SUMMARY
Disseminated Mycobacterium tuberculosis is a leading cause of bloodstream infection and severe sepsis in sub-Saharan African settings with a high burden of tuberculosis (TB) and human immunodeficiency virus (HIV) infection. Despite the high prevalence of M. tuberculosis bacteremia in these settings it is under-recognized. This is in part because timely diagnosis of M. tuberculosis bacteremia is difficult using traditional TB diagnostics. Novel triage algorithms and rapid diagnostic tests are needed to expedite the identification and treatment of patients with severe sepsis due to M. tuberculosis bacteremia. In this article, we emphasize the importance of M. tuberculosis bacteremia as an under-recognized etiology of severe sepsis, and discuss the potential role of two emerging rapid diagnostic tests in the triage and prognostication of critically ill patients with advanced HIV infection and suspected disseminated M. tuberculosis. We conclude with the recommendation that clinicians in high TB-HIV burden settings strongly consider empiric anti-tuberculosis treatment in patients with advanced HIV infection and severe sepsis in the appropriate clinical context. Future studies are needed to assess diagnostic and prognostic algorithms for severe sepsis caused by disseminated M. tuberculosis in these settings, and the safety, efficacy, and duration of empiric anti-tuberculosis treatment.
Keywords: tuberculosis diagnostics, critical illness, sepsis
OVER THE PAST TWO DECADES, disseminated Mycobacterium tuberculosis has been increasingly recognized as a leading cause of bloodstream infection (BSI) in sub-Saharan African settings with a high burden of tuberculosis (TB) and human immunodeficiency virus (HIV) infection.1–4 Despite the high prevalence of M. tuberculosis bacteremia in such settings, severe sepsis or septic shock (defined clinically as the presence of suspected infection plus hypotension and any one of the following: fever or hypothermia, tachycardia, or tachypnea), associated with localized or disseminated M. tuberculosis has been infrequently reported in the literature.5 However, large studies published over the past 2 years have identified M. tuberculosis as the most common cause of BSI among HIV-infected adults hospitalized in Uganda and Zambia with severe sepsis.6,7
Short-term mortality among patients with M. tuberculosis bacteremia, particularly those with severe sepsis, is high.2–4,6,8–10 Although evidence is limited, recently published data suggest that survival among adults with severe sepsis or septic shock due to M. tuberculosis, including those with disseminated disease, may be improved with early, empiric anti-tuberculosis treatment.6,11
Timely diagnosis of M. tuberculosis bacteremia is difficult, as clinical manifestations are often nonspecific. The utility of traditional diagnostic studies for TB is limited among patients with advanced HIV infection, who frequently manifest smear-negative or extra-pulmonary disease.12,13 The isolation of M. tuberculosis in blood requires laboratory infrastructure and personnel that are often lacking in district hospitals, and M. tuberculosis culture results can take 2–6 weeks.6,10 Novel triage algorithms and rapid diagnostic tests are needed to expedite the identification and treatment of critically ill HIV-infected patients with M. tuberculosis bacteremia in resource-limited settings.14,15
Here, we review the epidemiology and clinical features of M. tuberculosis bacteremia in sub-Saharan Africa and the role of traditional TB diagnostics in the diagnosis of M. tuberculosis bacteremia. We proceed to highlight trends in the outcomes of adults with M. tuberculosis bacteremia, including those receiving empiric anti-tuberculosis treatment. We then explore the potential role of two emerging rapid diagnostic tests (lateral-flow urinary lipoarabinomannan and whole-blood C-reactive protein) in the triage and prognostication of critically ill patients with disseminated M. tuberculosis. We conclude by reinforcing the need for clinicians in high TB-HIV burden settings to consider empiric anti-tuberculosis treatment in patients with advanced HIV infection and severe sepsis.
CLINICAL EPIDEMIOLOGY OF M. TUBERCULOSIS BACTEREMIA IN SUB-SAHARAN AFRICA
Multiple prospective studies over the past 20 years have reported M. tuberculosis to be the most common cause of community-acquired BSI in high TB-HIV burden settings in sub-Saharan Africa.1–4,6,7 The high prevalence of M. tuberculosis bacteremia reported in these settings contrasts sharply with the epidemiology of community-acquired BSI among severely ill patients in industrialised countries (Figure).6,7,11,16
Figure.
