Abstract
Choroidal osteoma is a benign ossifying tumor of the choroid, consisting of mature bone tissue. It has been described to enlarge and evolve at varying rates over time. Here, we report and quantify the progression of a unilateral choroidal osteoma in a 7-year-old boy by fundus photography, and document tumor remodeling by spectral domain optical coherence tomography images.
Key Words: Choroidal osteoma, Tumor progression, Bone remodeling, Fundus photographs, Spectral-domain optical coherence tomography
Introduction
Choroidal osteoma is a benign rare tumor consisting of normal cancellous bone located between the inner third and outer third of the choroidal layers [1]. The typical characteristics of choroidal osteomas are their unilaterality, juxta- or peripapillary location, predominance in young females [2], rapid or slow tumor progression [3,4,5], tumor involution [6] and decalcification [7,8]. Diagnosis, by convention, is made by clinical examination and fundus angiography, and confirmed by the presence of choroidal calcification on ultrasonography or other modes of imaging such as computed tomography. Spectral-domain optical coherence tomography (SD-OCT) has provided high-resolution cross-sectional images of the tumor and overlying retina [9,10,11], as well as a 3-dimensional view of the tumor [12]. Remodeling of the tumor has been suggested to explain the varying degrees of tumor reflectivity found on SD-OCT [10]. In this case report we objectively quantify the progression of a choroidal osteoma in a young boy, and demonstrate the effects of tumor remodeling documented by SD-OCT.
Case Report
An otherwise healthy 7-year-old boy, diagnosed with a right submacular choroidal osteoma as an incidental finding, was followed up over a period of 5 years. He had neither a preceding history of systemic illness nor of ocular trauma. His vision remained 20/20 in both eyes with minimal refractive correction throughout the years. There was varying central metamorphopsia. Anterior segment examination was unremarkable. Examination revealed a large round and well-demarcated orange-red-colored submacular lesion with central patchy depigmented areas with pigment mottling on the tumor surface (fig. 1a). Ultrasound B scan showed a highly reflective choroidal lesion with accompanying acoustic shadowing forming a pseudo-optic nerve appearance, classical of choroidal osteoma (fig. 2). SD-OCT images revealed disruption of the normal foveal architecture, with irregular retinal pigment epithelium (RPE) elevations (fig. 3). Fundus fluorescein angiography showed no evidence of subretinal neovascular membrane (fig. 4).
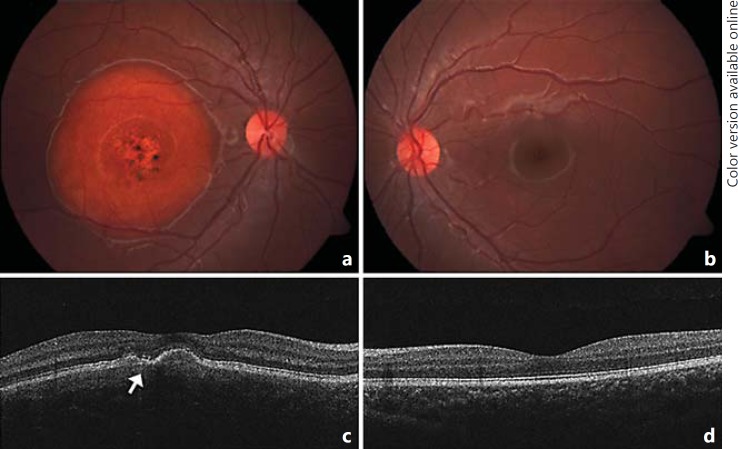
Fig. 1.
Fundus appearance on presentation showing a well-demarcated choroidal osteoma in the right macula with punctate dots of hyperpigmentation and central RPE thinning (a). SD-OCT images showed an irregularly elevated RPE layer with loss of normal foveal architecture. The RPE overlying the tumor showed some thinning. There was fragmentation of the photoreceptor inner and outer segment junction (arrow) suggesting photoreceptor damage, with irregular RPE elevations with overlying thinning (c). The left eye showed normal appearance on examination and SD-OCT scanning (b, d).
