Abstract
There is growing interest in anxiolytic and pro-social effects of the neuropeptide oxytocin (OXT), but the underlying intraneuronal mechanisms are largely unknown. Here we examined OXT-mediated anxiolysis in the hypothalamic paraventricular nucleus (PVN) of rats and effects of OXT administration on signaling events in hypothalamic primary and immortalized cells. In vivo, the application of SKF96365 prevented the anxiolytic activity of OXT in the PVN, suggesting that changes in intracellular Ca2+ mediate the acute OXT behavioral effects. In vitro, mainly in the neurons with autonomous Ca2+ oscillations, OXT increased intracellular Ca2+ concentration and oscillation amplitude. Pharmacological intervention revealed OXT-dependent changes in Ca2+ signaling that required activation of transient receptor potential vanilloid type-2 channel (TRPV2), mediated by phosphoinositide 3-kinase. TRPV2 induced the activation of the anxiolytic mitogen-activated protein kinase kinase (MEK1/2). In situ, immunohistochemistry revealed co-localization of TRPV2 and OXT in the PVN. Thus, functional and pharmacological analyses identified TRPV2 as a mediator of anxiolytic effects of OXT, conveying the OXT signal to MEK1/2 via modulation of intracellular Ca2+.
Introduction
The neuropeptide oxytocin (OXT) has received much attention due to its anxiolytic properties and its beneficial effects on social behavior and autism (Meyer-Lindenberg et al, 2011). OXT is produced in neurons of the hypothalamic supraoptic (SON) and paraventricular (PVN) nuclei, which project to the neurohypophysis and within the brain. It is released into the circulation to control peripheral physiological parameters (Gimpl and Fahrenholz, 2001), and also centrally from dendrites of magnocellular neurons (Ludwig et al, 2002) and from axons that target a variety of brain regions (Knobloch et al, 2012). Central OXT facilitates pro-social behaviors including pair-bonding and social preference (Lukas et al, 2008), and is anxiolytic in the amygdala (Bale et al, 2001) and PVN (Blume et al, 2008), but anxiogenic in the lateral septum (Guzman et al, 2013).
OXT exerts its effects by binding to the OXT receptor (OXTR), a G-protein-coupled receptor (GPCR), also expressed by the OXT-producing neurons themselves, and subsequent activation of several intracellular signaling pathways (Gimpl and Fahrenholz, 2001). One of these pathways involves transactivation of the epidermal growth factor receptor (EGFR), followed by the activation by phosphorylation of the mitogen-activated protein kinase kinase (MEK1/2). Phosphorylated MEK1/2 (pMEK1/2) mediates the anxiolytic activity of OXT in the PVN, in both male and female rats (Blume et al, 2008).
OXTR activation additionally recruits Ca2+ from thapsigargin-sensitive, but ryanodine-insensitive, intracellular stores (Jin et al, 2007). The increased intracellular Ca2+ concentration ([Ca2+]i) facilitates OXT release from the axons and dendrites of magnocellular SON and PVN neurons (Lambert et al, 1994), and therefore modulates the behavioral effects of OXT (Jin et al, 2007). In dendrites, elevated [Ca2+]i primes OXT-containing vesicles for immediate release on a subsequent stimulus (Ludwig et al, 2002). Voltage-dependent Ca2+ channels, although expressed in the somata of OXT-sensitive neurons in the SON, do not contribute to the OXT-induced rise of [Ca2+]i (Sabatier et al, 1997).
OXT neurons express additional plasma membrane Ca2+ channels, including the transient receptor potential (TRP), vanilloid type 2 (TRPV2) channel (Wainwright et al, 2004). It forms a Ca2+-conducting cation channel and was first found in sensory ganglia to be activated by heat (Caterina et al, 1999) and in aortic myocytes by cell swelling, the latter suggesting a role in osmoregulation (Muraki et al, 2003). TRPV2 channels are expressed both peripherally and centrally; their physiological functions in the brain, however, are not well understood (Ramsey et al, 2006). Their expression in OXT-producing neurons, demonstrated in macaques (Wainwright et al, 2004), makes it possible that they are involved in the modulation of [Ca2+]i in these cells, thus favoring a role of extracellular Ca2+ in OXT-induced intracellular signaling and behavior. Indeed, we found that influx of extracellular Ca2+ is necessary for MEK1/2 phosphorylation and the anxiolytic effect of OXT within the PVN. Subsequent Ca2+-imaging studies in primary hypothalamic neurons identified the TRPV2 channel as a mediator of Ca2+ entry in a phosphoinositide 3-kinase (PI3K)-dependent manner. In the light of the growing interest in OXT administration as psychotherapeutic option in humans, our data provide a further step toward therapy by unraveling new components of the underlying cellular signaling cascade.
Materials and Methods
Animals
Male Wistar rats (Charles River, Germany, 250–300 g body weight) were housed under standard laboratory conditions in groups of four (12 h light/dark cycle, 22–24 °C, lights on at 0600 h, food and water ad libitum). All experiments were performed between 0800 and 1100 h, approved by the government of the Oberpfalz, Germany, according with the Guide for Care and Use of Laboratory Animals by the National Institutes of Health, Bethesda, MD.
Surgeries
All surgical stereotaxic procedures were performed under isoflurane anesthesia and semi-sterile conditions. Following surgery, rats received a subcutaneous injection of antibiotic (0.03 ml enrofloxacin; 100 mg/1 ml Baytril, Bayer). Animals were housed singly after surgery for 7 days and were handled daily, to habituate them to the central infusion procedure and to avoid nonspecific stress responses during the experiment.
For intra-PVN infusions, indwelling bilateral guide cannulae (stainless steel, 23G, 12-mm long) were implanted 2 mm above both the left and the right PVN (AP: −1.4 mm bregma, ML: −1.8 and +2.1 mm lateral; DV: +6 mm below the surface of the skull; angle 10° Blume et al, 2008), and were anchored to two stainless-steel screws using dental acrylic. The guide cannulae were kept viable with dummy cannulae, which were removed daily and cleaned during the handling procedure.
Intra-PVN Infusions
For evaluation of the effect of SKF96365 hydrochloride (Tocris Bioscience) on OXT-induced anxiolysis in the PVN, conscious rats received two subsequent infusions, with a 5-min interval, in one of the following combinations: Vehicle/Vehicle, Vehicle/OXT, SKF96365/Vehicle, or SKF96365/OXT. Infusions were given directly into the left and the right PVN via an infusion cannula (31G, 14 mm long). After each infusion, the cannula was kept in place for 10 s to allow local substance diffusion. OXT and SKF96365 were diluted freshly from highly concentrated stock solutions in Ringer (Vehicle for both).
