Abstract
We isolated a Bacillus strain, RX7, with inhibitory activity against Listeria monocytogenes from soil and identified it as Bacillus amyloliquefaciens based on 16S rRNA gene sequencing. The inhibitory activity was stable over a wide range of pH and was fully retained after 30 min at 80°C, after which it decreased gradually at higher temperatures. The activity was sensitive to the proteolytic action of α-chymotrypsin, proteinase-K, and trypsin, indicating its proteinaceous nature. This bacteriocin was active against a broad spectrum of bacteria and the fungus Candida albicans. Direct detection of antimicrobial activity on a sodium dodecyl sulfate-polyacrylamide gel suggested an apparent molecular mass of approximately 5 kDa. Ammonium sulfate precipitation and anion-exchange and gel permeation chromatography integrated with reverse phase-high-performance liquid chromatography were used for bacteriocin purification. Automated N-terminal Edman degradation of the purified RX7 bacteriocin recognized the first 15 amino acids as NH2-X-Ala-Trp-Tyr-Asp-Ile-Arg-Lys-Leu-Gly-Asn-Lys-Gly-Ala, where the letter X in the sequence indicates an unknown or nonstandard amino acid. Based on BLAST similarity search and multiple alignment analysis, the obtained partial sequence showed high homology with the two-peptide lantibiotic haloduracin (HalA1) from Bacillus halodurans, although at least two amino acids differed between the sequences. A time-kill study demonstrated a bactericidal mode of action of RX7 bacteriocin.
1. Introduction
Bacteriocins are ribosomally synthesized antimicrobial peptides which are secreted to act against closely related bacterial species without affecting the producing strain [1]. To address increasing bacterial resistance to conventional antibiotics, bacteriocins are now considered as alternative antimicrobials for the treatment of human (and possibly animal) infections [2]. Furthermore, since minimally processed foods with no chemical preservatives are in demand by consumers, research into natural antimicrobial agents such as bacteriocins [3] has been increasing. Lactic acid bacteria (LAB) bacteriocins are studied widely due to their potential use as biopreservatives in the food industry because many strains have been “generally recognized as safe” (GRAS) status [4]. The lantibiotic nisin, which contains unusual amino acids such as lanthionine and β-methyllanthionine, is the most studied bacteriocin to date and is the only bacteriocin currently used as a food additive [5]; however, the use of nisin is limited due to its very low activity at neutral or alkaline pH. Therefore, the search for novel bacteriocins with improved biochemical properties, including stability over a wide pH range, thermostability, and a broad antimicrobial spectrum, is of great interest for applications in foods.
Members of the Bacillus group are considered to be good producers of antimicrobial substances such as peptide and lipopeptide antibiotics, as well as bacteriocins [6]. Interestingly, Bacillus represents an alternative genus for the identification of bacteriocins because it includes many industrial species and has a history of safe use in the food industry [5]. It is also considered to be the second most important bacteriocin producer following LAB [3]. Therefore, the ability to screen for antimicrobial Bacillus strains is of major interest in bacteriocin research since this genus produces a diverse array of antimicrobial peptides [6, 7].
Several bacteriocins and bacteriocin-like inhibitory substances (BLIS) produced by Bacillus amyloliquefaciens have been described, most of which are inhibitory to Gram-positive bacteria, but lack activity against Gram-negative bacteria. Here, we identified and characterized a novel bacteriocin from B. amyloliquefaciens RX7. This bactericidal protein exhibits a broad antimicrobial spectrum, inhibiting several Gram-negative and Gram-positive bacteria.
2. Materials and Methods
2.1. Isolation of Antimicrobial Microorganisms
Soil samples were collected from a farm in Cheonan city, Korea. They were mixed with sterile water (1 : 10, w/v), homogenized, and heated at 50°C for 60 min in a water bath. One mL of this suspension was inoculated into 100 mL of tryptic soy broth (Difco, USA) and incubated at 50°C for 24 h, after which microbial growth was monitored based on changes in turbidity of the cultures. Aliquots of the cultures were inoculated onto TSB plates, incubated at 30°C, and single colonies were isolated and screened for antimicrobial activity. The antimicrobial activity was performed according to Ramachandran et al. [8] with modifications. It was expressed as the diameter of the inhibition zones around the wells using agar well diffusion assays with Listeria monocytogenes ATCC 19114 as the indicator strain. Other indicator strains were propagated in appropriate media as indicated in Table 1.