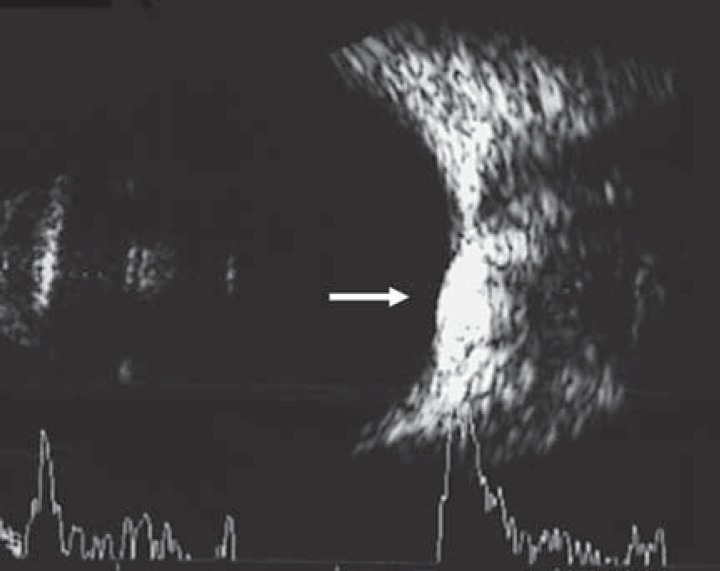
Fig. 2.
Ultrasonic B scan image showing a hyperechoic choroidal lesion with posterior acoustic shadowing forming a pseudo-optic nerve appearance (arrow), below the true optic nerve.
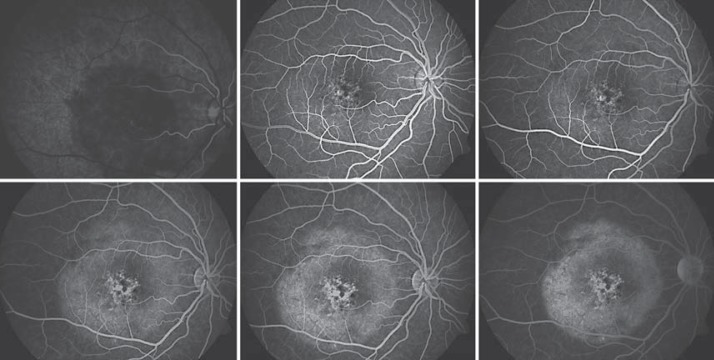
Fig. 3.
Fundus fluorescein angiogram revealed a round, well-defined hypofluorescent macular lesion, which causes hyperfluorescence in the late phases. Window defects representing RPE thinning or atrophy, punctuated with areas of hyperpigmented stippling caused by RPE clumping, were seen in the center of the tumor. There was no evidence of subretinal neovascular membrane.
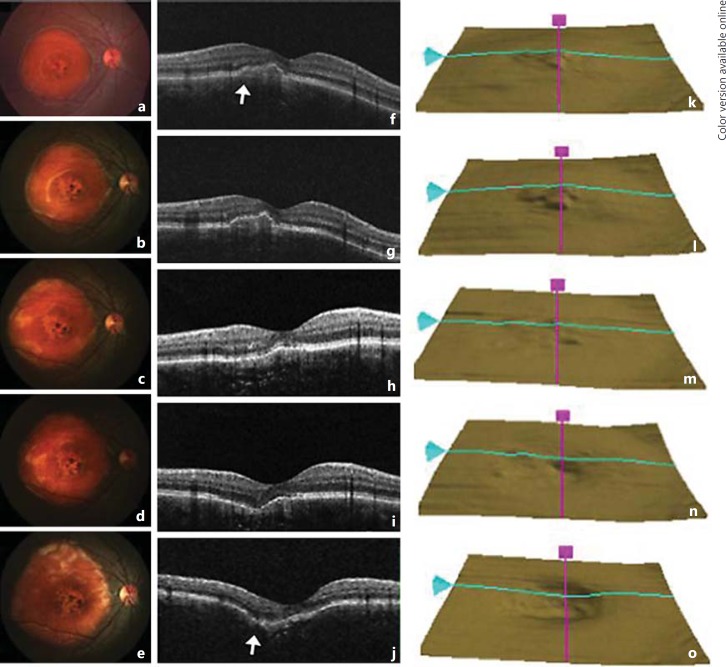
Fig. 4.
Fundus photos taken annually documented tumor progression, which showed the tumor size increasing in all directions. Over the period of 5 years, SD-OCT images showed gradual flattening and eventual depression over the area of RPE elevation (arrows), which resulted in a crater formation in the central macula, suggesting that tumor remodeling was taking place. a-e Fundus photographs of the right eye taken yearly. f-j Corresponding SD-OCT images of the right macula. k-o RPE layer images from SD-OCT scanning of the right eye.
