Abstract
The CB1 receptor represents a promising target for the treatment of several disorders including pain-related disease states. However, therapeutic applications of Δ9-tetrahydrocannabinol and other CB1 orthosteric receptor agonists remain limited because of psychoactive side effects. Positive allosteric modulators (PAMs) offer an alternative approach to enhance CB1 receptor function for therapeutic gain with the promise of reduced side effects. Here we describe the development of the novel synthetic CB1 PAM, 6-methyl-3-(2-nitro-1-(thiophen-2-yl)ethyl)-2-phenyl-1H-indole (ZCZ011), which augments the in vitro and in vivo pharmacological actions of the CB1 orthosteric agonists CP55,940 and N-arachidonoylethanolamine (AEA). ZCZ011 potentiated binding of [3H]CP55,940 to the CB1 receptor as well as enhancing AEA-stimulated [35S]GTPγS binding in mouse brain membranes and β-arrestin recruitment and ERK phosphorylation in hCB1 cells. In the whole animal, ZCZ011 is brain penetrant, increased the potency of these orthosteric agonists in mouse behavioral assays indicative of cannabimimetic activity, including antinociception, hypothermia, catalepsy, locomotor activity, and in the drug discrimination paradigm. Administration of ZCZ011 alone was devoid of activity in these assays and did not produce a conditioned place preference or aversion, but elicited CB1 receptor-mediated antinociceptive effects in the chronic constriction nerve injury model of neuropathic pain and carrageenan model of inflammatory pain. These data suggest that ZCZ011 acts as a CB1 PAM and provide the first proof of principle that CB1 PAMs offer a promising strategy to treat neuropathic and inflammatory pain with minimal or no cannabimimetic side effects.
Introduction
Endocannabinoids (N-arachidonoylethanolamine (anandamide, AEA) and 2-arachidoloylglycerol (2-AG)) are released on demand in response to various stimuli, including pain. Through their stimulation of CB1 receptors, they inhibit pain transmission at central, spinal, and peripheral synapses and may serve an auto-protective role (Walker et al, 1999). Although preclinical data indicate that Δ9-tetrahydrocannabinol (THC), the primary psychoactive constituent of Cannabis (Gaoni and Mechoulam, 1964), and other direct CB1 receptor agonists are also effective antinociceptive agents in laboratory animal models of neurodegenerative, neuroinflammatory, and pain-related disease states (Guindon and Hohmann, 2009; Pryce and Baker, 2012; Fagan and Campbell, 2014), their distinct cannabimimetic side-effect profile, which includes abuse, dependence, and memory impairment (Lichtman et al, 1995; Hutcheson et al, 1998; Hampson and Deadwyler, 2000; Justinova et al, 2003; Cooper and Haney, 2009), limits therapeutic use and further development. Direct agonists, including THC, target the orthosteric binding pocket on the CB1 receptor and initiate global activation of the receptor, which is heterogeneously expressed in brain, spinal cord, and periphery. Although endocannabinoids also bind orthosterically (Devane et al, 1992; Mechoulam et al, 1995; Sugiura et al, 1995), they are released on demand where needed and are quickly metabolized (Di Marzo et al, 1999); hence, their actions are more transient and selective with highly specific temporal and spatial regulation. Allosteric modulators may offer a similarly selective approach for alteration of CB1 receptor signaling, presumably with reduced pharmacodynamic-related side effects. Allosteric modulators bind to a distinct, non-orthosteric site on the receptor, and elicit conformational changes that alter ligand potency and/or efficacy (Kenakin, 2004, 2013). Accordingly, it has been hypothesized that CB1-positive allosteric modulators (PAMs) should enhance antinociceptive and other functional effects of endogenously released cannabinoids, but with limited cannabimimetic side effects (Pertwee, 2005; Ross, 2007a).
Initially reported CB1 receptor allosteric modulators were based on a series of Organon compounds, which enhanced orthosteric binding in a ligand-dependent manner, but paradoxically, inhibited signal transduction (Price et al, 2005). These, and other allosteric modulators of the CB1 receptor, have been characterized on the basis of their actions in radioligand-binding assays and other functional in vitro assays of CB1 receptor signal transduction (Horswill et al, 2007; Navarro et al, 2009; Pamplona et al, 2012; Piscitelli et al, 2012; Ahn et al, 2013; Baillie et al, 2013). In vivo, the purported negative allosteric modulator, PSNCBAM-1, reduced food intake (Horswill et al, 2007), an action consistent with CB1 orthosteric antagonism (Di Marzo et al, 2001), although CB1 receptor mediation of this anorectic effect was not ascertained. Another purported CB1-negative allosteric modulator, ORG27569, reduced food intake, but this effect was CB1 receptor independent (Gamage et al, 2014). Moreover, this compound generally failed to modify the pharmacological effects of CB1 orthosteric agonists in common rodent models indicative of CB1 receptor activity (Gamage et al, 2014). Likewise, ORG27569 generally did not perform as a CB1 receptor allosteric modulator in rats (Ding et al, 2014). Although it attenuated both cue- and drug-induced reinstatement of cocaine and methamphetamine-seeking behavior in rats, CB1 receptor involvement was not determined (Jing et al, 2014). The first compelling pharmacological evidence demonstrating the effectiveness of a CB1 receptor allosteric modulator in whole animals came from Pamplona et al (2012). They found that the endogenous anti-inflammatory mediator, lipoxin A4, enhanced the pharmacological effects of AEA at the CB1 receptor both in vitro and in vivo, as well as protected against β-amyloid (1-40)-induced performance deficits in the Morris water maze in mice (Pamplona et al, 2012).
In the present study, we examined a novel small-molecule CB1 PAM, ZCZ011 (Figure 1a), in in vitro and in vivo assays to evaluate whether it behaves as a CB1 PAM. In vitro, ZCZ011 increased the CB1 receptor agonist receptor binding and potentiated AEA-stimulated signaling in [35S]GTPγS binding, β-arrestin recruitment, and ERK phosphorylation assays. As there remains a tremendous need for new medications to treat chronic pain conditions (Nightingale, 2012), we tested whether this compound would reduce nociceptive behavior in the chronic constriction injury (CCI) model of neuropathic pain, as well as in the carrageenan model of inflammatory pain. Each of these assays is highly sensitive to the antinociceptive effects of orthosteric CB1 agonists and inhibitors of endocannabinoid catabolic enzymes (Lichtman et al, 2004; Russo et al, 2007; Kinsey et al, 2009; Ghosh et al, 2013). In addition, we examined ZCZ011 by itself or in combination with orthosteric CB1 receptor agonists in a range of common assays sensitive to cannabimimetic activity, including the tetrad tests (locomotor activity, antinociception, catalepsy, and hypothermia; (Little et al, 1988)) and drug discrimination (Jarbe et al, 1981). Finally, we tested whether systemically administered ZCZ011 was brain penetrant and whether it altered endocannabinoid levels in the brain. Here, we demonstrate the first evidence of a CB1 PAM that exhibits antinociceptive effects in neuropathic and inflammatory pain models with no associated cannabimimetic effects.
Figure 1.
