Abstract
Purpose
To determine the intra- and interobserver reproducibility of human amniotic fluid metabolite concentration measurements (including potential markers of fetal lung maturity) detectable by MR spectroscopy.
Materials and Methods
1H high-resolution magic angle spinning (HR-MAS) spectroscopy was performed at 11.7T on 23 third-trimester amniotic fluid samples. Samples were analyzed quantitatively using 3-(trimethylsilyl)propionic-2,2,3,3-d4 acid (TSP) as a reference. Four observers independently quantified eight metabolite regions (TSP, lactate doublet and quartet, alanine, citrate, creatinine, choline, and glucose) twice from anonymized, randomized spectra using a semiautomated software program.
Results
Excellent inter- and intraobserver reproducibility was found for all metabolites. Intraclass correlation as a measure of interobserver agreement for the four readers ranged from 0.654 to 0.995. A high correlation of 0.973 was seen for choline in particular, a major component of surfactant. Pearson correlation as a measure of intraobserver reproducibility ranged from 0.478 to 0.999.
Conclusion
Quantification of choline and other metabolite concentrations in amniotic fluid by high-resolution MR spectroscopy can be performed with high inter- and intraobserver reproducibility. Demonstration of reproducible metabolite concentration measurements is a critical first step in the search for biomarkers of fetal lung maturity.
Keywords: amniotic fluid, fetal lung maturity, reproducibility, high resolution magic angle spinning (HR-MAS)
Respiratory distress syndrome (RDS) is a major cause of neonatal morbidity and mortality due to insufficient surfactant production in the lungs (1–3). The disease primarily affects premature infants and is the seventh leading cause of death in infants under 1 year of age (1,2). Excreted surfactant aggregates to form a monolayer complex that reduces alveolar surface tension and facilitates alveolar inflation (4). Insufficient surfactant at birth results in collapse of the fetal alveoli and RDS. Surfactant is composed predominantly of phosphatidylcholine (lecithin) (70%) with lesser amounts of other phospholipids, including phosphatidylglycerol, phosphatidylethanolamine, and phosphatidylinositol (5).
Measurement of the lecithin-to-sphingomyelin (LS) ratio in amniotic fluid samples is traditionally considered to be the gold standard for fetal lung maturity testing, but the assay is time-consuming and technically challenging to perform. Consequently, many hospitals now measure the surfactant-to-albumin (SA) ratio, which is a faster and simpler test, although there is not good concordance between the two measurements (6). These traditional methods of evaluating fetal lung maturity have a number of disadvantages. Analysis of amniotic fluid requires amniocentesis, with the associated risks of this invasive procedure including infection and miscarriage (7,8). The tests are also associated with a high rate of false-positive results in fetuses with intermediate lung maturity, even when several different measurements are incorporated into a more comprehensive assessment (9–11). In these cases the laboratory tests can indicate fetal lung maturity yet the fetus may still develop RDS (12).
Choline, a major component of surfactant, can be easily detected and quantified by 1H MR spectroscopy ex vivo. Case reports of 1H MRS of amniotic fluid pockets in utero have generated interest in pursuing this technique for the potential noninvasive evaluation of fetal lung maturity in vivo (13–15). However, in vivo studies are lower in spectroscopic resolution and sensitivity, and are expensive and technically challenging to perform (16). Accordingly, a systematic study of amniotic fluid samples ex vivo would be useful to establish metabolic markers of fetal lung maturity (such as choline) and provide proof of concept to justify pursuing in vivo protocol development. High-resolution magic angle spinning (HR-MAS) spectroscopy is an ex vivo MRS technique that can be applied to intact tissues, cells, and biofluids. Although HR-MAS spectroscopy is normally used to improve spectral resolution, in the case of amniotic fluid, which contains mostly water, the small sample volume relative to the coil size allows for much better water suppression than a conventional liquids probe. Because reproducibility of measurements is an important fundamental parameter that needs to be established in such studies, the reproducibility of the MRS quantification technique first needs to be evaluated. Thus, the purpose of this study was to determine the intra- and interobserver reproducibility of metabolite concentration measurements (including choline concentration) detectable by 1H HR-MAS spectroscopy using a semiautomated software program for peak fitting.