As the child remained relatively asymptomatic over the 5-year period, tumor growth was documented by annual fundus photography and SD-OCT images. Using ImageJ [13], a free downloadable image processing and analysis software, the increase in tumor surface area was measured in square millimeters and the results are presented in table 1. The mean rate of tumor surface area growth was 4,396.2 mm2 over a period of 5 years. The maximal increase in growth was 7,349 mm2 between the ages of 10 and 11. For each measurement, the reference point was standardized on the optic disk, and the vertical diameter of the optic disk was calibrated to 1.5 mm on every fundus photograph. The tumor surface area was shown to increase by more than 180%, and the growth occurred in all directions (fig. 4). Corresponding SD-OCT images showed a gradual flattening followed by a depression of the tumor and eventual formation of a central excavation, which was clearly seen in the overlying RPE layer over a 5-year period. A sequential variation in metamorphopsia was also documented.
Table 1.
Calculation of tumor surface area growth
| Age, years | Surface area estimation, mm2 | Surface area growth per year, mm2 | Percentage of increase |
|---|---|---|---|
| 7 | 26,334 | NA | 100.0 |
| 8 | 30,953 | 4,619 | 117.5 |
| 9 | 36,400 | 5,447 | 138.2 |
| 10 | 40,966 | 4,566 | 155.6 |
| 11 | 48,315 | 7,349 | 183.5 |
The tumor surface area estimation was measured by calibrating the vertical diameter of the optic disk to 1.5 mm in each fundus photo using the ImageJ software. The surface area of the tumor from each fundus photo taken during annual visits is calculated in square millimeters. The percentage of increase in tumor surface area was measured by comparing the yearly increase to the baseline in the year 2009. The mean tumor surface area growth over the period of 5 years was 4,396.2 mm2/year.
Discussion
Choroidal osteoma is a benign bone tumor, and like all living mature bone tissues, undergoes an active remodeling process. This is supported by the histopathological findings of osteocytes, osteoclasts and osteoblasts by Williams et al. [14] in enucleated eyes with choroidal osteoma. SD-OCT findings of cavernous or sponge-like features with variable internal reflectivity [9,10] corroborate the presence of bony trabeculae and are suggestive of the various stages of the bone formation process occurring in choroidal osteomas. A common SD-OCT finding in choroidal osteoma is tumor elevation into the vitreous cavity [10,11,12,15]. In our case report, we used fundus images to monitor tumor surface area changes and SD-OCT to monitor changes in tumor thickness. We report a similar finding of tumor elevation in the early stages, progressively evolving to central excavation as the tumor edge enlarges. Central tumor depression has also been observed previously [1,6], and is believed to be caused by osteoclastic activity causing bone resorption. The increase in size and the formation of a central excavation suggests active bone remodeling in the underlying tumor in this case.
This remodeling process may or may not be associated with tumor progression over time. Progression of choroidal tumors has been documented to occur in 40-64% of cases [1,3,16]. In a series of 36 patients followed up for up to 22 years, Aylward et al. [3] observed that enlargement of choroidal tumors occurred in all directions in a uniform manner. No association was detected between growth of tumor and puberty. From our observation, the tumor growth spurt in this case occurred as the patient approached adolescence, suggesting that puberty may be a contributory factor. Possible reasons to consider hormonal influence in choroidal osteoma are its predominance in young females in the reproductive age group [1,2], and reports of tumor occurrences or progression during pregnancy [6,17] and in patients with thyroid diseases [18]. Transient elevation of serum parathyroid hormone and alkaline phosphatase levels has been reported during active growth of choroidal osteomas [19].
Choroidal osteoma is typically described as a slow-growing tumor [1,3,16], although there are several reported cases of rapid tumor growth [1,4,20]. Shields et al. [16] documented tumor growth at 0.37 mm per year by measuring the mean basal tumor diameter using indirect ophthalmoscopy. The tumor in this case showed a mean progression in surface area of 4,396.2 mm2 per year. The rate of tumor growth reported here is more reliable as it is based upon serial fundus photos and calculated using an easily available and widely accepted image processing and analysis software (ImageJ). This method allows a more reliable, reproducible and comparable measurement of tumor growth. The main limitation of this method is that it only measures change in tumor surface area but not tumor depth, and therefore may not necessarily reflect the true tumor growth.
