Abstract
Distinct environmental and conditioned stimuli influencing ethanol-associated appetitive and consummatory behaviors may jointly contribute to alcohol addiction. To develop an effective translational animal model that illuminates this interaction, daily seeking responses, maintained by alcohol-associated conditioned stimuli (CSs), need to be dissociated from alcohol drinking behavior. For this, we established a procedure whereby alcohol seeking maintained by alcohol-associated CSs is followed by a period during which rats have the opportunity to drink alcohol. This cue-controlled alcohol-seeking procedure was used to compare the effects of naltrexone and GSK1521498, a novel selective μ-opioid receptor antagonist, on both voluntary alcohol-intake and alcohol-seeking behaviors. Rederived alcohol-preferring, alcohol-nonpreferring, and high-alcohol-drinking replicate 1 line of rats (Indiana University) first received 18 sessions of 24 h home cage access to 10% alcohol and water under a 2-bottle choice procedure. They were trained subsequently to respond instrumentally for access to 15% alcohol under a second-order schedule of reinforcement, in which a prolonged period of alcohol-seeking behavior was maintained by contingent presentations of an alcohol-associated CS acting as a conditioned reinforcer. This seeking period was terminated by 20 min of free alcohol drinking access that achieved significant blood alcohol concentrations. The influence of pretreatment with either naltrexone (0.1−1−3 mg/kg) or GSK1521498 (0.1–1–3 mg/kg) before instrumental sessions was measured on both seeking and drinking behaviors, as well as on drinking in the 2-bottle choice procedure. Naltrexone and GSK1521498 dose-dependently reduced both cue-controlled alcohol seeking and alcohol intake in the instrumental context as well as alcohol intake in the choice procedure. However, GSK1521498 showed significantly greater effectiveness than naltrexone, supporting its potential use for promoting abstinence and preventing relapse in alcohol addiction.
Introduction
Alcohol is the most commonly used addictive substance worldwide, and alcohol use disorders place a major socioeconomic and public health burden on modern societies. Some 17.6 million people are estimated to abuse alcohol in United States (National Council on Alcoholism and Drug Dependence), whereas 1.6 million people are addicted to alcohol in the United Kingdom, where alcohol consumption has increased by 9% over the past 3 decades (Alcohol Concern, UK (http://www.alcoholconcern.org.uk/)). Despite a major need, there are however few effective pharmacological treatments for alcohol addiction, especially those that promote abstinence and prevent relapse.
Preclinical research on alcohol addiction and associated treatment development strategies have focused almost exclusively on the factors determining the propensity to maintain high levels of alcohol intake, reflecting the importance of intoxication and neuroadaptive changes during protracted withdrawal as the driving force to addiction (Koob, 2013). The commonly used strains of laboratory rats do not readily drink high quantities of alcohol unless sweet taste (eg, sucrose) fading techniques are used to induce it (see, eg, Samson, 1986; Czachowski and Samson, 2002), or animals are exposed intermittently to alcohol for a prolonged period of time under two- or three-bottle choice procedures (Wise, 1973; Simms et al, 2008; Carnicella et al, 2014), or are first made dependent by exposing them for long periods to alcohol in vapor chambers to induce drinking in withdrawal (Koob, 2013).
A complementary approach is to study rats that have, through selective breeding strategies, a spontaneous propensity to drink and prefer alcohol. Thus, alcohol-preferring (P) and high-alcohol-drinking (HAD) lines have been established on the basis of their preference for a 10% alcohol solution over water and their consumption of >5 g of alcohol/kg body weight/day. Both P and HAD lines have been shown to drink ethanol under free-choice conditions (McBride et al, 2014), to be highly motivated for ethanol compared with alcohol-nonpreferring (NP) rats, and to readily acquire instrumental responding for ethanol (Czachowski and Samson, 2002).
However, although alcohol drinking to intoxication is the major behavioral characteristic of those addicted to alcohol, such individuals also crave alcoholic beverages and spend time actively seeking alcohol, as well as drinking compulsively. Effective treatments for alcoholism may therefore not only be those reducing volumes drunk, such as the recently approved nalmefene (Gual et al, 2014), but may also be those that can reduce the craving and alcohol seeking that leads to drinking.
Alcohol-associated conditioned stimuli are known to elicit craving in alcohol-dependent individuals and cue-reactivity procedures have been used to identify relapse prevention treatments (Niaura et al, 1988). The invigorating impact of drug-associated conditioned stimuli (CSs) on seeking behavior has been operationalized in models of CS-induced relapse (Ciccocioppo et al, 2002; Gipson et al, 2013; Lee et al, 2006; Marchant et al, 2013) as well as cue-controlled cocaine- or heroin-seeking behavior (Arroyo et al, 1998; Economidou et al, 2011; Giuliano et al, 2013) and CS-dependent seeking of high incentive foods that is associated with obesity and binge eating (Giuliano et al, 2012). Although outbred rat strains will respond instrumentally for alcohol (Augier et al, 2014), and alcohol-associated CSs can elicit approach (Tomie and Sharma, 2014) and serve as conditioned reinforcers (Smith et al, 1977; Panlilio et al, 2004; Milton et al, 2012; Rodd et al, 2004), attempts to establish vigorous CS-dependent alcohol seeking that mediates delays to the opportunity to drink have proven difficult, probably because alcohol is an apparently weak reinforcer for rats with little or no propensity to drink.
Here we established a procedure in which P, and HAD rats seek alcohol for prolonged periods of time during which the response-contingent presentation of an alcohol-associated CS would bridge delays before animals eventually earned the opportunity freely to drink alcohol for 20 min (to ‘drink at the bar').
Having established this novel CS-controlled alcohol-seeking and drinking procedure, we then investigated the effects of a novel, putative treatment for alcohol dependence, GSK1521498. We further compared its effects with those of a clinically approved treatment for alcoholism, naltrexone, that has previously been shown to reduce ethanol drinking (Davidson and Amit, 1997; Froehlich et al, 1990; Henderson-Redmond and Czachowski, 2014) and cue-induced reinstatement (Ciccocioppo et al, 2002) in animal models, as well as to decrease cue-induced craving in human alcohol abusers (Rohsenow et al, 2000). Both compounds target the μ-opioid receptors (MORs), but GSK1521498 is a more selective MOR antagonist than naltrexone, and unlike naltrexone, has no partial agonist activity (Nathan et al, 2012; Kelly et al, 2014) and instead has, in some in vitro assays, minor inverse agonist activity (Ignar et al, 2011; Kelly et al, 2014). The effects of both treatments on CS-controlled alcohol-seeking behavior, alcohol intake during the free-access period earned by rats through making seeking responses, as well as in a home environment two-bottle choice procedure, were investigated.
Materials and Methods
Animals
Male rederived rP (~30 days old, n=63), rNP (n=20), and rHAD1 rats (n=20) were obtained from Indiana University Medical Center (Indiana). See Supplementary Information.