Table 1.
Antimicrobial spectra of the RX7 bacteriocin.
| Indicator organism | Media | Inhibition |
|---|---|---|
| zone (mm)∗ | ||
| Gram-positive | ||
| Bacillus cereus KCTC 1661 | LB | ++ |
| Bacillus licheniformis KCCM 12145 | NB | ++ |
| Enterococcus faecalis (VRE) CCARM 0011 | NB | ++ |
| Listeria monocytogenes ATCC 19114 | TSB | +++ |
| Streptococcus agalactiae ATCC 13813 | TSB | ++ |
| Staphylococcus aureus KFRI 00188 | NB | ++ |
| Staphylococcus aureus subsp. aureus (MRSA) KCCM 40510 | NB | + |
| Streptococcus mutans ATCC 25175 | BHI | + |
| Lactobacillus acidophilus KCCM 32820 | MRS | + |
| Lactobacillus delbrueckii KCCM 11357 | MRS | + |
| Lactobacillus johnsonii PF01 (our isolate) | MRS | + |
| Lactobacillus salivarius CPM7 (our isolate) | MRS | + |
| Lactobacillus plantarum KCCM 11322 | MRS | + |
|
| ||
| Gram-negative | ||
| Escherichia coli K88 | LB | ++ |
| Klebsiella pneumoniae subsp. pneumoniae KCCM 11418 | NB | + |
| Pseudomonas aeruginosa KCCM 11266 | NB | ++ |
| Pseudomonas aeruginosa CCARM 2003 | LB | ++ |
| Shigella flexneri KCCM 40414 | NB | ++ |
| Salmonella enteritidis KCCM 12021 | NB | ++ |
| Salmonella enteritidis KVCC-BA0700654 | NB | ++ |
| Salmonella gallinarum KVCC-BA0700722 | NB | ++ |
| Salmonella pullorum KVCC-BA0702509 | NB | + |
| Salmonella typhimurium KCCM 40253 | NB | ++ |
|
| ||
| Fungi | ||
| Candida albicans KCTC 7122 | PDA | ++ |
ATCC: American Type Culture Collection; CCARM: Culture Collection of Antimicrobial Resistant Microbes; KFRI: Kerala Forest Research Institute; KCCM: Korean Culture Center of Microorganisms; KCTC: Korean Collection for Type Culture.
TSB: tryptic soy broth; NB: nutrient broth; LB: Luria-bertani; BHI: brain heart infusion; MRS: de Man Rogosa and Sharpe.
∗Activity is expressed as the diameter of the inhibition zone around the well: +, less than 10 mm; ++, less than 20 mm; +++, less than 30 mm.
2.2. Antimicrobial Activity Assay
Antimicrobial activity was detected using the agar well diffusion assay and tested against all indicator strains (Table 1) grown in their respective media, as indicated (Difco, USA). An aliquot of culture was centrifugated at 6,000 g for 15 min at 4°C (Mega 21R, Hanil Co., Republic of Korea), filtered using 0.45 μm pore-size cellulose acetate syringe filters (Advantec Co., Japan) to remove cells, then applied to wells on agar plates. Experiments were done in no less than five individual trials having no less than three replications.
The plates were incubated at the optimal temperature of the test organism. The bacteriocin titer was determined using the serial twofold dilution method. Activity was defined as the reciprocal of the dilution after the last serial dilution giving a zone of inhibition and was expressed as activity units (AU) per milliliter. All measurements were done in triplicate and the means are shown as the results.