MATERIALS AND METHODS
Sample Collection
Twenty-three third trimester amniotic fluid samples were retrospectively identified from the Department of Laboratory Medicine. Samples were obtained by amniocentesis for measurement of the SA ratio, a standard clinical test for fetal lung maturity, after which the remaining sample was used for MR spectroscopic analysis. Samples were stored at −20°C prior to spectroscopic analysis. The SA ratios ranged from 14–124 mg/g with a mean value of 54 mg/g. At our institution, an SA ratio ≥50 mg/g is used to predict fetal lung maturity. Maternal age ranged from 18–39 years with a mean of 30 years. Maternal age was unknown for three patients. Fetal gestational age was unknown for these retrospectively identified samples. This study was approved by our Institutional Review Board with a waiver for informed consent.
HR-MAS Spectroscopy Technique
Ex vivo high-resolution spectroscopy was performed at 11.7T (500 MHz for 1H) using a Varian INOVA spectrometer (Palo Alto, CA) equipped with 4 mm gHX nano-probe with HR-MAS capabilities. The nanoprobe was used instead of a conventional “liquids” probe because it provided much better water suppression and consequently fewer baseline distortions that could affect quantitation. Aqueous samples were analyzed at 1°C and a 2250 Hz spin rate using custom-designed 35 μL leak-proof zirconium rotors following the method of Swanson et al (17) with minor modifications. To provide a lock, frequency, and concentration reference, 1.0-mL vials of deuterium oxide containing 0.75% wt/wt 3-(tri-methylsilyl)propionic-2,2,3,3-d4 acid (D2O+TSP) were purchased from Sigma-Aldrich (St. Louis, MO) and discarded daily to minimize absorption of atmospheric water. At sample preparation, 1.5 μL of D2O+TSP was pipetted into the bottom of the rotor and weighed to 0.01 mg, after which 30 μL of amniotic fluid was pipetted into the rotor and weighed. Means and standard deviations were 1.59 ± 0.46 mg for D2O+TSP and 31.95 ± 6.28 mg for amniotic fluid sample mass, respectively. Fully relaxed data were acquired with a 45° excitation pulse and a 6.5-second repetition time (4-second presaturation pulse and 2.5-second acquisition time). Data were processed using a 0.4 Hz matched exponential filter prior to Fourier transformation. A digital filter with a bandwidth of 25 Hz was applied to the water resonance prior to quantitative analysis.
Metabolite Concentration Measurement and Reproducibility Analysis
The 23 sample spectra were anonymized and randomized by a statistician. Four blinded observers were chosen with a range of experience in processing spectroscopy data, including one novice observer (Observer 4) with no prior experience. Observer 4 was trained by an experienced observer (Observer 1) on several test spectra prior to participating in this study. Observers independently processed the 23 randomized spectra offline using ACD/Labs 1D NMR processor (ACD/Labs, Toronto). Following phasing and frequency shifting, peaks were quantified using a semiautomated Lorentzian–Gaussian peak fitting algorithm and peak areas were exported to an Excel spreadsheet. Each observer quantified eight metabolite regions: TSP (reference standard), lactate (doublet and quartet), alanine, citrate, creatinine, total choline, and glucose. Chemical shift values (ppm) and the typical number of peaks fitted for each metabolite are listed in Table 1. Total choline included all of the choline-containing metabolites (ie, free choline, phosphocholine, and glycerophosphocholine) in the region from 3.15 to 3.3 ppm and is known to contain small contributions from other metabolites (17). Concentrations were calculated based on the peak areas and the masses of TSP and amniotic fluid in the rotor according to Eq. [1]:
| [1] |
where n1H/metabolite is the number of protons per metabolite resonance or multiplet used for quantitation (n for each metabolite given in Table 1). Each observer processed the anonymized spectra in a unique randomized order before and after a washout period ranging from 1 day to 2 weeks. Metabolite concentrations were decoded by another investigator who was not involved in the peak fitting process. Intraclass correlation (ICC) was used to measure interobserver agreement among all four observers resulting in one ICC value for each metabolite. Pearson correlation was used to measure intraobserver agreement, resulting in one value per metabolite for each observer. Processing times for each observer were tabulated.