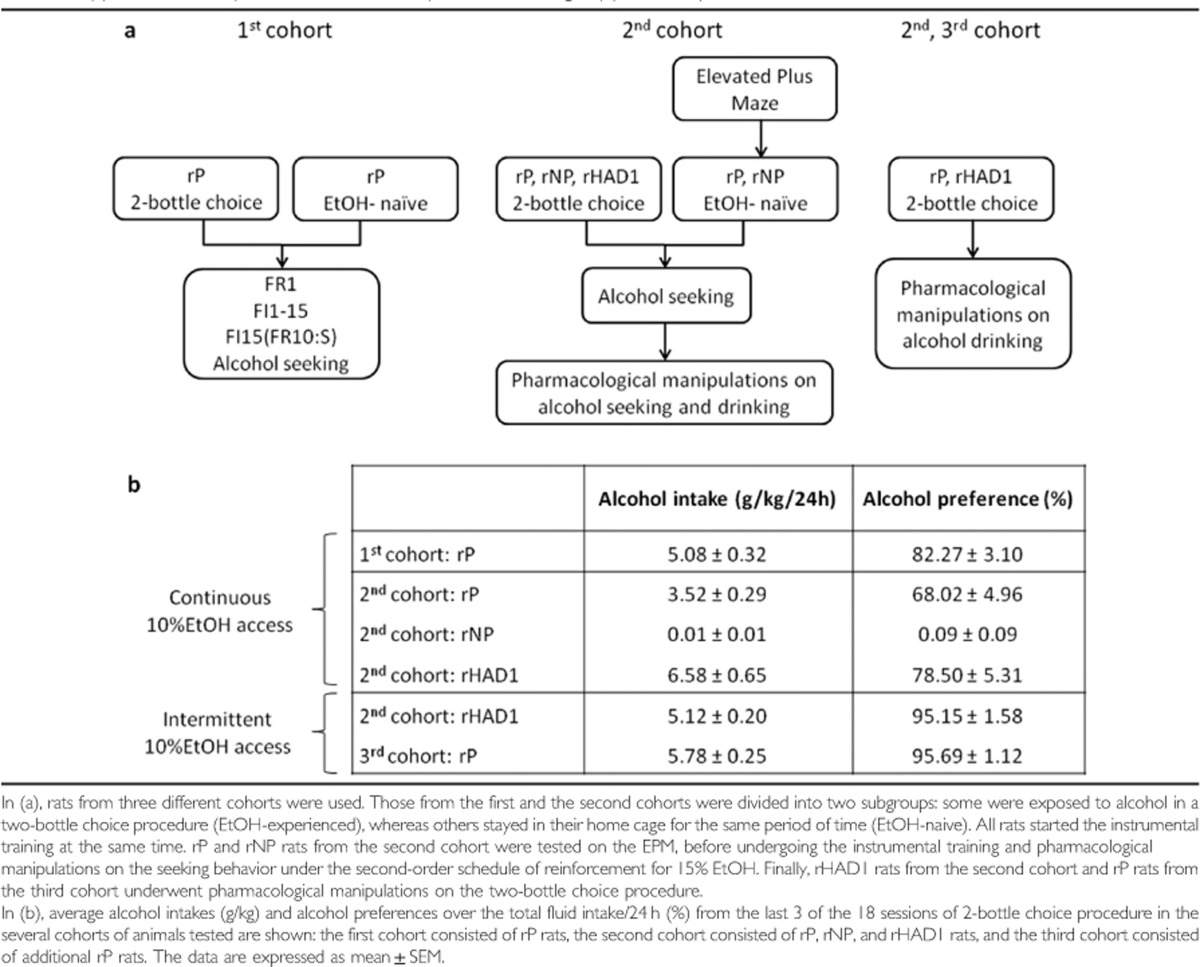
Rats from the first and the second cohorts were divided into two subgroups: some were exposed to alcohol in a two-bottle choice procedure (EtOH-experienced), whereas others were screened first for anxiety-like behavior and left in their home cages for the same period of time (EtOH-naive). All rats then began instrumental training at the same time. rP and rNP rats from the second cohort were first tested on the elevated plus maze (EPM) before undergoing instrumental training and pharmacological treatments under the second-order schedule of reinforcement for 15% EtOH to assess effects on alcohol-seeking behavior. The EPM tested the anxiety-like phenotype previously, but variably, shown in P rats (Stewart et al, 1993). Finally, rHAD1 rats from the second cohort and rP rats from the third cohort underwent pharmacological manipulations on the two-bottle choice procedure (Table 1a).
Table 1. (a) Schematic Representation of the Experimental Design. (b) Summary of Alcohol Intakes under Two-Bottle Choice Procedure.
Alcohol Consumption: Two-Bottle Choice Procedure
Animals were presented with either continuous or intermittent concurrent access (depending on the cohort of animals, see Table 1a) to 10% EtOH (v/v) and water in their home cage for a total of 18 sessions. Voluntary consumption was assessed every 24 h. See Supplementary Information.
Elevated Plus Maze
EtOH-naive animals were assessed for anxiety on the EPM as previously described (Dilleen et al, 2012). See Supplementary Information.
Alcohol Consumption: Instrumental Conditioning
Apparatus
Behavioral training was conducted in operant chambers as previously described (Giuliano et al, 2013). See Supplementary Information.
Alcohol seeking: second-order schedule of reinforcement for 15% EtOH
Rats were water-deprived for 22 h/day during the first 5 days of training that consisted of the following 4 phases.
Rats were initially trained to associate a light stimulus presentation with access to drinking EtOH (5 daily sessions): 1 min CS was illuminated during 1 min free access to a bottle containing 15% EtOH (v/v) in the operant chamber (Pavlovian conditioning) under a Random Time 60-s procedure. The rats received in average 38 CS-alcohol pairings in each 45 min session.
Rats were then trained to press a lever under a fixed-ratio 1 schedule (FR1) to have free access to 15% EtOH: each active lever press resulted in 20 s CS illumination above the active lever, retraction of both levers, free access to a 15% EtOH bottle and 20 s turning off of the house light. After these 20 s, the house light was again illuminated, the alcohol-associated CS was turned off, and the two levers again inserted into the chamber. Rats were limited to a maximum of 45 rewards/2 h session. Following acquisition of EtOH self-administration (7 daily sessions), a fixed-interval schedule (FI) of reinforcement was introduced.
The FI increased daily from 1 min to 2–4–6–8–10 min, before stabilizing at FI15 min for 3 consecutive sessions (total 9 daily sessions). The end of each FI was associated with a progressively longer drinking period of CS-paired free access to 15% EtOH: from 1 min under FI1 to 20 min under FI15.
Subsequently, a second-order schedule of reinforcement was introduced, in which every tenth active lever press during one FI15 resulted in a 1 s CS presentation (FI15(FR10:S)). At the end of FI15, rats earned 20 min of CS-paired free access to 15% EtOH in the operant chamber (minimum 25 daily sessions). Representative values of blood alcohol concentrations (BACs) following the 20 min free access to 15% EtOH were measured. See Supplementary Information.
After alcohol-seeking behavior had been acquired, dependence of seeking responses on contingent presentations of the alcohol-associated CS was assessed by omitting the CS in some sessions. As completion of each FR10 in these CS omission sessions had no consequence (in terms of CS presentation), these were effectively simple FI15 min sessions, as earlier in training. The CS omission sessions were followed by additional sessions in which the 1 s CS presentations were again contingent on completion of each FR10, that is, the FI15 (FR10:S) second-order schedule was reinstated.
Drugs
EtOH solutions were prepared by mixing 99.8% EtOH (Sigma, UK) in tap water to obtain 10% EtOH (v/v; 2-bottle choice procedure) or 15% EtOH (v/v; instrumental conditioning).
Naltrexone (Sigma, UK) and GSK1521498 (GlaxoSmithKline, UK) were prepared freshly on each test day following the protocol previously described (Giuliano et al, 2012, 2013). See Supplementary Information.
Statistical Analysis
Data were analyzed using repeated measures ANOVA (SPSS 21) with time, schedule, or dose as within-subject factors. Two-tailed values of P≤0.05 were considered statistically significant. Significant main effects and interactions were analyzed further using Dunnett's post hoc test where appropriate. See Supplementary Information.
Results
Alcohol Consumption: Two-Bottle Choice Procedure
When given free choice between 10% EtOH and water, rP and rHAD1, but not rNP, rats displayed a high propensity to drink alcohol and readily developed a marked preference for alcohol. Average intakes from the last 3 of the 18 total sessions in animals from all the cohorts used are presented in Table 1b. Alcohol intakes and preferences over 18 sessions in animals from the first and the second cohorts are presented in Figure 1a and b and Figure 2a and b, respectively.
Figure 1.