2.3. Bacterial Identification
Bacillus amyloliquefaciens strain RX7 was identified taxonomically based on phenotypic and physiological characteristics using the Analytical Profile Index (API) test system (Biomérieux, France) and analysis of a partial 16S rDNA sequence. RX7 genomic DNA was prepared from an overnight culture using the phenol-chloroform extraction method. Amplification of the 16S rDNA gene by polymerase chain reaction (PCR) using the F1 (5′-AGAGTTTCCTGGCTCAG-3′) and R3 (5′-AAGGAGGTGATCCAGCC-3′) primers was performed under the following conditions: initial denaturation at 94°C for 5 min, 35 cycles of 95°C for 1 min, 55°C for 1 min, and 72°C for 90 s and a final extension at 72°C for 7 min. In addition, a Bacillus subtilis-specific primer set, ytcP-F (5′-GCTTACGGGTTATCCCGC-3′) and ytcP-R (5′-CCGACCCCATTTCAGACATATC-3′), was used to differentiate between B. subtilis and B. amyloliquefaciens under the following conditions: initial denaturation at 94°C for 5 min, 30 cycles of denaturation at 94°C for 30 s, annealing at 50°C for 1 min, and primer extension at 72°C for 1 min. Purified PCR fragments were sequenced with both primers and compared with 16S rRNA gene sequences in the public database using BLAST. API CHB50 bacterial identification analysis was also performed according to the manufacturer's instructions.
2.4. Purification of Bacteriocin from the RX7 Strain
After aerobic cultivation of B. amyloliquefaciens RX7 in 500 mL Erlenmeyer flasks containing 200 mL of TSB at 30°C for 6 h, cell-free supernatant (CFS) was obtained by centrifugation at 8,000 ×g for 20 min and for 15 min at 4°C (Hanil Science Industrial Co., Republic of Korea). The resulting supernatant was passed through a 0.45 μm filter (Advantec Co., Japan). An ammonium sulfate precipitation at 60% saturation was performed in the culture filtrate. The resulting pellets were resuspended in 20 mM phosphate buffer (pH 7.0), followed by dialysis overnight (2-kDa cut-off, Sigma, USA). The dialyzed sample was applied to a Q Sepharose Fast-Flow resin anion-exchange column (GE Healthcare Life Sciences, USA) equilibrated with 20 mM phosphate buffer (pH 7) and eluted by a gradient of 2 M NaCl in the same buffer. Active fractions were collected, desalted, and concentrated using Sep-Pak C18 cartridges (Waters, USA). Active peptides were subsequently placed on a BioSep-SEC-S2000 gel permeation column (Phenomenex, USA) previously equilibrated with 50 mM phosphate buffer (pH 7.0) integrated in a HP1000 reverse phase-high-performance liquid chromatography (HPLC) system (Hewlett Packard, USA). The eluates were monitored based on their UV absorbance at 280 nm. Peptides were separated under isocratic conditions and subjected to N-terminal sequencing by Edman degradation.
2.5. Effects of pH, Temperature, Enzymes, and Organic Solvents on Antimicrobial Activity
To evaluate pH stability, the pH of crude bacteriocin solution was adjusted with 1 N HCl and 1 N NaOH and readjusted to pH 7 after incubation for 2 h at 37°C. To evaluate thermal stability, the crude bacteriocin solution was incubated at 4, 37, 50, 80, and 100°C for 15–30 minutes. All samples were cooled to room temperature before activity assays. To analyze sensitivity to various enzymes, the crude bacteriocin solution was treated at 37°C for 2 h with 10 mg/mL (final concentration) of the following enzymes: α-amylase, α-chymotrypsin, lipase, proteinase-K, pepsin, or trypsin. All enzymes used were from Sigma (USA) and Takara (Japan). The samples were then boiled for 2 min to denature the enzymes and cooled to room temperature. Samples were exposed to various organic solvents at a final concentration of 10% (v/v) to explore their effects on bacteriocin activity. After incubation for 2 h at 37°C, the residual activity was recorded. All treated samples were tested for residual activity against L. monocytogenes ATCC 19114, as described above.