Table 1.
Number of Peaks and ppm Locations for Selected Metabolite Regions Consistently Identified by Ex Vivo Spectroscopy of Amniotic Fluid
| Metabolite | Range (ppm) | # Peaks | # Protons (n) |
|---|---|---|---|
| TSP (reference) | 0 | 1 | 9 |
| Lactate-doublet | 1.3–1.35 | 2 | 3 |
| Alanine | 1.43–1.53 | 2 | 3 |
| Citrate | 2.45–2.75 | 4 | 4 |
| Creatinine | 3.03–3.05 | 1 to 3 | 3 |
| Choline | 3.15–3.3 | 4 to 6+ | 9 |
| Lactate-quartet | 4.08–4.18 | 4 | 1 |
| Glucose | 4.6–4.7 | 2 | 1 |
Number of protons (n) per metabolite shown in last column.
RESULTS
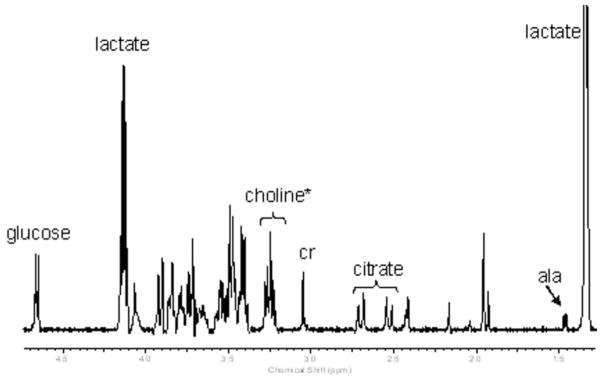
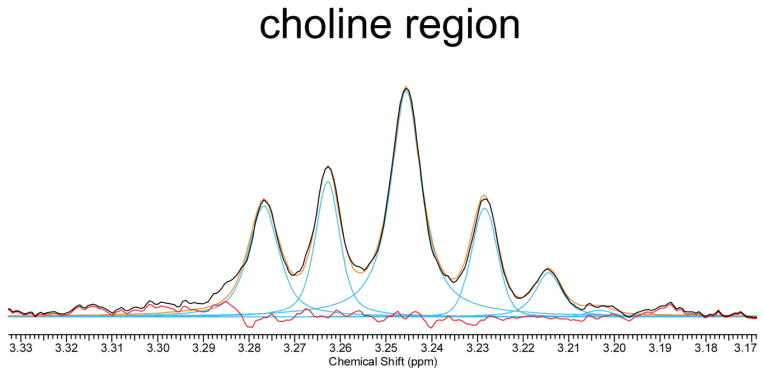
A sample spectrum demonstrating the relative quantities of the 8 amniotic fluid metabolites is shown in Fig. 1. Choline-containing metabolites (including surfactant) are observed from 3.20–3.25 ppm. The two resonances of lactate, a doublet at 1.33 ppm, and a quartet at 4.12 ppm are prominent in the spectrum. Also shown are alanine, citrate, creatinine, and glucose. The residual water resonance (~4.8 ppm) is beyond the scale of the graph. An example of the curve-fitting process used to calculate metabolite concentrations is shown in Fig. 2 for the choline-containing metabolites. Average processing times for each observer are summarized in Table 2 and ranged from 4.1 minutes (experienced observer) to 12.2 minutes (novice observer) to process each spectrum and tabulate eight metabolite peak areas.
Figure 1.
Representative 11.7 T HR-MAS spectrum obtained from a third trimester amniotic fluid sample showing the metabolites quantified in this study. *Choline = choline region.