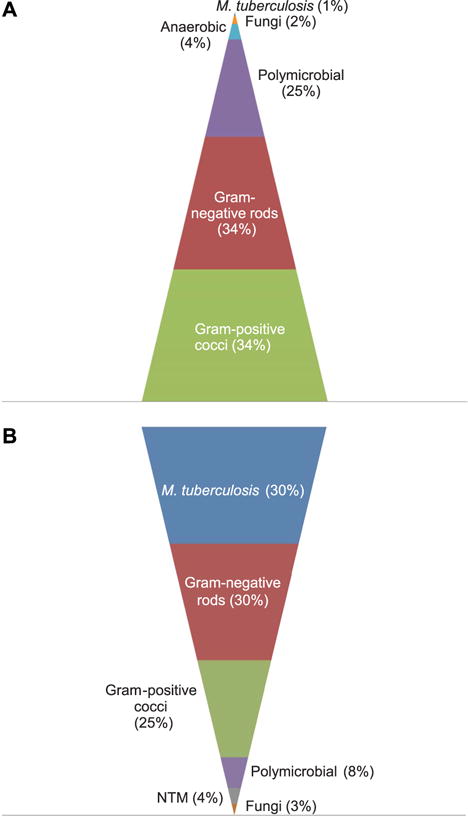
Pyramid figure demonstrating the estimated prevalence of A) community-acquired bloodstream infections in severely ill adults in industrialised countries vs. B) high TB-HIV burden settings in sub-Saharan Africa.1–4,6,7,11,16 NTM = non-tuberculous mycobacteria; TB = tuberculosis; HIV = human immunodeficiency virus. This image can be viewed online in color at http://www.ingentaconnect.com/content/iuatld/ijtld/2015/00000019/00000010/art00002
HIV infection and M. tuberculosis bacteremia: significance of advanced immunosuppression
The association between advanced HIV infection and M. tuberculosis bacteremia cannot be overemphasized. In five prospective investigations of community-acquired BSI in sub-Saharan Africa, M. tuberculosis bacteremia was identified only in patients with HIV infection.1,3,8,9,13 In a 2010 meta-analysis, M. tuberculosis bacteremia was significantly more likely to occur in the setting of HIV infection (odds ratio [OR] 23.4, P < 0.0001).17 Most HIV-infected patients with M. tuberculosis bacteremia have profound immunosuppression, typically meeting case definitions for acquired immune-deficiency syndrome (AIDS), with CD4 counts <50–100 cells/mm3.2–4,8,9,18–20 Most are not on antiretroviral therapy (ART).12,21 In two large prospective studies of HIV-infected adults with severe sepsis in Uganda and Zambia, M. tuberculosis was the most commonly identified BSI, accounting for nearly 25% and 40% of all positive blood cultures, respectively.6,7 Compared to patients with other BSI, septic patients with M. tuberculosis bacteremia in Uganda were significantly more likely to have lower CD4 counts (median 17 vs. 64 cells/mm3, P < 0.001).6 Patients with M. tuberculosis bacteremia were also significantly less likely to be on ART (P < 0.001).6
Clinical and laboratory characteristics associated with M. tuberculosis bacteremia
Prolonged fever and cough are more frequently identified among patients with M. tuberculosis bacteremia than in those with other BSI.1,3,4,6,8 Among septic patients in Uganda, those with M. tuberculosis bacteremia were significantly more likely to present with fever (P < 0.02) and to present following longer periods of illness than septic patients with other BSI (median 30 vs. 14 days, P < 0.05).6 Antecedent weight loss was significantly associated with M. tuberculosis bacteremia in studies from Tanzania and Malawi (P < 0.001 and P < 0.003, respectively).8,13 In Ivory Coast, 70% of HIV-infected adults with M. tuberculosis bacteremia had an antecedent wasting illness.9 Anemia (hemoglobin 8–10 g/dl) has been associated with M. tuberculosis bacteremia among HIV-infected patients in Malawi, Uganda, and Ivory Coast.3,4,6,9
SMEAR MICROSCOPY AND CHEST RADIOGRAPHY IN THE DIAGNOSIS OF M. TUBERCULOSIS BACTEREMIA
Although widely used, sputum smear microscopy for acid-fast bacilli (AFB) has poor sensitivity for the diagnosis of HIV-associated pulmonary and extra-pulmonary TB. The diagnostic yield when smear microscopy is used for the diagnosis of M. tuberculosis bacteremia is also limited.4,6,12,13 Respiratory samples may also be unobtainable in critically ill or sputum-scarce patients.