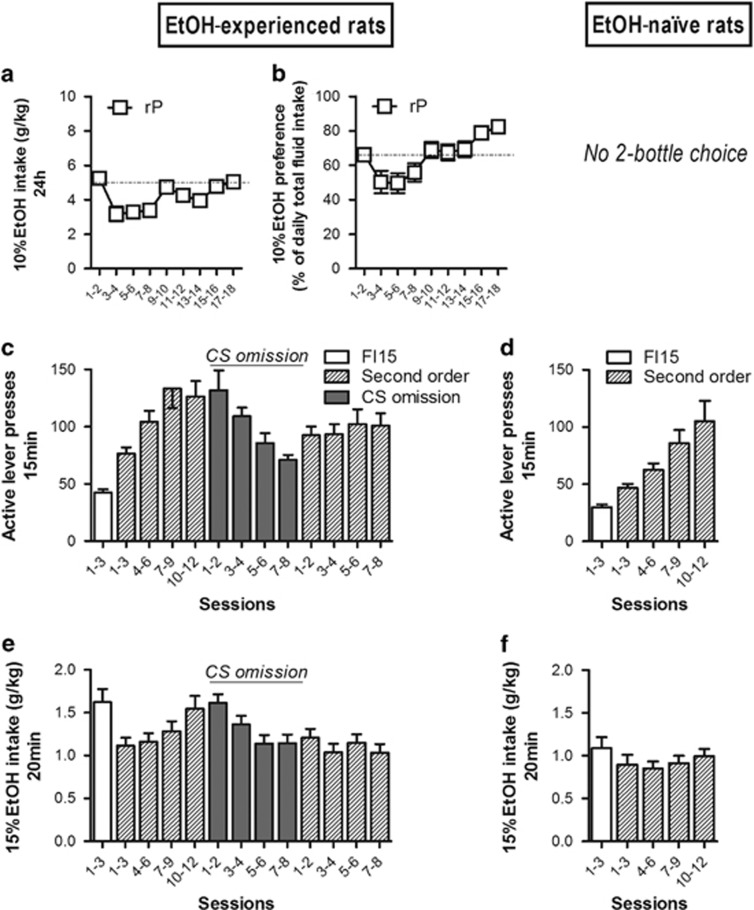
Voluntary alcohol intake, instrumental conditioning, and alcohol-seeking characterization in rP rats (first cohort). Rats from the first cohort were divided into two subgroups: some were exposed to alcohol in a two-bottle choice procedure (EtOH-experienced, on the left), whereas others stayed in their home cage for the same period of time (EtOH-naive, on the right). (a) The 10% alcohol intake (g/kg) and (b) percentage of alcohol preference over the total fluid intake (%) were assessed in rP (n=20) rats every 24 h using the 2-bottle choice procedure for 18 continuous days. All the animals (EtOH-experienced and EtOH-naive) were then instrumentally trained to seek 15% EtOH. Lever presses (mean±SEM) under FI15 (white bars) and under second-order schedule of reinforcement (diagonal bars) are shown in (c) and (d). Further 8 sessions, in which the CS was omitted (gray bars), were delivered to EtOH-experienced rats to assess the role of the CS as a conditioned reinforcer. Additional 8 sessions under second-order schedule of reinforcement were then delivered to reestablish CS-maintained seeking behavior (c). The acquisition of ethanol-seeking behavior in rats with no previous history of ethanol exposure was similar to that of EtOH-experienced rats (schedule × time × EtOH experience interaction (F(2, 64)=1.31; NS); EtOH experience effect (F(1, 32)=1.97; NS)). Alcohol intake (g/kg) during the 20 min drinking period earned by responding during the FI15 schedule (white bars) and the second-order schedule of reinforcement (diagonal bars) are shown in (e) and (f). EtOH intake in rP EtOH-naive rats was overall less than in rP EtOH-experienced rats (schedule × time × EtOH exposure interaction (F(2, 26)=4.34; P<0.05); EtOH experience effect (F(1, 32)=20.35; P<0.001)).
Figure 2.
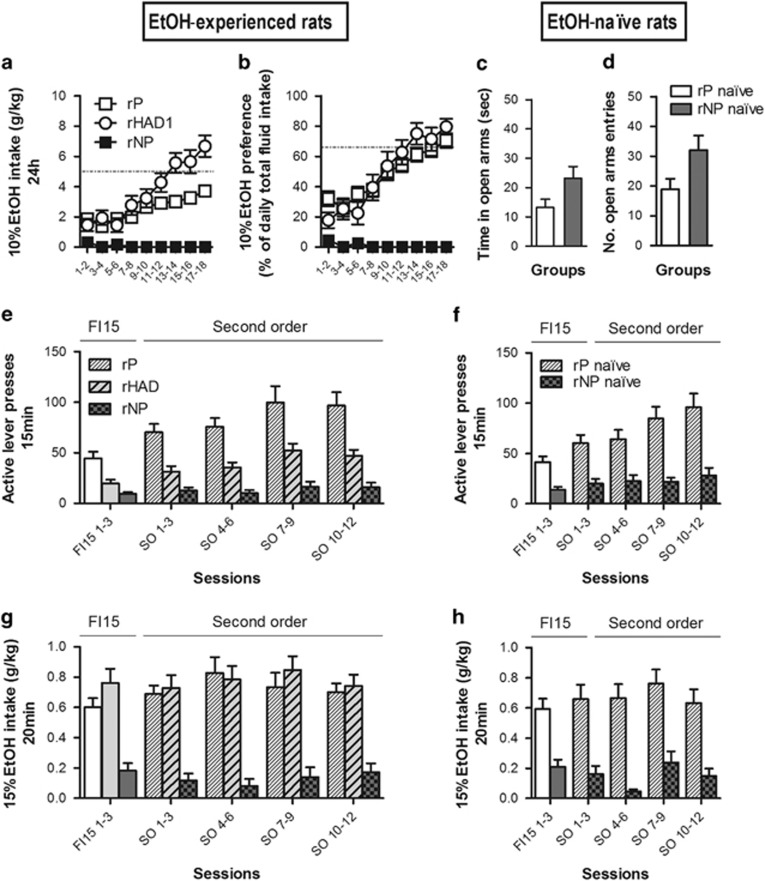
Voluntary alcohol intake, instrumental conditioning, and alcohol-seeking characterization in rP, rNP, and rHAD1 rats (second cohort). Rats from the second cohort were divided into two subgroups: some were exposed to alcohol in a 2-bottle choice procedure (EtOH-experienced, on the left), whereas others were screened for anxiety-like profile and stayed in their home cage for the same period of time (EtOH-naive, on the right). (a) The 10% alcohol intake (g/kg) and (b) percentage of alcohol preference over the total fluid intake (%) were assessed every 24 h using the 2-bottle choice procedure for 18 continuous days in rP (n=20), rNP (n=10), and rHAD1 (n=18) EtOH-experienced rats. In the meantime, EtOH-naive rats (n=10 rP rats; n=10 rNP rats) were assessed for anxiety-like behavior on the EPM. The % of time spent in the open arms (% time OA/(OA+CA), mean±SEM) and % of open arm entries (% entries OA/(OA+CA), mean±SEM) are shown in (c) and (d), respectively. All the animals (EtOH-experienced and EtOH-naive) were then instrumentally trained to seek 15% EtOH. Lever presses (mean±SEM) under FI15 (white bars) and under second-order schedule of reinforcement (diagonal bars) are shown in (e) and (f) in EtOH-experienced and EtOH-naive rats, respectively. Alcohol intake (g/kg) during the 20 min drinking period earned by responding during the FI15 schedule (white bars) and the second-order schedule of reinforcement (diagonal bars) are shown in (g) and (h) in EtOH-experienced and EtOH-naive rats, respectively. EtOH-naive rP rats were no different to EtOH-experienced rats in their acquisition of alcohol seeking (schedule × time × EtOH experience interaction (F(2, 36)=0.41; NS); EtOH experience effect (F(1, 18)=0.03; NS)).
rP rats Are More Anxious Than rNP Rats on the EPM
EtOH-naive rP rats showed more anxiety-like behavior (ie, more time in the open arms) on the EPM relative to EtOH-naive rNP rats (Figure 2a and d). See Supplementary Information.
Cue-Controlled Alcohol-Seeking Behavior under a Second-Order Schedule of 15% EtOH Reinforcement
First cohort
rP rats were divided into two subgroups before being trained in the cue-controlled alcohol-seeking task: (1) rP EtOH-experienced rats, exposed to 10% EtOH under the 2-bottle choice procedure; (2) rP EtOH-naive rats, screened first for anxiety-like behavior and left in the home cage for the same period of time on ad libitum food and water. All the animals then underwent instrumental seeking training (Table 1a).