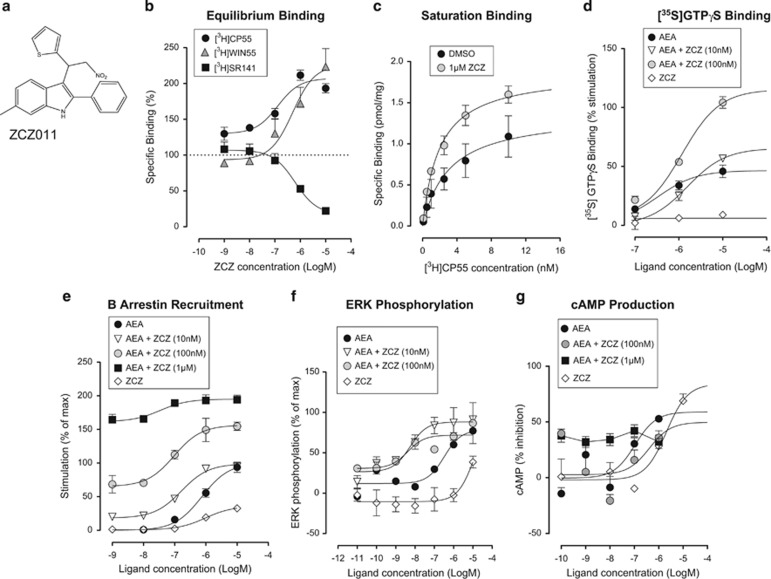
The CB1-positive allosteric modulator ZCZ011 enhances CB1 receptor binding and signaling. (a) Chemical structure of ZCZ011. (b) ZCZ011 significantly increased [3H]CP55,940 binding in mouse brain membranes. (c) ZCZ011 caused a significant increase in the Bmax for [3H]CP55,940 and [3H]WIN55212 while having no effect on the Kd. ZCZ011 caused an apparent displacement of [3H]SR141716A. (d) ZCZ011 caused a significant increase in the efficacy of AEA-stimulated [35S]GTPγS binding in mouse brain membranes. (e) ZCZ011 caused a significant increase in AEA-stimulated β-arrestin recruitment in hCB1 cells. (f) ZCZ011 caused a significant increase in the potency of AEA to stimulate ERK 1/2 phosphorylation in hCB1-expressing cells. (g) Effect of ZCZ011 on forskolin-stimulated cAMP production in hCB1-expressing cells; ZCZ011 alone inhibits forskolin-stimulated cAMP production. Symbols represent mean values±SEM from 2 to 7 independent experiments.
Materials and Methods
Animals
Male C57BL/6 J mice (Jackson Laboratory, Bar Harbor, ME) and male FAAH (−/−) mice, backcrossed onto a C57BL/6 J background for at least 13 generations served as subjects. FAAH (−/−) mice were employed in all experiments examining the in vivo actions of exogenously administered AEA to prevent its rapid hydrolysis to arachidonic acid, which is known to produce CB1 receptor-independent effects (Wiley et al., 2006). Although constitutively elevated levels of AEA and other lipids in FAAH (−/−) mice might complicate interpretation, it should be noted that these mice display normal CB1 receptor expression and function (Cravatt et al., 2001; Lichtman et al., 2002; Falenski et al., 2010). All animal protocols were approved by the respective Institutional Animal Care and Use Committees at Virginia Commonwealth University and West Virginia University and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Research Council, 2011). Other details are included in the Supplementary Information.
Materials
ZCZ011 (6-Methyl-3-(2-nitro-1-(thiophen-2-yl)ethyl)-2-phenyl-1H-indole) was synthesized at the University of Aberdeen (see synthesis below). AEA was provided by Organix Inc. (Woburn, MA). The pan CB1/CB2 receptor agonist CP55,940, the CB1 receptor antagonist rimonabant (N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide-HCl; SR141716A), and the CB2 receptor antagonist SR144528 (5-(4-chloro-3-methylphenyl)-1-((4-methylphenyl)methyl)–N-((1,2S,4R)-1,3,3-trimethylbicyclo [2.2.1] hept-2-yl]-1H-pyrazole-3-carboxamide) were obtained from the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD). Drugs were dissolved in a vehicle consisting of a mixture of ethanol, alkamuls-620 (Sanofi-Aventis, Bridgewater, NJ), and saline (0.9% NaCl) in a ratio of 1:1:18. Each drug was given via the intraperitoneal (i.p.) route of administration with exception of the discrimination studies, in which drugs were injected via subcutaneous (s.c.) route of administration. All drugs were administered at a volume of 10 μl/g body mass.
Synthesis of ZCZ011
See Supplementary Information for complete details.
Mouse Brain Membrane Preparation
Whole brains from adult male MF1 mice were suspended in centrifugation buffer (320 mM sucrose, 2 mM EDTA, 5 mM MgCl2) and the tissues were homogenized with an Ultra-Turrex homogenizer (see Supplementary Information). Tissue homogenates were centrifuged at 1600 g for 10 min and the resulting supernatant collected. This pellet was resuspended in centrifugation buffer centrifuged as before and the supernatant collected. Supernatants were combined before undergoing further centrifugation at 28 000 g for 20 min. The supernatant was discarded and the pellet resuspended in buffer A (50 mM Tris, 2 mM EDTA, 5 mM MgCl2 at pH 7.0) and incubated at 37 °C for 10 min. Following the incubation, the suspension was centrifuged for 20 min at 23 000 g. After resuspending the pellet in buffer A, the suspension was incubated for 40 min at room temperature before a final centrifugation for 15 min at 11 000 g. The final pellet was resuspended in buffer B (50 mM Tris, 1 mM EDTA, 3 mM MgCl2) and the final protein concentration, determined by Bio-Rad Dc kit, was 1 mg/ml. All centrifugation procedures were carried out at 4 °C. Prepared brain membranes were stored at −80 °C and defrosted on the day of the experiment.
CHO-hCB1R Cells
CHO cells stably transfected with cDNA encoding human cannabinoid CB1 receptors (see Baillie et al., 2013) were maintained in Dulbecco's modified Eagles's medium (DMEM) nutrient mixture F-12 HAM, supplemented with 2 mM L-glutamine, 10% fetal bovine serum, 0.6% penicillin–streptomycin, hygromycin B (300 μg/ml) and geneticin (600 μg/ml). All cells were maintained at 37 °C and 5% CO2 in their respective media and were passaged twice a week using non-enzymatic cell dissociation solution. The CHO-hCB1R-transfected cell line was used for cAMP and pERK1/2.
Equilibrium Binding Assays
Equilibrium binding assays were carried out using [3H]CP55,940, [3H]WIN55212, and [3H]SR141716A concentrations of 0.7, 1.2 and 1 nM, respectively. BSA (1 mg/ml) and 50 mM Tris buffer was used in a total assay volume of 500 μl containing 0.01% DMSO. Binding was initiated by adding 30 μg of mouse brain membranes, as previously described (Baillie et al, 2013). Assays were incubated at 37 °C for 60 min, and then the reaction was stopped by the addition of ice-cold wash buffer that contained 50 mM Tris buffer and 1 mg/ml BSA and vacuum filtration using a 24-well sampling manifold Brandel cell harvester (Gaithersburg, MD). Specific binding is defined as the difference between the binding that occurred in the presence and absence of 1 μM unlabeled ligand and varied between 70 and 90% of the total binding.
[35S]GTPγS-Binding Assay
Mouse brain membranes (5 μg protein) were preincubated for 30 min at 30 °C with adenosine deaminase (0.5 U/ml). The membranes were then incubated with the agonist±modulator or vehicle for 60 min at 30 °C in assay buffer (50 mM Tris; 5 mM MgCl2; 1 mM EDTA; 100 mM NaCl; 1 mM DTT; 0.1% BSA) in the presence of 0.1 nM [35S]GTPγS and 30 μM GDP. Binding was initiated by the addition of [35S]GTPγS. Nonspecific binding was measured in the presence of 30 μM GTPγS. The reaction was terminated by rapid vacuum filtration (50 mM Tris-HCl; 50 mM Tris-Base; 0.1% BSA) using a 24-well sampling manifold (cell harvester; Brandel, Gaitherburg, MD) and GF/B filters (Whatman, Maidstone, UK).
Data analysis
Raw data were presented as cpm. Basal level was defined as zero. Results were calculated as a percentage change from basal level of [35S]GTPγS binding (in the presence of vehicle). Data were analyzed by nonlinear regression analysis of sigmoidal dose response curves using GraphPad Prism 5.0 (GraphPad, San Diego, CA). The results of this analysis are presented as Emax with 95% confidence limits (CLs) and pEC50 (logEC50)±SEM.