OXT was infused at 0.01 nmol/0.5 μl per PVN to induce anxiolysis (Blume et al, 2008). The concentration of SKF96365 (0.5 nmol/0.5 μl) was selected following testing of several doses, while monitoring any signs of tremor, convulsions, wet-dog shakes, or grooming in the homecage. None of these symptoms were observed at the concentration used.
Behavioral Studies
Anxiety-related behavior and locomotor activity were tested in the light–dark box (LDB) and on the elevated plusmaze (EPM) as described (Jurek et al, 2012). Briefly, 10 min after the last intra-PVN infusion, rats were tested for 5 min between 0900 and 1200 h. The time spent in and the latency to the first entry into the light compartment of the LDB and the percentage of time spent in and the percentage of entries into the open arm of the EPM were recorded to assess anxiety-related behavior. The number of line crosses and rearings in the dark box (LDB) and the number of entries into the closed arms (EPM) were taken as locomotor activity. After the behavioral experiments, the animals were killed, brains were snap-frozen in isobutane cooled to −32 °C by dry ice, and the intra-PVN placement of cannulae was verified in Nissl-stained cryosections (Paxinos and Watson, 1998). Only animals with correctly positioned cannulae were included in statistical analyses.
Cell Culture
Primary hypothalamic cells were isolated from postnatal day 1–3 Wistar rat pups. Hypothalami were dissected posterior to the optic chiasma, anterior to the mammillary bodies, along both the lateral sulci and 3 mm ventrodorsal to exclude the thalamus. Tissue was digested in Hank's balanced salt solution containing 0.01% Papain (33 U/mg, Worthington), 0.1% Dispase II (0.5 U/mg, GIBCO), 0.01% DNAse (3555 U/mg; Worthington), and 12.4 mM MgSO4 at 37 °C for 30 min. After mechanical dispersion with a fire-polished Pasteur pipette, digestion was stopped with DMEM/HAM's 12 supplemented with 10% FCS, 2% B-27 supplement and 1% penicillin/streptomycin. The cell suspension was filtered through a 70-μm nylon filter and plated on poly-L-lysine (Sigma)-coated glass coverslips and kept at 37 °C and 5% CO2. Medium was changed to serum-free medium 24 h after isolation. Seventy-two hours after isolation, 5 μM cytosine-arabionfuranoside (Ara-C, Sigma) was added to inhibit glial cell proliferation. Ara-C was removed 24 h before experiments; cultures were used after 9–14 days in culture for experiments.
Rat-immortalized hypothalamic H32 cells (Mugele et al, 1993) were cultured in DMEM (Invitrogen, Carlsbad), supplemented with 10% fetal bovine serum, 10% horse serum, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37 °C and 5% CO2.
Intracellular Calcium Measurements
Cultured neurons were loaded with 2 μM fura-2 AM ester for 30 min at 37 °C in the dark in Ringer's solution. The cells were transferred to a recording chamber on the stage of an inverted microscope and continuously perfused with Ringer's solution at 10 ml/min. Fluorescence was elicited at 340- and 380-nm excitation wavelengths; emission was detected at 510 nm by a cooled charged-coupled camera (CoolSnap, Visitron Systems, Puchheim, Germany). Data were collected and analyzed with MetaFluor software (Visitron Systems). OXT (100 nM), the Ca2+ channel blockers lanthanum(III) chloride heptahydrate (La3+, Sigma), LY-294002 hydrochloride (Sigma), ruthenium red (Sigma), or SKF96365 hydrochloride were bath-applied. Drugs were diluted in water or DMSO; final DMSO concentration was <0.01% and was included in vehicle control solution.
Intracellular-free Ca2+ ([Ca2+]i) was calibrated by superfusing the cells with Ca2+-free Ringer's solution supplemented with 25 mM EGTA and 1 μM ionomycin, followed by Ringer's solution containing 1 μM ionomycin to achieve Ca2+ saturation of fura-2. [Ca2+]i was calculated according to Grynkiewicz et al (1985). All experiments were conducted at room temperature.
Reverse Transcriptase-PCR
RNA was isolated using the High Pure RNA Isolation Kit (Roche). Reverse transcription was performed using the iScriptcDNA Synthesis Kit (Biorad). Primer sequences were 5'-CAGTAGTGTCAAGCTTATCTCCA-3' and 5'-AAGAGCATGTAGATCCACGG-3' for the OXTR, and 5′-AGCGAAACTGTCAACCACG-3′ and 5′-TCTCCCAAGCAACCCAAT-3′ for the TRPV2 primers, forward and reverse primer, respectively. PCR analyses were performed at 94 °C for 2 min, followed by 35 cycles: denaturation at 94 °C for 30 s, annealing at 58 °C for 30 s, and elongation at 72 °C for 30 s. PCR products were visualized by agarose gel analysis. Negative controls consisted of PCR reactions without reverse transcription or template.
Immunohistochemistry
Five Wistar rats were anesthetized with isoflurane andperfused transcardially with PBS and 4% paraformaldehyde in PBS. Brains were collected and post fixed overnight at 4 °C, cryoprotected in 30% sucrose, and snap-frozen in isopentane cooled to −32 °C by dry ice. Cryosections, 40-μm thick and containing the PVN, were treated with PBS supplemented with 10% normal goat serum and 0.3% Triton-X, to block unspecific binding sites for 2 h, and then incubated in the same solution containing primary antibodies against TRPV2 (1 : 50; rabbit anti-TRPV2, Calbiochem/Merck Millipore), and one against OXT or vasopressin (both 1 : 400, p38 (OXT) and p41 (vasopressin) mouse monoclonal; generous gifts of Dr Gainer, NIH). After overnight incubation at 4 °C, sections were rinsed with PBS and incubated in an anti-mouse (Vector) antibody coupled to AlexaFluor564 (Vector) for 2 h, rinsed again in PBS, and incubated with a biotinylated anti-rabbit antibody (Vector) for 2 h. Finally, after rinsing in PBS, the sections were incubated with streptavidin conjugated with AlexaFluor 488 (Vector) for 1 h, washed, and mounted with Vectashield Hard Set Mounting Medium (Vector). Images were acquired with a LSM 510 Confocal Microscope (Zeiss, Germany).