The rP EtOH-experienced rats trained instrumentally to seek 15% EtOH under FI15 made 42±2.87 lever presses in the 15 min alcohol-free period (Figure 1c), whereas EtOH-naive rats displayed 29±2.38 alcohol-seeking responses in the same 15 min alcohol-free period (Figure 1d). When the brief 1 s CS presentation contingent on lever pressing was introduced (ie, a second-order schedule (FI15(FR10:S))), the alcohol-seeking lever presses during the 15 min alcohol-free period increased in EtOH-experienced and EtOH-naive rats to 126±13.65 (Figure 1c) and 104±17.91 (Figure 1d), respectively, during the last 3 of 12 sessions of training (P<0.001 vs FI15). A further 8 sessions in which the CS was omitted (in EtOH-experienced subjects only) resulted in a marked decrease in seeking responses during the 15 min alcohol-unaffected interval to 65±4.37 lever presses during the seventh and eighth sessions (P<0.01 vs (FI15(FR10:S)) (Figure 1c). This demonstrates that the vigorous alcohol-seeking behavior of EtOH-experienced rats depended on contingent presentations of the alcohol-associated CS. This control over alcohol seeking by alcohol-associated CSs was further confirmed by the reestablishment of high levels of seeking responses (105±13.06 lever presses in 15 min (P<0.05 vs FI15 and P=NS vs (FI15(FR10:S)) on subsequent reintroduction of contingent presentations of the CS in 8 additional sessions ((FI15(FR10:S)); overall schedule effect (F(3, 39)=27.48; P<0.001); Figure 1c).
At the end of the alcohol-unaffected seeking interval, under either FI15 or (FI15(FR10:S)), rP rats had earned 20 min free-drinking access to 15% EtOH in the operant chamber. EtOH-experienced and EtOH-naive rats consumed 1.62±0.16 g/kg (Figure 1e) and 1.09±0.13 g/kg (Figure 1f) of alcohol, respectively, under the FI15 schedule of reinforcement (ie, when no CS was presented). Their intake in the 20 min following seeking when CSs were presented was 1.54±0.15 g/kg (Figure 1e) and 0.99±0.09 g/kg (Figure 1f), respectively (EtOH exposure: (F(1, 32)=20.35; P<0.001); schedule × time × EtOH exposure: (F(2, 64)=4.34; P<0.05); no effect of schedule).
Second cohort
rP and rNP (but not rHAD1) rats were divided into two subgroups based on their EtOH experience (Table 1a).
rP rats
After concurrent access to 10% EtOH and water for 18 consecutive days in the home cage (Figure 2a and b), rP EtOH-experienced and rP EtOH-naive rats trained instrumentally to seek 15% EtOH under FI15 made respectively 44±6.51 (Figure 2e) and 41±5.78 (Figure 2f) lever presses in 15 min. When the brief 1 s CS presentation contingent on lever pressing was introduced, EtOH-experienced and EtOH-naive rats increased their lever presses to 96±13.25 (Figure 2e) and 98±13.67 (Figure 2f), respectively, in 15 min after 12 sessions of training (schedule × time interaction (F(2, 36)=11.91; P<0.001; no effect of EtOH exposure). Alcohol intake in the 20 min free-access period under either FI15 or (FI15(FR10:S)) was respectively 0.60±0.06 and 0.70±0.06 g/kg in EtOH-experienced rats (Figure 2g), and it was respectively 0.59±0.07 and 0.63±0.09 g/kg in EtOH-naive rats (Figure 2h) (no effect of schedule or time).
rNP rats
In marked contrast to rP rats, rNP EtOH-experienced and rNP EtOH-naive rats made respectively only 9±1.97 (Figure 2e) and 14±2.75 (Figure 2f) active lever presses in 15 min under FI15. They only increased their responding to respectively 16±4.45 (Figure 2e) and 28±7.62 (Figure 2f) when the alcohol-associated CS was introduced response contingently (schedule effect (F(1, 8)=3.35; NS)). Alcohol intake was very low under both schedules, reaching 0.18±0.05 g/kg under FI15 and 0.17±0.06 under (FI15(FR10:S)) in rNP EtOH-experienced rats (Figure 2g) and 0.21±0.05 g/kg under FI15 and 0.15±0.05 under (FI15(FR10:S)) in rNP EtOH-naive rats (Figure 2h).
rHAD1 rats
Under FI15, rHAD1 rats made 19±3.63 active lever presses (Figure 2e) and drank 0.76±0.09 g/kg of EtOH (Figure 2g) in the following 20 min drinking period. Once the response-contingent alcohol CS was introduced ((FI15(FR10:S))), active lever presses increased to 47±6.05 (Figure 2e), with an alcohol intake of 0.74±0.07 g/kg in the following 20 min (Figure 2g). Their seeking performance was significantly lower than rP rats, despite similar EtOH intake during the 20 min of earned free access (main effect of line (F(1, 18)=10.59; P<0.01); time × schedule × line interaction (F(2, 36)=5.60; P<0.01); no differences in EtOH intake (F(1, 18)=1.26; NS).
During the 20 min period of free access to 15% EtOH in the operant chamber after CS-controlled seeking, the rP and rHAD1 rats consumed rather large amounts of alcohol, reaching BACs in the range 15–79 mg% and 11–85 mg%, respectively, that were correlated with the amount of alcohol drunk during 20 min (R=0.52, P<0.05 and R=0.82, P<0.01 for rP and rHAD1 rats, respectively).
Pharmacological Manipulations
GSK1521498 decreased both cue-controlled alcohol seeking and alcohol drinking
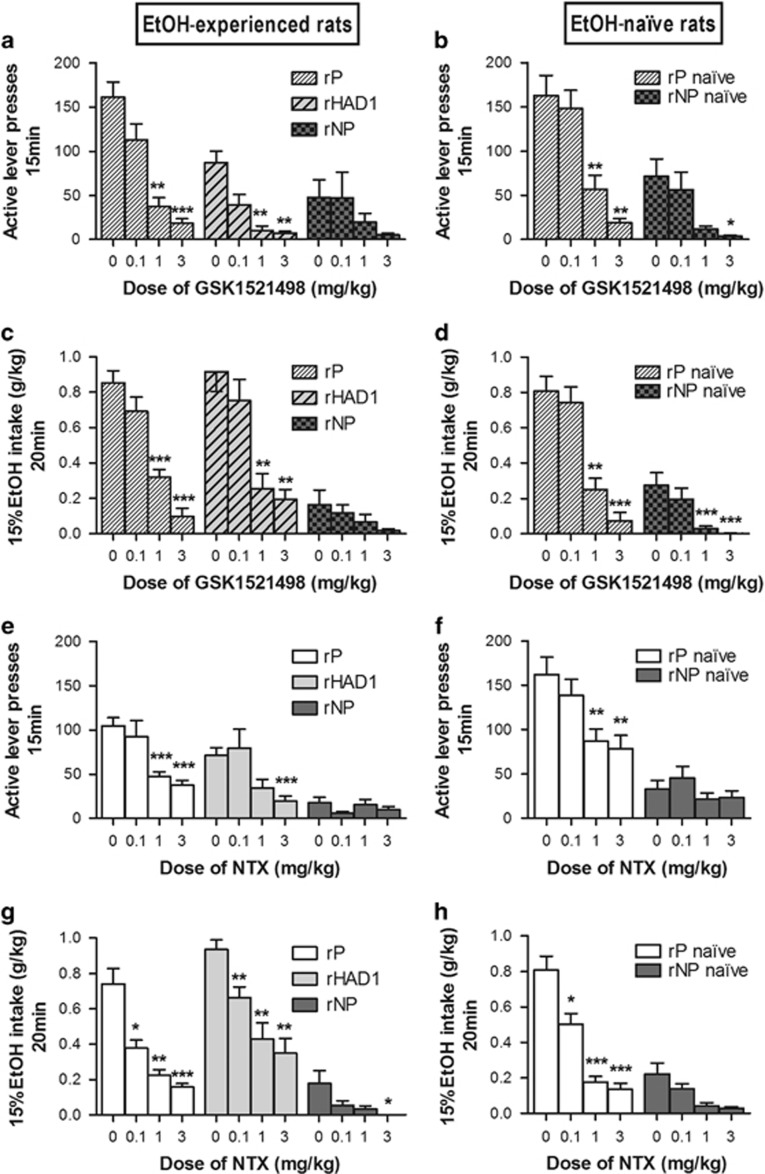
rP rats. GSK1521498 dose-dependently decreased seeking behavior (rP EtOH-experienced (F(3, 27)=20.98; P<0.001, partial effect size=0.70); Figure 3a; rP-naive (F(3, 27)=27.60; P<0.001, partial effect size=0.75); Figure 3b), and alcohol intake during the 20 min free-drinking period (rP EtOH-experienced (F(3, 27)=33.72; P<0.001, partial effect size=0.79); Figure 3c); rP-naive (F(3, 27)=29.65; P<0.001, partial effect size=0.77); Figure 3d).