PathHunter CB1 β-Arrestin Assays
PathHunter hCB1 β-arrestin cells were plated 48 h before use and incubated at 37 °C, 5% CO2. Compounds were dissolved in DMSO and diluted in OCC media. Five microliters of allosteric modulator or vehicle solution were added to each well and incubated for 60 min. Five microliters of agonist were added to each well followed by a 90-min incubation. Fifty-five microliters of detection reagent are then added followed by further 90 min incubation at room temperature. Chemiluminescence, indicated as relative light unit, was measured on a standard luminescence plate reader.
Data analysis
Raw data were relative light units. Basal level was defined as zero. Results were calculated as the percentage of CP55940 maximum effect. Data were analyzed by nonlinear regression analysis of sigmoidal dose–response curves using GraphPad Prism 5.0. The results of this analysis are presented as Emax with 95% CLs and pEC50 (logEC50)±SEM.
AlphaScreen SureFire ERK 1/2 phosphorylation assay
ERK1/2 MAP-kinase phosphorylation assay. For experimental studies of ERK1/2 MAP-kinase phosphorylation, hCB1R cells (40 000 cells/well) were plated onto 96-well plates and serum-starved for 24 h. Cells were then washed with DMEM before the addition of agonist±Org 27569 or vehicle at the desired concentration. After a 6-min incubation at 37 °C in a humidified atmosphere, ice-cold lysis buffer (provided with the AlphaScreen SureFire kit) was added to each well and the plate was placed at −80 °C for at least 1 h.
AlphaScreen SureFire ERK assay
The assay was performed in 384-well white Proxiplates according to the manufacturer's instructions. Briefly, 4 μl samples were incubated with 7 μl of mixture containing=1 part donor beads:1 part acceptor beads:10 parts activation buffer:60 parts reaction buffer. Plates were incubated for 3 h at 25 °C in the dark and read with the Envision system (PerkinElmer) using AlphaScreen settings.
Data analysis
Raw data were presented as ‘Envision units'. Basal level was defined as zero. Results were presented as means and variability as SEM or 95% CLs of the percent stimulation of phosphorylated ERK1/2 above the basal level (in the presence of vehicle). Data were analyzed by nonlinear analysis of log agonist vs response curves using GraphPad Prism 5.0. The results of this analysis were presented as Emax with 95% CLs and pEC50 (logEC50)±SEM.
DiscoverX cAMP Assays
For experimental studies of inhibition of cAMP formation, hCB1R cells (20 000 cells/well) were plated into 96-well plates and serum starved for 24 h. Cells were then washed with serum- and phenol-free DMEM before the addition of agonist with vehicle/allosteric modulator in the presence of 10 μM rolipram and 10 μM forskolin. Cells were stimulated for 30 min at 37 °C in a humidified atmosphere. The DiscoverX cAMP kit was then used and a standard curve was included in every assay. Antibody solution was added to each well followed by working solution: 1 part ED solution and 1 part combination of lysis (19), Emerald solution (5) and Gal (1). Plates were incubated at room temperature for 60 min. A final addition of EA reagent to each well was followed by incubation at room temperature for no less than 3 but no more than 18 h and plates were read using a luminescence plate reader.
Data analysis
Results were calculated as the percentage inhibition of forskolin-stimulated cAMP production. Data were analyzed by nonlinear regression analysis of sigmoidal dose–response curves using GraphPad Prism 5.0. The results of this analysis were presented as Emax with 95% CLs and pEC50 (logEC50)±SEM.
CCI Model of Neuropathic Pain
CCI nerve injury was induced according to the surgical procedures described previously (Kinsey et al, 2009), as detailed in Supplementary Methods. ZCZ011 (0, 10, 20, 40 mg/kg) was injected via the i.p. route of administration and mice were tested for mechanical and cold allodynia 75 min later. ZCZ011 was administered in a counterbalanced Latin square within subject design with at least a 5-day wash out period between tests. To assess the effects of repeated treatment of ZCZ011 (40 mg/kg, i.p.) on mechanical and cold allodynia induced by CCI, mice were divided into the following three experimental groups: (1) vehicle control (6 days of vehicle injections); (2) acute ZCZ011 (5 days of vehicle injections and injected with 40 mg/kg ZCZ011 on day 6); and (3) repeated ZCZ011 (6 days of injections of 40 mg/kg ZCZ011). In the repeated ZCZ011 group, mechanical and cold allodynia were assessed 1, 2, 4, 12, and 24 h following the first ZCZ011 injection to determine duration of acute anti-allodynic effects of ZCZ011. The subjects in all groups were tested 1 h following ZCZ011 administration on day 6. In experiments assessing cannabinoid receptor mechanism of action, rimonabant (3 mg/kg) or SR144528 (3 mg/kg) was administered 10 min before ZCZ011 or vehicle. Mechanical allodynia was assessed using von Frey filaments and the acetone flinching test was used to assess cold allodynia, as described previously (Kinsey et al, 2009) and detailed in Supplementary Information.
Carrageenan Model of Inflammatory Pain
Edema was induced via intraplantar injection of 0.3% carrageenan (Sigma, St Louis, MO) in a 20 μl volume using a 30-G needle into the hind left paw. Paw thickness was measured with an electronic digital micrometer (Traceable Calipers, Friendswood, TX) before and 5 h following carrageenan administration, which corresponds to peak edema (Wise et al, 2008). Paw edema data are expressed as the difference in paw thickness between the 5 h and pre-injection measures. Mechanical allodynia was assessed using von Frey filaments at the same peak time point (see Supplementary Information).
Tetrad Assay
The behavioral testing was conducted in the following order: bar test (catalepsy), tail withdrawal test, and rectal temperature. A separate group of mice was used to assess the effects of ZCZ011 on locomotor activity. Testing was performed according to the previously described procedures (Long et al, 2009b; Schlosburg et al, 2010). For a full description, see Supplementary Information.
Drug Discrimination
Male C57BL/6J and FAAH (−/−) mice (20–25 g) trained to discriminate CP55,940 (0.1 mg/kg) or AEA (6 mg/kg) from vehicle, respectively, were tested in a nose-poke operant task according to the procedures described previously (Long et al, 2009b) with minor modifications. For complete description of these procedures, see Supplementary Information.
ZCZ011 Place Conditioning
An unbiased mouse CPP paradigm was utilized, as previously described (Kota et al, 2008), in which vehicle, ZCZ011 (40 mg/kg), or cocaine (10 mg/kg; positive control) was randomly paired with one of two distinct chambers. On the test day, mice did not receive an injection and were allowed to roam freely for 15 min while the percentage of time spent in both chambers was scored as the dependent measure. For further details, see Supplementary Information.
Extraction and Quantification of Endocannabinoids by Liquid Chromatography-Tandem Mass Spectrometry
C57BL/6J mice were administered ZCZ011 (40 mg/kg) acutely and killed 45 min later (see Supplementary Information). Brains were harvested and the concentrations of 2-AG, AEA, palmitoylethanolamide, oleoylethanolamide, and arachidonic acid levels were quantified, as previously described (Ignatowska-Jankowska et al, 2014) and detailed in Supplementary Information.
Data Analyses
All in vivo data are presented as mean±standard error (SEM) or 95% CLs. In vitro data were analyzed using log agonist vs response curves in GraphPad Prism 5.0. The results of this analysis were presented as Emax with 95% CLs and pEC50 (logEC50)±SEM. In vivo data were analyzed using one-way or two-way analysis of variance (ANOVA). Dunnett's test was used for post hoc analysis in the dose–response experiments, and the Tukey test was used for post hoc analyses comparing different treatment groups. Multiple comparisons following two-way ANOVA were conducted with Bonferroni post hoc comparisons. Differences were considered significant at the level of P<0.05. Statistical analysis was performed with GraphPad Prism version 5.00.