HIV-infected patients with M. tuberculosis bacteremia commonly have non-specific parenchymal abnormalities and pleural effusions on chest radiography (CXR).1,4,6,8,12 Although highly sensitive, the specificity of an abnormal CXR for the diagnosis of M. tuberculosis bacteremia is poor, given the high prevalence of lung disease other than TB among patients with advanced HIV infection.4,6 In the absence of classic findings (e.g., miliary pattern, upper lobe cavitation and/or infiltrate, mediastinal lymphadenopathy), the role of CXR in the diagnosis of M. tuberculosis bacteremia is limited.
SHORT-TERM CLINICAL OUTCOMES OF ADULTS WITH M. TUBERCULOSIS BACTEREMIA IN SUB-SAHARAN AFRICA
Across the available literature, mean short-term mortality (30-day or in-hospital) for HIV-infected patients with M. tuberculosis bacteremia is 48% (range 25–80%), consistently above that of HIV-infected patients with other BSI.2–4,6,8–10,19,20 Compared to HIV-infected patients with sepsis due to other BSI in Uganda, those with M. tuberculosis bacteremia had significantly higher 30-day mortality (53% vs. 32%, P = 0.001), independently of CD4 count and active ART.6 Mortality risk among patients with M. tuberculosis bacteremia is likely related to advanced immunosuppression, specifically those with CD4 counts <50 cells/mm3 and wasting illness.2,8,19,20
Evidence on the efficacy of empiric anti-tuberculosis treatment in HIV-infected patients with M. tuberculosis bacteremia and severe sepsis or septic shock is very limited. Despite this, available data suggest that early treatment may improve outcomes. In patients with M. tuberculosis bacteremia and severe sepsis in Uganda, a non-significant trend towards increased 30-day survival was noted among those who received empiric anti-tuberculosis treatment within 24 h of hospital admission compared to those who did not (53.4% vs. 30.8%, P = 0.10).6 Although limited in generalizability to high TB-HIV burden developing countries, the results of a recent retrospective analysis of adults with M. tuberculosis septic shock in North America and Saudi Arabia suggest that survival may be improved with empiric anti-tuberculosis treatment.11 Among 53 patients, 15% of whom were HIV-infected, overall survival was significantly improved among patients who received empiric anti-tuberculosis treatment vs. those who did not (36.0% vs. 7.1%, P = 0.016).11 Over half of these patients had disseminated infection. In survivors, median time to initiation of anti-tuberculosis treatment after hospitalization was 10 h compared to 35 h in non-survivors (P < 0.02).11
EXPEDITING DIAGNOSIS OF M. TUBERCULOSIS BACTEREMIA AMONG PATIENTS WITH SEVERE SEPSIS
Despite the high prevalence of M. tuberculosis bacteremia in high TB-HIV burden settings, it is under-recognized, including among patients with severe sepsis.4,6,7,13 Outside of research settings, M. tuberculosis culture facilities are often unavailable.10,22 Even when analyzed using continuously monitored liquid culture systems (BacT/Alert® MB, bioMérieux, Marcy l’Etoile, France, or BACTEC Myco/F Lytic, BD, Sparks, MD, USA), M. tuberculosis blood culture can take 2–6 weeks to yield results.6,10,22 Given the often non-specific presentation of disseminated M. tuberculosis in HIV-infected patients, clinico-radiological screening strategies may result in missed diagnoses and treatment delays in severely ill patients.23
How can the identification of critically ill patients with disseminated M. tuberculosis be expedited? It is imperative that clinicians working in high TB-HIV burden settings maintain a high index of suspicion for disseminated M. tuberculosis among patients presenting with severe sepsis or septic shock.6,7 Novel triage algorithms are also needed to assist clinicians in the identification of critically ill patients likely to have disseminated M. tuberculosis. A clinical risk score for M. tuberculosis bacteremia among HIV-infected adults with severe sepsis has been developed, incorporating clinical and laboratory features identified in Uganda (sex, heart rate, CD4 count, antecedent ART, fever, hyponatremia, and anemia). Very good test characteristics were reported (receiver operator characteristics curve c-statistic 0.85).6 Unfortunately, the resources required for chemistry and hematological analyses are often lacking in resource-limited settings, and results may not be rapidly available to clinicians.