Figure 3.
GSK1521498 and naltrexone decreased EtOH seeking and voluntary consumption. Effects of GSK1521498 (first and second rows) and effects of naltrexone (third and fourth rows) on alcohol seeking and taking under a second-order schedule of reinforcement in EtOH-experienced rats (n=10 rP, n=9 rNP, n=9 rHAD1), on the left, and in EtOH-naive rats (n=10 rP, n=9 rNP), on the right, are shown. Number of active lever presses (a, b, e, f) and alcohol intake (g/kg) during the 20 min drinking period earned by responding for alcohol-associated CS presentation (c, d, g, h) are presented. Both GSK1521498 and naltrexone significantly reduced seeking behavior and alcohol drinking in rP rats regardless of their alcohol-experience (naltrexone: dose × alcohol experience (F(3, 57)=0.27; NS); GSK1521498: dose × alcohol experience (F(3, 54)=0.69; NS)). The data are expressed as mean±SEM. *P<0.05, **P<0.01, and ***P<0.001 compared with vehicle-treated animals.
rNP rats. GSK1521498 affected neither seeking behavior (F(3, 24)=1.91; NS, partial effect size=0.19; Figure 3a) nor alcohol intake (F(3, 24)=2.32; NS, partial effect size=0.22) in rNP EtOH-experienced rats (Figure 3c), but reduced the seeking behavior (F(3, 27)=7.70; P<0.01, partial effect size=0.46; Figure 3b) and alcohol intake (F(3, 27)=9.28; P<0.01, partial effect size=0.51) in rNP-naive rats (Figure 3d).
rHAD1 rats. GSK1521498 reduced both seeking behavior (F(3, 24)=17.21; P<0.001, partial effect size=0.68; Figure 3a) and alcohol intake (F(3, 24)=13.85; P<0.001, partial effect size=0.63; Figure 3c).
Naltrexone Decreased Both Cue-Controlled Alcohol Seeking and Alcohol Drinking
rP rats
Naltrexone reduced alcohol-seeking behavior (rP EtOH-experienced (F(3, 27)=9.02; P<0.01, partial effect size=0.50; Figure 3e; rP EtOH-naive (F(3, 27)=15.81; P<0.001, partial effect size=0.64; Figure 3f) and the 15% EtOH intake during the 20 min drinking period (rP EtOH-experienced (F(3, 27)=29.63; P<0.001, partial effect size=0.77; Figure 3g; rP EtOH-naive (F(3, 27)=45.69; P<0.001, partial effect size=0.83; Figure 3h).
rNP rats
Naltrexone reduced the low level of seeking behavior of rNP rats (rNP EtOH-experienced (F(3, 24)=3.95; P=0.05, partial effect size=0.33; Figure 3e; rNP-naive (F(3, 27)=5.28; P<0.05, partial effect size=0.37; Figure 3f) and the low level of alcohol intake (rNP EtOH-experienced (F(3, 24)=5.08; P<0.05, partial effect size=0.39; Figure 3g; rNP-naive (F(3, 27)=8.50; P<0.01, partial effect size=0.48; Figure 3h), but post -hoc pairwise comparisons revealed no effect of dose in rNP EtOH-experienced rats.
rHAD1 rats
Naltrexone reduced both EtOH seeking (F(3, 24)=5.67; P<0.05, partial effect size=0.79; Figure 3e) and alcohol intake (F(3, 24)=18.26; P<0.001, partial effect size=0.69; Figure 3g).
GSK1521498 Decreased the Consumption of EtOH in the Two-Bottle Choice Procedure
rP and rHAD1 rats
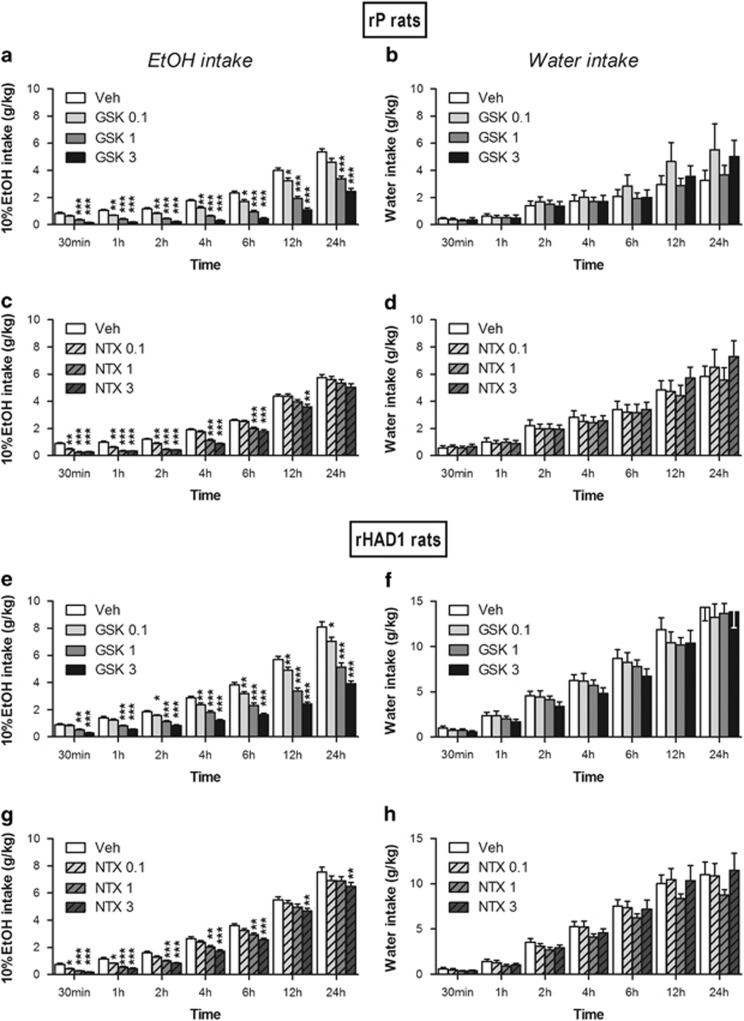
GSK1521498 was tested on EtOH consumption in the intermittent access two-bottle choice procedure at 30 min and 1–2–4–6–12–24 h after the beginning of the access period (30 min after GSK1521498 treatment). GSK1521498 dose-dependently reduced 10% EtOH intake during the 24 h alcohol vs water access (rP: (F(18, 396)=32.61; P<0.001, partial effect size for dose=0.77, partial effect size for dose × time=0.60; Figure 4a and b; rHAD1: (F(18, 342)=42.02; P<0.001, partial effect size for dose=0.77, partial effect size for dose × time=0.69; Figures 4e and f). See Supplementary Information.
Figure 4.
GSK1521498 and naltrexone decreased EtOH intake in the two-bottle choice procedure. (On the top) Effects of GSK1521498 (first row, a, b) and naltrexone (second row, c, d) on alcohol (a, c) and water (b, d) intake in the two-bottle choice procedure in rP rats (n=23). (On the bottom) Effects of GSK1521498 (third row, e, f) and naltrexone (fourth row, g, h) on alcohol (e, g) and water (f, h) intake in the two-bottle choice procedure in rHAD1 rats (n=23). Alcohol and water intake were assessed at different time points: 30 min and 1, 2, 4, 6, 12, and 24 h after the beginning of the access period, and 30 or 10 min after GSK1521498 or naltrexone treatment, respectively. The data are expressed as mean±SEM. *P<0.05, **P<0.01, and ***P<0.001 compared with vehicle-treated animals at each time point, following repeated measures ANOVA per time point.
Naltrexone Decreased the Consumption of EtOH in the Two-Bottle Choice Procedure
rP and rHAD1 rats
The effects of naltrexone on EtOH consumption were investigated at 30 min and 1–2–4–6–12–24 h after the beginning of the access period (10 min after naltrexone SC injection). Naltrexone dose-dependently decreased 10% EtOH intake during the 24 h alcohol vs water access (rP: treatment × time (F(18, 396)=3.88; P<0.001, partial effect size for dose × time=0.63; Figures 4c and d; rHAD1: treatment (F(3, 57)=14.90; P<0.001, partial effect size=0.44), time (F(6, 114)=550.61; P<0.001, partial effect size=0.97), treatment × time (F(18, 342)=1.45; NS; Figures 4g and h). See Supplementary Information.