Figure 2.
Magnified view of the choline region of the spectrum shown in Fig. 1, illustrating the curve-fitting process. Fitted curves to compute the area beneath the peaks are overlaid on the original spectra. (Fitted curves shown in blue, summation of fitted curves shown in orange, residual of fitted curves shown in red.)
Table 2.
Mean Processing Times and Standard Deviations (SD) for Each Observer
| Observer | Mean ± SD |
|---|---|
| 1 | 4.3 ± 2.3 |
| 2 | 10.8 ± 6.1 |
| 3 | 4.1 ± 2.8 |
| 4 | 12.2 ± 4.4 |
Processing times given in minutes.
Overall there was high inter- and intraobserver reproducibility for quantifying metabolite concentrations in amniotic fluid. Results of interobserver reproducibility based on ICC coefficients are given in Table 3. ICCs ranged from 0.654 for alanine to 0.995 for the lactate doublet. There was a high correlation of 0.9 for most metabolites and an ICC of 0.973 for choline-containing metabolites specifically. High intraobserver reproducibility was also observed across all measured metabolites. Table 4 lists ICCs for each metabolite. The intraobserver correlation coefficients for choline ranged from 0.95 to 0.99 for observers 1 to 4 (data shown in Fig. 3). In general, Pearson correlation coefficients were all higher than 0.65 except for alanine for one observer who fit different numbers of peaks (1 versus 3) between the first and second analyses, thus explaining the increased variability. Observer 3 mistakenly fit only 3 of 4 citrate peaks during one analysis but still had a high intraobserver correlation coefficient of 0.91 for citrate.
Table 3.
Intraclass Correlation Coefficients (Between 4 Blinded Observers) as a Measure of Interobserver Reproducibility for Quantifying Amniotic Fluid Metabolite Region Concentrations
| Metabolite | Intraclass correlation coefficient |
|---|---|
| Lactate (doublet) | 0.995 |
| Alanine | 0.654 |
| Citrate | 0.923 |
| Creatinine | 0.665 |
| Choline | 0.973 |
| Lactate (quartet) | 0.969 |
| Glucose | 0.875 |
Table 4.
Pearson Correlation Coefficients (for 4 Blinded Observers) as a Measure of Intraobserver Reproducibility for Quantifying Amniotic Fluid Metabolite Region Concentrations
| Metabolite | Observer 1 | Observer 2 | Observer 3 | Observer 4 |
|---|---|---|---|---|
| Lactate (doublet) | 0.991 | 0.999 | 0.996 | 0.997 |
| Alanine | 0.478a | 0.912 | 0.716 | 0.949 |
| Citrate | 0.930 | 0.920 | 0.910 | 0.991 |
| Creatinine | 0.679 | 0.757 | 0.738 | 0.931 |
| Choline | 0.983 | 0.947 | 0.988 | 0.996 |
| Lactate (quartet) | 0.990 | 0.925 | 0.991 | 0.980 |
| Glucose | 0.987 | 0.738 | 0.972 | 0.992 |
Observer 1 fit one peak in the alanine range at first sitting and three peaks at second sitting accounting for the increased intraobserver variability for alanine.
Figure 3.