2.6. Mode of Action
To determine the mode of action of the bacteriocin, the crude bacteriocin was added at a final concentration of 80 AU/mL to the culture at the mid-exponential growth phase of L. monocytogenes ATCC 19114 in 20 mL of TSB medium. Viable cells were counted at 1-hour intervals for the next 8 hours. Viable cell counting was repeated in triplicate and the mean values were calculated.
2.7. Molecular Weight Estimation
Antimicrobial activity was detected using tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as described previously [9]. Briefly, the crude bacteriocin sample (100 μg) was loaded onto a 16.5% polyacrylamide gel and electrophoresis was performed at 100 V for 5 h. After electrophoresis, half of the gel was stained with Coomassie blue R-250 and the other half was washed with sterile water for 5 h with a water change every 30 min to remove SDS. The washed gel was overlaid with soft agar (0.7%, w/v) containing L. monocytogenes ATCC 19114 (OD600 0.4–0.8) cells. The overlaid gel was incubated for 24 h at 37°C and the clear zone of inhibition was measured. Molecular weight standards were obtained from Sigma (USA).
2.8. N-Terminal Sequencing
N-terminal amino acid sequencing of the active peak obtained from the HPLC analysis was performed by Edman degradation on a Procise 492 Protein Sequencing System [10, 11] (Applied Biosystems, USA).
3. Results
3.1. Isolation and Taxonomical Identification of a Bacteriocin-Producing Strain
A bacterial strain designated RX7, which exhibited antimicrobial activity against L. monocytogenes, was isolated from a soil sample. The RX7 strain was a short rod-shaped, Gram-positive bacterium (data not shown). Taxonomical analysis of the RX7 isolate using the API 50CHB bacterial identification system and homology analysis of the nucleotide sequence of the 16S rRNA gene identified the RX7 strain as Bacillus subtilis or B. amyloliquefaciens. Further examination using polymerase chain reaction (PCR) analysis showed the absence of the B. subtilis-specific ytcP gene encoding a hypothetical ABC-type transporter [12], which confirmed that RX7 was B. amyloliquefaciens. The producer strain was identified as B. amyloliquefaciens and the nucleotide sequence of the 16S rDNA of the RX7 strain was deposited in GenBank under the accession number KU301791.
3.2. Antimicrobial Spectrum
We tested the antimicrobial activity of a cell-free supernatant (CFS) against Gram-positive and Gram-negative bacteria (Table 1). The RX7 bacteriocin exhibited its highest activity against L. monocytogenes. It was also active against both Gram-positive pathogenic and spoilage bacteria such as Bacillus cereus and Staphylococcus aureus, and against pathogenic Gram-negative bacteria including Escherichia coli, Pseudomonas aeruginosa, Salmonella enteritidis, and S. gallinarum. RX7 bacteriocin also inhibited the fungus Candida albicans. These findings demonstrated the broad inhibitory spectrum of the bacteriocin produced by B. amyloliquefaciens RX7.
3.3. Effects of pH, Temperature, Enzymes, and Organic Solvents
Table 2 summarizes the effects of various treatments and conditions on the activity of purified RX7 bacteriocin against L. monocytogenes ATCC 19114. The antimicrobial activity was sensitive to α-chymotrypsin, proteinase-K, and trypsin, indicative of the proteinaceous nature of the antimicrobial substance. Full activity was still observed when exposed to 80°C for 30 minutes. Activity remained stable up to 100°C, but only 20% activity remained when subjected to 121°C for 15 min. Likewise, RX7 bacteriocin activity was stable in the presence of organic solvents and even under a wide range of pH conditions. Full activity was observed in the range of pH 3–8.
Table 2.