Discussion
Alcohol-preferring, but not alcohol-nonpreferring, rats were shown to acquire high levels of CS-controlled alcohol-seeking behavior for 15% EtOH and to achieve blood alcohol concentrations up to 80 mg% in the immediately following 20 min earned drinking periods. Selective antagonism at the MOR by the novel compound GSK1521498 markedly reduced both cue-controlled alcohol seeking and alcohol drinking, effects that were both greater and longer lasting than those of naltrexone.
Cue-Controlled Alcohol-Seeking Behavioral Procedure
Alcohol-related cues, including the sight and smell of alcohol drinks, acquire conditioned incentive properties (Barker and Taylor, 2014; Field and Duka, 2002), can provoke intense craving in individuals that abuse or have lost control over alcohol (Ludwig, 1986; De Bruijn et al, 2004), and may be a predictor of relapse after abstinence (Schneekloth et al, 2012).
The first aim of this study, then, was to develop a procedure to quantify CS-controlled alcohol seeking over protracted periods of time in rats selectively bred for the propensity to drink alcohol. This was achieved by training rats daily to seek alcohol such that instrumental responding was under the control of alcohol-associated CSs that mediated delays to drinking and alcohol reinforcement by acting as conditioned reinforcers. This novel procedure therefore differs from extinction–reinstatement tasks by measuring the daily propensity for CS-elicited and maintained alcohol seeking and drinking that was only seen in rats predisposed to drink, rather than the reinstatement of instrumental responding after extinction that provides a low response baseline from which to measure CS effects on reinstatement (generally assumed to be a measure of relapse), but without the opportunity to drink (Marchant et al, 2013).
Even though a CS–alcohol Pavlovian association can be established in rats not genetically predisposed voluntarily to drink alcohol and this alcohol-associated CS can elicit approach (Tomie and Sharma, 2014), support Pavlovian-to-instrumental transfer (Milton et al, 2012), and act as a conditioned reinforcer (Samson et al, 2001; Milton et al, 2012), the present data show that an alcohol-associated CS cannot sustain prolonged and vigorous periods of alcohol-seeking behavior in animals not predisposed to drink alcohol (ie, in the NP selected line).
The rederived P, NP, and HAD rats used here have been bred over many generations as a rodent model to investigate the genetic basis of excessive alcohol consumption by humans (Czachowski and Samson, 2002; McBride et al, 2014). These lines have been both behaviorally and neurobiologically characterized and show phenotypic differences in their responses to ethanol that may be of relevance to the neurogenetic mechanisms underlying ethanol preference or nonpreference (McBride et al, 2014). For example, P rats have a lesser aversive response to ethanol in a conditioned taste aversion procedure (Froehlich et al, 1988) and may show more anxiety-like behavior in an EPM compared with NP rats (Stewart et al, 1993), the latter observation being confirmed in the rP cohorts studied here, although this high-anxiety phenotype is not always observed (Viglinskaya et al, 1995).
In the present study we established an alcohol-seeking procedure in alcohol-preferring rats by adapting the second-order schedule of reinforcement used previously to study not only cocaine and heroin seeking (Arroyo et al, 1998; Schindler et al, 1988; Everitt and Robbins, 2000; Giuliano et al, 2013), but also the seeking of high incentive foods (Giuliano et al, 2012) or sexual rewards (Everitt, 1990), in which seeking was measured before unrestrained access to, and consummatory interaction, with these rewards. Similarly, here rats were trained to make instrumental seeking responses over 15 min in order to have access to alcohol for an unrestricted 20 min drinking period in each daily session. The rats were never food deprived, nor did they undergo either sucrose fading or prior induction of dependence. Alcohol seeking was markedly dependent on response-contingent presentations of the alcohol-associated CS, as it decreased following CS omission and increased to pre-CS-omission levels following its reintroduction. This effect of CS omission is key evidence that seeking behavior depends on brief presentations of the CS that probably has conditioned reinforcing properties (Goldberg et al, 1981; Everitt and Robbins, 2000). It is well established that response-contingent, brief presentations of a CS paired with a drug reward results in significantly greater control over second-order responding than presentation of CSs explicitly not paired with drug, further supporting the conditioned reinforcement account (Goldberg et al, 1979). A related approach—that is, a period of instrumental responding followed by free alcohol drinking—has been used previously (Samson et al, 1999, 2001; Czachowski and Samson, 2002; Henderson-Redmond and Czachowski, 2014), in which seeking behavior measured over repeated extinction sessions (Czachowski and Samson, 1999) was suggested to be elicited by the Pavlovian context in which the rats had been trained (Conklin et al, 2008). In contrast, in the present experiments rats were always trained in the same alcohol-associated context, but the addition of response-contingent CS presentations resulted in more vigorous and sustained seeking responses that were maintained during repeated test sessions and always terminated by the attainment of an alcohol free-drinking period (ie, the animals were not under extinction conditions), thereby also making a clear distinction between appetitive and consummatory behavior (Czachowski and Samson, 2002; Kaminski et al, 2008; Samson et al, 2001).
Two cohorts of rP and rNP rats were trained to respond for 15% EtOH under cue-controlled seeking procedure either after having been allowed previously to drink 10% EtOH in a 2-bottle choice procedure, which confirmed the alcohol-preferring phenotype, or without such experience and therefore being EtOH naive at the time of instrumental training. Their seeking performance was very similar regardless of their alcohol-drinking history.
We also studied a cohort of rHAD1 rats following confirmation of their high alcohol-drinking phenotype in the 2-bottle choice procedure with 10% EtOH. They showed on initial exposure a higher level of ethanol intake than the rP rats, confirming previous findings (Czachowski and Samson, 2002), however, both lines subsequently showed similar levels of ethanol consumption (~1 g/kg/20 min) during the 20 min drinking period following CS-seeking behavior. However, rP rats that tended to drink less ethanol than rHAD1 rats in our studies, nevertheless responded more for the opportunity to drink. This observation is consistent with previous findings that P rats emit more appetitive lever press responding under ratio schedules than HAD rats (Czachowski and Samson, 2002). P rats have also been reported to show more activity in locomotor chambers and greater responsivity to a novel odor compared with HAD rats (Nowak et al, 2000), perhaps indicating that P rats display greater exploratory behavior or novelty seeking.
Effects of Manipulating the Opioid System on Alcohol Seeking and Drinking
Having established this novel cue-controlled alcohol-seeking and drinking procedure in alcohol-preferring rats, we investigated the effects on both appetitive and consummatory behavior of manipulating MOR activity. A large body of evidence indicates that alcohol, as well as other rewarding stimuli, may enhance the activity of the endogenous opioid system (Herz, 1997) and MOR levels (Krishnan-Sarin et al, 1998). Moreover, MOR antagonists can decrease drinking in individuals that abuse alcohol (Volpicelli et al, 1992; Drobes et al, 2003) and alcohol craving in the presence of alcohol-associated CSs (Monti et al, 1999).
Initially we investigated the effects of naltrexone on alcohol drinking in the two-bottle choice procedure as well as on CS-dependent alcohol seeking and on ethanol intake in the free drinking sessions earned by making seeking responses. As expected (Froehlich et al, 1990), pretreatment with naltrexone dose-dependently reduced voluntary alcohol intake in both two-bottle choice procedure and the instrumental context, but it also decreased alcohol-seeking responses (see also Czachowski and Delory, 2009; Henderson-Redmond and Czachowski, 2014). However, although used to treat alcoholism (Volpicelli et al, 1992), naltrexone is not universally viewed as an effective medication (Rösner et al, 2010) and the recent introduction of nalmefene has been to reduce the volume of alcohol consumed, rather than to maintain abstinence.