Results
ZCZ011 Enhanced CB1 Receptor Agonist Binding
In equilibrium binding experiments, ZCZ011 (Figure 1a) produced a significant and concentration-dependent increase in the specific binding of the CB1 receptor orthosteric agonist [3H]CP55,940 to mouse brain membranes with an Emax of 207% (95% CLs, 191–223) and a pEC50 of 6.90±0.23 (Figure 1b). ZCZ011 also produced a significant and concentration-dependent increase in the specific binding of the CB1 receptor orthosteric agonist [3H]WIN55212 to mouse brain membranes with an Emax of 225% (95% CLs, 182–269) and a pEC50 of 6.31±0.33 (Figure 1b). In contrast, ZCZ011 produced a significant and concentration-dependent decrease in the specific binding of the CB1 receptor orthosteric inverse agonist [3H]SR141716A to mouse brain membranes with an Emax of 17% (95% CLs, −2.7 to 37) and a pEC50 of 6.21±0.21 (Figure 1b).
In saturation binding experiments, [3H]CP55,940 bound in a saturable manner to the CB1 receptor with a Bmax of 1.37 pmol/mg (95% CLs, 1.042–1.704) and Kd of 3.10±0.9 nM (Figure 1c). ZCZ011 (1 μM) significantly increased the Bmax value of [3H]CP55,940 to 1.88 pmol/mg (95% CLs, 1.728–2.025) without significantly affecting binding affinity (Kd=1.97±0.2 nM; Figure 1c), suggesting an increase in the number of available binding sites for [3H]CP55,940.
ZCZ011 Enhanced CB1 Receptor Agonist Signalling
[35S]GTPγS binding
AEA stimulated [35S]GTPγS binding in mouse brain membranes with a pEC50 value of 6.5±0.2 and Emax (efficacy) of 46.5% (95% CLs, 40–54; Figure 1d). Addition of 100 nM ZCZ011, significantly enhanced AEA-stimulated [35S]GTPγS binding (Emax of 115.2% (95% CLs, 104–127)), but there was no significant change in the pEC50 value (potency). A concentration of 10 nM ZCZ011 enhanced AEA-stimulated [35S]GTPγS binding with an Emax of 65.4% (95% CLs, 51–80); however, this effect was not statistically significant. ZCZ011 alone caused no stimulation of [35S]GTPγS binding.
Similarly, CP55,940 stimulated [35S]GTPγS binding in mouse brain membranes with a pEC50 value of 8.34±0.25 and Emax of 61% (95% CLs, 50–72; Supplementary Figure 1a). Addition of 1 μM ZCZ011, significantly enhanced AEA-stimulated [35S]GTPγS binding (Emax of 100% (95% CLs, 82–112)), but there was no significant change in the pEC50 value (8.80±0.26).
PathHunter hCB1 β-Arrestin Recruitment Assay
In the PathHunter β-arrestin assay in hCB1 cells, AEA stimulated β-arrestin recruitment with a pEC50 of 6.1±0.10 and an Emax of 100% (95% CLs, 87–113; Figure 1e). ZCZ011 caused a concentration-dependent enhancement of AEA-stimulated β-arrestin recruitment at 10 nM, 100 nM, and 1 μM with Emax values of 99% (95% CLs, 90–107) and 157% (95% CLs, 138–175) and 195% (95% CLs, 185–205), respectively. There was no significant change to the pEC50 value of 6.78±0.11 and 7.02±0.12 in the presence of 10 nM and 100 nM ZCZ011, respectively. At 1 nM AEA, the maximum stimulation observed was 0.0% (95% CLs, −6.6 to 6.6); however, the addition of 10 nM, 100 nM and 1 μM ZCZ011 significantly increased stimulation to 18.1% (95% CLs, 10.8–25.4), 65.1% (95% CLs, 47–83), and 161.6% (95% CLs, 148–175), respectively. These findings indicate positive cooperativity between the endogenous cannabinoid and ZCZ011. When tested alone, ZCZ011 produced an increase in β-arrestin recruitment that was 35.9% (95% CLs, 33–39) of maximal stimulation.
AlphaScreen Surefire ERK 1/2 Phosphorylation Assay
Using an AlphaScreen surefire ERK 1/2 phosphorylation assay kit, we measured the effect of ZCZ011 on activation of ERK 1/2 phosphorylation by CB1 agonist, AEA in hCB1R cells (Figure 1f). AEA induced ERK 1/2 phosphorylation with an Emax of 98.2% (95% CLs, 79–118) and pEC50 of 6.5±0.3. Neither 10 nM nor 100 nM ZCZ011 significantly affect AEA Emax (efficacy), but pEC50 value (potency) was significantly increased to 8.3±0.3 and 8.4±0.5, respectively (one-way ANOVA, Dunnett's multiple comparison test, P<0.05). With the exception of the highest concentration (1 μM), ZCZ011 alone did not induce ERK 1/2 phosphorylation.
CP55,940 induced ERK 1/2 phosphorylation with an Emax of 101% (95% CLs, 86–116) and pEC50 of 7.85±0.25 (Supplementary Figure 1b). 1 μM ZCZ011 did not affect the Emax (efficacy) of CP55,940 (Emax of 119% (95% CLs, 107–132)) but pEC50 value (potency) was significantly increased to 8.95±0.3 (one-way ANOVA, Dunnett's multiple comparison test, P<0.05).
DiscoverX hCB1 cAMP Production Assay
Using a DiscoverX cAMP production assay, we measured the effect of ZCZ011 on activation of ERK 1/2 phosphorylation by CB1 agonist, AEA in hCB1 cells (Figure 1g). AEA inhibited forskolin-stimulated cAMP production with an Emax of 59% (95% CLs, 35–83) and pEC50 of 6.97±0.35. Alone, ZCZ011 acted as an agonist; inhibiting forskolin-stimulated cAMP production with an Emax of 84% (95% CLs, 46–122) and pEC50 of 5.68±0.33. 100 nM ZCZ011 did not significantly affect AEA. There was evidence positive cooperativity at a concentration of 1 μM ZCZ011.
CP55,940 inhibited forskolin-stimulated cAMP production with an Emax of 83% (95% CLs, 75–92) and pEC50 of 8.27±0.13. 100 nM ZCZ011 did not significantly affect AEA. 1 μM ZCZ011 did not significantly affect the Emax or pEC50 of CP55,940 (Supplementary Figure 1c).
ZCZ011 Does not Produce Psychoactive Effects in Mice
ZCZ011 (40 mg/kg) was detected in whole brain, 6.5±0.6 (mean±SEM) ng/wet (g), as determined by HPLC/MS/MS (Poklis et al, 2015). Given alone, 40 mg/kg ZCZ011 did not produce catalepsy (0 s immobility), hypothermia (P=0.4; Supplementary Figure 2a), antinociception in tail withdrawal (P=0.9; Supplementary Figure 2b) or hot-plate tests (P=0.8; Supplementary Figure 2c), or locomotor depression (P=0.9; Supplementary Figure 2d). ZCZ011 did not substitute for either CP55,940 (Supplementary Figure 2e) or AEA (Supplementary Figure 3a) in the drug discrimination assay and did not affect respective response rates for either training drug (P=0.9; Supplementary Figure 2f and P=0.9, P=0.1; Supplementary Figure 3b). Also, ZCZ011 (40 mg/kg) did not elicit a conditioned place preference or aversion compared with vehicle (P=0.2; Supplementary Figure 4).