Over the past decade, several emerging diagnostic tests have been explored as adjuncts to smear microscopy and CXR for the diagnosis of HIV-associated TB. Xpert® MTB/RIF (Cepheid, Sunnyvale, CA, USA) has excellent test characteristics in AFB smear-positive pulmonary TB. However, Xpert has reduced sensitivity for detecting certain forms of extra-pulmonary TB, such as pleural effusions.24 Nucleic acid amplification-based modalities have been investigated for the diagnosis of M. tuberculosis bacteremia; however, these require significant laboratory infrastructure, are costly, and low sensitivity has been reported (2–55%).22 Serologic tests have demonstrated poor accuracy for the diagnosis of active HIV-associated TB, with highly variable sensitivity and specificity reported across several antigenic targets.25
Detection of lipoarabinomannan (LAM), a glycolipid component of the M. tuberculosis cell wall, is one of the most promising rapid diagnostic tests for disseminated M. tuberculosis in HIV-infected patients. Through use of a rapid lateral-flow (LF) assay (Determine™ TB-LAM, Alere, Waltham, MA, USA), detection of LAM in urine has shown optimal diagnostic accuracy (sensitivity 60–70%, specificity 95–97%) among severely ill in-patients with advanced HIV-related immunosuppression (CD4 count <50–100 cells/mm3), and has been associated with M. tuberculosis bacteremia, higher illness severity, and death (Table 1).14,18,23,25–28 LF-LAM has also been shown to significantly improve diagnosis of culture-positive pulmonary and extra-pulmonary TB compared to a clinico-radiological strategy in patients with lower CD4 counts and higher illness severity.23 In the absence of oliguria, urine is often easy to obtain, the LF-LAM testing kit is straightforward to operate at the bedside without electricity, and results are available in 30 min for <US$4 per test.14 Despite limitations in sensitivity, prospective studies are urgently needed to examine the feasibility and accuracy of including LF-LAM in triage algorithms to be used among critically ill in-patients with suspected disseminated M. tuberculosis in high TB-HIV burden settings.
Table 1.
In-patient studies reporting the diagnostic accuracy of LF-LAM for HIV-associated tuberculosis
| Author, year, reference | Location, patient care setting | Sample size n | M. tuberculosis diagnostic standard | Sensitivity % (95%CI)* | Specificity % (95%CI)* | PPV % (95%CI)* | NPV % (95%CI)* |
|---|---|---|---|---|---|---|---|
| Peter et al., 201228 | South Africa, in-patient | 335 | Sputum, blood, body fluid, lymph node aspirate culture | Grade 1 cut-off: 66.4 (57.0–74.9); Grade 2 cut-off: 50.0 (40.6–59.2) |
Grade 1 cut-off: 65.6 (56.6–73.9); Grade 2 cut-off: 75.2 (66.7–82.5) |
Grade 1 cut-off: 64.2 (54.9–72.7); Grade 2 cut-off: 65.2 (54.3–75.0) |
Grade 1 cut-off: 67.8 (58.7–76.0); Grade 2 cut-off: 61.8 (53.6–69.6) |
| Peter et al., 201323 | South Africa, in-patient | 281 | Sputum, body fluid, lymph node aspirate culture | Grade 2 cut-off: 45.5 (38.2–52.9) | Grade 2 cut-off: 96.3 (81.0–99.4) | Grade 2 cut-off: 98.8 (93.7–99.8) | Grade 2 cut-off 20.3 (13.7–28.3) |
| Nakiyingi et al., 201426 | Uganda and South Africa, in-patient and out-patient | 1013 | Sputum or blood culture | Grade 2 cut-off: 37.1 (32.1–42.2) | Grade 2 cut-off: 97.6 (95.9–98.7) | Grade 2 cut-off: 90.7 (84.4–94.8) | Grade 2 cut-off: 70.8 (67.5–73.9) |
| Manabe et al., 201427 | Uganda, in-patient | 351 | Sputum or blood culture | Grade 1 cut-off: 62.1 (53.6–70.0) | Grade 1 cut-off: 81.1 (74.7–86.5) | Grade 1 cut-off: 72.0 (63.3–79.7) | Grade 1 cut-off: 73.2 (66.6–79.1) |
| Nakiyingi et al., 201518 | Uganda, in-patient and out-patient | 394 | Blood culture | Grade 2 cut-off: 70.7 (54.5–83.9) | Grade 2 cut-off: 93.5 (90.4–95.8) | Grade 2 cut-off: 55.8 (41.3–69.5) | Grade 2 cut-off: 96.5 (94.0–98.2) |
Grade cut-off refers to reference bands of graded intensity used to determine a positive or negative result.
LF-LAM = lateral-flow urinary lipoarabinomannan; HIV = human immunodeficiency virus; CI = confidence interval; PPV = positive predictive value; NPV = negative predictive value.