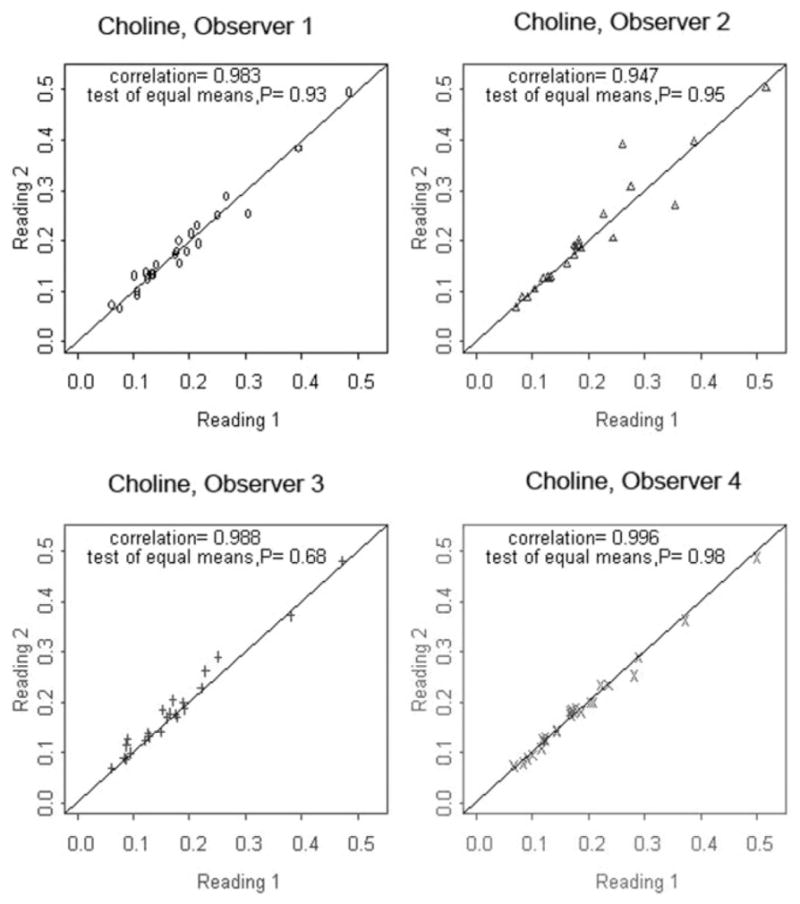
Intraobserver correlation coefficients for choline region concentration measurements in amniotic fluid using HR-MAS spectroscopy at 11.7T. Observers 1 to 4 independently analyzed 23 spectra in a blinded fashion. Reading 1 and Reading 2 were performed at separate sittings after a washout period to avoid recall bias.
DISCUSSION
Establishing the reproducibility of the metabolite quantification process in HR-MAS spectroscopy of amniotic fluid is an important fundamental step prior to embarking on a larger-scale study to identify amniotic fluid metabolites that may serve as biomarkers of fetal lung maturity. Overall, there was high inter- and intraobserver reproducibility for all the metabolites measured in this study. As expected, metabolites with small peak intensities such as alanine and creatinine had lower intraclass and Pearson correlation coefficients. In the case of alanine, much of the variability can be attributed to observer 1 fitting 1 peak versus 3 peaks between the first and second analyses. Although there were a variable number of peaks in the choline range, high inter- and intraobserver reproducibility was found for choline metabolite concentration measurements with all correlation coefficients above 0.94. Observers with varying experience levels from novice to highly experienced all had excellent intraobserver reproducibility, with the novice observer (Observer 4) actually showing the highest intraobserver reproducibility across all metabolites. This may in part relate to more time spent by the novice observer on processing each spectrum, who had the longest mean processing time of 12 minutes per spectrum. To our knowledge, the reproducibility of amniotic fluid metabolite quantitation has not yet been reported, although high analytic reproducibility of 1H NMR spectroscopy has been reported in other biologic fluids such as urine (18,19). These studies demonstrate the stability of modern NMR spectrometers in general, with a >98% reproducibility reported by Dumas et al (18). Saude et al (20) (1%–2% difference between analyses) reported similar quantitative accuracy among five different pulse sequences for NMR acquisition and minimal differences related to operator dependent phasing.
Establishing high inter- and intraobserver reproducibility for the choline region of metabolites is of particular interest because choline compounds are a major component of surfactant and could potentially be used as a noninvasive marker of fetal lung maturity detectable by in vivo MR spectroscopy. Several earlier studies have compared ex vivo amniotic fluid spectroscopy in normal and diseased populations such as cystic fibrosis, diabetes, and spina bifida (21–23). None of these studies looked specifically at choline metabolites or other possible indices of fetal lung maturity. An older study on 33 patients found that choline levels detected by 31P spectroscopy varied with gestational age in normal patients but did not look at disease states (24). No systematic study of amniotic fluid spectroscopy for fetal lung maturity in the ex vivo setting has yet been reported.
