Abstract
Background/Aims
Hyperhomocysteinemia (hHcys) has been reported to initiate Nod-like receptor protein 3 (NLRP3) inflammasome formation and activation in podocytes, leading to glomerular dysfunction and sclerosis. However, it remains unknown whether Nlrp3 gene is critical for the formation and activation of inflammasomes in glomeruli of hHcys mice.
Methods
Plasma homocysteine concentration was estimated utilizing HPLC, inflammasome formation and immunofluorescence expression from confocal microscopy, IL-1β production from ELISA.
Results
Uninephrectomized Nlrp3 knockout (Nlrp3-/-) and wild type (Nlrp3+/+) and intra renal Nlrp3 shRNA–transfected wild type mice (Nlrp3 shRNA) were fed a folate free (FF) diet or normal chow (ND) for 4 weeks to produce hHcys. The plasma Hcys levels were significantly elevated in both Nlrp3-/- and Nlrp3+/+ mice fed a FF diet compared to ND fed mice. The FF diet significantly increased the colocalization of Nlrp3 with apoptosis-associated speck-like protein (ASC) or caspase-1, caspase-1 activity and IL-1β production in glomeruli of Nlrp3+/+, but not in Nlrp3-/- mice and local Nlrp3 shRNA transfected mice. Correspondingly, the glomerular damage index (GDI) and urinary protein excretion were significantly higher in Nlrp3+/+ mice compared to ND fed mice. However, the hHcys-induced increase in GDI and proteinuria were significantly lower in Nlrp3-/- and local Nlrp3 shRNA transfected mice than in Nlrp3+/+ mice. Immunocytochemical analysis showed that hHcys decreased expression of podocin and nephrin, but increased desmin expression in glomeruli of Nlrp3+/+ mice compared to Nlrp3-/- mice.
Conclusion
Nlrp3 gene is an essential component of Nlrp3 inflammasomes and that targeting Nlrp3 may be important therapeutic strategy to prevent inflammasome activation and thereby protect podocytes and glomeruli from hHcys-induced injury
Keywords: hHcys, NLRP3, inflammasomes, glomerulosclerosis, end-stage renal disease
Introduction
Hyperhomocysteinemia (hHcys) is a pathological condition characterized by an increase in plasma concentration of total homocysteine [1-6]. Numerous clinical and epidemiological studies have demonstrated that hHcys is an independent risk factor for many diseases and pathological processes, including neurological, cardiovascular, and end stage renal diseases [7-9]. Recently we and others have demonstrated that hHcys initiates glomerular damage by inducing NADPH oxidase mediated local oxidative stress, ceramide production, podocyte injury and inhibition of extracellular matrix degradation contributing to the sclerotic process and eventually leading to the loss of kidney function [1-2, 5, 10-13]. In addition, we have also demonstrated that chronic elevations of plasma Hcys levels importantly contribute to the development of glomerular disease independent of hypertension in hHcys mice and rat models [13-14]. Despite extensive studies, the early mechanisms triggering the pathogenic actions of hHcys are not fully understood.
In this regard, the inflammasome is considered as an intracellular machinery of inflammation [15]. Among different kinds of inflammasomes, the Nlrp3 inflammasome is the most fully characterized one in a variety of mammalian cells with high abundance in glomerular podocytes [13, 16-17]. The Nlrp3 inflammasome is composed of three major proteins, including a NOD-like receptor Nlrp3, an adaptor protein apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), and caspase-1 [18-21]. Nlrp3 acts as the sensory component to recognize both endogenous and exogenous danger signals [22-24], when ASC and caspase-1 are recruited to form a protein complex, where caspase-1 is activated. The active caspase-1 not only proteolytically cleaves IL-1β and/or IL-18 into their biologically active form, but also produces other deleterious molecules like damage-associated molecular patterns (DAMPs). Both will act to turn on inflammatory response and induce cell dysfunction. This multiprotein complex was first characterized in monocytes [25-26], and its abnormality was attributed to the development of several autoinflammatory disorders including Familial cold autoinflammatory syndrome, Muckle-Wells syndrome and Sweet's syndrome [18, 27-28]. There is now increasing evidence that the derangement of this intracellular inflammatory machinery may not only contribute to autoinflammatory disorders, but also to a number of metabolic diseases including gout, silicosis, diabetes, acetaminophen liver toxicity, Alzheimer's disease and atherosclerosis [21, 29-36]. Correspondingly, the Nlrp3 inflammasome was found to be stimulated by various endogenous stress-associated danger signals such as DAMPs [37-38], ATP [39], monosodium urate crystals [31], obesity [40], β-amyloid [23], acute ischemia/reperfusion-induced kidney injury [41], unilateral ureteral obstruction [16-17]. Interestingly, inhibition of inflammasomes by either ASC gene silencing or pharmacological inhibition of casapase-1 has recently been shown to prevent the development of hHcys-induced glomerular injury and podocyte injury [13]. However, it remains unknown whether Nlrp3 gene is critical for the formation and activation of inflammasomes in glomeruli of hHcys mice
The present study hypothesized that Nlrp3 gene deficiency attenuates the glomeruli from hHcys-induced inflammasome formation and thereby ameliorate glomerulosclerosis and podocyte injury under such pathological condition. To test this hypothesis, we first performed a series of experiments using Nlrp3-/- and their wild type littermates on the normal chow or folate free diet to determine whether lack of Nlrp3 gene alters the formation and activation of Nlrp3 inflammasomes, glomerular injury and podocyte injury in mice during hHcys. Then, we locally silenced renal Nlrp3 gene using shRNA and observed the effects of renal Nlrp3 deficiency on hHcys-induced glomerular inflammasome formation and corresponding injury. Our results demonstrate that Nlrp3 gene is an essential component of Nlrp3 inflammasomes and that targeting Nlrp3 may be important therapeutic strategy to prevent inflammasome formation and activation and thereby protect podocytes and glomeruli from hHcys-induced injury
Materials and Methods
Animals
Eight weeks old male C57BL/6J WT, Nlrp3-/- mice and their wild type littermates were used in the present study. To speed up the damaging effects of hHcys on glomeruli, all mice were uninephrectomized as we described previously [1-2]. This model has been demonstrated to induce glomerular damage unrelated to the uninephrectomy and arterial blood pressure, but specific to hHcys. After a 1-week recovery period from uninephrectomy, mice were fed either normal chow or folate-free (FF) diet (Dyets Inc, Bethlehem, PA, USA) for 4 weeks to induce hHcys. In another series of C57BL/6J mice, Nlrp3 shRNA or a scrambled shRNA (Origen, Rockville, MD, USA) plasmid with a luciferase expression vector was co-transfected into the kidneys via intrarenal artery injection using the ultrasound microbubble system as we described previously [2, 13, 42]. After delivery of plasmids into the kidney, these uninephrectomized mice were maintained on a normal or a FF diet for 4 weeks. All protocols were approved by the Institutional Animal Care and Use Committee of the Virginia Commonwealth University.