C-reactive protein (CRP) is an acute phase protein that has been developed as an emerging rapid diagnostic test for HIV-associated TB. The sensitivity of CRP for HIV-associated TB in the appropriate setting is excellent, but specificity is poor, limiting the overall diagnostic accuracy (Table 2).15,29–31 However, marked elevations of CRP may be useful prognostically to identify patients with disseminated M. tuberculosis who are in need of urgent treatment.29–31 In a small population of HIV-infected adults in South Africa, 80% of patients with disseminated M. tuberculosis had a laboratory-based serum CRP >50 mg/l.29 Patients with CRP >50 mg/l were also more likely to have positive LF-LAM.29 This cut-off was associated with increased risk of death, presumably due to increased bacillary load, as patients with CRP >50 mg/l had shorter times to culture positivity.29 Serum CRP correlates well with whole-blood CRP; the latter is now the basis for a point-of-care testing modality (Nycocard™ CRP, Alere).15 Nycocard CRP results can be obtained within 5 min using whole-blood samples run on a portable, battery-powered device, with costs estimated at approximately US$3 per test.15 Further studies are warranted to examine the association between CRP and mortality risk in HIV-infected in-patients with disseminated M. tuberculosis presenting with severe sepsis.
Table 2.
Studies reporting the diagnostic accuracy of CRP for HIV-associated tuberculosis
| Author, year, reference | Location, patient care setting | Sample size | M. tuberculosis diagnostic standard | Sensitivity % (95%CI) | Specificity % (95%CI) | PPV % (95%CI) | NPV % (95%CI) |
|---|---|---|---|---|---|---|---|
| Wilson et al., 201131 | South Africa, out-patient | 364 | Sputum or body fluid culture or AFB with granulomata on histology | >1×ULN: 98.0 (94.0–99.0) >5×ULN: 88.0 (81.0–93.0) |
>1×ULN: 59.0 (50.0–68.0) >5×ULN: 85.0 (77.0–91.0) |
>1×ULN: 74.0 (67.0–80.0) >5×ULN: 87.0 (81.0–92.0) |
>1×ULN: 96.0 (88.0–99.0) >5×ULN: 86.0 (78.0–92.0) |
| Schleicher et al., 201130 | South Africa, in-patient | 67 | Sputum culture or smear microscopy for AFB | >10 mg/l: 100.0 (89.6–100.0) | >10 mg/l: 0.00 (0.00–10.7) | >10 mg/l: 50.8 (38.2–63.2) | >10 mg/l: not calculable |
| Lawn et al., 201329 | South Africa, out-patient | 496 | Sputum culture and Xpert MTB/RIF, urine Xpert | >10 mg/l: 85.2 (75.2–91.8) >50 mg/l: 55.6 (44.1–66.5) |
>10 mg/l: 57.6 (52.7–62.4) >50 mg/l: 88.7 (85.1–91.5) |
>10 mg/l: 28.2 (22.7–34.3) >50 mg/l: 48.9 (38.4–59.5) |
>10 mg/l: 95.2 (91.6–97.4) >50 mg/l: 91.1 (87.8–93.6) |
| Drain et al., 201415* | South Africa, out-patient | 93 | Sputum culture or smear microscopy for AFB | >8 mg/l: 94.9 (82.7–99.4) >50 mg/l: 59.0 (42.1–74.4) |
>8 mg/l: 51.1 (35.8–66.3) >50 mg/l: 86.7 (73.2–95.0) |
>8 mg/l: 62.7 (49.2–75.0) >50 mg/l: 79.3 (60.3–92.0) |
>8 mg/l: 92.0 (74.0–99.0) >50 mg/l: 70.9 (57.1–82.4) |
Whole-blood samples analyzed using a portable device.
CRP = C-reactive protein; HIV = human immunodeficiency virus; CI = confidence interval; PPV = positive predictive value; NPV = negative predictive value; AFB = acid-face bacilli; ULN = upper limit of normal.