Effects of enzymes, temperature, pH, and organic solvents on the antimicrobial activity of RX7 bacteriocin.
| Treatment | Residual activity (%)∗ |
|---|---|
| None (control) | 100 |
| Enzymes† | |
| α-Amylase | 100 |
| α-Chymotrypsin | 0 |
| Lipase | 100 |
| Proteinase-K | 0 |
| Pepsin | 100 |
| Trypsin | 0 |
| Heat | |
| 4°C/30 min | 100 |
| 37°C/30 min | 100 |
| 50°C/30 min | 100 |
| 80°C/30 min | 100 |
| 100°C/30 min | 80 |
| 121°C/15 min | 20 |
| pH | |
| 1 | 80 |
| 2 | 90 |
| 3 | 100 |
| 4 | 100 |
| 5 | 100 |
| 6 | 100 |
| 7 | 100 |
| 8 | 100 |
| 9 | 90 |
| 10 | 80 |
| Organic solvents‡ | |
| Acetone | 90 |
| Acetonitrile | 90 |
| Butanol | 80 |
| Chloroform | 80 |
| Ethanol | 90 |
| Methanol | 80 |
∗Residual activity compared with antimicrobial activity before the treatment.
†Enzyme concentrations were 10 mg/mL.
‡10% concentration.
3.4. Mode of Action
After addition of RX7 bacteriocin (80 AU/mL) to L. monocytogenes adjusted to 4.2 × 108 colony forming units (CFU)/mL in TSB agar culture medium, the CFU were counted following various incubation periods (Figure 1). After the addition of RX7 bacteriocin, the population of cells decreased quickly, suggestive of a bactericidal mode of action. The incubation time for complete bactericidal action (i.e., no colony formation on TSB agar) was 2 h.
Figure 1.
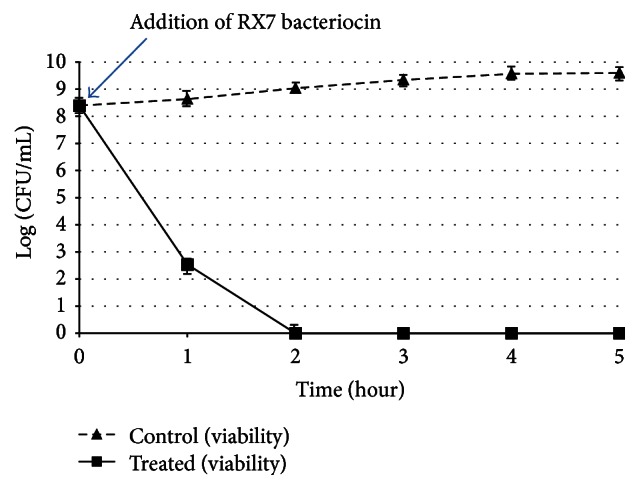
Bactericidal activity of RX7 bacteriocin against Listeria monocytogenes ATCC 19114. Viability of control (triangles) and treated (squares) cells was monitored. The time of RX7 bacteriocin addition is indicated by the arrow. Each point represents the mean of three independent experiments.
3.5. Molecular Weight Estimation and N-Terminal Amino Acid Determination
To estimate the molecular size of the bacteriocin, an ammonium sulfate precipitate of B. amyloliquefaciens RX7 culture supernatant was prepared as described in Section 2. The molecular size of RX7 bacteriocin was estimated based on tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The specific band associated with the antibacterial activity against L. monocytogenes was found at approximately 5 kDa, indicated by an observed single zone of inhibition from the sample after ammonium sulfate precipitation on the basis of its position (data not shown).
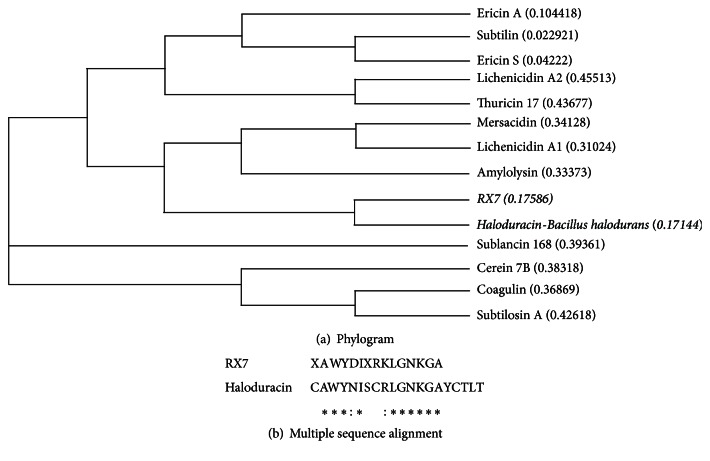
Automated N-terminal Edman degradation of the purified RX7 bacteriocin recognized the first 15 amino acids as NH2-X-Ala-Trp-Tyr-Asp-Ile-Arg-Lys-Leu-Gly-Asn-Lys-Gly-Ala, where the letter X in the sequence represents an unknown or nonstandard amino acid. Based on BLAST similarity search and multiple alignment analysis, the obtained partial sequence showed high homology with the lantibiotic and haloduracin, from Bacillus halodurans [13]; however, at least two amino acids differed based on sequence comparison (Figure 2).