The TRPV2 antibody was raised against a synthetic peptide (KNSASEEDHLPLQVLQSP) corresponding to amino acids 744–761 of rat TRPV2, conjugated to keyhole limpet hemocyanin. This antibody is highly specific, as all immunoreactivity is abolished in TRPV2 knockout mice (Nedungadi et al, 2012). The specificity of this and the OXT and vasopressin antibodies was further assessed by pre-incubation with their respective antigens, and by omission of the first antibody. In all cases, no immunoreactivity was observed. Pre-incubation of the OXT antibody with vasopressin, and vice versa, had no effect on immunoreactivity, further demonstrating the specificity of the antibodies (see also (Ben-Barak et al, 1985)).
Stimulation of H32 Cells with TGOT
To determine OXT-induced Ca2+/calmodulin-dependent kinase II (CaMKII) and MEK1/2 phosphorylation, we used the specific OXT receptor agonist TGOT to avoid nonspecific activation of AVP-R1A that are expressed in the H32 cells. Cells were cultured in serum-free DMEM with 0.1% bovine serum albumine for 1 h, and then stimulated with vehicle (serum-free DMEM), SKF96365 (100 μM) or ruthenium red (10 μM). Ten minutes later, TGOT (10 nM; Bachem) or its vehicle (serum-free DMEM) was added, and incubation continued for 10 min at 37 °C and 5% CO2. Next, the cells were placed on ice and washed with PBS containing protease and phosphatase inhibitors provided in the protein extraction kit used (Active Motif, Rixensart, Belgium), and were collected by scraping and pelleting at 4 °C and 525 g for 5 min.
Western Blot Analysis
Cytoplasmatic proteins were extracted using the protein extraction kit mentioned above and protein yield was determined using the Pierce BCA Protein Assay Kit (Thermo Scientific, Rockford). Thirty micrograms of each cytosolic protein extract were separated by SDS-polyacrylamide gel electrophoresis, and phosphorylated and total MEK1/2 and CaMKII were visualized by western blotting (Jurek et al, 2012), using antibodies against pMEK1/2 (S217/221, 1 : 1,000; Cell Signaling Technology), pCaMKII (Thr286, Santa Cruz Biotechnology), MEK1/2 (1 : 1,000, Cell Signaling Technology), CaMKII (Santa Cruz Biotechnology), and GAPDH (1 : 1,000; Abcam).
Statistical Analyses
For the analysis of the Ca2+ imaging data, the Student's t-test was used. Behavioral and western blotting data were analyzed using a two-way ANOVA (factors treatment 1 × treatment 2). Any overall statistical differences, set at P<0.05, were further analyzed using Bonferroni's post-hoc test. Data are expressed as group mean+SEM. Statistical analyses were performed using version 19 of SPSS.
Results
The Broad-Range Ca2+ Channel Blocker SKF96365 Prevents OXT-Induced Anxiolysis in the LDB and EPM
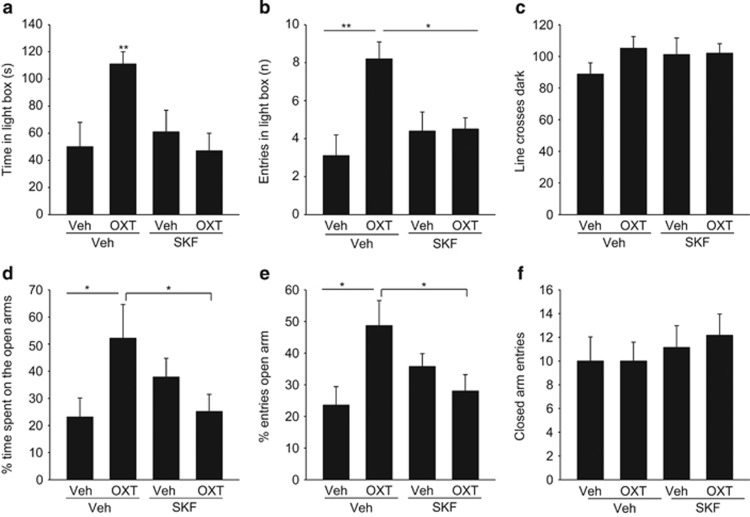
Infusion of Ca2+ channel blocker SKF96365 bilaterally into the PVN prevented the anxiolytic effect of a subsequent OXT infusion 5 min later, demonstrating that the influx of extracellular Ca2+ is necessary for anxiolysis (Figure 1).
Figure 1.
The local anxiolytic effect of oxytocin (OXT) is prevented by prior infusion of the transient receptor potential (TRP) channel blocker SKF96365 into the paraventricular nucleus (PVN). (a) Time spent in the lit compartment of the light–dark box, (b) number of entries into the lit compartment, and (c) number of line crosses in the dark compartment during the 5-min test period. (d) Percentage of time spent on the open arms of the elevated plus maze, (e) the percentage of open-arm entries, and (f) number of entries into the dark arms. Rats were pretreated with bilateral microinfusions into the PVN of either vehicle (Veh) or SKF96365 (SKF; 0.5 nmol/0.5 μl/PVN), followed by either Veh or OXT (0.01 nmol/0.5 μl/PVN). Data represent mean+SEM (n=6 or 7 per group). **P<0.01 vs all groups in a and vs Veh/Veh in b. *P<0.05 vs SKF/Veh and SKF/OXT in (b) and vs Veh/Veh and SKF/OXT in d and e.
In the LDB, we found a significant interaction between the first and second infusion on both the time the rats spent in the light box (F(1,26)=6.24; P=0.02) and the number of entries into the light box (F(1,26)=6.76; P=0.016) (Figure 1a–c). OXT increased both parameters in vehicle-pretreated rats (P<0.01; Bonferroni's post-hoc test), but not in SKF96365-pretreated rats (P<0.01 for time and P<0.05 for entries). SKF96365 alone did not influence anxiety-like behavior. OXT and SKF, alone and in combination, did not influence the latency to the first entry into the light box (Supplementary Figure S1). The effects of OXT were not paralleled by altered locomotor activity, as measured by the number of line crosses (Figure 1c) and rearings (Supplementary Figure S1) in the dark box.