ZCZ011 Potentiates the Pharmacological Effects of AEA and CP55,940 in Mice
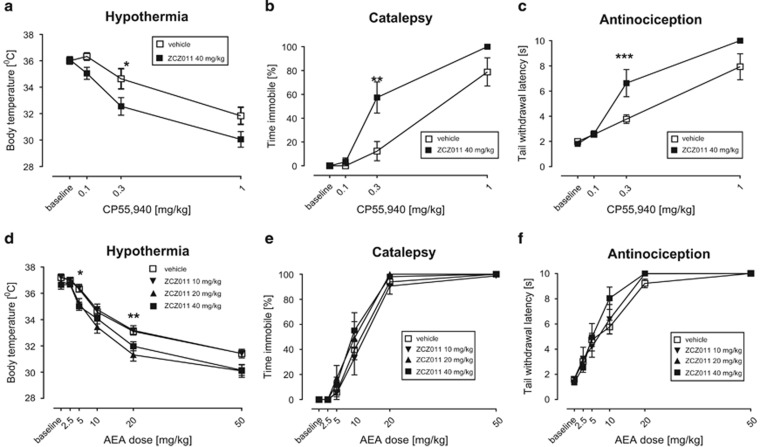
In contrast to its ineffectiveness to elicit cannabimimetic effects when administered alone, ZCZ011 significantly augmented the antinociceptive (F(1,14)=8.0, P<0.01), cataleptic (F(2,28)=3.84, P<0.05), and hypothermic (F(1,14)=5.5; P<0.05) effects of CP55,940 (Figure 2a–c). It also enhanced AEA-induced hypothermia (F(4,64)=2.93; P<0.05; Figure 2d), but did not affect the antinociceptive (P=0.8; Figure 2e) or cataleptic (P=1; Figure 2f) effects of AEA in FAAH (−/−) mice. Also, this compound did not alter the antinociceptive effects of 1 mg/kg nicotine (Supplementary Figure 5).
Figure 2.
ZCZ011 (40 mg/kg) potentiated the pharmacological effects of orthosteric CB1 receptor agonists CP55,940 and AEA. ZCZ011 significantly enhanced CP55,940-induced hypothermia (a), catalepsy (b), and antinociception (c) in C57BL/6J mice and AEA-induced hypothermia (d), but not AEA-induced catalepsy (e) or antinociception (f), in FAAH (−/−) mice. Data presented as mean±SEM; n=8–9 mice per group; *P<0.05, **P<0.01, ***P<0.001 vs vehicle.
In the drug discrimination assay, ZCZ011 (40 mg/kg) significantly increased the potency of the discriminative stimulus effects of AEA in FAAH (−/−) mice (F(4,48)=10.47, P<0.001; Supplementary Figure 3a). The respective ED50 (95% CI) values of AEA in the vehicle-pretreated mice and ZCZ011-pretreated mice were 4.0 (2.7–5.9) mg/kg and 1.4 (1.2–1.7) mg/kg. ZCZ011 increased AEA potency 2.2-fold compared with the vehicle-pretreated mice. ZCZ011 (20 mg/kg) also significantly enhanced the discriminative cue of AEA. Although ZCZ011 (40 mg/kg) given alone or in combination with AEA did not affect response rates of FAAH (−/−) mice in the drug discrimination paradigm (Supplementary Figure 3b), it potentiated the depressive effects of AEA (5.6 mg/kg) on operant responding for food in a separate group of mice (F(1,4)=6.88, P<0.05; Supplementary Figure 6).
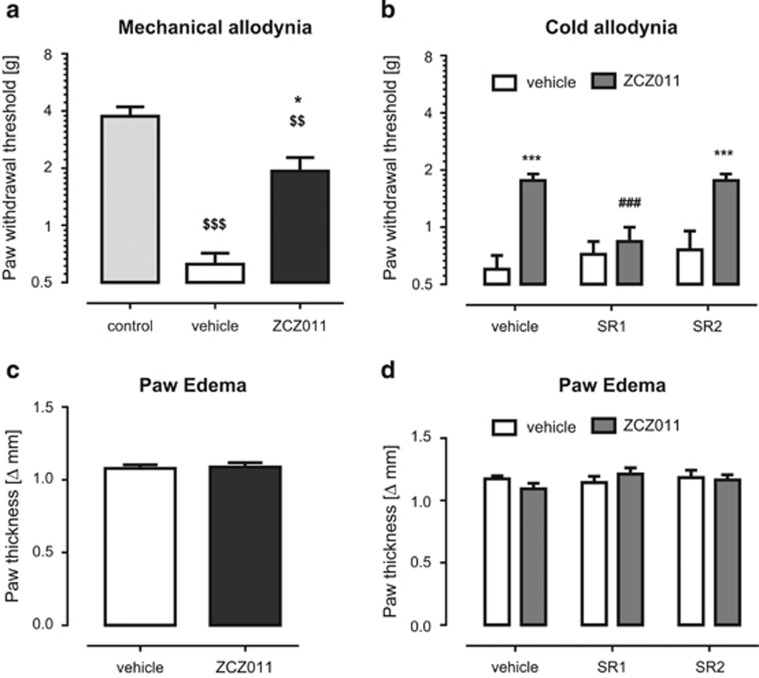
ZCZ011 Reverses Nociceptive Behavior in Neuropathic and Inflammatory Pain Models
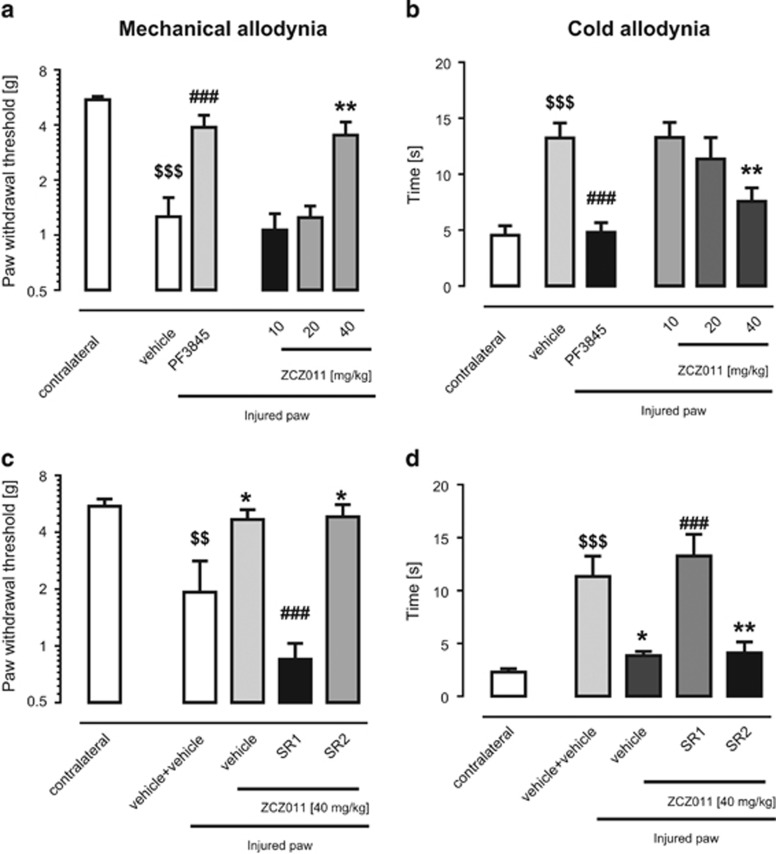
ZCZ011 enhanced the pharmacological effects of AEA and CP55,940 in in vitro and in behavioral assays, but did not produce common cannabimimetic effects on its own. Accordingly, we next investigated whether it would reverse nociceptive behavior in well-established models of neuropathic and inflammatory pain. As has been previously shown (Kinsey et al, 2010), the FAAH inhibitor, PF-3845, reversed mechanical (Figure 3a) and cold allodynia (Figure 3b) in the CCI model of neuropathic pain. These findings are consistent with the idea that AEA, which is rapidly hydrolyzed by FAAH, has an autoprotective role in this model. Similarly, ZCZ011 completely reversed mechanical (F(3,42)=7.6, P<0.001; Figure 3a) and cold allodynia (F(3,42)=3.6, P<0.05; Figure 3b) in the CCI model of neuropathic pain. Unlike endocannabinoid catabolic enzyme inhibitors; however, ZCZ011 (40 mg/kg) did not affect whole brain levels of 2-AG (P=0.3), AEA (P=0.3), palmitoylethanolamide (P=0.3), or oleoylethanolamide (P=1) in C57BL/6J mice (Supplementary Figure 7).
Figure 3.