We have previously shown significantly greater effects of the novel MOR antagonist GSK1521498, developed for overeating and substance dependence disorders (Nathan et al, 2012), over naltrexone in reducing highly palatable food-, cocaine-, and heroin-seeking behavior in rats (Giuliano et al, 2012, 2013), but it has no effect on locomotor activity (Supplementary Figure S1). Treatment with GSK1521498 dose-dependently reduced alcohol-seeking behavior and voluntary alcohol consumption in rP and rHAD1 rats more effectively than naltrexone and with a longer duration of effect significantly, reflecting its longer half-life. This apparently greater effectiveness of GSK1521498 is also convergent with prior results and is possibly because of the higher selectivity for the MOR (14–20-fold greater over δ- and κ-opioid receptors compared with 4–10-fold selectivity reported for naltrexone) or the 100-fold selectivity for MOR over nociceptin/orphanin receptors (Ignar et al, 2011; Kelly et al, 2014). Moreover, its more complete antagonist profile, and its lesser inverse agonist activity under some assay conditions, compared with the incomplete antagonism and partial agonist activity of naltrexone, also possibly contribute to the different behavioral profile of GSK1521498 (Ignar et al, 2011; Kelly et al, 2014).
Clinical Implications
In humans, GSK1521498 has been shown generally to be well tolerated compared with placebo, with no detectable deleterious effects on anxiety, mood, or other aspects of hedonic function, or on liver or other blood safety parameters. Importantly, its coadministration with ethanol did not affect its tolerability (Ziauddeen et al, 2013b).
That GSK1521498 treatment reduces the tendency to seek cocaine, heroin, high incentive chocolate, and, from the present results, also alcohol in rats with a propensity to drink, suggests effects on a common conditioned incentive motivational system, in addition to its effects to reduce the hedonic impact of the taste of chocolate and alcohol and hence reduce ingestion. In humans, GSK1521498 also reduced attentional bias to food-related stimuli in obese binge eaters, for whom the food cues had higher motivational value (Ziauddeen et al, 2013a). However, GSK1521498 did not reduce the attentional bias to alcohol-related cues in healthy social drinkers, for whom the motivational properties of alcohol cues may have been low (Ziauddeen et al, 2013b). Together, these observations suggest an important specificity in the behavioral effects of GSK1521498 in individuals having lost control over specific drugs or ingestive rewards. In addition, the present data suggest that administering GSK1521498 in an appropriate therapeutic context should decrease the propensity of alcohol-dependent patients, including those wanting to maintain abstinence, to seek or crave alcoholic drinks, especially when elicited by alcohol cues such as those in advertisements for drink products. It may also reduce the volume of alcohol consumed by patients seeking harm reduction treatments, as well as patients relapsing from abstinence. Thus, the novel behavioral procedure we have developed for evaluating alcohol seeking and consumption in rats can generate therapeutic predictions that may be important in guiding the clinical development of GSK1521498 as a new treatment for alcohol dependence.
Funding and Disclosure
TWR has consulted for GSK, Cambridge Cognition, Lilly, Lundbeck, and Merck. He also has research grants with GSK, Lilly, and Lundbeck. ETB is employed half-time by the University of Cambridge and half-time by GSK; he holds stock in GSK. CG, CRG, DE, MPG-P, DB, and BJE declare no conflict of interest.
Acknowledgments
This study was funded by Medical Research Council Programme Grant (no. G1002231) and by GlaxoSmithKline (GSK) that has a commercial interest in GSK1521498. The production of the P/NP and HAD rats was funded by the R24 Alcohol Research Resource Award grant (R24 AA015512) from NIAAA. Charles R Goodlett was funded by a grant from the IUPUI International Development Fund that supported his sabbatical leave at the University of Cambridge. Maria Pilar Garcia-Pardo was funded by Val+id para investigadores en formación (Conselleria de educacion, Generalitat Valenciana) that also supported her stay at the University of Cambridge (January–April 2014) as a visiting student. With this paper we would like to commemorate Daina Economidou who died in December 2012 after a heroic fight against lung cancer. The best way to remember her is to share with the scientific community her dedication and enthusiastic contribution to science through the data collected until a few months before her death, including the data in Figure 1. We thank Dr Larry Lumeng, Dr Richard Bell, and Rebecca Jane Smith at the Indiana University School of Medicine for facilitating the provision of the selected lines of rats; Dr Adam Mar for writing the program to analyze the EPM data; Dr Yolanda Peña-Oliver for analyzing the EPM data; Jing Xia for analyzing the blood samples; Sam Miller for excellent statistical advice; and Kristin Patterson and Ramprakash Govindarajan for providing the GSK1521498 solutions.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Arroyo M, Markou A, Robbins TW, Everitt BJ (1998). Acquisition, maintenance and reinstatement of intravenous cocaine self-administration under a second-order schedule of reinforcement in rats: effects of conditioned cues and continuous access to cocaine. Psychopharmacology (Berl) 140: 331–344. [DOI] [PubMed] [Google Scholar]
- Augier E, Flanigan M, Dulman RS, Pincus A, Schank JR, Rice KC et al (2014). Wistar rats acquire and maintain self-administration of 20% ethanol without water deprivation, saccharin/sucrose fading, or extended access training. Psychopharmacology (Berl) 9: 114727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Taylor JR (2014). Habitual alcohol seeking: modeling the transition from casual drinking to addiction. Neurosci Biobehav Rev 47: 281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ron D, Barak S (2014). Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol 48: 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, Weiss F (2002). Effect of selective blockade of mu(1) or delta opioid receptors on reinstatement of alcohol-seeking behavior by drug-associated stimuli in rats. Neuropsychopharmacology 27: 391–399. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Robin N, Perkins KA, Salkeld RP, McClernon FJ (2008). Proximal versus distal cues to smoke: the effects of environments on smokers' cue-reactivity. Exp Clin Psychopharmacol 16: 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czachowski CL, Delory MJ (2009). Acamprosate and naltrexone treatment effects on ethanol and sucrose seeking and intake in ethanol-dependent and nondependent rats. Psychopharmacology (Berl) 204: 335–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czachowski CL, Samson HH (1999). Breakpoint determination and ethanol self-administration using an across-session progressive ratio procedure in the rat. Alcohol Clin Exp Res 23: 1580–1586. [PubMed] [Google Scholar]
- Czachowski CL, Samson HH (2002). Ethanol- and sucrose-reinforced appetitive and consummatory responding in HAD1, HAD2, and P rats. Alcohol Clin Exp Res 26: 1653–1661. [DOI] [PubMed] [Google Scholar]
- Davidson D, Amit Z (1997). Naltrexone blocks acquisition of voluntary ethanol intake in rats. Alcohol Clin Exp Res 21: 677–683. [PubMed] [Google Scholar]
- De Bruijn C, Korzec A, Koerselman F, van Den Brink W (2004). Craving and withdrawal as core symptoms of alcohol dependence. J Nerv Ment Dis 192: 494–502. [DOI] [PubMed] [Google Scholar]
- Dilleen R, Pelloux Y, Mar AC, Molander A, Robbins TW, Everitt BJ et al (2012). High anxiety is a predisposing endophenotype for loss of control over cocaine, but not heroin, self-administration in rats. Psychopharmacology (Berl) 222: 89–97. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Anton RF, Thomas SE, Voronin K (2003). A clinical laboratory paradigm for evaluating medication effects on alcohol consumption: naltrexone and nalmefene. Neuropsychopharmacology 28: 755–764. [DOI] [PubMed] [Google Scholar]
- Economidou D, Dalley JW, Everitt BJ (2011). Selective norepinephrine reuptake inhibition by atomoxetine prevents cue-induced heroin and cocaine seeking. Biol Psychiatry 69: 266–274. [DOI] [PubMed] [Google Scholar]
- Everitt BJ (1990). Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci Biobehav Rev 14: 217–232. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW (2000). Second-order schedules of drug reinforcement in rats and monkeys: measurement of reinforcing efficacy and drug-seeking behaviour. Psychopharmacology (Berl) 153: 17–30. [DOI] [PubMed] [Google Scholar]