On the EPM, the anxiolytic effect of intra-PVN OXT was likewise prevented by pre-infusion of SKF96365, as assessed by the percentage of time the rats spent on the open arms (F(1,26)=6.27; P=0.02, Figure 1d) and the percentage of open-arm entries (F(1,26)=7.95; P=0.01, Figure 1e). Latency to first open-arm entry was not modulated by any treatment (Supplementary Figure S1). The inhibitory effect of SKF96365 on OXT-induced anxiolysis was not accompanied by changes in locomotor activity on the EPM, as the number of entries into the closed arms was similar in all groups (Figure 1f).
OXT Increases Ca2+ Concentration and Ca2+ Oscillation Amplitude in Primary Hypothalamic Neurons
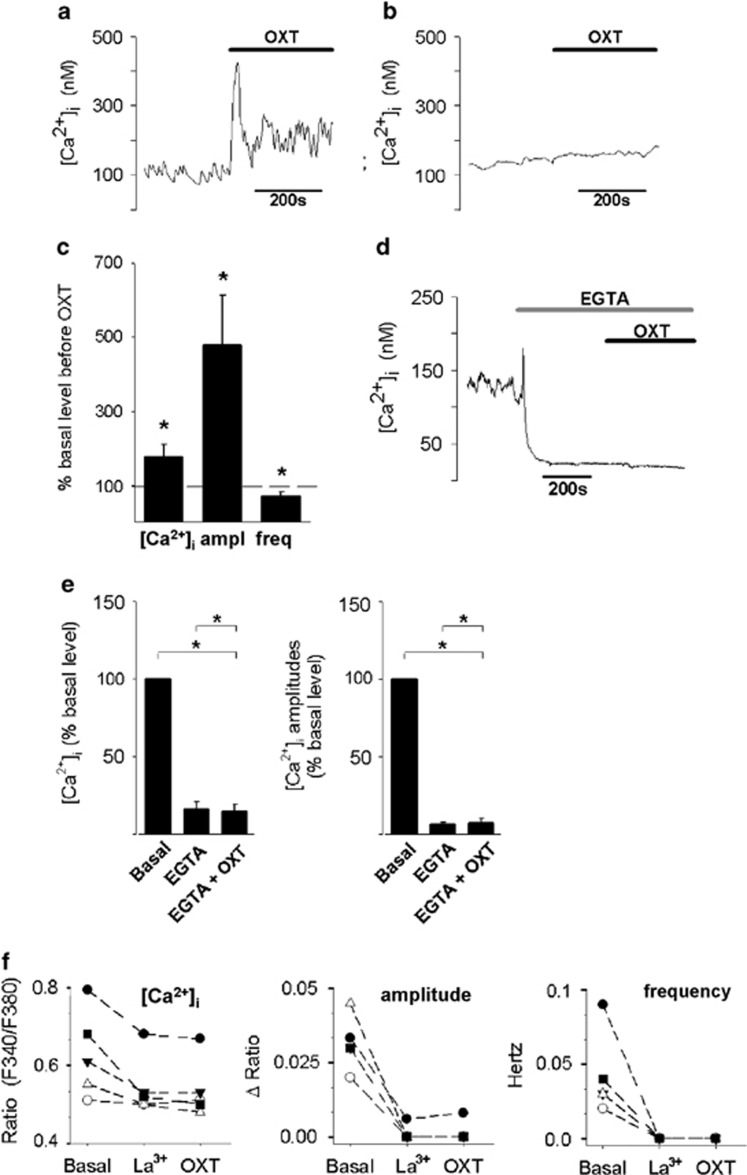
Identification of the Ca2+ channel mediating the OXT-induced Ca2+ influx and subsequent anxiolysis was performed in primary hypothalamic neurons from newborn rats. The cells showed neuron-like morphology, expressed fast inactivating voltage-dependent Na+ channels and outwardly rectifying K+ channels, and the OXTR (Supplementary Figure S2). One subpopulation of neurons showed spontaneous oscillations, which are characteristic for magnocellular neurons described in in-situ preparations (Tasker and Dudek, 1991), whereas another displayed stable basal [Ca2+]i (Figure 2a and b). OXT (100 nM) increased basal [Ca2+]i to 176±34.2% (P≤0.05) as well as oscillation amplitude to 479±133% (P≤0.05), while decreasing oscillation frequency to 70.0±10.7% (P≤0.05) in all of the 11 oscillating cells tested (Figure 2c). Only 10% of non-oscillating cells showed changes in cytosolic Ca2+ in response to OXT application.
Figure 2.
Oxytocin (OXT)-induced Ca2+ signaling in oscillating hypothalamic primary neurons is mediated by the plasma membrane ion channels. OXT effects on intracellular Ca2+ concentration ([Ca2+]i) of an oscillating (a) and a non-oscillating (b) neuron. Stimulation with 100 nM OXT evokes a [Ca2+]i response in the oscillating, but not in the non-oscillating cell. (c) OXT increases basal [Ca2+]i, increases amplitudes (ampl), and decreases frequency (freq) of oscillations. Data are presented as mean+SEM. *P<0.05 compared with control (100%). (d and e) Under extracellular Ca2+-free conditions (EGTA, 25 mM), Ca2+ oscillations disappear and OXT (100 nM) has no further effect on [Ca2+]i. (d) Representative trace. (e) Quantification of [Ca2+]i, oscillation amplitude, and frequency in response to chelating of extracellular Ca2+ and OXT application in eight cells. Data are expressed relative to pre-drug, basal levels (basal, 100%), and presented as mean+SEM. *P<0.05. (f) La3+-sensitive Ca2+channels mediate the Ca2+oscillations and the action of OXT on [Ca2+]i. Extracellular application of La3+ (100 μM) decreases [Ca2+]i, and oscillation amplitude and frequency. There is no further effect of OXT (100 nM) on these parameters. Each line represents a single neuron.