High-performance liquid chromatography (HPLC) analysis
Plasma and renal tissue Hcys levels were measured by HPLC method as we described previously [1, 43-44]. A 100 μL plasma or samples or standard solution mixed with 10 μL of internal standard, thionglycolic acid (2.0 mmol/L) solution, was treated with 10 μL of 10% tri-n-butylphosphine (TBP) solution in dimethylformamide at 4 °C for 30 minutes. Then, 80 μL of ice-cold 10% trichloro acetic acid (TCA) in 1 mmol/L EDTA was added and centrifuged to remove proteins in the sample. 100 μL of the supernatant was transferred into the mixture of 20 μL of 1.55 mol/L sodium hydroxide, 250 μL of 0.125 mol/L borate buffer (pH 9.5), and 100 μL of 1.0 mg/mL ABD-F solution. The resulting mixture was incubated at 60°C for 30 minutes to accomplish derivatization of thiols. HPLC was performed with a HP 1100 series equipped with a binary pump, a vacuum degasser, a thermo stated column compartment, and an auto sampler (Agilent Technologies, Waldbronn, Germany). Separation was carried out at an ambient temperature on an analytical column, Supelco LC-18-DB (1504.6 mm ID, 5 m) with a Supelcosil LC-18 guard column (204.6 mm ID, 5 m). Fluorescence intensities were measured with an excitation wavelength of 385 nm and emission wavelength of 515 nm by a Hewlett-Packard Model 1046A fluorescence spectrophotometer. The peak area of the chromatographs was quantified with a Hewlett-Packard 3392 integrator. The analytical column was eluted with 0.1 mol/L potassium dihydrogen phosphate buffer (pH 2.1) containing 6% acetonitrile (v/v) as the mobile phase with a flow rate of 2.0 mL/min.
Caspase-1 activity and IL-1β production
Caspase-1 activity in glomeruli was measured by a commercially available colorimetric assay kit (Biovision, Mountain View, CA). IL-1β production in glomeruli was measured by a commercially available ELISA kit (R&D System, Minneapolis, MN), according to the manufacturer's instructions [11, 13, 40].
Morphological examinations
The fixed kidneys were paraffin-embedded, and sections were prepared and stained with Periodic acid–Schiff stain. Glomerular damage index (GDI) was calculated from 0 to 4 on the basis of the degree of glomerulosclerosis and mesangial matrix expansion as described previously [40, 42, 45]. In general, we counted 50 glomeruli in total in each kidney slice under microscope, when each glomerulus was graded level 0-4 damages. 0 represents no lesion, 1+ represents sclerosis of <25% of the glomerulus, while 2+, 3+, and 4+ represent sclerosis of 25% to 50%, >50% to 75%, and >75% of the glomerulus. A whole kidney average sclerosis index was obtained by averaging scores from counted glomeruli. This observation was examined by two independent investigators who were blinded to the treatment of the experimental groups [2, 11, 13, 42, 45].
Urinary total protein and albumin excretion measurements
The 24-hour urine samples were collected using metabolic cages and subjected to total protein and albumin excretion measurements, respectively [2, 42, 45-46]. Total protein content in the urine was detected by Bradford method using a UV spectrophotometer. Urine albumin was detected using a commercially available albumin ELISA kit (Bethyl Laboratories, Montgomery, TX).
Delivery of Nlrp3 shRNA into the kidneys by ultrasound-microbubble technique
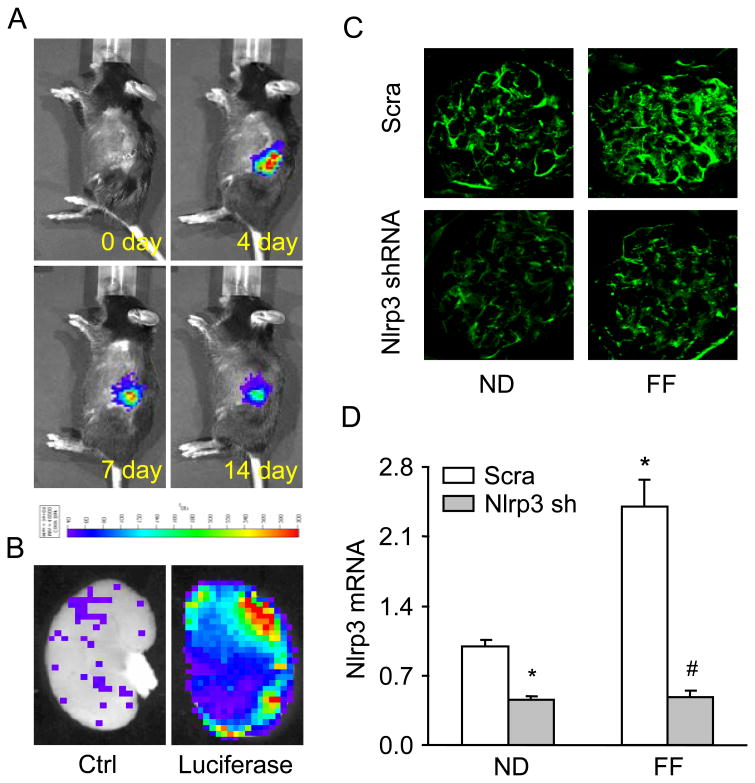
Nlrp3 shRNA or a scrambled shRNA plasmid with a luciferase expression vector was used to co-transfect the kidneys via intrarenal artery injection using the ultrasound-microbubble system. These experiments were performed to test whether local silencing Nlrp3 gene expression in podocytes alters hHcys-induced glomerular injury. A full description of the procedures for the ultrasound-microbubble gene transfer technique can be found in our previous studies [2, 13, 42]. To monitor the efficiency of gene expression through somatic plasmid transfection daily, mice were anesthetized with isoflurane, and an aqueous solution of luciferin (150 mg/kg) was injected intraperitoneally 5 minutes before imaging. The anesthetized mice were imaged using the IVIS200 in vivo molecular imaging system (Xenogen, Hopkinton, MA, USA). Photons emitted from luciferase-expressing cells within the animal body and transmitted through tissue layers were quantified over a defined period of time ranging up to 5 minutes using the software program Living Image as program (Xenogen). The inhibitory efficiency of gene expression by Nlrp3 shRNA was further confirmed by detection of Nlrp3 level in mouse renal cortex using real-time RT-PCR and Immunofluorescence analysis.
Real-time reverse transcription polymerase chain reaction (RT-PCR)
Total RNA from isolated mouse renal tissue was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the protocol as described by the manufacturer. RNA samples were quantified by measurement of optic absorbance at 260 nm and 280 nm in a spectrophotometer. The concentrations of RNA were calculated according to A260. Aliquots of total RNA (1 μg) from each sample were reverse-transcribed into cDNA according to the instructions of the first strand cDNA synthesis kit manufacturer (Bio-Rad, Hercules, CA). Equal amounts of the reverse transcriptional products were subjected to PCR amplification using SYBR Green as the fluorescence indicator on a Bio-Rad iCycler system (Bio-Rad, Hercules, CA) [2, 13, 42]. The primers used in this study were synthesized by Operon (Huntsville, AL, USA) and the sequences were: for Nlrp3 sense TCACAACTCGCCCAAGGAGGAA, antisense AAGAGACCACGGCAGA-AGCTAG and for β-actin sense TCGCTGCGCTGGTCGTC, antisense GGCCTCGTCACCCACATAGGA.