- Field M, Duka T (2002). Cues paired with a low dose of alcohol acquire conditioned incentive properties in social drinkers. Psychopharmacology (Berl) 159: 325–334. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Harts J, Lumeng L, Li TK (1988). Differences in response to the aversive properties of ethanol in rats selectively bred for oral ethanol preference. Pharmacol Biochem Behav 31: 215–222. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Harts J, Lumeng L, Li TK (1990). Naloxone attenuates voluntary ethanol intake in rats selectively bred for high ethanol preference. Pharmacol Biochem Behav 35: 385–390. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, Kalivas PW (2013). Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron 77: 867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano C, Robbins TW, Nathan PJ, Bullmore ET, Everitt BJ (2012). Inhibition of opioid transmission at the μ-opioid receptor prevents both food seeking and binge-like eating. Neuropsychopharmacology 37: 2643–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano C, Robbins TW, Wille DR, Bullmore ET, Everitt BJ (2013). Attenuation of cocaine and heroin seeking by μ-opioid receptor antagonism. Psychopharmacology (Berl) 227: 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SR, Kelleher RT, Goldberg DM (1981). Fixed-ratio responding under second-order schedules of food presentation or cocaine injection. J Pharmacol Exp Ther 218: 271–281. [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD, Kelleher RT (1979). Enhancement of drug-seeking behavior by environmental stimuli associated with cocaine or morphine injections. Neuropharmacology 18: 1015–1017. [DOI] [PubMed] [Google Scholar]
- Gual A, Bruguera P, López-Pelayo H (2014). Nalmefene and its use in alcohol dependence. Drugs Today (Barc) 50: 347–355. [DOI] [PubMed] [Google Scholar]
- Henderson-Redmond A, Czachowski C (2014). Effects of systemic opioid receptor ligands on ethanol- and sucrose seeking and drinking in alcohol-preferring (P) and Long Evans rats. Psychopharmacology (Berl) 231: 4309–4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz A (1997). Endogenous opioid systems and alcohol addiction. Psychopharmacology (Berl) 129: 99–111. [DOI] [PubMed] [Google Scholar]
- Ignar DM, Goetz AS, Noble KN, Carballo LH, Stroup AE, Fisher JC et al (2011). Regulation of ingestive behaviors in the rat by GSK1521498, a novel micro-opioid receptor-selective inverse agonist. J Pharmacol Exp Ther 339: 24–34. [DOI] [PubMed] [Google Scholar]
- Kaminski BJ, Goodwin AK, Wand G, Weerts EM (2008). Dissociation of alcohol-seeking and consumption under a chained schedule of oral alcohol reinforcement in baboons. Alcohol Clin Exp Res 32: 1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly E, Mundell SJ, Sava A, Roth AL, Felici A, Maltby K et al (2014). The opioid receptor pharmacology of GSK1521498 compared to other ligands with differential effects on compulsive reward-related behaviours. Psychopharmacology (Berl) 232: 305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2013). Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci 13: 3–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Wand GS, Li X-W, Portoghese PS, Froehlich JC (1998). Effect of mu opioid receptor blockade on alcohol intake in rats bred for high alcohol drinking. Pharmacol Biochem Behav 59: 627–635. [DOI] [PubMed] [Google Scholar]
- Lee JLC, Milton AL, Everitt BJ (2006). Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci 26: 5881–5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig AM (1986). Pavlov's “bells” and alcohol craving. Addict Behav 11: 87–91. [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Li X, Shaham Y (2013). Recent developments in animal models of drug relapse. Curr Opin Neurobiol 23: 675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Rodd ZA, Bell RL, Lumeng L, Li T-K (2014). The alcohol-preferring (P) and high-alcohol-drinking (HAD) rats—animal models of alcoholism. Alcohol 48: 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton AL, Schramm MJW, Wawrzynski JR, Gore F, Oikonomou-Mpegeti F, Wang NQ et al (2012). Antagonism at NMDA receptors, but not β-adrenergic receptors, disrupts the reconsolidation of pavlovian conditioned approach and instrumental transfer for ethanol-associated conditioned stimuli. Psychopharmacology (Berl) 219: 751–761. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Hutchison KE, Swift RM, Mueller TI, Colby SM et al (1999). Naltrexone's effect on cue-elicited craving among alcoholics in treatment. Alcohol Clin Exp Res 23: 1386–1394. [PubMed] [Google Scholar]
- Nathan PJ, O'Neill BV, Bush MA, Koch A, Tao WX, Maltby K et al (2012). Opioid receptor modulation of hedonic taste preference and food intake: a single-dose safety, pharmacokinetic, and pharmacodynamic investigation with GSK1521498, a novel μ-opioid receptor inverse agonist. J Clin Pharmacol 52: 464–474. [DOI] [PubMed] [Google Scholar]
- National Council on Alcoholism and Drug Dependence. Welcome to NCADD, at <http://ncadd.org/index.php>.
- Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB (1988). Relevance of cue reactivity to understanding alcohol and smoking relapse. J Abnorm Psychol 97: 133–152. [DOI] [PubMed] [Google Scholar]
- Nowak KL, Ingraham CM, Mckinzie DL, Mcbride WJ, Lumeng L, Li TK et al (2000). An assessment of novelty-seeking behavior in alcohol-preferring and nonpreferring rats. Pharmacol Biochem Behav 66: 113–121. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Yasar S, Nemeth-Coslett R, Katz JL, Henningfield JE, Solinas M et al (2004). Human cocaine-seeking behavior and its control by drug-associated stimuli in the laboratory. Neuropsychopharmacology 30: 433–443. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Sable HJK, Murphy JM, McBride WJ (2004). Recent advances in animal models of alcohol craving and relapse. Pharmacol Biochem Behav 79: 439–450. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Hutchison KE, Swift RM, Colby SM, Kaplan GB (2000). Naltrexone's effects on reactivity to alcohol cues among alcoholic men. J Abnorm Psychol 109: 738–742. [PubMed] [Google Scholar]
- Rösner S, Hackl-Herrwerth A, Leucht S, Vecchi S, Srisurapanont M, Soyka M (2010). Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev (12): CD001867. [DOI] [PubMed] [Google Scholar]
- Samson HH (1986). Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res 10: 436–442. [DOI] [PubMed] [Google Scholar]
- Samson HH, Chappell A, Czachowski C, Sharpe A (2001). Measuring ethanol-seeking behavior: the effect of using repeated extinction trials. Alcohol 24: 205–209. [DOI] [PubMed] [Google Scholar]
- Samson HH, Sharpe AL, Denning C (1999). Initiation of ethanol self-administration in the rat using sucrose substitution in a sipper-tube procedure. Psychopharmacology (Berl) 147: 274–279. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Katz JL, Goldberg SR (1988). The use of second-order schedules to study the influence of environmental stimuli on drug-seeking behavior. NIDA Res Monogr 84: 180–195. [PubMed] [Google Scholar]
- Schneekloth TD, Biernacka JM, Hall-Flavin DK, Karpyak VM, Frye MA, Loukianova LL et al (2012). Alcohol craving as a predictor of relapse. Am J Addict Am Acad Psychiatr Alcohol Addict 21: S20–S26. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R et al (2008). Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res 32: 1816–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SG, Werner TE, Davis WM (1977). Alcohol-associated conditioned reinforcement. Psychopharmacology (Berl) 53: 223–226. [DOI] [PubMed] [Google Scholar]
- Stewart RB, Gatto GJ, Lumeng L, Li TK, Murphy JM (1993). Comparison of alcohol-preferring (P) and nonpreferring (NP) rats on tests of anxiety and for the anxiolytic effects of ethanol. Alcohol 10: 1–10. [DOI] [PubMed] [Google Scholar]
- Tomie A, Sharma N (2014). Pavlovian sign-tracking model of alcohol abuse. Curr Drug Abuse Rev 6: 201–219. [DOI] [PubMed] [Google Scholar]
- Viglinskaya IV, Overstreet DH, Kashevskaya OP, Badishtov BA, Kampov-Polevoy AB, Seredenin SB et al (1995). To drink or not to drink: tests of anxiety and immobility in alcohol-preferring and alcohol-nonpreferring rat strains. Physiol Behav 57: 937–941. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O'Brien CP (1992). Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry 49: 876–880. [DOI] [PubMed] [Google Scholar]
- Wise RA (1973). Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia 29: 203–210. [DOI] [PubMed] [Google Scholar]
- Ziauddeen H, Chamberlain SR, Nathan PJ, Koch A, Maltby K, Bush M et al (2013. a). Effects of the mu-opioid receptor antagonist GSK1521498 on hedonic and consummatory eating behaviour: a proof of mechanism study in binge-eating obese subjects. Mol Psychiatry 18: 1287–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziauddeen H, Nathan PJ, Dodds C, Maltby K, Miller SR, Waterworth D et al (2013. b). The effects of alcohol on the pharmacokinetics and pharmacodynamics of the selective mu-opioid receptor antagonist GSK1521498 in healthy subjects. J Clin Pharmacol 53: 1078–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.