ZCZ011 significantly reduced mechanical (a) and cold (b) allodynia induced by chronic constriction nerve injury (CCI). The anti-allodynic effects of ZCZ011 (40 mg/kg, i.p.) were blocked by the CB1 receptor antagonist rimonabant (SR141716A, SR1; 3 mg/kg; c), but not by the CB2 receptor antagonist SR144528 (SR2; 3 mg/kg; d). The FAAH inhibitor, PF-3845 (10 mg/kg, i.p.) was included for comparison. Results presented as mean±SEM (n=9–12 mice per group); *P<0.05, **P<0.01, ###P<0.001 vs vehicle; $$P<0.01, $$$P<0.001 vs contralateral paw.
These anti-allodynic actions of ZCZ011 were prevented by the CB1 receptor antagonist, rimonabant (3 mg/kg), but not by the CB2 receptor antagonist, SR144528 (3 mg/kg; F(3,28)=8.9, P<0.001; Figure 3c and d), indicating a CB1 receptor-mediated mechanism of action.
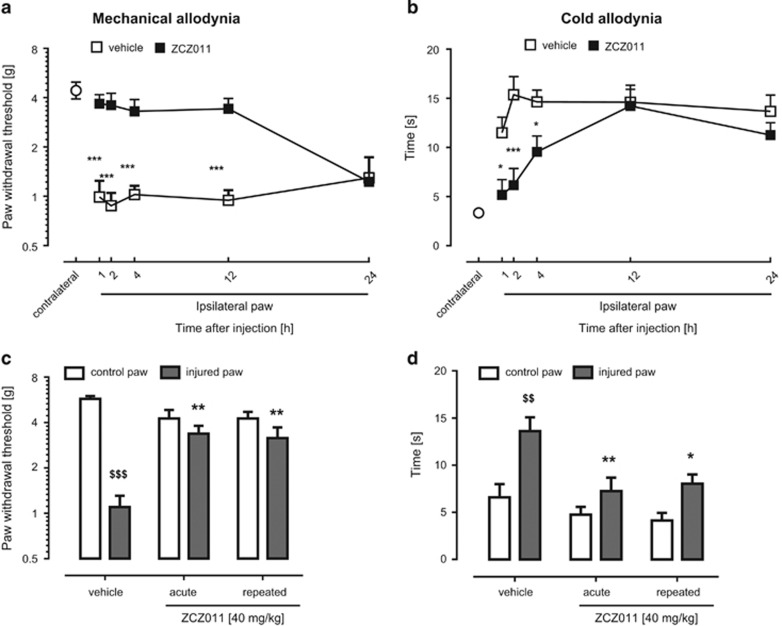
ZCZ011 (40 mg/kg) blocked mechanical allodynia (F(4,56)=6.0, P<0.001) and cold allodynia (F(4,56)=4.44, P<0.01; Figure 4a and b) for durations of 12 and 4 h, respectively. The anti-allodynic effects of ZCZ011 (40 mg/kg) to mechanical (F(2,21)=8.9, P<0.01; Figure 4c) and cold (F(2,21)=7.0, P<0.01; Figure 4d) stimuli were retained following 6 days of daily injections and did not differ from the antinociceptive effects produced by acute ZCZ011. Thus, the antinociceptive effects of ZCZ011 were resistant to tolerance.
Figure 4.
The anti-allodynic effects of ZCZ011 (40 mg/kg) are of long duration and do not undergo tolerance after 6 days of daily injections in the CCI model of neuropathic pain. ZCZ011 significantly reversed mechanical allodynia for up to 12 h (a) and cold allodynia for up to 4 h (b). The anti-allodynic effects of ZCZ011 are retained following 6 days of repeated administration in response to tactile (c) and cold (d) stimulation. Values represent mean±SEM, n=8 mice per group, *P<0.05, **P<0.01, ***P<0.001 vs vehicle, $$P<0.01, $$$P<0.001 vs contralateral paw.
Likewise, ZCZ011 (40 mg/kg) partially reversed carrageenan-induced mechanical allodynia (F(2,21)=22.3, P<0.001; Figure 5a), which required CB1 receptors, but not CB2 receptors (F(1,16)=14.7, P<0.01; Figure 5b). Consistent with its lack of CB2 receptor action, ZCZ011 did not reduce carrageenan-induced paw edema (Figure 5c and d; P=0.8).
Figure 5.
ZCZ011 partially reversed mechanical allodynia in the carrageenan model of inflammatory pain. The CB1 receptor antagonist rimonabant (SR141716A, SR1; 3 mg/kg; (a), but not the CB2 receptor antagonist SR144528 (SR2; 3 mg/kg; b) blocked the anti-allodynic effects of ZCZ011. Results presented as mean±SEM (n=9–12 mice per group). ZCZ011 (40 mg/kg, i.p.) did not attenuate carrageenan-induced paw edema (c and d). Values represent mean±SEM, n=8 mice per group.*P<0.05, ***P<0.001, ###P<0.001 vs vehicle; $$P<0.01, $$$P<0.001 vs contralateral paw.
Discussion
Here we report the development and pharmacological characterization of the novel synthetic CB1 receptor PAM, ZCZ011. This compound increased equilibrium binding of the potent CB1 receptor orthosteric agonist, CP55,940. In addition, ZCZ011 enhanced the efficacy of AEA in stimulating [35S]GTPγS binding in whole brain as well as β-arrestin recruitment and the potency of AEA in ERK phosphorylation assays in hCB1 cells. In mice, ZCZ011 potentiated CP55,940-induced catalepsy, hypothermia, and antinociception. It also increased the potency of AEA in several in vivo assays employing FAAH (−/−) mice, including the discriminative stimulus effects of AEA, AEA-induced depression of operant responding for food, and AEA-induced hypothermia. Most strikingly, when administered alone, ZCZ011 completely reversed allodynia in the CCI model of neuropathic pain and partially reversed carrageenan-induced allodynia, but did not elicit any apparent cannabimimetic side effects. Its actions in the CCI model required CB1 receptors, were of long duration (ie, up to 12 h), and did not undergo tolerance after 6 days of treatment. Accordingly, we hypothesize that ZCZ011 blocked neuropathic pain, without eliciting general cannabimimetic activity, by augmenting the actions of endocannabinoids at CB1 receptors in pathways mediating nociceptive responses following sciatic nerve injury. Likewise, ZCZ011 reduced carrageenan-induced allodynia through a CB1 receptor mechanism of action, and did not reduce the edematous effects of carrageenan. Thus, this study provides compelling parallel in vitro and in vivo evidence that ZCZ011 acts as a CB1 receptor PAM. Accordingly, ZCZ011 represents a valuable pharmacological tool for mechanistic studies as well as for exploring potential therapeutic applications of CB1 receptor allosteric target(s).
Our in vivo observations showing that ZCZ011 potentiated the pharmacological effects of either CP55,940 or AEA in behavioral assays confirm in vitro observations showing that ZCZ011 acts as a CB1 PAM and enhances the signalling of the bound agonist. ZCZ011 (1 μM) caused an increase in the Bmax of [3H]CP55,940 and [3H]WIN55212, which implies an increase in the number of available receptors for CP55,940 to bind. Intriguingly, ZCZ011 elicited an apparent displacement of the CB1 receptor inverse agonist, SR141716A. With the exception of cAMP activity, ZCZ011 increased CB1 orthosteric agonist potency and/or efficacy in all the functional assays performed. Specifically, ZCZ011 potentiated AEA and CP55,940 signaling efficacy in the [35S]GTPγS binding in mouse brain membranes and increased the potency of AEA and CP55,940 in the ERK phosphorylation assay in hCB1 cells, and enhanced the potency of AEA-mediated β-arrestin recruitment. This evidence strongly suggests that ZCZ011 acts as a CB1 PAM both in vitro and in vivo. In the cAMP assay, ZCZ011 acted as an agonist alone, but did not affect the potency or efficacy of AEA or CP55,940 at a concentration of 1 μM.