Figure 2.
(a) Phylogram based on amino acid sequence homology of bacteriocins produced by Bacillus species. (b) Sequence alignment of RX7 bacteriocin and haloduracin A1 produced by Bacillus halodurans generated using MUSCLE 3.8 and Clustal 2.1 software.
4. Discussion
Bacteriocins from Bacillus species, together with those from LAB, are gaining considerable attention for applications in human and animal health. We isolated the RX7 strain, which is active against the food pathogen L. monocytogenes, from a local soil sample and identified it as B. amyloliquefaciens based on biochemical profiling and 16S rDNA sequencing. DNA-based identification methods such as 16S rRNA gene sequencing have been used widely for the purpose of identification and typing of microorganisms isolated from natural environments [12]. However, identification based on rRNA gene sequences sometimes fails to distinguish one species from the other if they share highly similar rRNA genes, as in the case of B. subtilis and B. amyloliquefaciens. In this work, a primer pair specific for ytcP gene, which B. subtilis contains and B. amyloliquefaciens does not, was used to distinguish these species. However, further confirmation experiments are needed in the future.
The sensitivity of the antimicrobial substance from B. amyloliquefaciens RX7 to proteases was indicative of its proteinaceous nature, leading us to classify it as a bacteriocin. This compound exhibited broad spectrum antibacterial activity. In addition, RX7 bacteriocin inhibits the growth of various species of Gram-positive bacteria, including L. monocytogenes, B. cereus, and S. aureus, and Gram-negative bacteria, including E. coli, P. aeruginosa, S. enteritidis, and S. gallinarum. This characteristic is atypical of bacteriocins in that bacteriocins produced by Gram-positive bacteria are mostly inhibitory towards Gram-positive bacteria and are less effective against Gram-negative bacteria [14].
To date, several bacteriocin or bacteriocin-like inhibitory substances (BLIS) from B. amyloliquefaciens have been reported: BLIS RC-2, which is antagonistic to bacterial and fungal plant pathogens [15]; BLIS 5006, which is antagonistic to food-spoilage bacteria with declining activity at alkaline pH [16]; and BLIS 5940, which is active against Clostridium perfringens [17]. Other well-characterized bacteriocins from B. amyloliquefaciens are amylolysin [18] and amylocyclicin [19], which are primarily active against Gram-positive bacteria including food pathogenic Listeria. Subtilosin A, a posttranslationally modified peptide (Class I) produced by B. subtilis and B. amyloliquefaciens, has antimicrobial activity against Gram-positive and Gram-negative bacteria [20].
However, N-terminal sequencing and sequence alignment of the bacteriocin from RX7 showed no homology to subtilosin A, but significant similarity with haloduracin A1, a lantibiotic, which is a posttranslationally modified peptide (Class I) produced by B. halodurans [13]. In addition, the estimated molecular size of RX7 bacteriocin and its stability at high temperature and in solvents are similar to those of haloduracin. However, at least two amino acids were different based on sequence comparison. Moreover, haloduracin inhibits only Gram-positive bacteria and is alkaliphilic in nature unlike RX7 bacteriocin, indicating that RX7 bacteriocin from B. amyloliquefaciens differs from the lantibiotic haloduracin.