OXT-Induced Intracellular Ca2+ Changes Depend on the Influx of Extracellular Ca2+ Through TRPV2 Channels
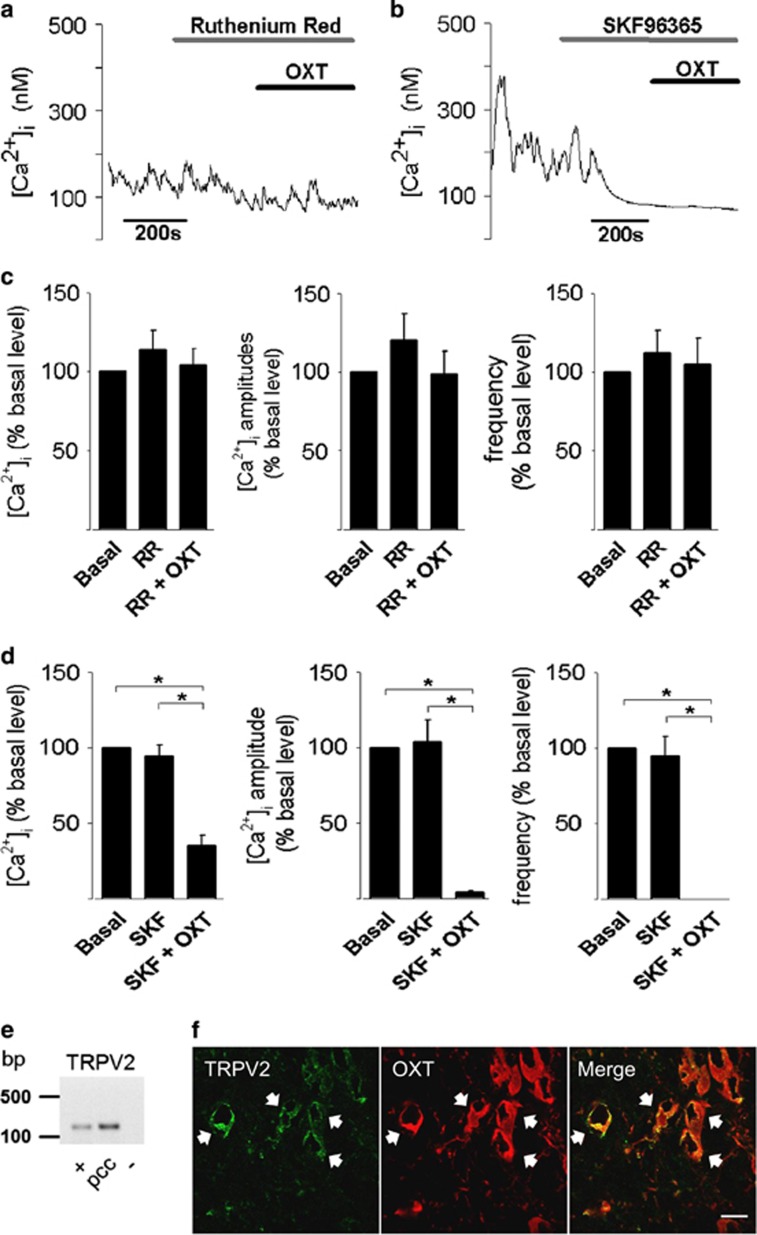
To gain further insight into OXTR-mediated Ca2+ signaling, OXT was applied under extracellular Ca2+-free conditions. Removal of extracellular Ca2+ (Ca2+-free solution with 25 mM EGTA) stopped the Ca2+ oscillations and reduced basal [Ca2+]i by 83.7±4.48% (P<0.01, n=8) (Figures 2d and e). Furthermore, OXT failed to change [Ca2+]i under these conditions (Figures 2d and e). Thus, plasma membrane Ca2+-conducting ion channels contribute to OTX-dependent Ca2+ signaling. In support of this, the broad-range Ca2+ channel blocker lanthanum (La3+; 100 μM) reduced basal [Ca2+]i in all cells and this was accompanied by a reduction of both amplitude and frequency of the cytosolic Ca2+ oscillations (Figure 2f). Application of OXT in the presence of La3+ failed to change either the frequency or the amplitude of the Ca2+ oscillations (Figure 2f). Considering that TRP channels are sensitive to La3+ (Evans, 1983) and are often coupled to GPCR (Cornell et al, 2008), it is likely to be that OXT mediates its effects through TRP channels. Indeed, OXT failed to change both the amplitude and frequency of the Ca2+ oscillations in the presence of the TRP channel blockers ruthenium red (10 μM, blocks TRPC3 and TRPV1-V6; Figure 3a) or SKF96365 (100 μM; blocks TRPC6, TRPC7 and TRPV2; Figure 3c). Furthermore, OXT reduced basal ([Ca2+]i by 65.1±7.20% (P<0.01) and arrested Ca2+ oscillations completely, following application of SKF96365 in seven out of nine cells tested (Figure 3b and d). As TRPV2 is the only TRP channel that is blocked by both SKF96365 and ruthenium red (Boels et al, 2001), we identified the TRPV2 channel as the one that mediates the OXT-dependent influx of extracellular Ca2+. This conclusion is corroborated by the expression of the TRPV2 channel in OXT-positive cells, as revealed by PCR (Figures 3e and Supplementary Figure S3) and immunohistochemical analyses (Figure 3f). TRPV2 immunoreactivity was mostly somatic and punctate, suggesting a vesicular localization (Figure 3f). TRPV2 was further expressed in vasopressin-positive cells, confirming the results by Nedungadi et al (2012).
Figure 3.
Oxytocin (OXT) action on intracellular Ca2+ concentration ([Ca2+]i) is mediated via transient receptor potential vanilloid type-2 channel (TRPV2) channels in primary hypothalamic cells. (a and c) The TRPV channel blocker ruthenium red (10 μM; RR) has no influence on [Ca2+]i, but prevents the effects of 100 nM OXT on [Ca2+]i and oscillation amplitude and frequency (see Figure 2c). (b and d) The TRPC/TRPV2 channel blocker SKF96365 (100 μM) has no effect on [Ca2+]i, but additional application of OXT (100 nM) significantly decreases [Ca2+]i and stops the oscillations. Data represent mean+SEM. *P<0.05 (e) TRPV2 expression in the hypothalamic primary cell culture. +, positive control (hypothalamic tissue from adult rats); pcc, primary cell culture; −, negative control (without reverse transcription). (f) TRPV2 immunoreactivity in OXT-positive cells. Left: TRPV2 staining; middle: OXT staining; right panel: merged. White arrows show double-positive cells. Scale bar: 20 μm.