EMPIRIC ANTI-TUBERCULOSIS TREATMENT IN HIV-INFECTED PATIENTS WITH SEVERE SEPSIS
In addition to volume resuscitation, provision of supplemental oxygen, and control of an infectious source, prompt initiation of appropriate antimicrobial therapy is an evidence-based cornerstone in the initial management of patients with severe sepsis.5 At present, emerging triage algorithms and diagnostic tests require further evaluation of their accuracy and feasibility for use in high TB-HIV burden settings, and may not be sensitive enough to rule out disseminated M. tuberculosis. Early empiric anti-tuberculosis treatment should thus be strongly considered in patients with advanced HIV infection (e.g., known CD4 count <50–100 cells/mm3 or fulfilling the clinical case definition for AIDS) and suspected disseminated M. tuberculosis (e.g., prolonged fever, night sweats, antecedent weight loss or wasting, hepatosplenomegaly, lymphadenopathy), who present with severe sepsis or septic shock in these settings. Empiric treatment strategies for smear-negative patients have been criticized, with medication toxicity, potential interaction with ART agents, and poor treatment adherence as potential risks.32 However, in the sub-Saharan African context, where TB incidence is extremely high and diagnostic capacity is lacking, empiric treatment for patients with suspected HIV-associated TB has been recommended as a rational approach in the appropriate clinical context.32 Criticisms of empiric treatment were also not focused on patients with critical illness, the population in which the risk-benefit ratio of empiric therapy may be more favorable.
In 2007, the World Health Organization (WHO) released revised management guidelines aiming to expedite the diagnosis and treatment of TB in HIV-infected adults with ‘serious illness’ (defined based on the presence of vital sign abnormalities similar to those included in the definition of severe sepsis).33 These guidelines, primarily focused on presumed pulmonary TB patients, suggest sputum smear microscopy and CXR as initial diagnostic studies. However, respiratory samples and CXR may be unobtainable in critically ill and sputum-scarce patients, and the diagnostic yield of these studies for disseminated M. tuberculosis is limited. Prompt sampling of body fluid or lymph node aspiration in presumed extra-pulmonary patients may not be feasible in resource-limited settings. The WHO guidelines therefore emphasize that anti-tuberculosis treatment should be initiated immediately, preferably with input from a senior clinician, for HIV-infected patients with evidence of disseminated M. tuberculosis and rapid clinical deterioration. Given the potential for bacterial co-infection among patients with advanced immunosuppression and disseminated M. tuberculosis, concurrent initiation of additional broad-spectrum parenteral antimicrobials (preferably non-fluoroquinolones) is also suggested.33,34 This recommendation to consider early empiric anti-tuberculosis treatment in combination with other broad-spectrum antimicrobials in HIV-infected adults presenting with severe sepsis or septic shock is reinforced in recently released guidelines for the management of critical illness in resource-limited environments, and is supported by available evidence.6,7,11,35 Future studies are needed to assess the safety, efficacy, and duration of empiric anti-tuberculosis treatment among patients with severe sepsis in high TB-HIV burden settings. Given the risk of drug malabsorption among patients with severe sepsis, future investigations are also needed to assess the pharmacokinetics of anti-tuberculosis drugs among critically ill patients.
CONCLUSIONS
M. tuberculosis bacteremia is an under-recognized cause of severe sepsis in high TB-HIV burden settings in sub-Saharan Africa, and is associated with high mortality. In patients with advanced HIV infection, M. tuberculosis bacteremia is difficult to diagnose promptly using traditional tools. LF-LAM and whole-blood CRP show potential to improve the triage and prognostication of critically ill patients with advanced HIV infection and disseminated M. tuberculosis in resource-limited settings. As novel rapid diagnostics and triage algorithms undergo further evaluation, we strongly encourage clinicians in high TB-HIV burden settings to consider the initiation of empiric anti-tuberculosis treatment in patients with advanced HIV infection and severe sepsis in the appropriate clinical context.
Acknowledgments
MO is supported by National Institutes of Health/National Institute of Allergy and Infectious Diseases, Bethesda, MD, USA (K23 AI098479-01A1).
Footnotes
Conflicts of interest: none declared.