Recently, sonorensin was isolated from a marine isolate of Bacillus sonorensis and it exhibited broad-spectrum antibacterial activity towards both Gram-positive and Gram-negative bacteria [21]. Sonorensin had significant similarity to the putative thiazole-containing heterocyclic bacteriocin of Bacillus licheniformis ATCC 14580 (accession number YP_006712426.1), and its N-terminal sequence is homologous to the reader sequences of protoxins from various Bacillus strains. This suggested that sonorensin belongs to a new subfamily of bacteriocin, heterocycloanthracin, a group of putative peptides containing oxazole and/or thiazole heterocycles [22].
RX7 bacteriocin is similar to subtilosin A in that it has broad antimicrobial activity, thermostability, and antimicrobial activity under a broad range of pH conditions. However, the two bacteriocins exhibit amino acid sequence differences; RX7 bacteriocin is similar to haloduracin in the N-terminal sequences. The characteristics of RX7 bacteriocin are in common with Class I bacteriocins (lantibiotics). Further study to identify gene clusters involved in the biosynthesis and mechanism of RX7 bacteriocin is required for structural characterization.
Here, we identified and characterized a potentially novel bacteriocin produced by a strain of B. amyloliquefaciens. With its broad spectrum inhibitory properties, thermostability, and tolerance to a broad range of pH conditions and various solvents, it may be applicable to food preservation and human and animal health.
Acknowledgments
This work was supported by the Ministry of Trade, Industry and Energy (MOTIE) and Korea Institute for Advancement of Technology (KIAT) (R0000576) and by a grant from the Next-Generation BioGreen 21 Program (PJ01115903), Rural Development Administration, Republic of Korea.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Klaenhammer T. R. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiology Reviews. 1993;12(1–3):39–85. doi: 10.1016/0168-6445(93)90057-g. [DOI] [PubMed] [Google Scholar]
- 2.Lawton E. M., Cotter P. D., Hill C., Ross R. P. Identification of a novel two-peptide lantibiotic, Haloduracin, produced by the alkaliphile Bacillus halodurans C-125. FEMS Microbiology Letters. 2007;267(1):64–71. doi: 10.1111/j.1574-6968.2006.00539.x. [DOI] [PubMed] [Google Scholar]
- 3.Abriouel H., Franz C. M. A. P., Omar N. B., Galvez A. Diversity and applications of Bacillus bacteriocins. FEMS Microbiology Reviews. 2011;35(1):201–232. doi: 10.1111/j.1574-6976.2010.00244.x. [DOI] [PubMed] [Google Scholar]
- 4.O'Sullivan L., Ross R. P., Hill C. Potential of bacteriocin-producing lactic acid bacteria for improvements in food safety and quality. Biochimie. 2002;84(5-6):593–604. doi: 10.1016/s0300-9084(02)01457-8. [DOI] [PubMed] [Google Scholar]
- 5.Delves-Broughton J., Blackburn P., Evans R. J., Hugenholtz J. Applications of the bacteriocin, nisin. Antonie van Leeuwenhoek. 1996;69(2):193–202. doi: 10.1007/bf00399424. [DOI] [PubMed] [Google Scholar]
- 6.Stein T. Bacillus subtilis antibiotics: structures, syntheses and specific functions. Molecular Microbiology. 2005;56(4):845–857. doi: 10.1111/j.1365-2958.2005.04587.x. [DOI] [PubMed] [Google Scholar]
- 7.Gebhardt K., Schimana J., Müller J., et al. Screening for biologically active metabolites with endosymbiotic bacilli isolated from arthropods. FEMS Microbiology Letters. 2002;217(2):199–205. doi: 10.1016/S0378-1097(02)01065-0. [DOI] [PubMed] [Google Scholar]
- 8.Ramachandran R., Chalasani A. G., Lal R., Roy U. A broad-spectrum antimicrobial activity of Bacillus subtilis RLID 12.1. The Scientific World Journal. 2014;2014:10. doi: 10.1155/2014/968487.968487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhunia A. K., Johnson M. C., Ray B. Direct detection of an antimicrobial peptide of Pediococcus acidilactici in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Journal of Industrial Microbiology. 1987;2(5):319–322. doi: 10.1007/bf01569434. [DOI] [Google Scholar]