OXT Recruits TRPV2 Channels in a PI3K-Dependent Manner
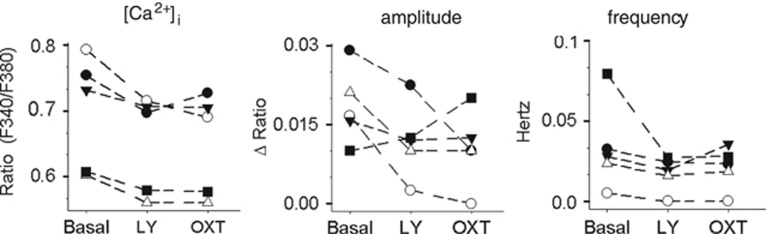
Ruthenium red and SKF96365 had no effect on basal [Ca2+]i and Ca2+ oscillations when applied alone, suggesting that TRPV2 channels are only active following OXTR activation. For a variety of TRP channels, PI3K has a prominent role in GPCR-dependent activation of TRP channels (Bezzerides et al, 2004). To study the contribution of PI3K activation to the OXTR-dependent modulation of Ca2+ oscillations, the PI3K inhibitor LY294002 (30 μM) was extracellularly applied, before OXT. LY294002 itself had minor effects on basal [Ca2+]i and Ca2+ oscillation (Figure 4). Importantly, application of OXT in the presence of LY294002 failed to change the amplitude and frequency of Ca2+ oscillations (Figure 4). These results demonstrate that PI3K is involved in OXT-induced [Ca2+]i changes.
Figure 4.
Oxytocin (OXT) induces Ca2+ signaling in primary hypothalamic cells by activating phosphoinositide 3-kinase (PI3K). Application of the PI3K inhibitor LY294002 (LY; 30 μM) weakly reduced intracellular Ca2+ concentration ([Ca2+]i), oscillation amplitude, as well as oscillation frequency in four of five cells tested. OXT is without effect on any of these parameters in the presence of LY294002. Each line represents a single neuron.
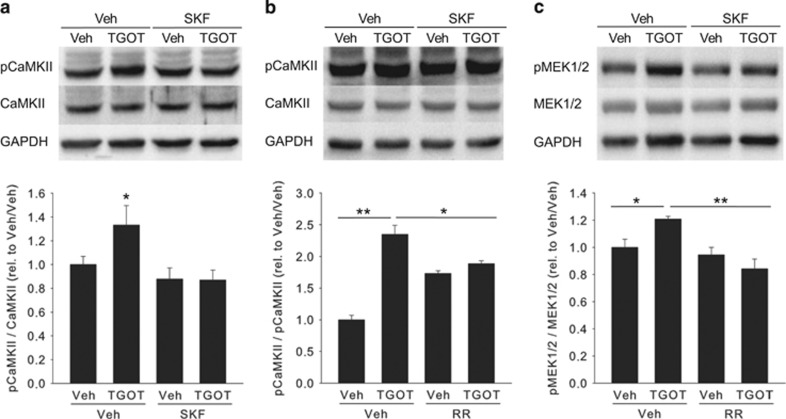
The Specific OXTR Agonist TGOT Stimulates CaMKII and MEK1/2 Phosphorylation Through TRPV2 Channels
To identify downstream effectors of Ca2+ influx through TRPV2 channels, we determined the phosphorylation state of CaMKII, which is strictly Ca2+-dependent, and MEK1/2, known to mediate the anxiolytic effect of OXT in the PVN (Blume et al, 2008). Western blot analysis revealed that stimulation of immortalized rat hypothalamic H32 cells with the specific OXTR agonist TGOT (10 nM, 10 min) increased pCaMKII levels significantly up to more than twofold (Figure 5a and b). This increase was prevented by pre-incubation with either SKF96563 (two-way ANOVA; F(3,21)=3.75; P<0.05) or ruthenium red, with the latter exerting a small but significant effect on CaMKII phosphorylation when applied alone (two-way ANOVA; F(3,15)=21.1; P<0.001; Figures 5a and b). Importantly, TGOT application in the presence of ruthenium red did not increase pCaMKII levels to those observed when TGOT was applied alone (P<0.05; Bonferroni post-hoc test), indicating that TGOT-induced CaMKII phosphorylation depends on TRPV2 channel activity.
Figure 5.
Oxytocin (OXT)-induced Ca2+/calmodulin-dependent kinase II (CaMKII) and mitogen-activated protein kinase kinase (MEK1/2) phosphorylation is at least partially prevented by transient receptor potential (TRP) channel blockers in the hypothalamic cell line H32. (a) CaMKII is phosphorylated by the specific OXT receptor agonist TGOT; this is prevented by prior incubation with SKF96563 (SKF), but not vehicle (Veh). SKF alone is without effect. (b) CaMKII phosphorylation induced by TGOT is partially prevented by ruthenium red (RR); RR alone has a slight but significant stimulatory effect on CaMKII phosphorylation. (c) RR prevents TGOT-induced phosphorylation of MEK1/2. Shown are representative western blottings of fout to six independent experiments; GAPDH is used as total protein loading control. *P<0.05; **P<0.01.
Interestingly, TGOT-induced MEK1/2 phosphorylation in H32 cells was attenuated by pretreatment with ruthenium red (two-way ANOVA; F(3,15)=7.81; P=0.004; Figure 5c), indicating that Ca2+-influx through TRPV2 channels is upstream of MEK1/2 activation, thus an early event in the intracellular signaling pathway underlying the anxiolytic activity of OXT in the PVN.
Discussion
This study describes the influx of extracellular Ca2+ as an important mediator of OXT-induced intracellular signaling in acute anxiolysis in the PVN of male rats. More specifically, OXT-mediated anxiolysis is blocked by SKF96365 by inhibiting Ca2+ influx through TRPV2 channels in OXT-sensitive hypothalamic neurons. This prevents increase of [Ca2+]i and Ca2+ oscillation amplitude induced by OXT, as well as the activation of MEK1/2.
As a basic paradigm to study OXT-dependent effects on anxiety, we used injections of OXT into the PVN. Co-application of SKF96365 prevented the anxiolytic OXT effects. We have previously demonstrated, by using a specific OXTR antagonist in the same tests as used here, that the anxiolytic effect of OXT in the PVN is exclusively mediated by the OXTR, and not the closely related AVP-R1A (Blume et al, 2008). Thus, the TRPV2 channels expressed in the AVP system (Nedungadi et al, 2012 and this study) are not likely to have contributed to OXT-induced anxiolysis. Furthermore, AVP exerts an anxiogenic, not an anxiolytic, activity (Murgatroyd et al, 2004; Landgraf et al, 2007; Bunck et al, 2009), again arguing against the involvement of TRPV2 channels expressed by AVP-producing cells.