Confocal microscopic detection of inflammasome protein complexes
Indirect immunofluorescent staining was used to determine colocalization of the inflammasome proteins in glomeruli of the mouse kidney, which indicate the formation of inflammasome molecular complex. Frozen kidney tissue slides were fixed in acetone and then incubated overnight at 4°C with either goat anti- Nalp3 (1:200) and rabbit anti-Asc (1:50), or goat anti- Nalp3 (1:200) and mouse anti-caspase-1 (1:100). To further confirm the presence of the inflammasomes specifically in podocytes of the mouse glomeruli, Nalp3 was co incubated with a podocin antibody (1:400; Sigma, St. Louis, MO). Double immunofluorescent staining was achieved by incubating with either Alexa-488 or Alexa-555-labeled secondary antibodies for 1 hour at room temperature. After washing, slides were mounted with a DAPI-containing mounting solution, and then observed with a confocal laser scanning microscope (Fluoview FV1000, Olympus, Japan). As previously described [13, 42], images were analyzed by the Image Pro Plus 6.0 software (Media Cybernetics, Bethesda, MD), where colocalization was measured and expressed as the Pearson Correlation Coefficient (PCC).
Immunofluorescent staining
Immunofluorescent staining was performed using frozen slides of mouse kidneys. After fixation with acetone, the slides were incubated with anti-podocin (Sigma, St. Louis, MO, USA, 1: 100), anti-desmin (BD Biosciences, San Jose, CA, 1: 50), anti-nephrin (Abcam, Cambridge, MA, USA, 1:50) antibodies overnight at 4°C. Then, these slides were washed and incubated with corresponding Texas Red-labeled secondary antibodies. Finally, the slides were washed, mounted and subjected to fluorescent microscopy examination. The images were captured with a spot CCD camera and a pseudocolor was added to fluorescence showing in this slide (Diagnostic Instruments Inc., Sterlin Heights, MI, USA). All exposure settings were kept constant for each group of kidneys.
Immunohistochemistry
Kidneys were embedded with paraffin and 5 mm sections were cut from the embedded blocks. After heat-induced antigen retrieval, wash with 3% H2O2 and 30 min blocking with serum, slides were incubated with primary antibody diluted in phosphate-buffered saline (PBS) with 4% serum. Anti-IL-1β (Abcam, Cambridge, MA, USA) antibody was used in this study. After incubation with primary antibody overnight, the sections were washed in PBS and incubated with biotinylated IgG (1:200) for 1 h and then with streptavidin-HRP for 30 min at room temperature. 50 μl of DAB was added to each kidney section and stained for 1 min. After washing, the slides were counterstained with hematoxylin for 5 min. The slides were then mounted and observed under a microscope in which photos were taken.
2.12. Statistical Analysis
Data are provided as arithmetic means ± SEM; n represents the number of independent experiments. All data were tested for significance using ANOVA or paired and unpaired Student's t-test as applicable. The glomerular damage index was analysed using a nonparametric Mann-Whitney rank sum test. Only results with p<0.05 were considered statistically significant.
Results
Nlrp3 gene deletion abolished hHcys-induced inflammasome formation and activation in glomeruli
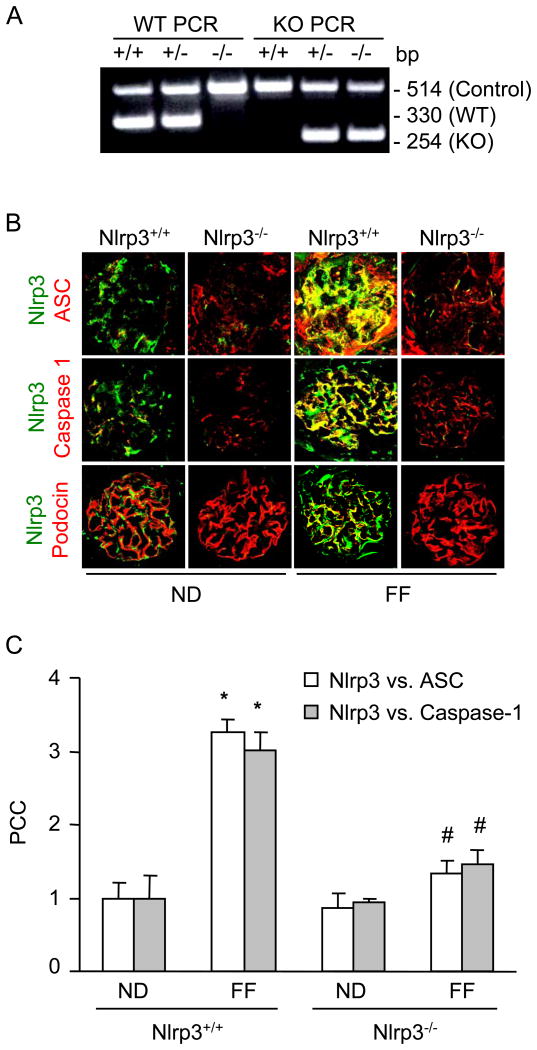
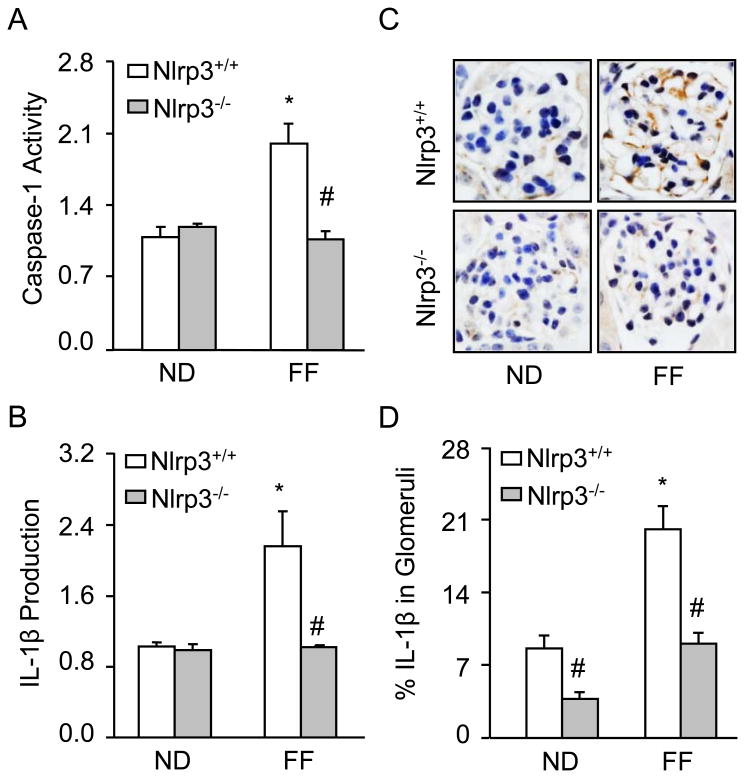
The plasma Hcys concentration was similar in Nlrp3+/+ and Nlrp3-/- mice fed a normal diet (Nlrp3+/+: 4.9 ± 0.5 vs Nlrp3-/-: 7.0 ± 0.6). However, the FF diet significantly increased the plasma Hcys concentration in both mouse strains compared to the normal diet fed mice (Nlrp3+/+: 39.5 ± 12 vs Nlrp3-/-: 44 ± 7). As shown in Fig. 1A, Nlrp3 knockout (Nlrp3-/-) and wild type (Nlrp3+/+) mice were genotyped using PCR. Detection of a PCR product at 254 bp indicates Nlrp3-/-, while a PCR product of 330 bp indicates Nlrp3+/+ mice. Next, we tested whether Nlrp3 mediates the hHcys-induced inflammasome formation and activation in glomeruli of mice. Using confocal microscopy, we demonstrated that hHcys increased the colocalization of Nlrp3 with ASC or Nlrp3 with caspase-1 in glomeruli of mice compared to normal diet fed mice. However, Nlrp3-/- mice attenuated the hHcys-induced colocalization of Nlrp3 with ASC or Nlrp3 with caspase-1 in glomeruli of mice (Fig. 1A). Furthermore, colocalization of Nlrp3 with podocin (podocyte marker) indicates enrichment of Nlrp3 in podocytes. The summarized data of quantitative colocalization of Nlrp3 with Asc or Nlrp3 with caspase-1 in glomeruli of mice were shown in Fig. 1B. Biochemical analysis showed that hHcys significantly increased the caspase activity and IL-1 β production in glomeruli of Nlrp3+/+ mice. However, Nlrp3-/- mice attenuated the hHcys-induced caspase-1 activity and IL-1β production (Fig. 2). Taken together, these results suggest that Nlrp3 mediates the hHcys-induced the NLRP3 inflammasome formation and activation in glomeruli of mice.