This study did not include enough samples to perform a statistically significant correlation with the SA ratio, the current standard clinical test, and patients were not followed to determine the presence or absence of RDS after birth because this was not a prospective study. Additionally, because all samples were from third trimester fetuses, we were unable to correlate choline and other metabolite concentrations with gestational age. The question of whether choline and other metabolites measured by MR spectroscopy may be useful markers of fetal lung maturity and how the metabolites vary with gestational age is the subject of ongoing work. This study demonstrated that metabolite concentrations in fetal amniotic fluid can be reproducibly measured from HR-MAS spectroscopy data with minimal training, as evidenced by the results for the novice observer. These findings encourage future investigations to determine if choline compounds and other metabolites in amniotic fluid may be potential MR-detectable biomarkers of fetal lung maturity.
Acknowledgments
Contract grant sponsor: RSNA Research and Education Foundation (Berlex Laboratories/RSNA Research Scholar Grant); Contract grant sponsor: UCSF Research Evaluation and Allocation Committee; Contract grant sponsor: UCSF Academic Senate-Committee on Research.
References
- 1.Anderson RN, Smith BL. National Vital Statistics Reports. Vol. 53. Hyattsville, MD: National Center for Health Statistics; 2005. Deaths: leading causes for 2002. [PubMed] [Google Scholar]
- 2.Minino AM, Heron MP, Smith BL. National Vital Statistics Reports. Vol. 54. Hyattsville, MD: National Center for Health Statistics; 2006. Deaths: preliminary data for 2004; pp. 1–50. [PubMed] [Google Scholar]
- 3.American Lung Association Web Site. American Lung Association; 2006. Lung disease data at a glance: respiratory distress syndrome (RDS) [Google Scholar]
- 4.Kattwinkel J. Surfactant. Evolving issues. Clin Perinatol. 1998;25:17–32. [PubMed] [Google Scholar]
- 5.Lacaze-Masmonteil T. Exogenous surfactant therapy: newer developments. Semin Neonatol. 2003;8:433–440. doi: 10.1016/S1084-2756(03)00120-9. [DOI] [PubMed] [Google Scholar]
- 6.Karcher R, Sykes E, Batton D, et al. Gestational age-specific predicted risk of neonatal respiratory distress syndrome using lamellar body count and surfactant-to-albumin ratio in amniotic fluid. Am J Obstet Gynecol. 2005;193:1680–1684. doi: 10.1016/j.ajog.2005.03.080. [DOI] [PubMed] [Google Scholar]
- 7.Hughes ML, Bartholomew D, Paluzzi M. Abdominal wall endometriosis after amniocentesis. A case report. J Reprod Med. 1997;42:597–599. [PubMed] [Google Scholar]
- 8.Fines B, Ben-Ami TE, Yousefzadeh DK. Traumatic prenatal sigmoid perforation due to amniocentesis. Pediatr Radiol. 2001;31:440–443. doi: 10.1007/s002470100452. [DOI] [PubMed] [Google Scholar]
- 9.Ragosch V, Jurgens S, Lorenz U, Stolowsky C, Arabin B, Weitzel HK. Prediction of RDS by amniotic fluid analysis: a comparison of the prognostic value of traditional and recent methods. J Perinat Med. 1992;20:351–360. doi: 10.1515/jpme.1992.20.5.351. [DOI] [PubMed] [Google Scholar]
- 10.Hacker NF, Moore JG. Essentials of obstetrics and gynecology. Philadelphia: WB Saunders; 1998. [Google Scholar]