- 10.Smith J. B. Encyclopedia of Life Sciences. Mac Millan Publishers Ltd, Nature Publishing Group; 2012. Peptide sequencing by Edman degradation. [Google Scholar]
- 11.Edman P., Högfeldt E., Sillén L. G., Kinell P. Method for determination of the amino acid sequence in peptides. Acta Chemica Scandinavica. 1950;4:283–293. doi: 10.3891/acta.chem.scand.04-0283. [DOI] [Google Scholar]
- 12.Kwon G.-H., Lee H.-A., Park J.-Y., et al. Development of a RAPD-PCR method for identification of Bacillus species isolated from Cheonggukjang. International Journal of Food Microbiology. 2009;129(3):282–287. doi: 10.1016/j.ijfoodmicro.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 13.McClerren A. L., Cooper L. E., Quan C., Thomas P. P., Kelleher N. L., van der Donk W. A. Discovery and in vitro biosynthesis of haloduracin, a two-component lantibiotic. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(46):17243–17248. doi: 10.1073/pnas.0606088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray E. J., Lee K. D., Souleimanov A. M., et al. A novel bacteriocin, thuricin 17, produced by plant growth promoting rhizobacteria strain Bacillus thuringiensis NEB17: isolation and classification. Journal of Applied Microbiology. 2006;100(3):545–554. doi: 10.1111/j.1365-2672.2006.02822.x. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida S., Hiradate S., Tsukamoto T., Hatakeda K., Shirata A. Antimicrobial activity of culture filtrate of Bacillus amyloliquefaciens RC-2 isolated from mulberry leaves. Phytopathology. 2001;91(2):181–187. doi: 10.1094/phyto.2001.91.2.181. [DOI] [PubMed] [Google Scholar]
- 16.Lisboa M. P., Bonatto D., Bizani D., Henriques J. A. P., Brandelli A. Characterization of a bacteriocin-like substance produced by Bacillus amyloliquefaciens isolated from the Brazilian Atlantic forest. International Microbiology. 2006;9(2):111–118. [PubMed] [Google Scholar]
- 17.Lei X., Piao X., Ru Y., Zhang H., Péron A., Zhang H. Effect of Bacillus amyloliquefaciens-based direct-fed microbial on performance, nutrient utilization, intestinal morphology and cecal microflora in broiler chickens. Asian-Australasian Journal of Animal Sciences. 2015;28(2):239–246. doi: 10.5713/ajas.14.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arias A. A., Ongena M., Devreese B., Terrak M., Joris B., Fickers P. Characterization of amylolysin, a novel lantibiotic from Bacillus amyloliquefaciens GA1. PLoS ONE. 2013;8(12) doi: 10.1371/journal.pone.0083037.e83037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scholz R., Vater J., Budiharjo A., et al. Amylocyclicin, a novel circular bacteriocin produced by Bacillus amyloliquefaciens FZB42. Journal of Bacteriology. 2014;196(10):1842–1852. doi: 10.1128/jb.01474-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shelburne C. E., An F. Y., Dholpe V., Ramamoorthy A., Lopatin D. E., Lantz M. S. The spectrum of antimicrobial activity of the bacteriocin subtilosin A. Journal of Antimicrobial Chemotherapy. 2007;59(2):297–300. doi: 10.1093/jac/dkl495. [DOI] [PubMed] [Google Scholar]
- 21.Chopra L., Singh G., Choudhary V., Sahoo D. K. Sonorensin: An antimicrobial peptide, belonging to the heterocycloanthracin subfamily of bacteriocins, from a new marine isolate, Bacillus sonorensis MT93. Applied and Environmental Microbiology. 2014;80(10):2981–2990. doi: 10.1128/aem.04259-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haft D. H. A strain-variable bacteriocin in Bacillus anthracis and Bacillus cereus with repeated Cys-Xaa-Xaa motifs. Biology Direct. 2009;4, article 15 doi: 10.1186/1745-6150-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]