A participation of an influx of extracellular Ca2+ into the cytosol to mediate the anxiolytic activity of OXT in the PVN was evidenced by the lack of effect of an OXT infusion following that of SKF96365 in two behavioral tests for anxiety, the LDB and the EPM. There was, however, a tendency to an anxiolytic effect of SKF96365 in the absence of OXT in the EPM, but not LDB. We have, at present, no explanation for this, but the lack of any anxiolysis when both SKF96563 and OXT were infused clearly indicates that SKF96563 prevents the anxiolytic activity of OXT. In two previous papers, we have shown that application of OTX directly into the PVN induces anxiolysis, and that infusions outside of the PVN exert no effect (Blume et al, 2008; Jurek et al, 2012). The anxiolytic effects of OXT are thus restricted to the PVN, implying that SKF96365, applied using the same techniques as described earlier, is locally active within the PVN as well.
SKF96365 blocks some TRP channels as well as T-type channels (Boels et al, 2001; Clapham, 2007). Our observation that SKF96365, when applied alone, did not significantly influence anxiety-like behavior, excludes a role of voltage-dependent T-type Ca2+ channels in the anxiolytic activity of OXT, because T-type channels should be active in the absence of OXT. The Ca2+ channel mediating the OXT effects should therefore be one of the SKF96365-sensitive TRP-channel family members TRPC6, TRPC7, and TRPV2 (Clapham, 2007).
Our observation that La3+, SKF96365, and ruthenium red prevented the effects of OXT on intracellular Ca2+ dynamics in cultured primary hypothalamic cells demonstrates that the OXT-sensitive channel is TRPV2, as this is the only channel that is blocked by all of the three blockers tested (Boels et al, 2001). Experiments with siRNA to inhibit TRPV2 synthesis for further verification appeared to be impossible, as the neurons in our cell cultures form a network as can be seen by synchronous Ca2+ oscillations, but leading to false-positive results in TRPV2 knockdown studies. As SKF96563 and ruthenium red, when applied in the absence of OXT, did not influence basal intracellular Ca2+ and Ca2+ oscillations, one can conclude that T-type channels are not involved, and furthermore that TRPV2 channels do not contribute to the membrane conductance without OXT. This is consistent with the outcome of the behavioral tests, where SKF96365 was without effect in the absence of OXT. Indeed, TRPV2 activity depended on PI3K, which likely promoted the surface expression of the channel on the cell membrane, as has been described before for TRPV2 incorporation in the cell membrane following insulin-like growth factor-1 stimulation (Caterina et al, 1999). The punctate distribution and intracellular localization of TRPV2 that we found in the rat hypothalamus suggests that TRPV2 is stored in vesicles (Everaerts et al, 2010), which fuse with the membrane when OXT is released. This subcellular arrangement supports the scenario of TRPV2 cell surface expression following an OXT surge. Interestingly, the TRPV2-immunopositive cells not only included OXT-positive cells but also vasopressin-positive cells, thus confirming the results by Nedungadi et al (2012) who used the same antibody as we did in our study.
OXT failed to phosphorylate MEK1/2 in H32 hypothalamic cells when ruthenium red was applied in the bath. This is of particular interest, as MEK1/2 phosphorylation is necessary for the anxiolytic activity of OXT (Blume et al, 2008). Thus, TRPV2 activation and MEK1/2 phosphorylation are part of one and the same intracellular pathway that mediates OXT-induced anxiolysis. This anxiolytic pathway likely starts with OXTR-induced G protein β/γ-subunit activation, which permits binding of PI3K to the G protein (Chen et al, 2008). Consequently, PI3K is activated and incorporates TRPV2 channels in the plasma membrane, leading to Ca2+ influx and MEK1/2 phosphorylation. How the latter two events are coupled is not precisely known, but a Ca2+-activated calmodulin-dependent activation of the EGFR is likely to be involved (Blume et al, 2008).
Apart from the stimulatory effects on axon outgrowth in spinal motor neurons and dorsal root ganglia during development (Shibasaki et al, 2010), and calcitonin gene-related peptide release from dorsal root ganglion neurons in culture (Qin et al, 2008), little is known about the physiological functions of TRPV2 in the central nervous system. Here we describe for the first time that TRPV2 channels have a role in emotion regulation and, more specifically, are involved in anxiolytic effects when activated by OXT. Two other TRP channels have been described to be involved in the control of anxiety and fear, namely TRPC5 and TRPV1. In contrast to the anxiolytic effect of TRPV2 in the PVN, however, TRPC5 seems to be anxiogenic in the amygdala, as TRPC5−/− mice exhibit diminished innate fear in tests for general and social anxiety (Riccio et al, 2009). Likewise, TRPV1 knockout mice show decreased anxiety-like behavior in the LDB and EPM, as well as reduced conditioned fear, suggesting that TRPV1 activity promotes anxiogenic behavior (Marsch et al, 2007).
In summary, our in vivo and in vitro studies identified TRPV2 channels in the PVN of rats as mediators of OXT-induced anxiolysis. This implies an until now unrecognized role of extracellular Ca2+ in OXT signaling in the PVN, in addition to the previously described roles of Ca2+ from intracellular stores, especially in female rats during labor and lactation (Israel et al, 2008). In the light of growing interest in intranasally applied OXT as a therapeutic tool for the treatment of various psychopathologies, including anxiety- and stress-related disorders, as well as social dysfunctions, it is essential to understand especially the acute consequences of OXT application at the neuronal level. As both the anxiolytic (amygdala, PVN; (Bale et al, 2001; Blume et al, 2008) and the anxiogenic (lateral septum; (Guzman et al, 2013) effects of OXT application have been reported, a thorough understanding of the molecular, cellular, and behavioral consequences of intranasal OXT application in the brain is a prerequisite for the efficient therapeutic use of synthetic OXT in humans.
Funding and Disclosure
The authors declare no conflict of interest.
Acknowledgments
With great emphasis we thank Marisa Brockmann whose work gave important impulses for the hypothesis leading to this study. We thank Dr J Navarro (University of Regensburg) for assistance with confocal microscopy, and Mr R Maloumby and Mrs A Havasi for excellent technical assistance. This study was supported by DFG grant NE 465/19-1 (EHvdB, IDN). The OXT antibody was generously supplied by Dr H Gainer (NIH, Bethesda, MD, USA).
