin bundles during Drosophila egg chamber elongation. Nature Communications. 2014;5:5511. doi: 10.1038/ncomms6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XJ, Squarr AJ, Stephan R, Chen B, Higgins TE, Barry DJ, Martin MC, Rosen MK, Bogdan S, Way M. Ena/VASP proteins cooperate with the WAVE complex to regulate the actin cytoskeleton. Dev Cell. 2014;30:569–584. doi: 10.1016/j.devcel.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frydman H, Spradling A. The receptor-like tyrosine phosphatase lar is required for epithelial planar polarity and for axis determination within drosophila ovarian follicles. Development. 2001;128:3209–3220. doi: 10.1242/dev.128.16.3209. [DOI] [PubMed] [Google Scholar]
- Galic M, Matis M. Polarized trafficking provides spatial cues for planar cell polarization within a tissue. Bioessays. 2015;37:678–686. doi: 10.1002/bies.201400196. [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138:1877–1892. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray RS, Roszko I, Solnica-Krezel L. Planar cell polarity: coordinating morphogenetic cell behaviors with embryonic polarity. Dev Cell. 2011;21:120–133. doi: 10.1016/j.devcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigo SL, Bilder D. Global tissue revolutions in a morphogenetic movement controlling elongation. Science. 2011;331:1071–1074. doi: 10.1126/science.1199424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harumoto T, Ito M, Shimada Y, Kobayashi TJ, Ueda HR, Lu B, Uemura T. Atypical cadherins Dachsous and Fat control dynamics of noncentrosomal microtubules in planar cell polarity. Dev Cell. 2010;19:389–401. doi: 10.1016/j.devcel.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S, Ito K, Sado Y, Taniguchi M, Akimoto A, Takeuchi H, Aigaki T, Matsuzaki F, Nakagoshi H, Tanimura T, et al. GETDB, a database compiling expression patterns and molecular locations of a collection of Gal4 enhancer traps. Genesis. 2002;34:58–61. doi: 10.1002/gene.10137. [DOI] [PubMed] [Google Scholar]
- Heemskerk IJ, Streichan SJ. Developmental cartography: Compressing Bio-Image Data by Dimensional Reduction. Nature Methods. 2015 doi: 10.1038/nmeth.3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner DW, McCoy D, Isabella AJ, Mahowald AP, Gerlach GF, Chaudhry TA, Horne-Badovinac S. A Rab10-Dependent Mechanism for Polarized Basement Membrane Secretion during Organ Morphogenesis. Dev Cell. 2013;24:159–168. doi: 10.1016/j.devcel.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewellyn L, Cetera M, Horne-Badovinac S. Misshapen decreases integrin levels to promote epithelial motility and planar polarity in Drosophila. J. Cell Biol. 2013;200:721–729. doi: 10.1083/jcb.201209129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MA, Alls JD, Avancini RM, Koo K, Godt D. The large Maf factor Traffic Jam controls gonad morphogenesis in Drosophila. Nat. Cell Biol. 2003;5:994–1000. doi: 10.1038/ncb1058. [DOI] [PubMed] [Google Scholar]
- Matis M, Russler-Germain DA, Hu Q, Tomlin CJ, Axelrod JD. Microtubules provide directional information for core PCP function. eLife. 2014;3:e02893. doi: 10.7554/eLife.02893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller MJ, Soofi A, Braun GS, Li X, Watzl C, Kriz W, Holzman LB. Protocadherin FAT1 binds Ena/VASP proteins and is necessary for actin dynamics and cell polarization. Embo J. 2004;23:3769–3779. doi: 10.1038/sj.emboj.7600380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystul TG, Spradling A. Regulation of epithelial stem cell replacement and follicle formation in the Drosophila ovary. Genetics. 2010;184:503–515. doi: 10.1534/genetics.109.109538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson J, Sharp KA, Matis M, Cho B, Axelrod JD. Prickle/spiny-legs isoforms control the polarity of the apical microtubule network in planar cell polarity. Development. 2014;141:2866–2874. doi: 10.1242/dev.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepich DS, Usmani M, Pawlicki S, Solnica-Krezel L. Wnt/PCP signaling controls intracellular position of MTOCs during gastrulation convergence and extension movements. Development. 2011;138:543–552. doi: 10.1242/dev.053959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada Y, Yonemura S, Ohkura H, Strutt D, Uemura T. Polarized transport of Frizzled along the planar microtubule arrays in Drosophila wing epithelium. Dev Cell. 2006;10:209–222. doi: 10.1016/j.devcel.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Singh J, Mlodzik M. Planar cell polarity signaling: coordination of cellular orientation across tissues. Wiley Interdiscip Rev Dev Biol. 2012;1:479–499. doi: 10.1002/wdev.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling AC. Oogenesis. In: Bate CMAM-AA, editor. The Development ofDrosophila Melanogaster. Cold Spring Harbor Press; Cold Spring Harbor: 1993. [Google Scholar]
- Squarr AJ, Brinkmann K, Chen B, Steinbacher T, Ebnet K, Rosen MK, Bogdan S. Fat2 acts through the WAVE regulatory complex to drive collective cell migration during tissue rotation. J. Cell Biol. 2016;212:591–603. doi: 10.1083/jcb.201508081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanoue T, Takeichi M. Mammalian Fat1 cadherin regulates actin dynamics and cell-cell contact. J. Cell Biol. 2004;165:517–528. doi: 10.1083/jcb.200403006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viktorinova I, Konig T, Schlichting K, Dahmann C. The cadherin Fat2 is required for planar cell polarity in the Drosophila ovary. Development. 2009;136:4123–4132. doi: 10.1242/dev.039099. [DOI] [PubMed] [Google Scholar]
- Viktorinova I, Pismen LM, Aigouy B, Dahmann C. Modelling planar polarity of epithelia: the role of signal relay in collective cell polarization. J R Soc Interface. 2011 doi: 10.1098/rsif.2011.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viktorinová I, Dahmann C. Microtubule polarity predicts direction of egg chamber rotation in Drosophila. Current Biology. 2013;23:1472–1477. doi: 10.1016/j.cub.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Vladar EK, Antic D, Axelrod JD. Planar Cell Polarity Signaling: The Developing Cell's Compass. Csh Perspect Biol. 2009;1:a00296. doi: 10.1101/cshperspect.a002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford JB. Planar cell polarity and the developmental control of cell behavior in vertebrate embryos. Annu. Rev. Cell Dev. Biol. 2012;28:627–653. doi: 10.1146/annurev-cellbio-092910-154208. [DOI] [PubMed] [Google Scholar]
- Zallen JA. Planar polarity and tissue morphogenesis. Cell. 2007;129:1051–1063. doi: 10.1016/j.cell.2007.05.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.