References
- 1.Archibald LK, McDonald LC, Nwanyanwu O, et al. A hospital-based prevalence survey of bloodstream infections in febrile patients in Malawi: implications for diagnosis and therapy. J Infect Dis. 2000;181:1414–1420. doi: 10.1086/315367. [DOI] [PubMed] [Google Scholar]
- 2.Arthur G, Nduba VN, Kariuki SM, Kimari J, Bhatt SM, Gilks CF. Trends in bloodstream infections among human immunodeficiency virus-infected adults admitted to a hospital in Nairobi, Kenya, during the last decade. Clin Infect Dis. 2001;33:248–256. doi: 10.1086/321820. [DOI] [PubMed] [Google Scholar]
- 3.Ssali FN, Kamya MR, Wabwire-Mangen F, et al. A prospective study of community-acquired bloodstream infections among febrile adults admitted to Mulago Hospital in Kampala, Uganda. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:484–489. doi: 10.1097/00042560-199812150-00007. [DOI] [PubMed] [Google Scholar]
- 4.Lewis DK, Peters RP, Schijffelen MJ, et al. Clinical indicators of mycobacteraemia in adults admitted to hospital in Blantyre, Malawi. Int J Tuberc Lung Dis. 2002;6:1067–1074. [PubMed] [Google Scholar]
- 5.Jacob ST, Lim M, Banura P, et al. Integrating sepsis management recommendations into clinical care guidelines for district hospitals in resource-limited settings: the necessity to augment new guidelines with future research. BMC Med. 2013;11:107. doi: 10.1186/1741-7015-11-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacob ST, Pavlinac PB, Nakiyingi L, et al. Mycobacterium tuberculosis bacteremia in a cohort of HIV-infected patients hospitalized with severe sepsis in Uganda-high frequency, low clinical sand derivation of a clinical prediction score. PLOS ONE. 2013;8:e70305. doi: 10.1371/journal.pone.0070305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrews B, Muchemwa L, Kelly P, Lakhi S, Heimburger DC, Bernard GR. Simplified severe sepsis protocol: a randomized controlled trial of modified early goal-directed therapy in Zambia. Crit Care Med. 2014;42:2315–2324. doi: 10.1097/CCM.0000000000000541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crump JA, Ramadhani HO, Morrissey AB, et al. Bacteremic disseminated tuberculosis in sub-Saharan Africa: a prospective cohort study. Clin Infect Dis. 2012;55:242–250. doi: 10.1093/cid/cis409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vugia DJ, Kiehlbauch JA, Yeboue K, et al. Pathogens and predictors of fatal septicemia associated with human immunodeficiency virus infection in Ivory Coast, West Africa. J Infect Dis. 1993;168:564–570. doi: 10.1093/infdis/168.3.564. [DOI] [PubMed] [Google Scholar]
- 10.Archibald LK, den Dulk MO, Pallangyo KJ, Reller LB. Fatal Mycobacterium tuberculosis bloodstream infections in febrile hospitalized adults in Dar es Salaam, Tanzania. Clin Infect Dis. 1998;26:290–296. doi: 10.1086/516297. [DOI] [PubMed] [Google Scholar]
- 11.Kethireddy S, Light RB, Mirzanejad Y, et al. Mycobacterium tuberculosis septic shock. Chest. 2013;144:474–482. doi: 10.1378/chest.12-1286. [DOI] [PubMed] [Google Scholar]
- 12.von Reyn CF, Kimambo S, Mtei L, et al. Disseminated tuberculosis in human immunodeficiency virus infection: ineffective immunity, polyclonal disease and high mortality. Int J Tuberc Lung Dis. 2011;15:1087–1092. doi: 10.5588/ijtld.10.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonald LC, Archibald LK, Rheanpumikankit S, et al. Unrecognised Mycobacterium tuberculosis bacteraemia among hospital in-patients in less developed countries. Lancet. 1999;354:1159–1163. doi: 10.1016/s0140-6736(98)12325-5. [DOI] [PubMed] [Google Scholar]
- 14.Lawn SD, Dheda K, Kerkhoff AD, et al. Determine TB-LAM lateral flow urine antigen assay for HIV-associated tuberculosis: recommendations on the design and reporting of clinical studies. BMC Infect Dis. 2013;13:407. doi: 10.1186/1471-2334-13-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drain PK, Mayeza L, Bartman P, et al. Diagnostic accuracy and clinical role of rapid C-reactive protein testing in HIV-infected individuals with presumed tuberculosis in South Africa. Int J Tuberc Lung Dis. 2014;18:20–26. doi: 10.5588/ijtld.13.0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valles J, Ferrer R. Bloodstream infection in the ICU. Infect Dis Clin N Am. 2009;23:557–569. doi: 10.1016/j.idc.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Reddy EA, Shaw AV, Crump JA. Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:417–432. doi: 10.1016/S1473-3099(10)70072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakiyingi L, Ssengooba W, Nakanjako D, et al. Predictors and outcomes of mycobacteremia among HIV-infected smear-negative presumptive tuberculosis patients in Uganda. BMC Infect Dis. 2015;15:62. doi: 10.1186/s12879-015-0812-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munseri PJ, Talbot EA, Bakari M, Matee M, Teixeira JP, von Reyn CF. The bacteraemia of disseminated tuberculosis among HIV-infected patients with prolonged fever in Tanzania. Scand J Infect Dis. 2011;43:696–701. doi: 10.3109/00365548.2011.577802. [DOI] [PubMed] [Google Scholar]