Figure 1. hHcys-induced Nlrp3 inflammasome formation in glomeruli of Nlrp3+/+ and Nlrp3-/- mice fed a normal diet or folate free diet.
A: Genotyping, B. Colocalization of Nlrp3 (green) with ASC (red), Nlrp3 (green) with caspase-1 (red) and Nlrp3 (green) with podocin (red) in mouse glomeruli. C. Summarized data shows the fold changes of pearson coefficient correlation (PCC) for the colocalization of Nlrp3 with ASC and Nlrp3 with caspase-1 in glomeruli of Nlrp3+/+ and Nlrp3-/- mice fed with ND or FF diet. * Significant difference (P<0.05) compared to the values from control Nlrp3+/+ mice, # Significant difference (P<0.05) compared to the values from Nlrp3+/+ mice receiving the FF diet.
Fig. 2. Effects of normal diet and folate free diet on caspase-1 activity and IL-1β production in Nlrp3+/+ and Nlrp3-/- mice.
Values are arithmetic means ± SEM (n=6 each group) of caspase-1 activity (A), IL-1B production (B-C) in glomeruli of Nlrp3+/+ and Nlrp3-/- mice fed with ND or FF diet. Significant difference (P<0.05) compared to the values from control Nlrp3+/+ mice, # Significant difference (P<0.05) compared to the values from Nlrp3+/+ mice receiving the FF diet.
Mice lacking Nlrp3 gene protects against hHcys-induced glomerular injury and podocyte injury
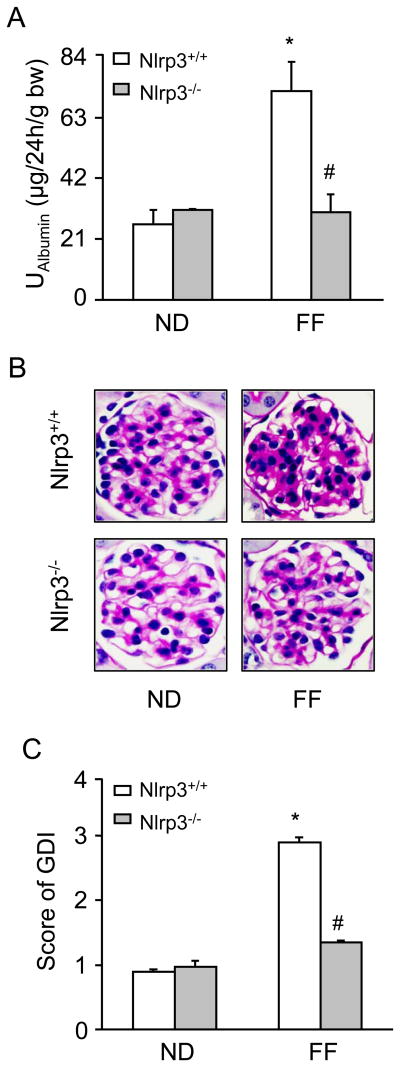
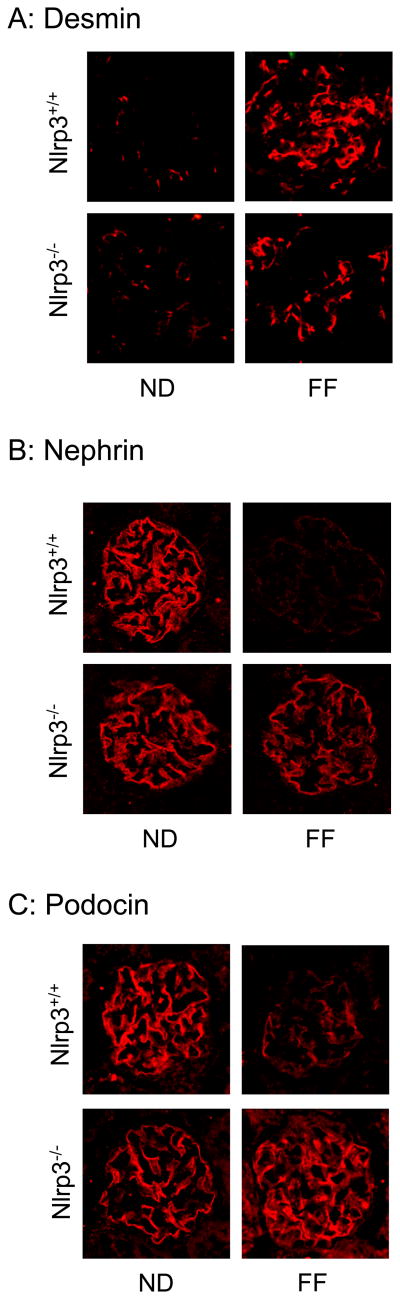
As shown in Figure 3A, urinary albumin excretion were similar in Nlrp3+/+ and Nlrp3-/- mice fed a normal diet. However, hHcys significantly increased the urinary albumin excretion in Nlrp3+/+ mice, but not in Nlrp3-/- mice. By PAS staining, we observed a typical pathological change in glomerular sclerotic damage in Nlrp3+/+ mice on the folate free diet such as glomerular capillary collapse and mesangial expansion. This pathology was not observed in Nlrp3-/- mice. The glomerular damage index (GDI) was significantly higher in Nlrp3+/+ mice fed a FF diet compared to ND fed mice. However, the hHcys-induced glomerular damage index was significantly attenuated in Nlrp3-/- mice (Fig. 3B and 3C). Further experiments were performed to test whether inflammasome-mediated glomerular injury is due to podocyte dysfunction or injury. By immunofluorescent microscopic analysis, desmin was found to have more profound abundance in glomeruli of FF diet-fed mice compared to that in normal diet-fed mice. The lack of Nlrp3 gene abrogated the FF diet-induced increase in desmin expression (Figure 4A). In contrast, nephrin and podocin were markedly reduced in glomeruli from FF diet-fed mice compared to those in normal diet-fed mice. However, the hHcys Nlrp3-/- mice completely attenuated the decrease in nephrin and podocin staining (Figure 4B and 4C).