- 11.Bonebrake RG, Towers CV, Rumney PJ, Reimbold P. Is fluorescence polarization reliable and cost efficient in a fetal lung maturity cascade? Am J Obstet Gynecol. 1997;177:835–841. doi: 10.1016/s0002-9378(97)70278-3. [DOI] [PubMed] [Google Scholar]
- 12.Pinette MG, Blackstone J, Wax JR, Cartin A. Fetal lung maturity indices-a plea for gestational age-specific interpretation: a case report and discussion. Am J Obstet Gynecol. 2002;187:1721–1722. doi: 10.1067/mob.2002.128089. [DOI] [PubMed] [Google Scholar]
- 13.Clifton MS, Joe BN, Zektzer AS, et al. Feasibility of magnetic resonance spectroscopy for evaluating fetal lung maturity. J Pediatr Surg. 2006;41:768–773. doi: 10.1016/j.jpedsurg.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 14.Fenton BW, Lin CS, Ascher S, Macedonia C. Magnetic resonance spectroscopy to detect lecithin in amniotic fluid and fetal lung. Obstet Gynecol. 2000;95:457–460. doi: 10.1016/s0029-7844(99)00566-9. [DOI] [PubMed] [Google Scholar]
- 15.Fenton BW, Lin CS, Macedonia C, Schellinger D, Ascher S. The fetus at term: in utero volume-selected proton MR spectroscopy with a breath-hold technique—a feasibility study. Radiology. 2001;219:563–566. doi: 10.1148/radiology.219.2.r01ma29563. [DOI] [PubMed] [Google Scholar]
- 16.Kim DH, Vahidi K, Caughey AB, et al. 1.5T in vivo 1H MRS for evaluation of fetal lung maturity: technical feasibility study. Proc ISMRM; Berlin. 2007; p. 569. [Google Scholar]
- 17.Swanson MG, Zektzer AS, Tabatabai ZL, et al. Quantitative analysis of prostate metabolites using 1H HR-MAS spectroscopy. Magn Reson Med. 2006;55:1257–1264. doi: 10.1002/mrm.20909. [DOI] [PubMed] [Google Scholar]
- 18.Dumas ME, Maibaum EC, Teague C, et al. Assessment of analytical reproducibility of 1H NMR spectroscopy based metabonomics for large-scale epidemiological research: the INTERMAP Study. Anal Chem. 2006;78:2199–2208. doi: 10.1021/ac0517085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keun HC, Ebbels TM, Antti H, et al. Analytical reproducibility in (1)H NMR-based metabonomic urinalysis. Chem Res Toxicol. 2002;15:1380–1386. doi: 10.1021/tx0255774. [DOI] [PubMed] [Google Scholar]
- 20.Saude EJ, Slupsky CM, Sykes BD. Optimization of NMR analysis of biological fluids for quantitative accuracy. Metabolomics. 2006;2:113–123. [Google Scholar]
- 21.Le Moyec L, Muller F, Eugene M, Spraul M. Proton magnetic resonance spectroscopy of human amniotic fluids sampled at 17–18 weeks of pregnancy in cases of decreased digestive enzyme activities and detected cystic fibrosis. Clin Biochem. 1994;27:475–483. doi: 10.1016/0009-9120(94)00051-v. [DOI] [PubMed] [Google Scholar]
- 22.Groenen PM, Engelke UF, Wevers RA, et al. High-resolution 1H NMR spectroscopy of amniotic fluids from spina bifida fetuses and controls. Eur J Obstet Gynecol Reprod Biol. 2004;112:16–23. doi: 10.1016/s0301-2115(03)00279-3. [DOI] [PubMed] [Google Scholar]
- 23.McGowan PE, Lawrie WC, Reglinski J, et al. 1H NMR as a non-invasive probe of amniotic fluid in insulin dependent diabetes mellitus. J Perinat Med. 1999;27:404–408. doi: 10.1515/JPM.1999.056. [DOI] [PubMed] [Google Scholar]
- 24.Pearce JM, Krone JT, Pappas AA, Komoroski RA. Analysis of saturated phosphatidylcholine in amniotic fluid by 31P NMR. Magn Reson Med. 1993;30:476–484. doi: 10.1002/mrm.1910300410. [DOI] [PubMed] [Google Scholar]