- 20.Gilks CF, Brindle RJ, Mwachari C, et al. Disseminated Mycobacterium avium infection among HIV-infected patients in Kenya. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:195–198. [PubMed] [Google Scholar]
- 21.Heysell SK, Thomas TA, Gandhi NR, et al. Blood cultures for the diagnosis of multidrug-resistant and extensively drug-resistant tuberculosis among HIV-infected patients from rural South Africa: a cross-sectional study. BMC Infect Dis. 2010;10:344. doi: 10.1186/1471-2334-10-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feasey NA, Banada PP, Howson W, et al. Evaluation of Xpert MTB/RIF for detection of tuberculosis from blood samples of HIV-infected adults confirms Mycobacterium tuberculosis bacteremia as an indicator of poor prognosis. J Clin Microbiol. 2013;51:2311–2316. doi: 10.1128/JCM.00330-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peter JG, Theron G, Dheda K. Can point-of-care urine LAM strip testing for tuberculosis add value to clinical decision making in hospitalised HIV-infected persons? PLOS ONE. 2013;8:e54875. doi: 10.1371/journal.pone.0054875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denkinger CM, Schumacher SG, Boehme CC, Dendukuri N, Pai M, Steingart KR. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2014;44:435–446. doi: 10.1183/09031936.00007814. [DOI] [PubMed] [Google Scholar]
- 25.Achkar JM, Lawn SD, Moosa MY, Wright CA, Kasprowicz VO. Adjunctive tests for diagnosis of tuberculosis: serology, ELISPOT for site-specific lymphocytes, urinary lipoarabinomannan, string test, and fine needle aspiration. J Infect Dis. 2011;204(Suppl 4):S1130–S1141. doi: 10.1093/infdis/jir450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakiyingi L, Moodley VM, Manabe YC, et al. Diagnostic accuracy of a rapid urine lipoarabinomannan test for tuberculosis in HIV-infected adults. J Acquir Immune Defic Syndr. 2014;66:270–279. doi: 10.1097/QAI.0000000000000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manabe YC, Nonyane BA, Nakiyingi L, et al. Point-of-care lateral flow assays for tuberculosis and cryptococcal antigenuria predict death in HIV infected adults in Uganda. PLOS ONE. 2014;9:e101459. doi: 10.1371/journal.pone.0101459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peter JG, Theron G, van Zyl-Smit R, et al. Diagnostic accuracy of a urine lipoarabinomannan strip-test for TB detection in HIV-infected hospitalised patients. Eur Respir J. 2012;40:1211–1220. doi: 10.1183/09031936.00201711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawn SD, Kerkhoff AD, Vogt M, Wood R. Diagnostic and prognostic value of serum C-reactive protein for screening for HIV-associated tuberculosis. Int J Tuberc Lung Dis. 2013;17:636–643. doi: 10.5588/ijtld.12.0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schleicher GK, Herbert V, Brink A, et al. Procalcitonin and C-reactive protein levels in HIV-positive subjects with tuberculosis and pneumonia. Eur Respir J. 2005;4:688–692. doi: 10.1183/09031936.05.00067604. [DOI] [PubMed] [Google Scholar]
- 31.Wilson D, Badri M, Maartens G. Performance of serum C-reactive protein as a screening test for smear-negative tuberculosis in an ambulatory high HIV prevalence population. PLOS ONE. 2011;6:e15248. doi: 10.1371/journal.pone.0015248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawn SD, Ayles H, Egwaga S, et al. Potential utility of empirical tuberculosis treatment for HIV-infected patients with advanced immunodeficiency in high TB-HIV burden settings. Int J Tuberc Lung Dis. 2011;15:287–295. [PubMed] [Google Scholar]
- 33.World Health Organization. Improving the diagnosis and treatment of smear-negative pulmonary and extra-pulmonary tuberculosis: recommendations for HIV-prevalent and resource-constrained settings. Geneva, Switzerland: WHO; 2007. [Google Scholar]
- 34.Wong EB, Omar T, Setlhako GJ, et al. Causes of death on antiretroviral therapy: a post-mortem study from South Africa. PLOS ONE. 2012;10:e47542. doi: 10.1371/journal.pone.0047542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. Integrated Management of Adolescent and Adult Illness district clinician manual: hospital care for adolescents and adults. Geneva, Switzerland: WHO; 2011. [Google Scholar]