A highly novel finding in the present study was that ZCZ011 produced anti-allodynic effects in CCI model of neuropathic pain. Previous studies have also demonstrated that the anti-allodynic effects of FAAH inhibitors (Kinsey et al, 2009, 2010), as well as MAGL inhibitors JZL184 and KML29 (Kinsey et al, 2009, 2010; Ignatowska-Jankowska et al, 2014) elicited anti-allodynic effects in the CCI assay. Repeated administration of high-dose ZCZ011 retained its antinociceptive effects, which is similar to the finding that the antinociceptive effects produced by FAAH inhibition also do not undergo tolerance (Schlosburg et al, 2010). Although repeated high doses of MAGL inhibitors leads to antinociceptive tolerance associated with CB1 receptor downregulation and desensitization (Schlosburg et al, 2010; Ignatowska-Jankowska et al, 2014), CB1 receptor function is retained following repeated low doses of the MAGL inhibitor JZL184 (Sciolino et al, 2011; Kinsey et al, 2013). The most notable difference between ZCZ011 and endocannabinoid catabolic enzyme inhibitors is that ZCZ011 did not alter the concentration of endocannabinoids or other N-acylethanoloamines in the brain (Supplementary Figure 7). In contrast, FAAH and MAGL inhibitors produce increased brain levels of AEA (Kathuria et al, 2003) and 2-AG (Long et al, 2009a), respectively. The anti-allodynic effects of ZCZ011 in the CCI assay were CB1, but not CB2, receptor dependent. In contrast, FAAH inhibitors require both CB1 and CB2 receptors to reverse CCI-induced allodynia (Kinsey et al, 2009, 2010). In addition, FAAH (Holt et al, 2005) and MAGL (Ghosh et al, 2013) inhibitors produce anti-edematous actions in the carrageenan assay, which in each case was completely blocked by a CB2 receptor antagonist and not a CB1 receptor antagonist. Thus, a CB1 PAM would not be expected to reduce carrageenan-induced paw edema, as the case in the present study. Collectively, these findings are consistent with the idea that the anti-allodynic effects of ZCZ011 in the CCI and carrageenan assays are mediated through its actions as a CB1 PAM by enhancing the activity of endocannabinoids at CB1 receptors.
An important observation from a drug development perspective is that ZCZ011 did not produce cannabimimetic side effects (ie, catalepsy, hypothermia, thermal antinociception, or hypomotility) and did not substitute for AEA or CP55,940 in the drug discrimination paradigm. However, ZCZ011 enhanced many pharmacological effects produced by these orthosteric agonists, consistent with in vitro data showing that it acts as a CB1 receptor PAM. Although it is interesting that ZCZ011 augmented more of the measured actions produced by CP55,940 than those elicited by AEA, it is known that allosteric modulators affect orthosteric agonists in a ligand-dependent manner. The failure of ZCZ011 to affect nicotine-induced antinociception in the tail withdrawal and hot-plate tests (Supplementary Figure 5) shows that its effects are selective to cannabinergic ligands.
Pamplona et al. (2012) presented the first in vivo and in vitro evidence demonstrating that the endogenous anti-inflammatory mediator, lipoxin A4, acts as a CB1 PAM. This naturally occurring lipid enhanced both CB1 receptor binding of AEA and AEA-induced cAMP inhibition. Moreover, when given via the i.c.v. route of administration, lipoxin A4 produced cannabimimetic effects (ie, catalepsy, hypothermia, hypomotility, and antinociceptive effects in the hot-plate test). Notably, systemic administration of an inhibitor of 5-lipoxygenase, the primary biosynthetic enzyme of lipoxin A4, attenuated the cataleptic effects of i.c.v administered AEA, suggesting that this endogenous lipid contributes to the behavioral actions of CB1 orthosteric agonists. In addition, i.c.v. administration of lipoxin A4 protected mice from impaired spatial memory performance in the Morris water maze task elicited by i.c.v. injection of β-amyloid (1-40) protein. This protective effect was blocked by rimonabant, indicating a CB1 receptor-mediated mechanism of action. The data presented here with ZCZ011, together with those previously published with lipoxin A4 (Pamplona et al, 2012), provide compelling proof of principle that CB1 PAMs offer promise as therapeutic strategies for neurodegenerative diseases and pain states related to nerve injury. The identification of the putative binding site(s) of ZCZ011 and lipoxin A4 at the CB1 receptor will be a crucial step for future development of allosteric modulators of the CB1 receptor as well as better understanding of the physiological function of CB1 allosteric modulation for the development of novel pharmacotherapies based on this mechanism. Nonetheless, the present study demonstrates that the synthetic CB1 PAM, ZCZ011, produces CB1-mediated anti-allodynic effects in murine models of neuropathic and inflammatory pain without the development of tolerance or the occurrence of cannabimimetic side effects.
Funding and Disclosure
RAR, IRG, and MZ are inventors on patent applications filed by the Universities of Toronto and Aberdeen, which disclose pharmaceutical agents targeting molecular pathways described in the present article. RAR, IRG and MZ have an equity share in Signal Pharma Ltd, a University spin-out company developing CB1-positive allosteric modulators. Research was supported by NIH grants DA-009789, DA-026449, DA-003672 and CIHR Proof of Principal Phase 1 grant 288645. The remaining authors declare no competing financial interests.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Ahn KH, Mahmoud MM, Samala S, Lu D, Kendall DA (2013). Profiling two indole-2-carboxamides for allosteric modulation of the CB1 receptor. J Neurochem 124: 584–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillie GL, Horswill JG, Anavi-Goffer S, Reggio PH, Bolognini D, Abood ME et al (2013). CB(1) receptor allosteric modulators display both agonist and signaling pathway specificity. Mol Pharmacol 83: 322–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Haney M (2009). Actions of delta-9-tetrahydrocannabinol in cannabis: relation to use, abuse, dependence. Int Rev Psychiatry 21: 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR et al (2001). Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA 98: 9371–9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G et al (1992). Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258: 1946–1949. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L, Bisogno T, Meick D (1999). Metaboloism of anadamide and 2-arachidonoylglycerol: an historical overview and some recent developments. Lipids 34: S319–S325. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z et al (2001). Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 410: 822–825. [DOI] [PubMed] [Google Scholar]
- Ding Y, Qiu Y, Jing L, Thorn DA, Zhang Y, Li JX (2014). Behavioral effects of the cannabinoid CB1 receptor allosteric modulator ORG27569 in rats. Pharmacol Res Perspect 2: e00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falenski KW1, Thorpe AJ, Schlosburg JE, Cravatt BF, Abdullah RA, Smith TH et al (2010). FAAH-/- mice display differential tolerance, dependence, and cannabinoid receptor adaptation after delta 9-tetrahydrocannabinol and anandamide administration. Neuropsychopharmacology 35: 1775–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan SG, Campbell VA (2014). The influence of cannabinoids on generic traits of neurodegeneration. Br J Pharmacol 171: 1347–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamage TF, Ignatowska-Jankowska BM, Wiley JL, Abdelrahman M, Trembleau L, Greig IR et al (2014). In-vivo pharmacological evaluation of the CB1-receptor allosteric modulator Org-27569. Behav Pharmacol 25: 182–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R (1964). Isolation, structure, and partial synthesis of an active constituent of hashish. J Amer Chem Soc 86: 1646–1647. [Google Scholar]