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM (2001). CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J Neurosci 21: 2546–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Barak Y, Russell JT, Whitnall MH, Ozato K, Gainer H (1985). Neurophysin in the hypothalamo-neurohypophysial system. I. Production and characterization of monoclonal antibodies. J Neurosci 5: 81–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE (2004). Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol 6: 709–720. [DOI] [PubMed] [Google Scholar]
- Blume A, Bosch OJ, Miklos S, Torner L, Wales L, Waldherr M et al (2008). Oxytocin reduces anxiety via ERK1/2 activation: local effect within the rat hypothalamic paraventricular nucleus. Eur J Neurosci 27: 1947–1956. [DOI] [PubMed] [Google Scholar]
- Boels K, Glassmeier G, Herrmann D, Riedel IB, Hampe W, Kojima I et al (2001). The neuropeptide head activator induces activation and translocation of the growth-factor-regulated Ca(2+)-permeable channel GRC. J Cell Sci 114: 3599–3606. [DOI] [PubMed] [Google Scholar]
- Bunck M, Czibere L, Horvath C, Graf C, Frank E, Kessler MS et al (2009). A hypomorphic vasopressin allele prevents anxiety-related behavior. PLoS One 4: e5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D (1999). A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 398: 436–441. [DOI] [PubMed] [Google Scholar]
- Chen S, Lin F, Shin ME, Wang F, Shen L, Hamm HE (2008). RACK1 regulates directional cell migration by acting on G betagamma at the interface with its effectors PLC beta and PI3K gamma. Mol Biol Cell 19: 3909–3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE (2007). SnapShot: mammalian TRP channels. Cell 129: 220. [DOI] [PubMed] [Google Scholar]
- Cornell RA, Aarts M, Bautista D, Garcia-Anoveros J, Kiselyov K, Liman ER (2008). A double TRPtych: six views of transient receptor potential channels in disease and health. J Neurosci 28: 11778–17784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CH (1983). Interesting and useful biochemical properties of lanthanides. Trends Biochem Sci 8: 445–449. [Google Scholar]
- Everaerts W, Vriens J, Owsianik G, Appendino G, Voets T, De Ridder D et al (2010). Functional characterization of transient receptor potential channels in mouse urothelial cells. Am J Physiol Renal Physiol 298: F692–F701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F (2001). The oxytocin receptor system: structure, function, and regulation. Physiol Rev 81: 629–683. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY (1985). A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450. [PubMed] [Google Scholar]
- Guzman YF, Tronson NC, Jovasevic V, Sato K, Guedea AL, Mizukami H et al (2013). Fear-enhancing effects of septal oxytocin receptors. Nat Neurosci 16: 1185–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel J-M, Poulain DA, Oliet SHR (2008). Oxytocin-induced postinhibitory rebound firing facilitates bursting activity in oxytocin neurons. J Neurosci 28: 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D, Liu HX, Hirai H, Torashima T, Nagai T, Lopatina O et al (2007). CD38 is critical for social behaviour by regulating oxytocin secretion. Nature 446: 41–45. [DOI] [PubMed] [Google Scholar]
- Jurek B, Slattery DA, Maloumby R, Hillerer K, Koszinowski S, Neumann ID et al (2012). Differential contribution of hypothalamic MAPK activity to anxiety-like behaviour in virgin and lactating rats. PLoS One 7: e37060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH et al (2012). Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 73: 553–566. [DOI] [PubMed] [Google Scholar]
- Lambert RC, Dayanithi G, Moos FC, Richard P (1994). A rise in the intracellular Ca2+ concentration of isolated rat supraoptic cells in response to oxytocin. J Physiol 478: 275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R, Kessler MS, Bunck M, Murgatroyd C, Spengler D, Zimbelmann M et al (2007). Candidate genes of anxiety-related behavior in HAB/LAB rats and mice: focus on vasopressin and glyoxalase-I. Neurosci Biobehav Rev 31: 89–102. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Sabatier N, Bull PM, Landgraf R, Dayanithi G, Leng G (2002). Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature 418: 85–89. [DOI] [PubMed] [Google Scholar]
- Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID (2008). The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology 36: 2159–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsch R, Foeller E, Rammes G, Bunck M, Kössl M, Holsboer F et al (2007). Reduced anxiety, conditioned fear, and hippocampal long-term potentiation in transient receptor potential vanilloid type 1 receptor-deficient mice. J Neurosci 27: 832–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M (2011). Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci 12: 524–538. [DOI] [PubMed] [Google Scholar]
- Mugele K, Kügler H, Spiess J (1993). Immortalization of a fetal rat brain cell line that expresses corticotropin-releasing factor mRNA. DNA Cell Biol 12: 119–126. [DOI] [PubMed] [Google Scholar]
- Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M et al (2003). TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res 93: 829–838. [DOI] [PubMed] [Google Scholar]
- Murgatroyd C, Wigger A, Frank E, Singewald N, Bunck M, Holsboer F et al (2004). Impaired repression at a vasopressin promoter polymorphism underlies overexpression of vasopressin in a rat model of trait anxiety. J Neurosci 24: 7762–7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedungadi TP, Dutta M, Bathina CS, Caterina MJ, Cunningham JT (2012). Expression and distribution of TRPV2 in rat brain. Exp Neurol 237: 223–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1998) The Rat Brain in Sterotaxic Coordinates, 4th edn. Academic Press: New York. [Google Scholar]
- Qin N, Neeper MP, Liu Y, Hutchinson TL, Lubin ML, Flores CM (2008). TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J Neurosci 28: 6231–6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey IS, Delling M, Clapham DE (2006). An introduction to TRP channels. Annu Rev Physiol 68: 619–647. [DOI] [PubMed] [Google Scholar]
- Riccio A, Li Y, Moon J, Kim K-S, Smith KS, Rudolph U et al (2009). Essential role for TRPC5 in amygdala function and fear-related behavior. Cell 137: 761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier N, Richard P, Dayanithi G (1997). L-, N- and T- but neither P- nor Q-type Ca2+ channels control vasopressin-induced Ca2+ influx in magnocellular vasopressin neurones isolated from the rat supraoptic nucleus. J Physiol 503: 253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki K, Murayama N, Ono K, Ishizaki Y, Tominaga M (2010). TRPV2 enhances axon outgrowth through its activation by membrane stretch in developing sensory and motor neurons. J Neurosci 30: 4601–4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasker JG, Dudek FE (1991). Electrophysiological properties of neurones in the region of the paraventricular nucleus in slices of rat hypothalamus. J Physiol 434: 271–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright A, Rutter AR, Seabrook GR, Reilly K, Oliver KR (2004). Discrete expression of TRPV2 within the hypothalamo-neurohypophysial system: Implications for regulatory activity within the hypothalamic-pituitary-adrenal axis. J Comp Neurol 474: 24–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.