Figure 3. Effects of the normal diet and FF Diet on glomerular injury in Nlrp3+/+ and Nlrp3-/- mice.
Values are arithmetic means ± SEM (n=6 each group) of urinary albumin (A) excretion in Nlrp3+/+ and Nlrp3-/- mice with or without the FF diet. B: Photomicrographs show typical glomerular structure (original magnification, ×400) in Nlrp3+/+ and Nlrp3-/- mice fed with or without FF diet, C: Summarized data of glomerular damage index (GDI) by semi-quantitation of scores in 4 different groups of mice (n=6 each group). For each kidney section, 50 glomeruli were randomly chosen for the calculation of GDI. Significant difference (P<0.05) compared to the values from control Nlrp3+/+ mice, # Significant difference (P<0.05) compared to the values from Nlrp3+/+ mice receiving the FF diet.
Fig. 4. Immunofluorescence staining and expression of desmin, nephrin and podocin in glomeruli from Nlrp3-/- and Nlrp3+/+ mice fed a ND or folate free diet.
A. Typical images of desmin (A), nephrin (B) and podocin (C) staining in glomeruli from Nlrp3-/- and Nlrp3+/+ mice with or without folate free diet.
Efficiency of in vivo local transfection of Nlrp3 shRNA into the kidney
As shown in Figure 5A, we used an IVIS in vivo molecular imaging system to detect the expression of co-transfected luciferase gene, which insures an efficient delivery of target gene into the mouse kidney. The luciferase reporter gene was monitored in to the kidney of the living mouse after the injection of plasmid mixed with microbubbles under ultrasound force. Starting on day 3, the expression of luciferase gene persisted for 4 weeks. In the hemi-dissected kidney, all of the cortical regions were observed to exhibit efficient gene transfection as own in green and red fluorescence (Fig. 5B). As illustrated in Fig. 5C and 5D, Nlrp3 expression was significantly decreased in C57BL/6J WT mice transfected with Nlrp3 shRNA compared to control mice (scramble shRNA) fed a normal diet. Compared to the normal diet fed mice, the hHcys significantly increased the Nlrp3 expression in glomeruli from mice receiving scrambled shRNA, but it had no effect on in mice receiving Nlrp3 shRNA.
Figure 5. Effects of local Nlrp3 gene silencing on hHcys-induced Nlrp3 inflammasome formation and activation in C57BL/6J mice with or without the FF diet.
Nlrp3 shRNA (Nlrp3 sh) and luciferase cDNA plasmids were locally delivered into the kidney. A: Daily imaging confirmation of gene transfection by detection of cotransfected luciferase gene expression by using an IVIS in vivo molecular imaging system. B: Localization of luciferase gene expression in hemidissected kidney on day 12 after gene delivery. C: Nlrp3 Immunofluorescent staining, D: Values are arithmetic means ± SEM (n=5-6 each group) of Nlrp3 mRNA expression in C57BL/6J mice on the normal or folate free diet with or without Nlrp3 shRNA transfection. Scra: Scramble, Nlrp3 sh: Nlrp3 shRNA, * Significant difference (P<0.05) compared to the values from control mice on the normal diet, # Significant difference (P<0.05) compared to the values from mice on the folate free diet.
Attenuation of hHcys-induced inflammasome formation, glomerular injury and O2.- production by Nlrp3 gene silencing
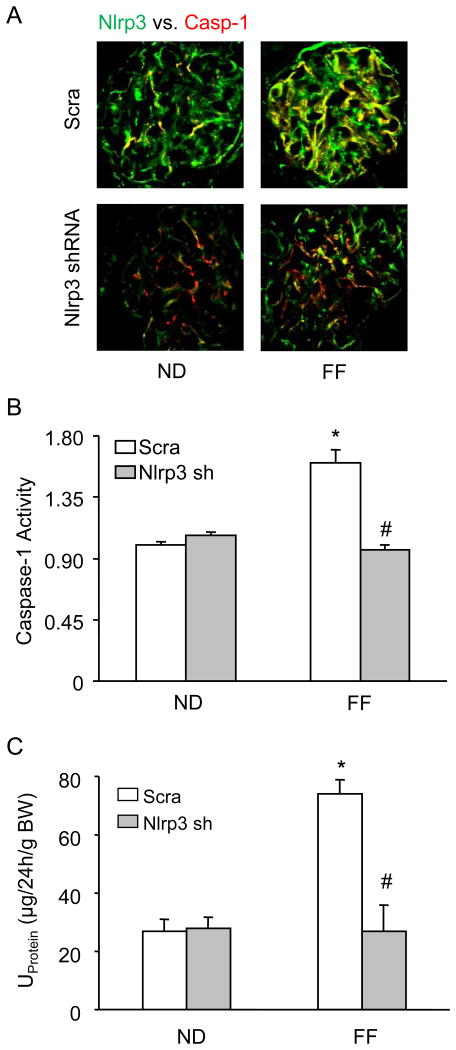
Further we determined whether Nlrp3 gene silencing locally in the kidney may attenuate the hHcys-induced Nlrp3 inflammasome formation and protects against the glomerular injury. As shown in Figure 6A, hHcys increased the colocalization of Nlrp3 with caspase-1 in glomeruli of scrambled shRNA transfected mice. However, such colocalization was not observed in glomeruli of Nlrp3 shRNA transfected mice, suggesting the attenuation of inflammasome formation in glomeruli. In consistent with the decreased Nlrp3 inflammasome formation, caspase-1 activity was attenuated in Nlrp3 shRNA transfected mice (Fig. 6B). The urinary protein excretion was similar in both scrambled and Nlrp3 shRNA transfected mice fed a normal diet. hHcys significantly increased the urinary total protein excretion when compared to the normal diet-fed mice. However, the Nlrp3 shRNA transfection significantly attenuated hHcys-induced urinary total protein excretion (Fig. 6C).
Figure 6. Effects of renal Nlrp3 gene silencing on glomerular injury in C57BL/6J mice with or without the folate free diet.
A: Colocalization of Nlrp3 (green) with caspase-1 (red) in mouse glomeruli, B: caspase-1 activity, C: Values are arithmetic means ± SEM (n=5-6 each group) of urinary total protein excretion in C57BL/6J mice on the normal or folate free diet with or without Nlrp3 shRNA transfection. Scra: Scramble, Nlrp3 sh: Nlrp3 shRNA, * Significant difference (P<0.05) compared to the values from control mice on the normal diet, # Significant difference (P<0.05) compared to the values from mice on the folate free diet.
Discussion
The major goal of the present study is to determine the role Nlrp3 gene in the formation and activation of NLRP3 inflammasomes in glomeruli of hHcys mice. We found that hHcys-induced the Nlrp3 inflammasome formation and activation and contributes to the podocyte injury and glomerular injury/sclerosis in Nlrp3+/+ mice. The deficiency of Nlrp3 gene completely abolished the hHcys-induced inflammasome formation, glomerular injury and podocyte injury. Our results for the first time demonstrate that NLRP3 gene is a critical component in mediating the hHcys-induced NLRP3 inflammasome formation and glomerular injury.