References
- 1.Gass JD. New observations concerning choroidal osteomas. Int Ophthalmol. 1979;1:71–84. doi: 10.1007/BF00154194. [DOI] [PubMed] [Google Scholar]
- 2.Gass JD, Guerry RK, Jack RL, Harris G. Choroidal osteoma. Arch Ophthalmol. 1978;96:428–435. doi: 10.1001/archopht.1978.03910050204002. [DOI] [PubMed] [Google Scholar]
- 3.Aylward GW, Chang TS, Pautler SE, Gass JD. A long-term follow-up of choroidal osteoma. Arch Ophthalmol. 1998;116:1337–1341. doi: 10.1001/archopht.116.10.1337. [DOI] [PubMed] [Google Scholar]
- 4.Mizota A, Tanabe R, Adachi-Usami E. Rapid enlargement of choroidal osteoma in a 3-year-old girl. Arch Ophthalmol. 1998;116:1128–1129. doi: 10.1001/archopht.116.8.1128. [DOI] [PubMed] [Google Scholar]
- 5.Shields JA, Shields CL, de Potter P, Belmont JB. Progressive enlargement of a choroidal osteoma. Arch Ophthalmol. 1995;113:819–820. doi: 10.1001/archopht.1995.01100060145049. [DOI] [PubMed] [Google Scholar]
- 6.Buettner H. Spontaneous involution of a choroidal osteoma. Arch Ophthalmol. 1990;108:1517–1518. doi: 10.1001/archopht.1990.01070130019009. [DOI] [PubMed] [Google Scholar]
- 7.Trimble SN, Schatz H. Decalcification of a choroidal osteoma. Br J Ophthalmol. 1991;75:61–63. doi: 10.1136/bjo.75.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trimble SN, Schatz H, Schneider GB. Spontaneous decalcification of a choroidal osteoma. Ophthalmology. 1988;95:631–634. doi: 10.1016/s0161-6420(88)33144-1. [DOI] [PubMed] [Google Scholar]
- 9.Freton A, Finger PT. Spectral domain-optical coherence tomography analysis of choroidal osteoma. Br J Ophthalmol. 2012;96:224–228. doi: 10.1136/bjo.2011.202408. [DOI] [PubMed] [Google Scholar]
- 10.Pellegrini M, Invernizzi A, Giani A, Staurenghi G. Enhanced depth imaging optical coherence tomography features of choroidal osteoma. Retina. 2014;34:958–963. doi: 10.1097/IAE.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 11.Yoshikawa T, Takahashi K. Decalcified choroidal osteoma found in the retina. Clin Ophthalmol. 2012;6:1823–1825. doi: 10.2147/OPTH.S37952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sayanagi K, Morimoto Y, Ikuno Y, Tano Y. Spectral-domain optical coherence tomographic findings in myopic foveoschisis. Retina. 2010;30:623–628. doi: 10.1097/iae.0b013e3181ca4e7c. [DOI] [PubMed] [Google Scholar]
- 13.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams AT, Font RL, Van Dyk HJ, Riekhof FT. Osseous choristoma of the choroid simulating a choroidal melanoma. Association with a positive 32P test. Arch Ophthalmol. 1978;96:1874–1877. doi: 10.1001/archopht.1978.03910060378017. [DOI] [PubMed] [Google Scholar]
- 15.Erol MK, Coban DT, Ceran BB, Bulut M. Enhanced depth imaging optical coherence tomography and fundus autofluorescence findings in bilateral choroidal osteoma: a case report. Arq Bras Oftalmol. 2013;76:189–191. doi: 10.1590/s0004-27492013000300012. [DOI] [PubMed] [Google Scholar]
- 16.Shields CL, Sun H, Demirci H, Shields JA. Factors predictive of tumor growth, tumor decalcification, choroidal neovascularization, and visual outcome in 74 eyes with choroidal osteoma. Arch Ophthalmol. 2005;123:1658–1666. doi: 10.1001/archopht.123.12.1658. [DOI] [PubMed] [Google Scholar]
- 17.McLeod BK. Choroidal osteoma presenting in pregnancy. Br J Ophthalmol. 1988;72:612–614. doi: 10.1136/bjo.72.8.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warrasak S, Suvaranamani C, Euswas A, Sumetpimolchai V, Laothamatas J. Choroidal osteoma in Oriental patients. J Med Assoc Thai. 2003;86:562–572. [PubMed] [Google Scholar]
- 19.Katz RS, Gass JD. Multiple choroidal osteomas developing in association with recurrent orbital inflammatory pseudotumor. Arch Ophthalmol. 1983;101:1724–1727. doi: 10.1001/archopht.1983.01040020726012. [DOI] [PubMed] [Google Scholar]
- 20.Pamer Z, Kovacs B. A case of a fast-growing bilateral choroidal osteoma. Retina. 2001;21:657–659. doi: 10.1097/00006982-200112000-00015. [DOI] [PubMed] [Google Scholar]