- Ghosh S, Wise LE, Chen Y, Gujjar R, Mahadevan A, Cravatt BF et al (2013). The monoacylglycerol lipase inhibitor JZL184 suppresses inflammatory pain in the mouse carrageenan model. Life Sci 92: 498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon J, Hohmann AG (2009). The endocannabinoid system and pain. CNS Neurol Disorders Drug Targets 8: 403–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA (2000). Cannabinoids reveal the necessity of hippocampal neural encoding for short-term memory in rats. J Neurosci 20: 8932–8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S, Comelli F, Costa B, Fowler CJ (2005). Inhibitors of fatty acid amide hydrolase reduce carrageenan-induced hind paw inflammation in pentobarbital-treated mice: comparison with indomethacin and possible involvement of cannabinoid receptors. Br J Pharmacol 146: 467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horswill JG, Bali U, Shaaban S, Keily JF, Jeevaratnam P, Babbs AJ et al (2007). PSNCBAM-1, a novel allosteric antagonist at cannabinoid CB1 receptors with hypophagic effects in rats. Br J Pharmacol 152: 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson DM, Tzavara ET, Smadja C, Valjent E, Roques BP, Hanoune J et al (1998). Behavioural and biochemical evidence for signs of abstinence in mice chronically treated with delta-9-tetrahydrocannabinol. Br J Pharmacol 125: 1567–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatowska-Jankowska BM, Ghosh S, Crowe MS, Kinsey SG, Niphakis MJ, Abdullah RA et al (2014). In vivo characterization of the highly selective monoacylglycerol lipase inhibitor KML29: antinociceptive activity without cannabimimetic side effects. Br J Pharmacol 171: 1392–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarbe TU, Swedberg MD, Mechoulam R (1981). A repeated test procedure to assess onset and duration of the cue properties of (−) delta 9-THC, (−) delta 8-THC-DMH and (+) delta 8-THC. Psychopharmacology 75: 152–157. [DOI] [PubMed] [Google Scholar]
- Jing L, Qiu Y, Zhang Y, Li JX (2014). Effects of the cannabinoid CB1 receptor allosteric modulator ORG 27569 on reinstatement of cocaine- and methamphetamine-seeking behavior in rats. Drug Alcohol Depend. 143: 251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Redhi GH, Goldberg SR (2003). Self-administration of delta9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology 169: 135–140. [DOI] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A et al (2003). Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med 9: 76–81. [DOI] [PubMed] [Google Scholar]
- Kenakin T (2004). Principles: receptor theory in pharmacology. Trends Pharmacol Sci 25: 186–192. [DOI] [PubMed] [Google Scholar]
- Kenakin T (2013). Analytical pharmacology and allosterism: the importance of quantifying drug parameters in drug discovery. Drug Discov Today Technol 10: e229–e235. [DOI] [PubMed] [Google Scholar]
- Kinsey SG, Long JZ, Cravatt BF, Lichtman AH (2010). Fatty acid amide hydrolase and monoacylglycerol lipase inhibitors produce anti-allodynic effects in mice through distinct cannabinoid receptor mechanisms. J Pain 11: 1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Long JZ, O'Neal ST, Abdullah RA, Poklis JL, Boger DL et al (2009). Blockade of endocannabinoid-degrading enzymes attenuates neuropathic pain. J Pharmacol Exp Ther 330: 902–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Wise LE, Ramesh D, Abdullah R, Selley DE, Cravatt BF et al (2013). Repeated low-dose administration of the monoacylglycerol lipase inhibitor JZL184 retains cannabinoid receptor type 1-mediated antinociceptive and gastroprotective effects. J Pharmacol Exp Ther 345: 492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota D, Martin BR, Damaj MI (2008). Age-dependent differences in nicotine reward and withdrawal in female mice. Psychopharmacology 198: 201–210. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Dimen KR, Martin BR (1995). Systemic or intrahippocampal cannabinoid administration impairs spatial memory in rats. Psychopharmacology 119: 282–290. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Hawkins EG, Griffin G, Cravatt BF (2002). Pharmacological activity of fatty acid amides is regulated, but not mediated, by fatty acid amide hydrolase in vivo. J Pharmacol Exp Ther 302: 73–79. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Leung D, Shelton C, Saghatelian A, Hardouin C, Boger D et al (2004). Reversible inhibitors of fatty acid amide hydrolase that promote analgesia: evidence for an unprecedented combination of potency and selectivity. J Pharmacol Exp Ther 311: 441–448. [DOI] [PubMed] [Google Scholar]
- Little PJ, Compton DR, Johnson MR, Melvin LS, Martin BR (1988). Pharmacology and stereoselectivity of structurally novel cannabinoids in mice. J Pharmacol Exp Ther 247: 1046–1051. [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE et al (2009. a). Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol 5: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Nomura DK, Vann RE, Walentiny DM, Booker L, Jin X et al (2009. b). Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc Natl Acad Sci USA 106: 20270–20275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR et al (1995). Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 50: 83–90. [DOI] [PubMed] [Google Scholar]
- National Research Council (2011). Institutional Animal Care and Use Committees at Virginia Commonwealth University and West Virginia University and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals..
- Navarro HA, Howard JL, Pollard GT, Carroll FI (2009). Positive allosteric modulation of the human cannabinoid (CB) receptor by RTI-371, a selective inhibitor of the dopamine transporter. Br J Pharmacol 156: 1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nightingale S (2012). The neuropathic pain market. Nat Rev Drug Discov 11: 101–102. [DOI] [PubMed] [Google Scholar]
- Pamplona FA, Ferreira J, Menezes de Lima O Jr, Duarte FS, Bento AF, Forner S et al (2012). Anti-inflammatory lipoxin A4 is an endogenous allosteric enhancer of CB1 cannabinoid receptor. Proc Natl Acad Sci USA 109: 21134–21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG (2005). The therapeutic potential of drugs that target cannabinoid receptors or modulate the tissue levels or actions of endocannabinoids. AAPS J 7: E625–E654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piscitelli F, Ligresti A, La Regina G, Coluccia A, Morera L, Allara M et al (2012). Indole-2-carboxamides as allosteric modulators of the cannabinoid CB(1) receptor. J Med Chem 55: 5627–5631. [DOI] [PubMed] [Google Scholar]
- Poklis J, Clay D, Ignatowska-Jankowska B, Zanato C, Ross R, Greig I et al (2015). HPLC/MS/MS Determination of ZCZ-011, a Novel Pharmacological Tool for Investigation the Cannabinoid Receptor in Mouse Brain Using Clean Screen FAStTM Column Extraction. J Chromatography B 39: 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MR, Baillie GL, Thomas A, Stevenson LA, Easson M, Goodwin R et al (2005). Allosteric modulation of the cannabinoid CB1 receptor. Mol Pharmacol 68: 1484–1495. [DOI] [PubMed] [Google Scholar]
- Pryce G, Baker D (2012). Potential control of multiple sclerosis by cannabis and the endocannabinoid system. CNS Neurol Disord Drug Targets 11: 624–641. [DOI] [PubMed] [Google Scholar]
- Ross RA (2007. a). Allosterism and cannabinoid CB(1) receptors: the shape of things to come. Trends Pharmacol Sci 28: 567–572. [DOI] [PubMed] [Google Scholar]
- Ross RA (2007. b). Tuning the endocannabinoid system: allosteric modulators of the CB1 receptor. Br J Pharmacol 152: 565–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo R, Loverme J, La Rana G, Compton TR, Parrott J, Duranti A et al (2007). The fatty acid amide hydrolase inhibitor URB597 (cyclohexylcarbamic acid 3'-carbamoylbiphenyl-3-yl ester) reduces neuropathic pain after oral administration in mice. J Pharmacol Exp Ther 322: 236–242. [DOI] [PubMed] [Google Scholar]
- Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG et al (2010). Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci 13: 1113–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciolino NR, Zhou W, Hohmann AG (2011). Enhancement of endocannabinoid signaling with JZL184, an inhibitor of the 2-arachidonoylglycerol hydrolyzing enzyme monoacylglycerol lipase, produces anxiolytic effects under conditions of high environmental aversiveness in rats. Pharmacol Res 64: 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K et al (1995). 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun 215: 89–97. [DOI] [PubMed] [Google Scholar]
- Walker JM, Huang SM, Strangman NM, Tsou K, Sanudo-Pena MC (1999). Pain modulation by release of the endogenous cannabinoid anandamide. Proc Natl Acad Sci USA 96: 12198–12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Razdan RK, Martin BR (2006). Evaluation of the role of the arachidonic acid cascade in anandamide's in vivo effects in mice. Life Sci 80: 24–35. [DOI] [PubMed] [Google Scholar]
- Wise LE, Cannavacciulo R, Cravatt BF, Martin BF, Lichtman AH (2008). Evaluation of fatty acid amides in the carrageenan-induced paw edema model. Neuropharmacology 54: 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.