It has been reported that NLRP3 inflammasome, an inflammatory machinery that when stimulated results in IL-1β maturation and triggers the early innate immunity cascade to initiate an immune response, is comprised of three major proteins – NLRP3, considered to be the sensory component, the adaptor protein ASC, and the effector caspase-1. Deficiencies in this multiprotein complex have been associated with autoimmune disorders such as familial Mediterranean fever and Muckle-Wells syndrome, but aberrant NLRP3 inflammasome activation has extended to many traditionally considered non-inflammatory disorders including diabetes, obesity, silicosis, acute myocardial infarction, liver toxicity, and kidney diseases [21, 29, 31, 47-50]. Most recently we have demonstrated that inhibition of NLRP3 inflammasomes either by apoptosis associated speck-like protein (ASC) gene silencing or by pharmacological inhibition of caspase-1 attenuated the hHcys-induced glomerular injury [13]; however, the role of NLRP3 gene in hHcys-induced glomerular injury/sclerosis has not been elucidated. Therefore, we used Nlrp3 deficient mice to explore the role of Nlrp3 gene in mediating the hHcys-induced glomerular injury or sclerosis. It was found that FF diet treatment for 4 weeks significantly increased the plasma Hcys levels in both Nlrp3+/+ and Nlrp3-/- mice, which indicates a successful establishment of the hHcys and also suggests that Nlrp3 gene is not involved in the global metabolism of Hcys. The increased plasma Hcys concentration induced the formation of NLRP3 inflammasomes in glomeruli as shown by colocalization of NLRP3 with ASC or NLRP3 with caspase in Nlrp3+/+ mice. Using podocin as a podocyte marker, our confocal observations demonstrated that hHcys-induced inflammasome formation in glomeruli was mostly located in podocytes as demonstrated by the colocalization of Nlrp3 with podocin. These colocalizations were substantially blocked in mice lacking Nlrp3 gene. Moreover, the biochemical analysis showed that hHcys increased the caspase-1 activity and IL-1β production in Nlrp3+/+ mice but not in Nlrp3-/- mice, suggesting the essential role of NLRP3 gene in mediating the hHcys-induced inflammasome formation and activation in glomeruli of mice.
In previous studies, Nlrp3 inflammasome has been implicated in the regulation of kidney function [16-17, 41]. Previous reports from our laboratory and others demonstrated the role of inflammasome in hHcys or obesity-induced glomerular injury [11, 13, 40], acute ischemia/reperfusion-induced kidney injury [41], unilateral ureteral obstruction [16-17], and renal biopsies from patients with non-diabetic kidney disease [17]. Inhibition of inflammasomes attenuated the proteinuria and kidney function. In this study, we tested, whether NLRP3 gene is critically involved in the hHcys-induced glomerular injury. In accordance with decreased inflammasome formation in hHcys Nlrp3-/- mice, urinary albumin excretion and glomerular injury/sclerosis were significantly blocked compared with Nlrp3+/+ mice on the FF diet suggesting that that inflammasome formation and activation are an important early mechanism responsible for glomerular injury in hHcys mice. In particular, activation of podocyte or glomerular inflammasomes is critical for the development of glomerular injury associated with hHcys. Furthermore, we explored the mechanism mediating the protective action of NLRP3 gene in glomerular injury, we observed the changes in podocyte function in both Nlrp3+/+ and Nlrp3-/- mice, given that podocyte dysfunction or effacement is considered the initiating mechanism to produce glomerular injury or sclerosis, leading to proteinuria with possible progression to ESRD [36, 51]. The present study showed that podocin and nephrin protein were markedly decreased in FF diet-fed Nlrp3+/+ mice, but not in mice lacking Nlrp3. While another podocyte injury marker, desmin was increased in hHcys Nlrp3+/+ mice, which was abolished in Nlrp3-/- mice. These results support the view that podocyte inflammasomes as an intracellular inflammatory machinery or responder to endogenous danger factors could be a target of therapeutic strategy for hHcys-induced glomerular injury and podocyte injury.
To further explore the role of NLRP3 gene in mediating hHcys induced-inflammasome formation and glomerular injury, we locally modulated gene expression of Nlrp3 in mouse kidney and observed changes in NLRP3 inflammasomes colocalization in glomeruli of mice. To reach this goal, we combined an ultrasound technique and microbubble wrapping for introduction of plasmids into kidney. Several recent reports from our laboratory and by others have shown that the ultrasound-microbubble system is a very effective method to deliver plasmids into cells of different organs in vivo with a transfection rate over 90% [52-55]. Combination of the ultrasound technique and microbubble wrapping for introduction of plasmids greatly enhances transgene expression with a 300-fold increase over naked DNA alone without toxicity [56-57]. The present study using in vivo molecular imaging demonstrated that plasmids (Nlrp3 shRNA plasmids co-transfected with luciferase cDNA plasmid) were successfully delivered into the kidney and co-transfected luciferase expression persisted for up to 4 weeks. In such local Nlrp3 gene silenced kidney, we found that mRNA expression of Nlrp3, colocalization of Nalp3 with caspase-1 in mouse glomeruli and caspase 1 activity were significantly decreased. It was also demonstrated that silencing the Nlrp3 gene in the kidney ameliorates proteinuria. These results from mice with local renal Nlrp3 gene silencing further support the conclusion above drawn from studies using Nlrp3-/- mice that Nlrp3 inhibition or gene silencing abolishes hHcys-induced action of inflammasome and thereby protect the kidney from hHcys-induced glomerular injury. In conclusion, the present study demonstrated that Nlrp3 gene deficiency attenuates the hHcys-induced inflammasome formation, glomerular injury and podocyte injury. Therefore, targeting Nlrp3 may be an important therapeutic strategy to prevent inflammasome activation and thereby protect podocytes and glomeruli from hHcys-induced injury.
Acknowledgments
This work was supported by grants DK54927, HL091464, HL57244 and HL075316 from the National Institutes of Health (to P.L.) and VCU's CTSA (UL1TR000058 from the National Center for Advancing Translational Sciences) and the CCTR Endowment Fund of Virginia Commonwealth University (to K.B.).
References
- 1.Boini KM, Xia M, Abais JM, Xu M, Li CX, Li PL. Acid sphingomyelinase gene knockout ameliorates hyperhomocysteinemic glomerular injury in mice lacking cystathionine-beta-synthase. PLoS One. 2012;7:e45020. doi: 10.1371/journal.pone.0045020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boini KM, Xia M, Li C, Zhang C, Payne LP, Abais JM, Poklis JL, Hylemon PB, Li PL. Acid sphingomyelinase gene deficiency ameliorates the hyperhomocysteinemia-induced glomerular injury in mice. Am J Pathol. 2011;179:2210–2219. doi: 10.1016/j.ajpath.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grenz A, Hermes M, Hammel P, Roll JB, Osswald H, Kloor D. Hyperhomocysteinemia is associated with decreased erythropoietin expression in rats. Cell Physiol Biochem. 2010;26:449–456. doi: 10.1159/000320568. [DOI] [PubMed] [Google Scholar]
- 4.Li CX, Xia M, Han WQ, Li XX, Zhang C, Boini KM, Liu XC, Li PL. Reversal by growth hormone of homocysteine-induced epithelial-to-mesenchymal transition through membrane raft-redox signaling in podocytes. Cell Physiol Biochem. 2011;27:691–702. doi: 10.1159/000330078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yi F, Chen QZ, Jin S, Li PL. Mechanism of homocysteine-induced rac1/nadph oxidase activation in mesangial cells: Role of guanine nucleotide exchange factor vav2. Cell Physiol Biochem. 2007;20:909–918. doi: 10.1159/000110451. [DOI] [PubMed] [Google Scholar]
- 6.Austin RC, Lentz SR, Werstuck GH. Role of hyperhomocysteinemia in endothelial dysfunction and atherothrombotic disease. Cell Death Differ. 2004;11(Suppl 1):S56–64. doi: 10.1038/sj.cdd.4401451. [DOI] [PubMed] [Google Scholar]
- 7.Cavalca V, Cighetti G, Bamonti F, Loaldi A, Bortone L, Novembrino C, De Franceschi M, Belardinelli R, Guazzi MD. Oxidative stress and homocysteine in coronary artery disease. Clin Chem. 2001;47:887–892. [PubMed] [Google Scholar]
- 8.Ientile R, Curro M, Ferlazzo N, Condello S, Caccamo D, Pisani F. Homocysteine, vitamin determinants and neurological diseases. Front Biosci (Schol Ed) 2010;2:359–372. doi: 10.2741/s70. [DOI] [PubMed] [Google Scholar]
- 9.Robinson K, Gupta A, Dennis V, Arheart K, Chaudhary D, Green R, Vigo P, Mayer EL, Selhub J, Kutner M, Jacobsen DW. Hyperhomocysteinemia confers an independent increased risk of atherosclerosis in end-stage renal disease and is closely linked to plasma folate and pyridoxine concentrations. Circulation. 1996;94:2743–2748. doi: 10.1161/01.cir.94.11.2743. [DOI] [PubMed] [Google Scholar]
- 10.Abais JM, Xia M, Li G, Gehr TW, Boini KM, Li PL. Contribution of endogenously produced reactive oxygen species to the activation of podocyte nlrp3 inflammasomes in hyperhomocysteinemia. Free Radic Biol Med. 2014;67:211–220. doi: 10.1016/j.freeradbiomed.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abais JM, Zhang C, Xia M, Liu Q, Gehr T, Boini KM, Li PL. Nadph oxidase-mediated triggering of inflammasome activation in mouse podocytes and glomeruli during hyperhomocysteinemia. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2012.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sen U, Munjal C, Qipshidze N, Abe O, Gargoum R, Tyagi SC. Hydrogen sulfide regulates homocysteine-mediated glomerulosclerosis. Am J Nephrol. 2010;31:442–455. doi: 10.1159/000296717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang C, Boini KM, Xia M, Abais JM, Li X, Liu Q, Li PL. Activation of nod-like receptor protein 3 inflammasomes turns on podocyte injury and glomerular sclerosis in hyperhomocysteinemia. Hypertension. 2012;60:154–162. doi: 10.1161/HYPERTENSIONAHA.111.189688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li N, Chen L, Muh RW, Li PL. Hyperhomocysteinemia associated with decreased renal transsulfuration activity in dahl s rats. Hypertension. 2006;47:1094–1100. doi: 10.1161/01.HYP.0000219634.83928.6e. [DOI] [PubMed] [Google Scholar]
- 15.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors asc and ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 16.Anders HJ, Muruve DA. The inflammasomes in kidney disease. J Am Soc Nephrol. 2011;22:1007–1018. doi: 10.1681/ASN.2010080798. [DOI] [PubMed] [Google Scholar]
- 17.Vilaysane A, Chun J, Seamone ME, Wang W, Chin R, Hirota S, Li Y, Clark SA, Tschopp J, Trpkov K, Hemmelgarn BR, Beck PL, Muruve DA. The nlrp3 inflammasome promotes renal inflammation and contributes to ckd. J Am Soc Nephrol. 2010;21:1732–1744. doi: 10.1681/ASN.2010020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinon F, Mayor A, Tschopp J. The inflammasomes: Guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 19.Gross O, Thomas CJ, Guarda G, Tschopp J. The inflammasome: An integrated view. Immunological reviews. 2011;243:136–151. doi: 10.1111/j.1600-065X.2011.01046.x. [DOI] [PubMed] [Google Scholar]
- 20.Tschopp J, Schroder K. Nlrp3 inflammasome activation: The convergence of multiple signalling pathways on ros production? Nature reviews Immunology. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 21.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 22.Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, Ojcius DM. Atp activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem. 2007;282:2871–2879. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The nalp3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nour AM, Yeung YG, Santambrogio L, Boyden ED, Stanley ER, Brojatsch J. Anthrax lethal toxin triggers the formation of a membrane-associated inflammasome complex in murine macrophages. Infect Immun. 2009;77:1262–1271. doi: 10.1128/IAI.01032-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinon F, Burns K, Tschopp J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proil-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 26.Srinivasula SM, Poyet JL, Razmara M, Datta P, Zhang Z, Alnemri ES. The pyrin-card protein asc is an activating adaptor for caspase-1. J Biol Chem. 2002;277:21119–21122. doi: 10.1074/jbc.C200179200. [DOI] [PubMed] [Google Scholar]
- 27.Aganna E, Martinon F, Hawkins PN, Ross JB, Swan DC, Booth DR, Lachmann HJ, Bybee A, Gaudet R, Woo P, Feighery C, Cotter FE, Thome M, Hitman GA, Tschopp J, McDermott MF. Association of mutations in the nalp3/cias1/pypaf1 gene with a broad phenotype including recurrent fever, cold sensitivity, sensorineural deafness, and aa amyloidosis. Arthritis Rheum. 2002;46:2445–2452. doi: 10.1002/art.10509. [DOI] [PubMed] [Google Scholar]
- 28.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. Nalp3 forms an il-1beta-processing inflammasome with increased activity in muckle-wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 29.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, Flavell RA, Mehal WZ. Acetaminophen-induced hepatotoxicity in mice is dependent on tlr9 and the nalp3 inflammasome. J Clin Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the nalp3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 32.Menu P, Pellegrin M, Aubert JF, Bouzourene K, Tardivel A, Mazzolai L, Tschopp J. Atherosclerosis in apoe-deficient mice progresses independently of the nlrp3 inflammasome. Cell Death Dis. 2011;2:e137. doi: 10.1038/cddis.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freigang S, Ampenberger F, Spohn G, Heer S, Shamshiev AT, Kisielow J, Hersberger M, Yamamoto M, Bachmann MF, Kopf M. Nrf2 is essential for cholesterol crystal-induced inflammasome activation and exacerbation of atherosclerosis. Eur J Immunol. 2011;41:2040–2051. doi: 10.1002/eji.201041316. [DOI] [PubMed] [Google Scholar]
- 34.Rajamaki K, Lappalainen J, Oorni K, Valimaki E, Matikainen S, Kovanen PT, Eklund KK. Cholesterol crystals activate the nlrp3 inflammasome in human macrophages: A novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5:e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salminen A, Ojala J, Suuronen T, Kaarniranta K, Kauppinen A. Amyloid-beta oligomers set fire to inflammasomes and induce alzheimer's pathology. J Cell Mol Med. 2008;12:2255–2262. doi: 10.1111/j.1582-4934.2008.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis TL, Cao D, Lu H, Mans RA, Su YR, Jungbauer L, Linton MF, Fazio S, LaDu MJ, Li L. Overexpression of human apolipoprotein a-i preserves cognitive function and attenuates neuroinflammation and cerebral amyloid angiopathy in a mouse model of alzheimer disease. J Biol Chem. 2010;285:36958–36968. doi: 10.1074/jbc.M110.127829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savage CD, Lopez-Castejon G, Denes A, Brough D. Nlrp3-inflammasome activating damps stimulate an inflammatory response in glia in the absence of priming which contributes to brain inflammation after injury. Front Immunol. 2012;3:288. doi: 10.3389/fimmu.2012.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosin DL, Okusa MD. Dangers within: Damp responses to damage and cell death in kidney disease. J Am Soc Nephrol. 2011;22:416–425. doi: 10.1681/ASN.2010040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vasileiou E, Montero RM, Turner CM, Vergoulas G. P2x(7) receptor at the heart of disease. Hippokratia. 2010;14:155–163. [PMC free article] [PubMed] [Google Scholar]
- 40.Boini KM, Xia M, Abais JM, Li G, Pitzer AL, Gehr TW, Zhang Y, Li PL. Activation of inflammasomes in podocyte injury of mice on the high fat diet: Effects of asc gene deletion and silencing. Biochim Biophys Acta. 2014;1843:836–845. doi: 10.1016/j.bbamcr.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, Ulland TK, Eisenbarth SC, Florquin S, Flavell RA, Leemans JC, Sutterwala FS. Necrotic cells trigger a sterile inflammatory response through the nlrp3 inflammasome. Proc Natl Acad Sci U S A. 2009;106:20388–20393. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boini KM, Xia M, Xiong J, Li C, Payne LP, Li PL. Implication of cd38 gene in podocyte epithelial-to-mesenchymal transition and glomerular sclerosis. J Cell Mol Med. 2012;16:1674–1685. doi: 10.1111/j.1582-4934.2011.01462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen YF, Li PL, Zou AP. Effect of hyperhomocysteinemia on plasma or tissue adenosine levels and renal function. Circulation. 2002;106:1275–1281. doi: 10.1161/01.cir.0000027586.64231.1b. [DOI] [PubMed] [Google Scholar]
- 44.Zhang C, Hu JJ, Xia M, Boini KM, Brimson CA, Laperle LA, Li PL. Protection of podocytes from hyperhomocysteinemia-induced injury by deletion of the gp91phox gene. Free Radic Biol Med. 2010;48:1109–1117. doi: 10.1016/j.freeradbiomed.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boini KM, Zhang C, Xia M, Poklis JL, Li PL. Role of sphingolipid mediator ceramide in obesity and renal injury in mice fed a high-fat diet. J Pharmacol Exp Ther. 2010;334:839–846. doi: 10.1124/jpet.110.168815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boini KM, Amann K, Kempe D, Alessi DR, Lang F. Proteinuria in mice expressing pkb/sgk-resistant gsk3. Am J Physiol Renal Physiol. 2009;296:F153–159. doi: 10.1152/ajprenal.90398.2008. [DOI] [PubMed] [Google Scholar]
- 47.Watanabe H, Gaide O, Petrilli V, Martinon F, Contassot E, Roques S, Kummer JA, Tschopp J, French LE. Activation of the il-1beta-processing inflammasome is involved in contact hypersensitivity. J Invest Dermatol. 2007;127:1956–1963. doi: 10.1038/sj.jid.5700819. [DOI] [PubMed] [Google Scholar]
- 48.Nishi Y, Satoh M, Nagasu H, Kadoya H, Ihoriya C, Kidokoro K, Sasaki T, Kashihara N. Selective estrogen receptor modulation attenuates proteinuria-induced renal tubular damage by modulating mitochondrial oxidative status. Kidney Int. 2013;83:662–673. doi: 10.1038/ki.2012.475. [DOI] [PubMed] [Google Scholar]
- 49.Wang C, Pan Y, Zhang QY, Wang FM, Kong LD. Quercetin and allopurinol ameliorate kidney injury in stz-treated rats with regulation of renal nlrp3 inflammasome activation and lipid accumulation. PLoS One. 2012;7:e38285. doi: 10.1371/journal.pone.0038285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang W, Wang X, Chun J, Vilaysane A, Clark S, French G, Bracey NA, Trpkov K, Bonni S, Duff HJ, Beck PL, Muruve DA. Inflammasome-independent nlrp3 augments tgf-beta signaling in kidney epithelium. J Immunol. 2013;190:1239–1249. doi: 10.4049/jimmunol.1201959. [DOI] [PubMed] [Google Scholar]
- 51.Dressler GR. The cellular basis of kidney development. Annu Rev Cell Dev Biol. 2006;22:509–529. doi: 10.1146/annurev.cellbio.22.010305.104340. [DOI] [PubMed] [Google Scholar]
- 52.Hou CC, Wang W, Huang XR, Fu P, Chen TH, Sheikh-Hamad D, Lan HY. Ultrasound-microbubble-mediated gene transfer of inducible smad7 blocks transforming growth factor-beta signaling and fibrosis in rat remnant kidney. Am J Pathol. 2005;166:761–771. doi: 10.1016/s0002-9440(10)62297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koike H, Tomita N, Azuma H, Taniyama Y, Yamasaki K, Kunugiza Y, Tachibana K, Ogihara T, Morishita R. An efficient gene transfer method mediated by ultrasound and microbubbles into the kidney. J Gene Med. 2005;7:108–116. doi: 10.1002/jgm.632. [DOI] [PubMed] [Google Scholar]
- 54.Lan HY, Mu W, Tomita N, Huang XR, Li JH, Zhu HJ, Morishita R, Johnson RJ. Inhibition of renal fibrosis by gene transfer of inducible smad7 using ultrasound-microbubble system in rat uuo model. J Am Soc Nephrol. 2003;14:1535–1548. doi: 10.1097/01.asn.0000067632.04658.b8. [DOI] [PubMed] [Google Scholar]
- 55.Sheyn D, Kimelman-Bleich N, Pelled G, Zilberman Y, Gazit D, Gazit Z. Ultrasound-based nonviral gene delivery induces bone formation in vivo. Gene Ther. 2008;15:257–266. doi: 10.1038/sj.gt.3303070. [DOI] [PubMed] [Google Scholar]
- 56.Bekeredjian R, Chen S, Frenkel PA, Grayburn PA, Shohet RV. Ultrasound-targeted microbubble destruction can repeatedly direct highly specific plasmid expression to the heart. Circulation. 2003;108:1022–1026. doi: 10.1161/01.CIR.0000084535.35435.AE. [DOI] [PubMed] [Google Scholar]
- 57.Tsunoda S, Mazda O, Oda Y, Iida Y, Akabame S, Kishida T, Shin-Ya M, Asada H, Gojo S, Imanishi J, Matsubara H, Yoshikawa T. Sonoporation using microbubble br14 promotes pdna/sirna transduction to murine heart. Biochem Biophys Res Commun. 2005;336:118–127. doi: 10.1016/j.bbrc.2005.08.052. [DOI] [PubMed] [Google Scholar]